Abstract
NETosis (NET generation), a programmed death pathway initiated in mature neutrophils by pathogens and inflammatory mediators, can be a protective process that sequesters microbes and prevents spread of infection, but can also be a pathological process that causes inflammation and serious tissue injury. Little is known about the regulatory mechanism. Previously we demonstrated that serpinb1-deficient mice are highly susceptible to pulmonary bacterial and viral infections due to inflammation and tissue injury associated with increased neutrophilic death. Here we used in vitro and in vivo approaches to investigate whether SerpinB1 regulates NETosis. We found that serpinb1-deficient bone marrow and lung neutrophils are hyper-susceptible to NETosis induced by multiple mediators in both NADPH-dependent and independent manner, indicating a deeply rooted regulatory role in NETosis. This role is further supported by increased nuclear expansion (representing chromatin decondensation) of PMA-treated serpinb-1-deficient neutrophils compared to wild-type, by migration of SerpinB1 from the cytoplasm to the nucleus of human neutrophils coincident with, or before, early conversion of lobulated (segmented) nuclei to delobulated (spherical) morphology, and by finding that exogenous rSerpinB1 abrogates NET production. NETosis of serpinb1-deficient neutrophils is also increased in vivo during Pseudomonas aeruginosa lung infection. The findings identify a previously unrecognized regulatory mechanism involving SerpinB1 that restricts the production of NETs.
Keywords: NETosis, serpins, neutrophil nucleus, inflammation
SerpinB1 (previously called MNEI) is an ancient protein abundant in neutrophil cytoplasm. Its best known function is inhibition of the neutrophil serine proteases (NSPs): elastase, cathepsin G (cat-G) and proteinase-3 (PR3), major agents of human inflammatory injury (1, 2). Mice with germline ablation of serpinb1 are viable and are grossly normal. On infection with bacterial and viral pathogens, their immune responses are initially normal, but transition over time to tissue injury, protracted inflammation and early and excess mortality (2, 3). While studying the highly pro-inflammatory IL-17-skewed lung pathology of influenza virus infected serpinb1−/− mice, we noted excess death of infiltrating leukocytes (3). The magnitude of this pathology raised the question of whether serpinb1−/− neutrophils, when present in an inflammatory environment, preferentially die by a mechanism other that apoptosis or spontaneous necrosis. This led us to examine here the possible link between SerpinB1 and NETosis.
NETosis is a programmed death pathway involving complex intracellular signaling induced in terminally-differentiated neutrophils on activation by pathogens and inflammatory mediators and in vitro by phorbol myristate acetate (PMA) (4). Late events of NETosis include chromatin decondensation, nuclear expansion and extrusion of linearized DNA decorated with elastase, cat-G and PR-3 (the NSPs) and other anti-microbial proteins including histones and myeloperoxidase (MPO). NETs can ensnare and kill pathogens, and at the time of the discovery, NETosis was considered protective, a final act by dying neutrophils to prevent or delay systemic infection. However, mounting evidence shows that NETs, or more specifically excess NETs, can inflict serious local inflammatory damage including endothelial cell death as seen in a wide disease range including cystic fibrosis (CF), thrombosis, and most recently fatal murine influenza (5–7). Excess NETs also exacerbate the pathology of autoinflammatory and autoimmune diseases: rheumatoid arthritis, systemic lupus erythematosus and small-vessel vasculitis (8). Because NETs consist of DNA and RNA decorated with self-proteins, they can be targeted by self-antibodies, endocytosed as immune complexes by plasmacytoid dendritic cells (pDCs) to signal via TLR-7/9 and induce type-I IFN responses that propagate autoimmunity (9, 10).
The best-characterized NETosis model is activation of isolated neutrophils by PMA. This is an active process mechanistically distinct from apoptosis and necrosis; it has been found to require reactive oxygen species (ROS) generated by NADPH oxidase, the protease elastase but not caspases, and includes decondensation of nuclear chromatin (11–13). NET generation also requires peptidyl-arginine deiminase-4 (PAD-4), a nuclear enzyme that deiminates histone tail arginine residues, i.e., converts positively charged arginines to uncharged citrullines and thereby mediates chromatin decondensation (14–16). Currently, there is only minimal understanding of how NETosis is regulated.
To examine a role for SerpinB1 in NETosis, we studied lung and bone marrow neutrophils of serpinb1−/− and wild-type (WT) mice stimulated in vitro with PMA and physiological mediators. We evaluated NET production in vivo in infection and we examined the effect on NETosis of exogenous rSerpinB1. The findings, some of which were reported in an abstract1, identify SerpinB1 as a non-redundant regulator of NETosis. The findings indicate that SerpinB1, which migrates to the nucleus early in NETosis, functions downstream of NADPH oxidase and ROS to regulate NETosis by restricting chromatin decondensation.
Materials and Methods
Reagents
Human recombinant SerpinB1 (rSerpinB1) was expressed in insect cells, purified and stored in aliquots (2 mg/ml) in PBS at −80°C (17).
Mice
Serpinb1-deficient mice serpinb1−/−) generated in129S6/SvEv/Tac (129S6) background were studied or, where indicated, serpinb1−/− mice backcrossed to C57BL/6 for 10 generations (2). Wild-type (WT) 129S6 and C57Bl/6 mice from Taconic Labs or Jackson Labs, respectively, were maintained with serpinb1−/− mice in the animal facility of the Immune Disease Institute or Boston Children’s Hospital for at least several weeks or were bred in house. Animal studies were approved by the Institutional Animal Care and Use Committee of the Immune Disease Institute and/or Children’s Hospital Boston.
Neutrophil isolation
Lung neutrophils for in vitro study of NET production were recruited by intranasal delivery of 30μg LPS (P. aeruginosa) Sigma-Aldrich, Saint Louis, MO). The mice were sacrificed 24h later and the lungs lavaged with PBS. The cells, which were pelleted, suspended in RPMI, counted by use of the Hemavet 950FS (Drew Scientific, Waterbury, CT), were >90% neutrophils by flow cytometry. Following an established protocol for isolating morphologically mature bone marrow neutrophils, cells were flushed from femurs and fractionated by Percoll gradient (78, 69, 52%) centrifugation (18). Washed interface cells were 75–80% neutrophils.
Neutrophils were purified from whole blood of healthy donors obtained with informed written consent and approval of the Institutional Review Board of the Immune Disease Institute. The blood was collected in EDTA-coated vacutainers (BD Biosciences, San Diego, CA) and purified (>90% neutrophils) as described (4).
Production of NETs
Murine lung neutrophils (1–2.2 × 105 in 200µl RPMI) were allowed to adhere to 96-well culture plates coated with 0.001% poly-L-lysine for 1h at 37°C. PMA (100nM) was added and cells were cultured for 12h (19). Bone marrow neutrophils (2.5×105 in 200µl) in RPMI with 10mM HEPES and 50µM mercapthanol were allowed to adhere to 8-well (uncoated) glass chamber slides (Thermo Fisher Scientific, Waltham, MA) for 1h at 37°C. Activating agents were added: PMA (100nM) for 4 h; platelet activating factor (PAF) (10μM) (Sigma-Aldrich) for 30 min; macrophage inflammatory protein-2/cxcl2 (MIP-2) (R&D systems, Minneapolis, MN)(10nM) for 45 min, hydrogen peroxide (100μM) for 4h or LPS (P. aeruginosa) (10μg/ml) for 4h. Human neutrophils (5 × 104 in 200µl) in RPMI with 10 mM HEPES, 50µM mercapthanol and 2% fetal calf serum (heated at 70°C for 30 min to inactivate DNase) (20) were allowed to adhere to 8-well glass chamber slides for 1h at 37°C and were stimulated with 50nM PMA for 4h or as indicated. When neutrophils were treated with diphenylene iodonium (DPI) (10μM), carbobenzoxy-Val-Ala-Asp-fluoromethylketone (Z-VAD-fmk) (50μM), aminoethylbenzene sulfonylfluoride (AEBSF)(2 mM), phenylmethyl sulfonylfluoride (PMSF) (2mM) or diisopropyl fluorophosplate (DFP) (2 mM), or rSerpinB1 (10 μg/ml), the agents were added at the start of the 1h preincubation/adherence step unless otherwise indicated.
Fluorescence microscopy
To quantify NETs, the cells at termination of NET induction were washed once with warm PBS, and Sytox orange or Sytox green (0.1μM) was added. Where indicated, the cells were stained prior to Sytox addition with Alexa-Fluor-488 labeled Gr-1 antibody for 30 min at ambient temperature. The unfixed NETs were imaged with a fluorescence microscope, Zeiss Axiovert (Carl Zeiss Inc., Thornwood, NY) or Nikon Eclipse TS100 (Nikon Instruments, Inc., Melville NY), and non-overlapping random images were obtained with a 20x objective. NETs were manually identified on digitized images as Sytox-positive structures emanating from cells with overall length greater than 2x cell diameter and were counted for at least 3 fields of view (FOV) per variable using IMAGE-J software (NIH, Bethesda, MD). Sytox-positive cells were similarly counted. Total cells were quantified by analysis of bright field images or Gr-1 stained images using IMAGE-J software. Results are expressed as percent of NETs per total number of cells. Based on independent blinded enumeration of WT and serpinb1−/− NETs and Sytox-positive cells by a second evaluator, the intraassay coefficient of variability was <10%.
For antibody staining, cytospins and slides of neutrophils and NETs were fixed with paraformaldehyde (4% for cell staining, 2% for NETs staining) and were treated with 0.5% Triton-X for 10 min (this step was omitted for staining of NETs), blocked with 2% normal goat serum in PBS-0.1% Tween-20 for 1h, stained with 1μg/ml of primary rabbit antibodies to human neutrophil elastase (Calbiochem, Rockland, MA; Abcam, Cambridge, MA), or human SerpinB1 (in house) or guinea pig antibodies to LBR amino acids 1-228 (provided by Monika Zwerger, German Cancer Research Center, Heidelberg) for 1h, washed and incubated for 1h with 2μg/ml secondary antibodies (Invitrogen). For MPO, cells were stained with FITC-labeled anti-mouse MPO (Hycult Biotech, Plymouth Meeting, PA). The slides were washed and mounted with DAPI-labeled mounting medium (Vector Laboratories, Burlingame, CA) and evaluated by fluorescent microscopy on the Zeiss Axiovert microscope with Zeiss Axiovision LE software or an Olympus Fluoview 1000 confocal microscope (Olympus America Inc., Center Valley, PA) with Olympus software.
DNA fluorimetry assays for NETs
NETs were induced in lung neutrophils in 96-well black plates (Thermo Fisher Scientific, Waltham, MA. Sytox green (5 μM) (Invitrogen) was added, and the fluorescence was detected (485nm excitation, 535nm emission) by Fluoroskan Ascent microplate fluorimeter (Thermo Labsystems, Helsinki, Finland. Alternatively, NETs induced in chamber slides were quantified as released DNA by adding micrococcal nuclease (Worthington Biochemical Co., Lakewood NJ) to 1 U/ml, incubating at 37°C for 10 min, stopping the reaction with EDTA and clarifying the supernatant (15,000 rpm for 5 min at 4°C) prior to Sytox addition.
Pseudomonas aeruginosa lung infection and LPS model
Groups of weight-matched female WT and serpinb1−/− mice were sedated with 100 mg/kg ketamine and 10 mg/kg xylazine and intranasally inoculated with 3 × 106 CFU of P. aeruginosa PAO1 as described (2). Mice were sacrificed 24h later, and the lungs were lavaged once with 1 ml cold sterile PBS. Aliquots of the fluid were centrifuged to produce cell-free bronchoalveolar lavage fluid (BALF), which was stored at −80°C for DNA and elastase assays or held briefly on ice for MPO assay. To quantify NETs produced in vivo, NETs and cells in non-centrifuged lavage fluid were allowed to adhere to 8-well glass chamber slides (200µl/well) at 37°C for 3h in the presence of DFP (10 mM) to inhibit ex vivo NETosis. The slides were stained with Sytox and evaluated for content of NETs as described above. Aliquots of the fluid were also incubated without additive to allow additional NET generation ex vivo. The number of NETs produced ex vivo was calculated as the difference between total NETs without DFP and NETs in DFP-treated incubations.
Reactive oxygen species
Neutrophils (2 × 105 in 200μl) were allowed to adhere in 24-well plates at 37°C for 1h. Dihydrorhodamine-123 (DHR; Sigma-Aldrich) (1 µM) was added for 5 min at 37°C. Supernatant was removed, and the cells were washed once. PMA (100nM), MIP-2 (10nM) or PAF (10µM) was added in 1 ml medium, and the cells were incubated for 15 min at 37°C. After one wash, the cells were removed from the plate by cell scrapers and resuspended in PBS with 1% fetal calf serum for detection of ROS-induced rhodamine-123 on a FACS-Calibur (BD Biosciences). Neutrophils were identified on the basis of forward and side scatter properties.
Myeloperoxidase (MPO) and Elastase
MPO activity was measured by spectrophotometric assay in 50 mM potassium phosphate, pH 6 with 0.17 mg/ml o-dianisidine and 0.0005% hydrogen peroxide using human leukocyte MPO (Sigma-Aldrich) as standard. Elastase was measured by hydrolysis of N-methoxysuccinyl-Ala-Ala-Pro-Ala-p-nitroanilide (2 mM) (Sigma-Aldrich) in 20 mM Tris-HCl, pH 7.4, 500 mM NaCl, 0.05% Tween, 4 mM dithiothreitol as change of OD450 nm over time at 22°C with human neutrophil elastase (Elastin Products, Owensville, MO) as standard.
Statistical analysis
Means ± SEM are presented except for bacterial CFU, for which log transformed individual data points are shown and medians indicated. For pairwise comparison, differences between means (or medians) were analyzed by Student’s t-test; differences among multiple means were analyzed by one-way ANOVA followed by Tukey’s post-hoc test. Analyses were performed using Prism software (GraphPad, La Jolla, CA). P<0.05 was considered statistically significant.
Results
Increased NET generation by serpinb1−/− neutrophils
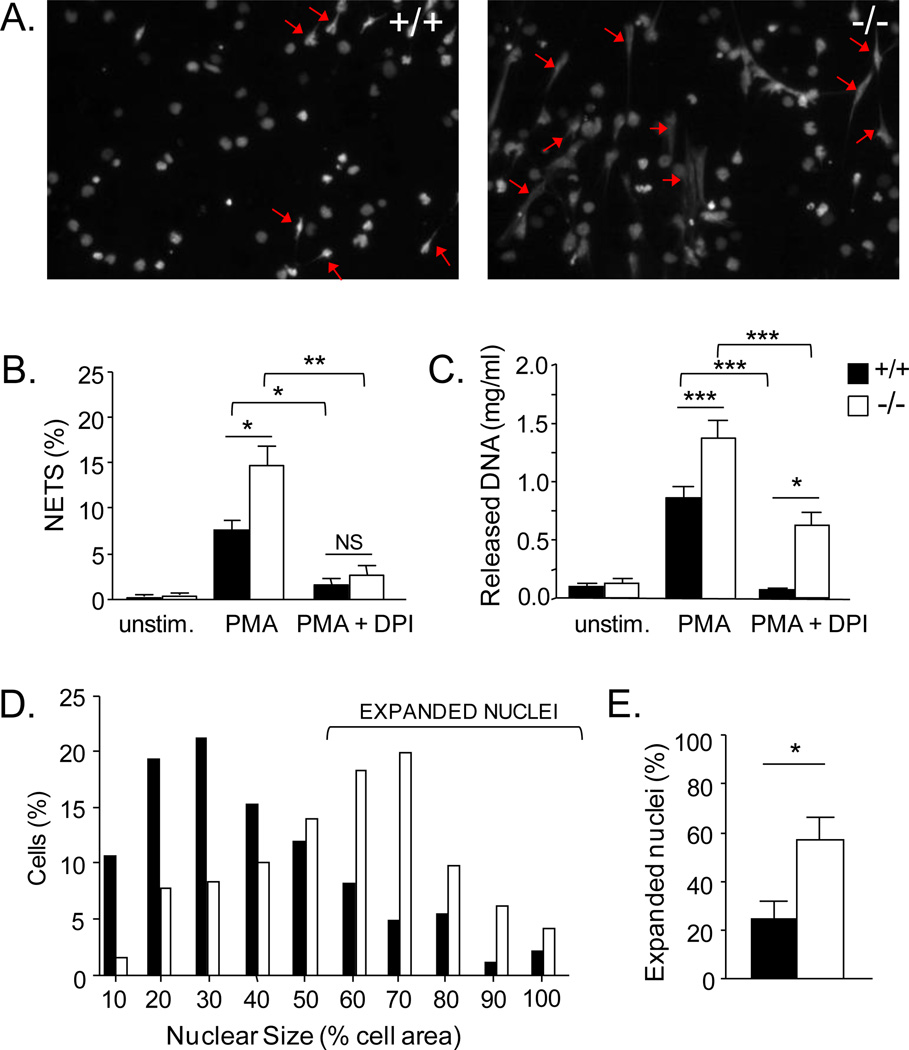
On culture of lung neutrophils with PMA, NETs were generated that could be detected in fluorescence micrographs as DNA-based structures emanating from cells (Fig. 1A). On counting, we found increased numbers of NETs in serpinb1−/− micrographs compared with WT (Fig. 1B). A similar increase of serpinb1−/− NETs compared with WT was found when quantitation was based on fluorimetry of Sytox-stained DNA (Fig. 1C) or by treatment with micrococcal nuclease to release DNA (Supplemental Fig. 1). The increase of serpinb1−/− NETs was not associated with an increase of adherent cells remaining at the end of PMA culture (WT:173 ± 23; KO:146 ± 23 cells/field of view (FOV), n=6) or an increase of total dead cells (plasma membrane-permeable cells) (WT:45 ± 6%; KO:57 ± 4%). This eliminates genotype differences of adherence and overall cell death as explanations for the extra increment of serpinb1−/− NETs. PMA-induced NET production by serpinb1−/− and WT neutrophils was abrogated by the NADPH oxidase inhibitor DPI, consistent with findings for human NETs (11). Other known features of NETosis that were not qualitativley different between serpinb1−/− and WT include staining of the NETs for elastase and MPO, sensitivity of the NETs to DNase, and insensitivity of the NETosis process to the pan-caspase inhibitor Z-VAD-fmk (Supplemental Fig. 1).
FIGURE 1.
Increased production of NETs and expansion of nuclei of serpinb1−/− lung neutrophils. A-C, NET production. WT (+/+) and serpinb1-, deficient (−/−) lung neutrophils were cultured in poly-L-lysine coated tissue culture wells with no additive, PMA, or PMA plus DPI. Staining with Sytox green, a plasma membrane impermeable DNA dye, revealed NETs as Sytox-positive structures emanating from cells with length greater than 2x cell diameter. A, Representative fluorescence images of PMA-treated neutrophils; examples of NETs are indicated by arrows. B, NETs enumerated in micrographs. C, NETs quantified by DNA fluorimetry. D,E, Nuclear size. D, Distribution of PMA-treated Sytox-stained WT and serpinb1−/− neutrophils on the basis of nuclear area (as percent of cell area). E, Frequency of neutrophils with expanded nuclei (>50% of cell area). Means ± SEM for 3-4 experiments (each 2-3 mice/group); *, P<0.05; **, P<0.001, ***, P<0.0001.
Because NET generation requires chromatin decondensation (15), we tested whether nuclear expansion was altered. Although the range was broad, the distribution of nuclear size of PMA-treated serpinb1−/− lung neutrophils was highly skewed toward larger values compared to WT neutrophils (Fig. 1D). By using ‘50% of cell area’ to define an expanded nucleus (Fig. 1D), we found that the frequency of serpinb1−/− neutrophils with expanded nuclei was double that of WT neutrophils (Fig. 1E).
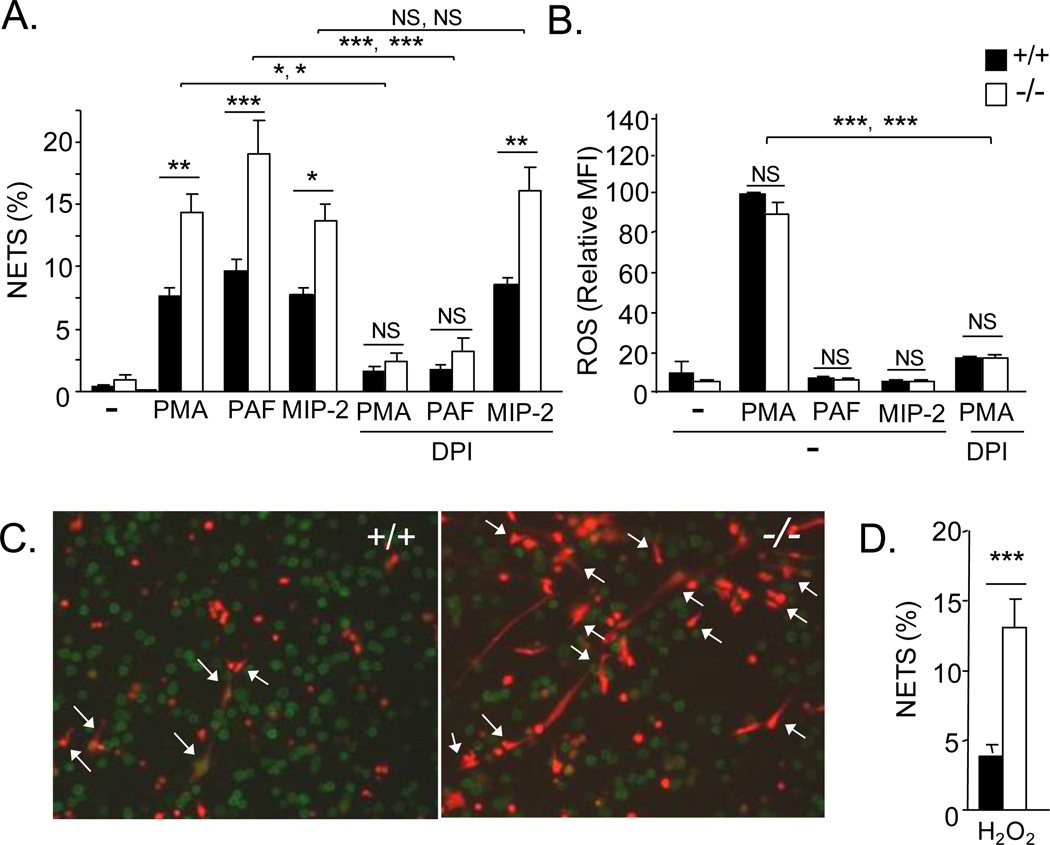
To study lung neutrophils (as in the above experiments), mice must be instilled with chemokines or LPS, which initiates the process of recruitment (extravasation) that substantially alters neutrophil properties (21, 22). To test whether the effects of serpinb1 on NETosis are limited to neutrophils that have undergone this process, we examined bone marrow neutrophils of both genotypes. Indeed, increased NETs were induced in serpinb1−/− bone marrow neutrophils compared with WT in response to PMA and also in response to the phospholipid mediator platelet activating factor (PAF), the chemokine macrophage inflammatory protein-2 (MIP-2/cxcl2) (Fig. 2A, left) and LPS (Supplementary Fig.2), indicating that the enhancing effect of serpinb1 deletion extends to bone marrow neutrophils and to multiple NETosis inducing mediators. Treatment with DPI to inhibit NADPH oxidase, the major neutrophil ROS generating system, blocked PMA-induced NET production by bone marrow neutrophils of both genotypes as anticipated and also blocked PAF-induced NET production; however, MIP-2 induced NET production was not inhibited by DPI (Fig. 2A, right). The findings indicate that (i) the MIP-2 induced NETosis pathway differs from the PMA and PAF pathways and (ii) at least for MIP-2, the additional increment of NETs produced by serpinb1−/− neutrophils is not due to increased ROS. Consistent with there being different pathways for different inducers, the time for optimal NET production also differed: 4h for PMA, 30 min for PAF and 45 min for MIP-2.
FIGURE 2.
Production of NETs and ROS by WT and serpinb1−/− bone marrow neutrophils. A, Neutrophils were cultured in uncoated glass chamber slides with the indicated agents, and the resulting NETs were quantified in fluorescence images after Sytox staining. B, ROS. Neutrophils pre-labeled with dihydrorhodamine were stimulated for 15 min, and accumulated ROS was measured as MFI. C,D, NETs produced by neutrophils treated with H2O2. C, Representative WT and serpinb1−/− neutrophils stained with Gr-1 antibody (green) and Sytox orange. D, Quantitation of NETs. (A,B,D) Means ± SEM for 2 experiments (each 3 mice/genotype). *, P<0.05; **, P<0.001, ***, P<0.0001; two symbols show the results for the WT with WT comparison followed by the KO with KO comparison. (D)
To further evaluate the role of ROS, we measured oxidation of the intracellular dye dihydrorhodamine. PMA caused DPI-sensitive production of ROS by serpinb1−/− and WT neutrophils as anticipated, but there was no difference between the genotypes (Fig. 2B), indicating that the NETosis enhancing effect of serpinb1 deletion in the PMA pathway is not due to increased ROS. Neither PAF nor MIP-2 induced ROS as measured by dihydrorhodamine (Fig. 2B) and by luminol-based chemiluminescence (data not shown). This finding for PAF is consistent with reports that PAF primes neutrophils, but does not activate NADPH oxidase (23). The DPI-sensitivity of PAF-induced NETosis in the absence of NADPH oxidase activation suggests involvement of a different DPI-sensitive enzyme that still needs to be determined; DPI is known to inhibit a range of flavoenzymes including mitochondrial oxidase and nitric oxide synthase (24, 25). NET production was also increased for serpinb1−/− neutrophils compared with WT on treatment with H2O2, a direct source of ROS. (Fig. 2 C,D), and again there was no genotype-difference of adherent neutrophils (WT: 517 ± 27; KO: 457 ± 27 cells/field of view (FOV), n=6; P=0.16) or total neutrophil death (WT: 31.0 ± 3.2%; KO: 39.5 ± 3.2%, P=0.13). Thus, although ROS is required to produce NETs when the activating agent is PMA, the results eliminate ROS as the cause of the increased NET production of serpinb1−/− neutrophils. The findings also indicate that PMA, PAF and MIP-2 activate mechanistically distinguishable pathways of NET production, each subject to regulation by serpinb1. The relatively rapid ROS-independent PAF- and MIP-2 induced NETosis pathways may be mechanistically related to the rapid ROS-independent, vesicular mechanism of NET release induced by Staphylococcus aureus (26), but this was not investigated.
SerpinB1 migrates to the nucleus during NETosis
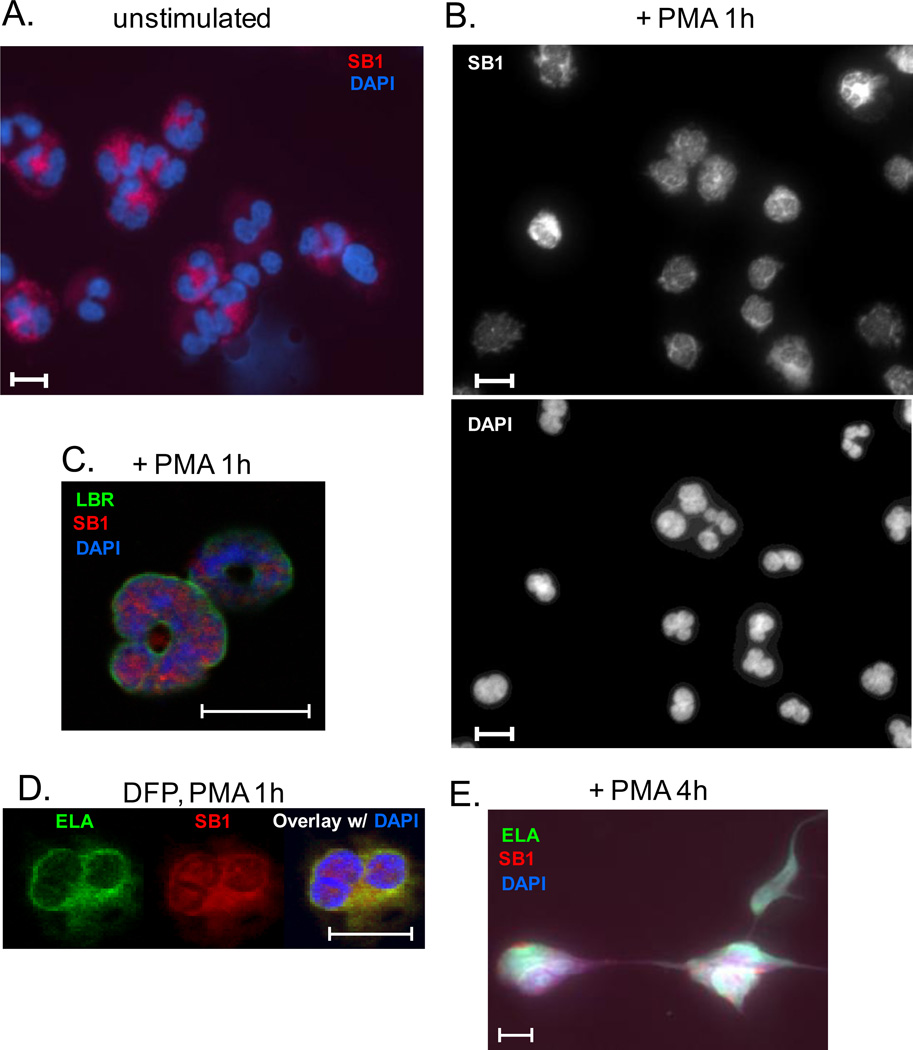
During granulopoiesis, post-mitotic progenitor neutrophils progressively acquire the abundance of heterochromatin and segmented (lobular) nuclear shape that characterize the mature cells (27). Here we used fluorescence imaging to examine terminally differentiated human neutrophils during NETosis. In the resting state, SerpinB1 was exclusively in the cytoplasm (Fig. 3A) consistent with current understanding of clade B serpins. Clade B serpins, an ancient group also known as ovalbumin serpins, are found in the nucleus as well as the cytoplasm (nucleocytoplasmic location) despite their lack of classical nuclear localization signals, and a few including SerpinB1 are also found extracellularly (28–30). A study of human neutrophils undergoing PMA-induced NETosis characterized step-wise changes of the cells. The first event, observed in 1/3 of neutrophils after 15 min, was the transformation from lobulated to delobulated nuclei, a change accompanied by minimal alteration of nuclear area (31). On treating human neutrophils with PMA, we found a mixture of neutrophils with fully delobulated and partially delobulated nuclei at 1 h at which time nuclear expansion was minimal. Importantly, SerpinB1 had entered the nucleus of almost all neutrophils (Fig. 3B). Localization within the nucleus was verified in confocal micrographs using lamin B receptor (LBR) as nuclear envelope marker (Fig. 3C). Migration of SerpinB1 into the nucleus coincident with or prior to the first detectable morphological change indicates that SerpinB1 may regulate events in the nucleus.
FIGURE 3.
Translocation of SerpinB1 to the nucleus during NETosis of human neutrophils. Resting neutrophils (A) and neutrophils treated with PMA for 1h (B,C) or 4h (E) or with PMA for 1h after DFP pretreatment (D) were stained as indicated for SerpinB1 (SB1), elastase (ELA), lamin B receptor (LBR), or DAPI. Cells were examined by fluorescence microscopy, original magnification 40X (A,B) or confocal microscopy, original magnification 63X (C-E). Bars indicate 10 microns.
Previous studies had shown that elastase also enters the nucleus during PMA-induced NETosis (12). We thus questioned whether SerpinB1 translocation requires formation of a complex with elastase, as occurs in protease inhibition (1). The same study had shown that small molecule inhibitors of elastase abrogate PMA-induced elastase migration to the nucleus and NET production (12). Consistent with this report, we found that PMA-induced NET production was abrogated when neutrophils were treated with potent inhibitors of elastase: DFP, PMSF and AEBSF (data not shown). On evaluation of DFP-pretreated neutrophils after 1h with PMA, elastase remained confined to the cytoplasmic region as predicted, but SerpinB1 entered the nucleus of almost all cells (Fig. 3D), indicating that complex formation with elastase is not required for SerpinB1 translocation. Finally, after 4h with PMA, SerpinB1 was localized to NETs along with DNA and elastase (Fig. 3E).
Increased NET production in vivo in serpinb1−/− mice
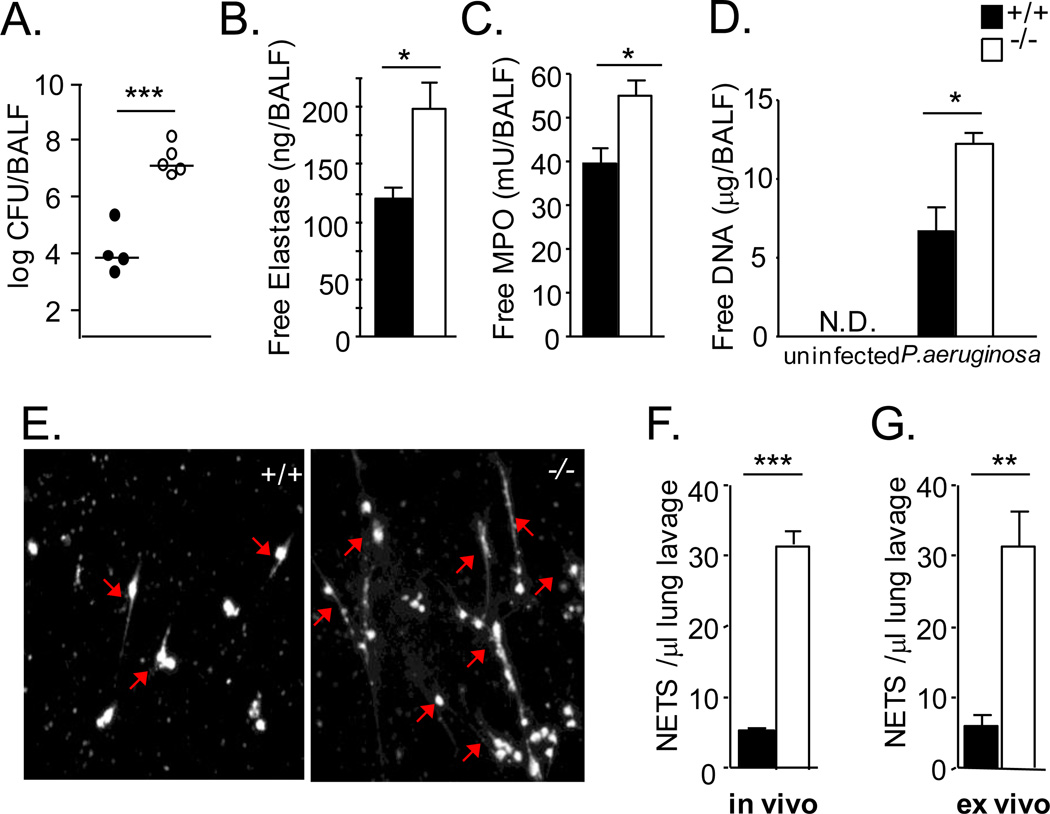
To determine whether serpinb1−/− NET production is increased in vivo, we used the previously characterized Pseudomonas aeruginosa lung infection model. Findings generated with this model strongly indicate that SerpinB1 protects pulmonary anti-bacterial capacity and prevents lung inflammation and injury (2). On intranasal infection with P. aeruginosa, the immune response of serpinb1−/− mice was initially normal, i.e., normal levels of cytokines/chemokines were generated, normal numbers of neutrophils recruited, and bacteria restricted normally at 6 hr, but by 24 hr when WT mice had largely cleared the infection and restored cytokines toward baseline, serpinb1−/− mice had logs higher bacterial numbers, increased levels of inflammatory cytokines, proteolytic injury and unexplained excess neutrophil death (2). To replicate the model, groups of mice were inoculated with P. aeruginosa strain PAO1 and were sacrificed 24h later. As anticipated, WT mice largely cleared the infection, but bacterial numbers were increased in serpinb1−/− mice (Fig. 4A). The specific neutrophil proteins MPO and elastase, which were measured in cell-free bronchoalveolar lavage fluid (BALF), were increased as anticipated in serpinb1−/− mice compared with WT mice (Fig. 4B,C). The increase of free elastase and MPO indicates increased death of serpinb1−/− neutrophils. Free DNA, which is frequently used as a measure of NETosis, was assayed in cell-free lavage fluid and was increased in serpinb1−/− mice compared with WT and not detected in the absence of infection (Fig. 4D). These findings are consistent with increased NETosis in infected serpinb1−/− mice. However, there are other possible sources of free DNA in lungs of infected mice. Therefore, to directly quantify in vivo generated NETs, we incubated non-centrifuged lavage fluid in chamber slides to allow NETs to adhere and be subsequently quantified by Sytox staining and microscopy. DFP was present during incubation to block ex vivo NET generation The number of NETs in DFP-treated lavage fluid, i.e., in vivo generated NETs, was increased for infected serpinb1−/− mice compared with WT mice (Fig. 4E,F). The increase did not reflect a corresponding increase of neutrophils. Rather, the number of cells (primarily neutrophils) recovered from infected serpinb1−/− mice was decreased, although not significantly, compared with WT mice (WT: 3.2 ± 0.9 × 106 cells/mouse; KO: 2.0 ± 0.5 × 106 cells/mouse), consistent with previous reports (2). We also incubated (non-centrifuged) lavage fluid without DFP and without further additive and found that the neutrophils continued to generate additional NETs. This ex vivo NET production by neutrophils in lung fluid of infected mice was also increased for serpinb1−/− mice compared with WT (Fig. 4D). These findings demonstrate that NET production occurs in vivo in the setting of infection and that both in vivo NETosis and ex vivo NETosis are substantially increased for infected serpinb1−/− mice.
FIGURE 4.
Increased in vivo NET production in Pseudomonas aeruginosa-infected serpinb1−/− mice. WT (solid symbols) and serpinb1-deficient (open symbols) mice were intranasally inoculated with P. aeruginosa (3 × 106 CFU/mouse) and were sacrificed 24h later. (A) Lung bacteriology. CFU/mouse. Symbols represent individual mice; horizontal lines indicate the medians. (B) Elastase and (C) MPO per mouse in cell-free bronchoalveolar lavage fluid (BALF). (D) Free DNA per mouse in cell-free lavage fluid. (E, F) In vivo generated NETs in non-clarified lavage fluid detected in the presence of DFP. (E) Representative Sytox-stained images; examples of NETs are indicated by arrows. In vivo generated NETs were not all cell-associated. (F) Quantitation. (G) Additional NETs generated ex vivo (in the absence of DFP) by neutrophils in equal volumes of lavage fluid. Means ± SEM for representative experiment out of 2 (4–5 mice/group per experiment) *, P<0.05; **, P<0.001, ***, P<0.0001. ND, not detected (<0.002 μg DNA/ml BALF/mouse)
Abrogation of NET generation by exogenous rSerpinB1
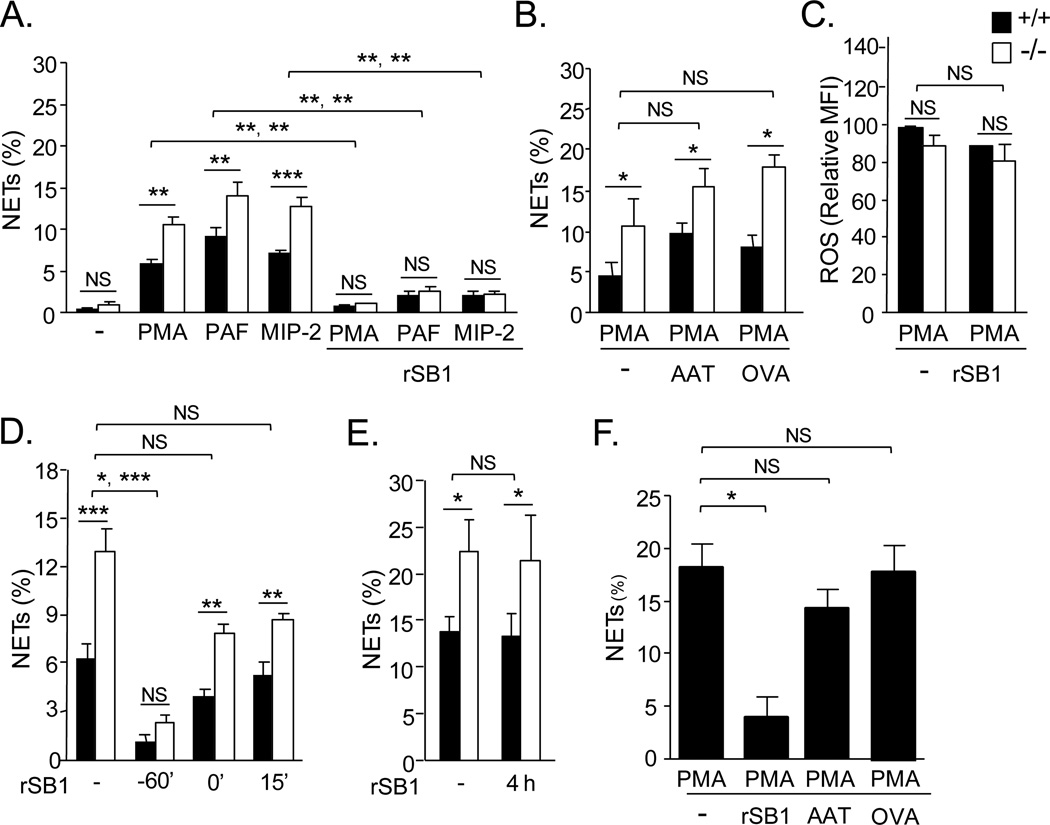
To further probe the role of SerpinB1 in NET generation, we tested whether exogenous SerpinB1 affects the process. Inclusion of recombinant human SerpinB1 (rSerpinB1) was found to abrogate or substantially decrease NET production in response to PMA and also PAF and MIP-2 (Fig. 5A) and LPS (supplemental Fig. 2). Abrogation of NETosis was found for both WT and serpinb1−/− neutrophils (Fig. 5A), and inclusion of rSerpinB1 did not alter neutrophil recovery or total neutrophil death (not shown). To assess the specificity of rSerpinB1 as an anti-NETosis agent, we tested closely related proteins: α1-antitrypsin (AAT) (SerpinA1), which largely replicates the protease inhibitory specificity of SerpinB1 (1), and ovalbumin (serpinB14), a non-protease inhibitor serpin of the same clade (29). Neither α1-antitrypsin nor ovalbumin (Fig. 5B) nor bovine serum albumin (not shown) altered PMA-induced NET production. By contrast to its effect on NET production, exogenous rSerpin1 did not alter PMA-induced ROS production (Fig. 5C). To determine whether delaying addition of rSerpinB1 alters its effect, we varied the time of addition. Compared to addition at the start of preincubation (- 60 min), the inhibitory effect was only partial for rSerpinB1 added at the time of, or 15 min after, PMA (Fig. 5D), indicating that rSerpinB1 is more efficient at inhibiting NETosis if present during the preincubation and adherence step prior to NETosis induction. There was no inhibitory effect when rSerpinB1 was present only during the final 1h with PMA (Fig. 5E), verifying that the recombinant protein acts by blocking NET generation rather than inducing NET degradation. Finally, NETs were induced as anticipated when human neutrophils were treated with PMA, and human NET production was inhibited by rSerpinB1 and not by α1-antitrypsin or ovalbumin (Fig. 5F).
FIGURE 5.
Abrogation of NET generation by exogenous rSERPINB1. Bone marrow neutrophils of WT (+/+) and serpinb1-deficient (−/−) mice (A-E) or human blood neutrophils (F) were pre-incubated without or with rSERPINB1 (rSB1) (10µg/ml) for 1 h (A-C,F) or the indicated time (D,E) and stimulated with PMA, PAF and MIP-2 (A) or PMA (B-F). NETs were quantified by microscopy after Sytox staining. A, NET generation by both genotypes of murine neutrophils stimulated with PMA, PAF or MIP-2 and its inhibition by rSERPINB1. B, Lack of inhibition of PMA-stimulated NET generation by α1-antitrypsin (AAT, SERPINA1) and ovalbumin (ova, serpinB14). Protocol as in A except ovalbumin or AAT replaced rSerpinB1. C, ROS generation by PMA-stimulated neutrophils and lack of inhibition by rSERPINB1. ROS detected as in Fig. 2B. D,E, Time course of inhibition by rSERPINB1 of PMA-stimulated NET generation. -60’ indicates the start of preincubation; 0’ indicates the time of PMA addition. NETs were evaluated at 4h (D) or 5h (E). F, NET production by human neutrophils treated with PMA and inhibition by rSERPINB1 but not by AAT or ovalbumin. A-E, Means ± SEM for 2-4 experiments (each 3 mice/group). Data in A and B are from the same series of experiments; data in E and F are from another series. *, P<0.05; **, P<0.001, ***, P<0.0001. Two symbols show the results for the WT with WT comparison followed by the KO with KO comparison. F, Means ± SEM for cells of 3-4 normal healthy donors.
Discussion
In this paper, we identified a novel NETosis regulatory mechanism involving SerpinB1, an intracellular clade B serpin. A number of lines of evidence support this conclusion. First, isolated serpinb1−/− neutrophils generated substantially more NETs than WT neutrophils. Second, the increase of serpinb1−/− NETs was induced by unrelated activating agents (PMA, PAF, MIP-2, LPS) and found for bone marrow neutrophils and recruited lung neutrophils. Third, increased serpinb1−/− NET production in response to PMA, PAF and MIP-2 was found in two genetic backgrounds, 129S6 (Fig.1,2,4,5) and C57Bl/6 (Supplemental Fig. 3), indicating that the link of SerpinB1 and Netosis is highly penetrant. Fourth, the frequency of expanded nuclei, indicative of chromatin decondensation, was increased in serpinb1−/− neutrophils. Fifth, exogenous rSerpinB1 abrogated NET production. Lastly, increased NETs were found in vivo in P. aeruginosa infected serpinb1−/− mice.
The finding that excess NETs are produced (i) in vitro by highly purified serpinb1−/− neutrophils and (ii) in vivo in infected lungs of serpinb1−/− mice strongly suggests that the in vitro and in vivo outcomes have their basis in the same NETosis regulatory mechanism. This non-redundant restriction of NET production by SerpinB1 occurs despite expression in neutrophils of other clade B serpin inhibitors (32) and the clade A serpin α1-antitrypsin (33), which shares the SerpinB1 inhibitory specificity. Factors eliminated as cause of the enhanced NET production associated with SerpinB1 deletion include altered neutrophil adherence and overall cell death. A mechanism involving caspases is unlikely because NET production by neutrophils of both genotypes is insensitive to the pan-caspase inhibitor Z-VAD-fmk (Supplemental Fig. 1). ROS was required to produce NETs when the activating agent was PMA, but ROS was not different in serpinb1−/− and WT neutrophils, and MIP-2, PAF and also LPS (supplemental Fig. 2) induced increased serpinb1−/− NETs without requiring ROS. Thus, the NETosis enhancing effect of serpinb1 deletion is ROS-independent. Three NET generating pathways, each regulated by serpinb1, were distinguished: PMA, MIP-2, which is NADPH/ROS-independent, and PAF, which is NADPH oxidase/ROS-independent but requires a DPI-sensitive enzyme. Thus, SerpinB1 acts at a signaling step common to the PMA, MIP-2 and PAF pathways and located downstream of ROS in the PMA pathway.
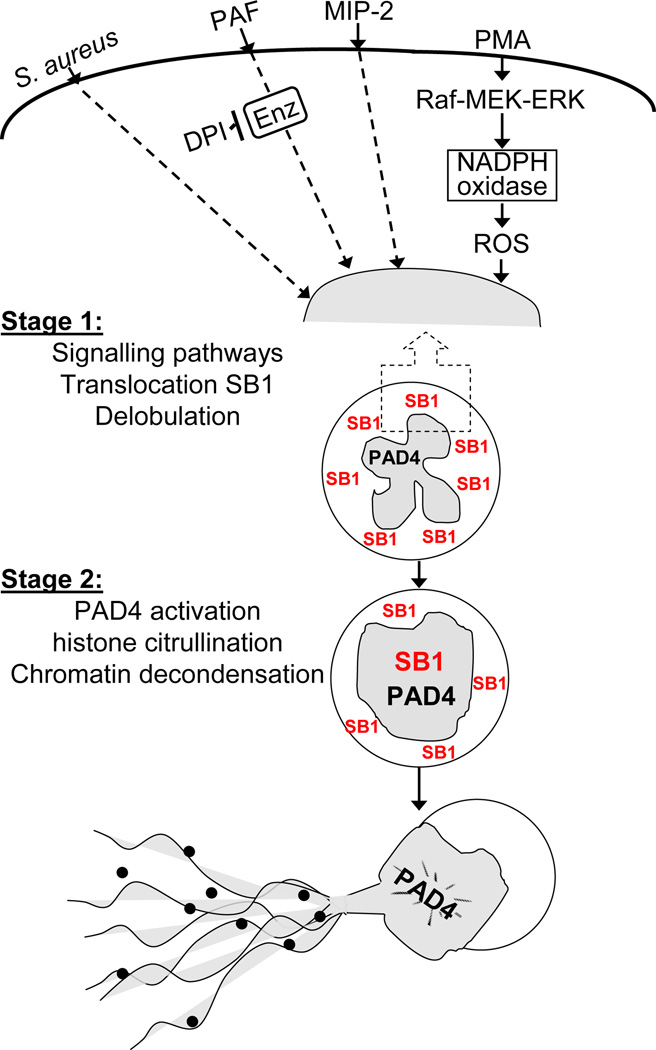
At this time we can only speculate about the mechanism of NETosis inhibition by added recombinant SerpinB1; however the current findings suffice to place the endogenous protein within a schematic of NETosis regulation (Fig. 6). NETosis occurs in two stages: an initial primarily cell surface/cytoplasmic stage and a subsequent nuclear stage. Initial events include signaling cascades specific to the inducing agent, which in the case of PMA involves the Raf-MEK-ERK pathway and leads to NADPH oxidase activation and ROS (31). Multiple other agents induce NETosis including bacteria, fungi, protozoa, TNFα, LPS, MIP-2 and PAF (4, 13, 26, 31, 34–36); these are represented in the Fig. 6 schematic by S. aureus, MIP-2 and PAF, all of which are ROS-independent (26) and one of which, PAF, requires a DPI-sensitive enzyme. Elastase, which can be released from damaged granules, was reported to participate in the PMA-pathway (12), and thus SerpinB1 might restrict NETosis by inhibiting cytoplasmic elastase. Importantly, SerpinB1 translocates independently to the nucleus coincident with or prior to the early PMA-induced transformation of the nucleus from segmented to spherical shape, strongly suggesting a regulatory role in the nucleus. A rapid conversion of the polymorphonuclear nucleus to a condensed sphere occurs also in S. aureus induced NETosis (26), suggesting that this transformation of the nucleus may be a common feature of NETosis.
FIGURE 6.
Schematic of proposed NETosis pathways induced by PMA and other mediators, represented by MIP-2, PAF and S. aureus as described in text. Four upstream pathways are shown, but additional diversity is likely. Dashed lines indicate uncertainties. SerpinB1 (SB1) is exclusively in the cytoplasm of resting neutrophils and translocates into the nucleus early during NETosis. Both PMA-induced nuclear envelope disintegration (11) and non-lytic vesicular exocytosis (26) are compatible with the proposed stage 1 and 2 schematic.
The dominant subsequent nuclear is decondensation of chromatin, which requires that histone tail arginines be deiminated (converted to citrulline) by peptidyl arginine deiminase-4 (PAD-4), an event that involves loss of positive charges. Histone citrullination is required for NET production in response to PMA, LPS, TNF-α, and IL-8 plus Shigella flexneri (14–16, 36), strongly indicating that multiple pathways have converged at or before this step. The responsible enzyme, PAD-4, is expressed at high levels in neutrophils and localized to the nucleus (37); its mechanism of activation in vivo has not been studied. We hypothesize that SerpinB1 regulates events at the level of chromatin decondensation, possibly by restricting histone citrullination. Putative mechanisms might include, e.g., blocking PAD4 access to histone tails or preventing dissociation or destruction of a PAD4 inhibitory protein (Fig. 6).
A role for SerpinB1 in regulating chromatin decondensation was suggested also by an independent study, which showed that SerpinB1 associates tightly with condensed chromatin of human neutrophils prepared so that SerpinB1 was localized in the nucleus (38). That study also showed the unusual composition of mature neutrophil nuclei, in particular the presence in heterochromatin regions of the repressive marker H3K9, but depletion of heterochromatin protein-1 (HP1)-α, -β and –γ, which normally binds H3K9 and maintains heterochromatin. Nuclear envelope stabilizing proteins, lamins A/C, B1 and B2, LAP2β and emerin, are also depleted in mature neutrophils, and the resulting nucleus, although malleable, is also fragile (39), perhaps increasingly fragile after conversion to spherical shape. These findings led Olins et al to speculate that chromatin binding of SerpinB1 contributes to maintaining the fragile nuclear shape (39). Importantly, the proposed role of SerpinB1 in regulating chromatin decondensation of mammalian neutrophils is not without precedent. The closely related protein serpinb10b, also known as MENT (mature erythrocyte nuclear termination-stage specific protein), a specialized clade B serpin in avian species, has been found to enforce chromatin condensation in terminally differentiated chicken neutrophils (40).
Overall, the in vitro and in vivo findings identify a regulatory mechanism involving SerpinB1 that restricts NET production in response to multiple inflammatory mediators. The findings document a previously unrecognized layer of regulation that protects the host from excess NET production. This control mechanism is (i) deeply embedded in NETosis and (ii) localized downstream of signaling events shared with other neutrophil functional pathways. For these reasons, further study of the SerpinB1 regulatory step may identify targets suitable for pharmacological dampening of NETosis.
Supplementary Material
Acknowledgements
We thank Monika Zwerger (German Cancer Research Center, Heidelberg) for reagents; Don and Ada Olins (University of New England, Portland ME), Maren von Koeckritz-Blickwede (University of California, La Jolla), and Saurabh Ghosh-Roy and Hongbo Luo (Boston Children's Hospital) for advice on methodology; and Maziar Divangahi (McGill University), Peter Nigrovic (Boston Children’s Hospital), Heinz Remold (Brigham and Women’s Hospital) and Alan Sher (National Institutes of Allergy and infectious Diseases) for critical comments on the manuscript.
This work was supported by the National Heart, Lung and Blood Diseases grant HL-066548 and the National Institute of Allergy and Immunology grant AI-072552.
Abbreviations used in this article
- AEBSF
4-(2-aminoethyl)benzene-sulfonylfluoride
- BALF
clarified bronchoalveolar lavage fluid
- DFP
diisopropyl fluorophosphate
- DPI
diphenylene iodonium
- FOV
field of view
- MIP-2
macrophage inflammatory protein-2 (cxcl2)
- MPO
myeloperoxidase
- NET
neutrophil extracellular trap(s)
- PAD-4
peptidyl-arginine-deiminase-4
- PAF
platelet activating factor
- PBS
phosphate buffered saline
- PMA
phorbol myristate acetate
- PMSF
phenylmethylsulfonyl fluoride
- ROS
reactive oxygen species
- WT
wild-type
- zVAD-fmk
carbobenzoxy-Val-Ala-Asp-fluoromethylketone
Footnotes
Farley K, Stolley M, Cooley J, Remold-O’Donnell, E, SerpinB1 functions in generating neutrophil extracellular traps. J Immunol, 186, 111a, 2011.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Cooley J, Takayama TK, Shapiro SD, Schechter NM, Remold-O'Donnell E. The serpin MNEI inhibits elastase-like and chymotrypsin-like serine proteases through efficient reactions at two active sites. Biochemistry. 2001;40:15762–15770. doi: 10.1021/bi0113925. [DOI] [PubMed] [Google Scholar]
- 2.Benarafa C, Priebe GP, Remold-O'Donnell E. The neutrophil serine protease inhibitor serpinb1 preserves lung defense functions in Pseudomonas aeruginosa infection. J Exp Med. 2007;204:1901–1909. doi: 10.1084/jem.20070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong D, Farley K, White M, Hartshorn KL, Benarafa C, Remold-O'Donnell E. Critical Role of SerpinB1 in Regulating Inflammatory Responses in Pulmonary Influenza Infection. J Infect Dis. 2011;204:592–600. doi: 10.1093/infdis/jir352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 5.Papayannopoulos V, Staab D, Zychlinsky A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One. 2011;6:e28526. doi: 10.1371/journal.pone.0028526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 7.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Romo SG, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 17.Cooley J, Mathieu B, Remold-O'Donnell E, Mandle RJ. Production of recombinant human monocyte/neutrophil elastase inhibitor (rM/NEI) Protein Expr Purif. 1998;14:38–44. doi: 10.1006/prep.1998.0951. [DOI] [PubMed] [Google Scholar]
- 18.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 19.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1:181–193. doi: 10.1159/000205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Kockritz-Blickwede M, Chow OA, Nizet V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 2009;114:5245–5246. doi: 10.1182/blood-2009-08-240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theilgaard-Monch K, Knudsen S, Follin P, Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol. 2004;172:7684–7693. doi: 10.4049/jimmunol.172.12.7684. [DOI] [PubMed] [Google Scholar]
- 22.Drewniak A, van Raam BJ, Geissler J, Tool AT, Mook OR, van den Berg TK, Baas F, Kuijpers TW. Changes in gene expression of granulocytes during in 21 vivo granulocyte colony-stimulating factor/dexamethasone mobilization for transfusion purposes. Blood. 2009;113:5979–5998. doi: 10.1182/blood-2008-10-182147. [DOI] [PubMed] [Google Scholar]
- 23.Sheppard RF, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 25.Stuehr DJ, Fasehun OA, Kwon NS, Gross SS, Gonzalez JA, Levi R, Nathan CF. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 1991;5:98–103. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]
- 26.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 27.Bainton DF, Ullyot JL, Farquhar MG. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971;134:907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bird CH, Blink EJ, Hirst CE, Buzza MS, Steele PM, Sun J, Jans DA, Bird PI. Nucleocytoplasmic distribution of the ovalbumin serpin PI-9 requires a nonconventional nuclear import pathway and the export factor Crm1. Mol Cell Biol. 2001;21:5396–5407. doi: 10.1128/MCB.21.16.5396-5407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benarafa C, Remold-O'Donnell E. The ovalbumin serpins revisited: perspective from the chicken genome of clade B serpin evolution in vertebrates. Proc Natl Acad Sci U S A. 2005;102:11367–11372. doi: 10.1073/pnas.0502934102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remold-O'Donnell E. The ovalbumin family of serpin proteins. FEBS Lett. 1993;315:105–108. doi: 10.1016/0014-5793(93)81143-n. [DOI] [PubMed] [Google Scholar]
- 31.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 32.Scott FL, Hirst CE, Sun J, Bird CH, Bottomley SP, Bird PI. The intracellular serpin proteinase inhibitor 6 is expressed in monocytes and granulocytes and is a potent inhibitor of the azurophilic granule protease, cathepsin G. Blood. 1999;93:2089–2097. [PubMed] [Google Scholar]
- 33.du Bois RM, Bernaudin JF, Paakko P, Hubbard R, Takahashi H, Ferrans V, Crystal RG. Human neutrophils express the alpha 1-antitrypsin gene and produce alpha 1-antitrypsin. Blood. 1991;77:2724–2730. [PubMed] [Google Scholar]
- 34.von Kockritz-Blickwede M, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J Mol Med (Berl) 2009;87:775–783. doi: 10.1007/s00109-009-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guimaraes-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceicao-Silva F, Saraiva EM. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci U S A. 2009;106:6748–6753. doi: 10.1073/pnas.0900226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcos V, Zhou Z, Yildirim AO, Bohla A, Hector A, Vitkov L, Wiedenbauer EM, Krautgartner WD, Stoiber W, Belohradsky BH, Rieber N, Kormann M, Koller B, Roscher A, Roos D, Griese M, Eickelberg O, Doring G, Mall MA, Hartl D. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat Med. 2010;16:1018–1023. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidyl-arginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277:49562–49568. doi: 10.1074/jbc.M208795200. [DOI] [PubMed] [Google Scholar]
- 38.Popova EY, Claxton DF, Lukasova E, Bird PI, Grigoryev SA. Epigenetic heterochromatin markers distinguish terminally differentiated leukocytes from incompletely differentiated leukemia cells in human blood. Exp Hematol. 2006;34:453–462. doi: 10.1016/j.exphem.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Olins AL, Zwerger M, Herrmann H, Zentgraf H, Simon AJ, Monestier M, Olins DE. The human granulocyte nucleus: Unusual nuclear envelope and heterochromatin composition. Eur J Cell Biol. 2008;87:279–290. doi: 10.1016/j.ejcb.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irving JA, Shushanov SS, Pike RN, Popova EY, Bromme D, Coetzer TH, Bottomley SP, Boulynko IA, Grigoryev SA, Whisstock JC. Inhibitory activity of a heterochromatin-associated serpin (MENT) against papain-like cysteine proteinases affects chromatin structure and blocks cell proliferation. J Biol Chem. 2002;277:13192–13201. doi: 10.1074/jbc.M108460200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.