Abstract
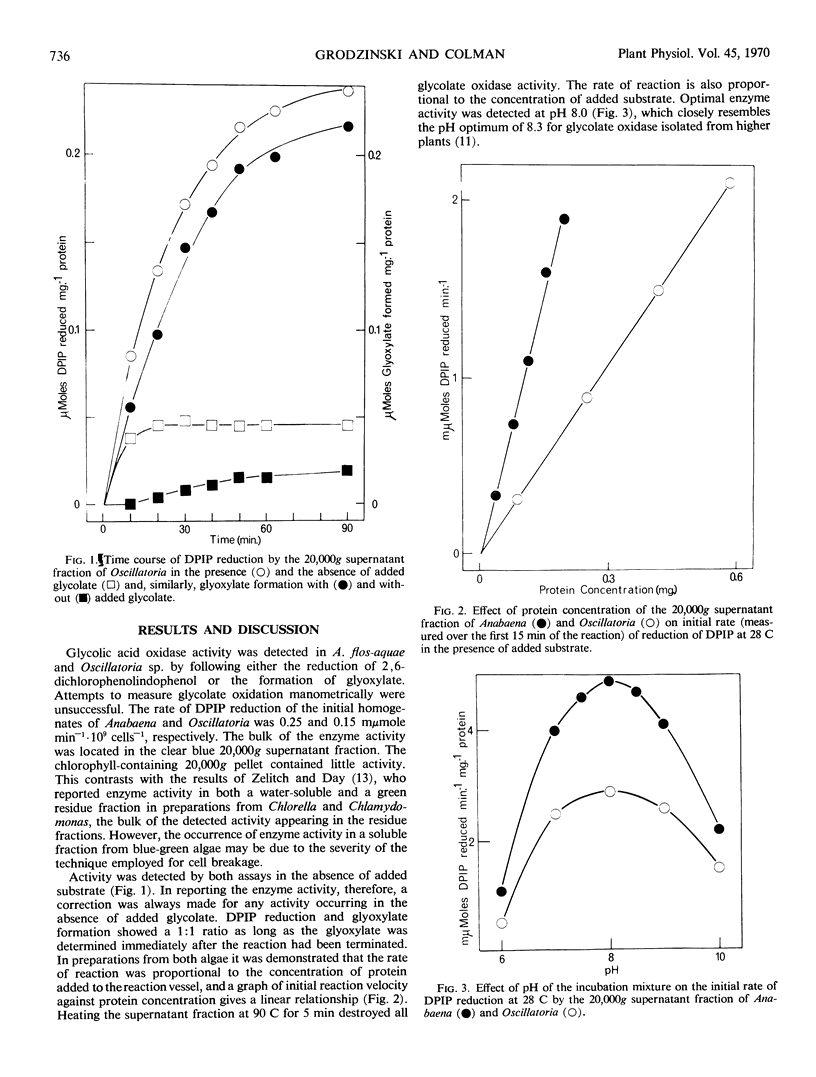
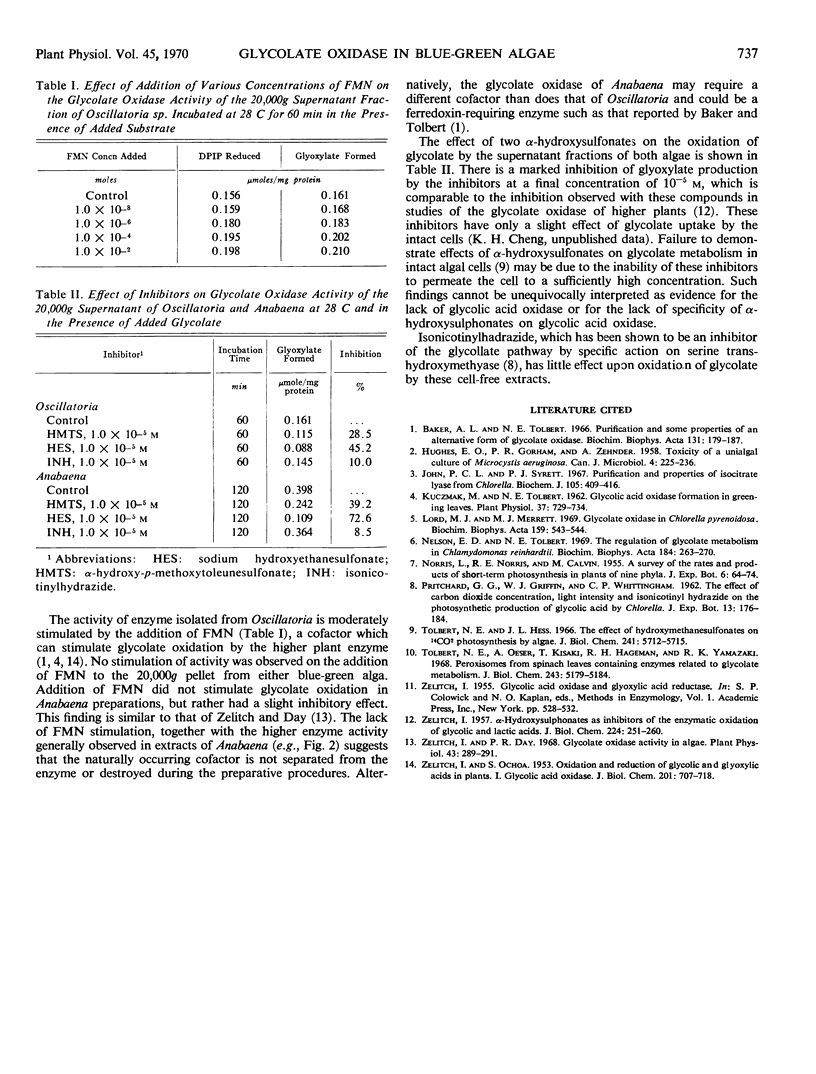
Glycolic acid oxidase activity was detected in cell-free preparations of Anabaena flos-aquae and Oscillatoria sp. by the reduction of 2,6-dichlorophenolindophenol and by the formation of glyoxylate. Enzyme activity was localized in the 20,000 times gravity supernatant fraction, and optimal activity was obtained at pH 8.0. Activity was lost on storing the preparation at 4 C and could not be restored by addition of flavin mononucleotide.
Oxidase activity of the supernatant fraction of Oscillatoria sp. was slightly stimulated by addition of flavin mononucleotide, but that of A. flos-aquae was unaffected by addition of this cofactor. The formation of glyoxylate by supernatant fractions of either alga was markedly inhibited by the addition of α-hydroxysulfonates.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HUGHES E. O., GORHAM P. R., ZEHNDER A. Toxicity of a unialgal culture of Microcystis aeruginosa. Can J Microbiol. 1958 Jun;4(3):225–236. doi: 10.1139/m58-024. [DOI] [PubMed] [Google Scholar]
- John P. C., Syrett P. J. The purification and properties of isocitrate lyase from Chlorella. Biochem J. 1967 Oct;105(1):409–416. doi: 10.1042/bj1050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmak M., Tolbert N. E. Glycolic acid oxidase formation in greening leaves. Plant Physiol. 1962 Nov;37(6):729–734. doi: 10.1104/pp.37.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M. J., Merrett M. J. Glycollate oxidase in Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Jul 9;159(3):543–544. doi: 10.1016/0005-2744(68)90140-x. [DOI] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Hess J. L. The effect of hydroxymethanesulfonates on 14-CO-2 photosynthesis by algae. J Biol Chem. 1966 Dec 10;241(23):5712–5715. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- ZELITCH I., OCHOA S. Oxidation and reduction of glycolic and glyoxylic acids in plants. I. Glycolic and oxidase. J Biol Chem. 1953 Apr;201(2):707–718. [PubMed] [Google Scholar]
- ZELITCH I. alpha-Hydroxysulfonates as inhibitors of the enzymatic oxidation of glycolic and lactic acids. J Biol Chem. 1957 Jan;224(1):251–260. [PubMed] [Google Scholar]
- Zelitch I., Day P. R. Glycolate oxidase activity in algae. Plant Physiol. 1968 Feb;43(2):289–291. doi: 10.1104/pp.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]



