Abstract
We present OnTheFly (http://bhapp.c2b2.columbia.edu/OnTheFly/index.php), a database comprising a systematic collection of transcription factors (TFs) of Drosophila melanogaster and their DNA-binding sites. TFs predicted in the Drosophila melanogaster genome are annotated and classified and their structures, obtained via experiment or homology models, are provided. All known preferred TF DNA-binding sites obtained from the B1H, DNase I and SELEX methodologies are presented. DNA shape parameters predicted for these sites are obtained from a high throughput server or from crystal structures of protein–DNA complexes where available. An important feature of the database is that all DNA-binding domains and their binding sites are fully annotated in a eukaryote using structural criteria and evolutionary homology. OnTheFly thus provides a comprehensive view of TFs and their binding sites that will be a valuable resource for deciphering non-coding regulatory DNA.
INTRODUCTION
Specific interactions between transcription factors (TFs) and their DNA binding sites (TFBSs) play a critical role in the control of transcriptional regulation. To decipher the molecular mechanisms underlying these interactions, it is important to collect and analyze known TFs and their corresponding TFBSs. The first studies of TF DNA-binding specificities used biochemical methods such as DNase I footprinting to identify individual binding sites in known target regulatory sequences. Compilation of these sites (1,2) has provided a rich, albeit crude, source of binding-site preferences. Subsequently, a variety of additional methods have been developed to study binding specificities more systematically (3), including systematic evolution of ligands by exponential enrichment (SELEX) (4), SELEX with deep sequencing (5,6) and protein-binding microarrays (PBMs) (7). In addition, the bacterial one-hybrid (B1H) system was developed (8), allowing TF specificities to be determined without the need for protein purification.
Databases that store collections of TF DNA-binding information can be classified by three major criteria (Supplementary Table S1): the species represented in the data set; the type of data stored for each TF (i.e. the sequence or structure of the TF or the TFBS); and the techniques used for collecting the DNA-binding sites (e.g. DNase I or B1H). The commercial database Transfac (2) and the publically accessible database JASPAR (1) include matrix descriptions of recognition motifs for TFs across multiple species. These were generated through a variety of methodologies used to collect the DNA-binding sites, including compiled sequences, B1H, DNase I, SELEX and PBMs. The Uniprobe database provides specificity information for TFs derived from a single technique, PBM, which allows investigators to directly reveal binding site sequence preferences from a diverse collection of organisms including human, mouse and yeast (9).
Several databases focus on TFs encoded in the Drosophila melanogaster genome. Of these, FlyBase (10) is the primary database for integrated genetic and genomic data. Information in FlyBase originates from a variety of sources ranging from a large-scale genome projects to the primary research literature. Another D. melanogaster TF database is FlyTF (11), which is a manually annotated catalogue of site-specific TFs in the genome. The REDfly database provides an extensive compilation of published experimental data identifying TFBSs (12), while FlyReg (13) comprises a DNase I footprint database and presents a systematic genome annotation of D. melanogaster TFBSs. The latter two databases fully merged in 2007 to provide one portal for D. melanogaster TFBSs. The FlyFactorSurvey (14) database summarizes a project that used the B1H method to systematically describe the binding site preferences of D. melanogaster TFs. A smaller database is the Berkeley D. melanogaster Transcription Network Project (BDNTP) (15), which focuses on deciphering the transcriptional information contained in the extensive cis-acting DNA sequences that control the patterns of gene expression during embryogenesis. Components of this effort include in vivo DNA-binding sequences using either the ChIP–chip or the ChIP–seq methods, as well as in vitro DNA-binding sequences using the SELEX protocol.
Three-dimensional structural information for TFs and their binding sites in existing databases is limited, although several D. melanogaster databases store and present structural annotations for TFs. For example, FlyTF classifies TFs based on the DNA Binding Domains database (DBD) (16). FlyFactorSurvey classifies D. melanogaster TFs using Interpro classification (17). Currently, there is no database that contains TF structural models or structural information about the TF-binding sites. Recent studies suggest that an improved understanding of protein–DNA recognition requires that, in addition to the information contained in the linear sequence of nucleotides, DNA shape must also be taken into account (18–21). To integrate sequence and structural information for a single organism, we created OnTheFly (http://bhapp.c2b2.columbia.edu/OnTheFly/index.php), a database for D. melanogaster TFs and TFBSs. OnTheFly currently houses DNA recognition motifs for >387 genes encoding TFs (>50% of the predicted Drosophila TF genes), and it extracts binding sites based on multiple data sources (e.g. DNase I, B1H and SELEX). OnTheFly also provides structural information for both TFs and their binding sites whenever possible. We believe that the scope of its coverage and its integration of both sequence and structural information renders it as an important tool in the study of the interactions between TFs and their DNA-binding sites.
MATERIALS AND METHODS
Annotating and classifying D. melanogaster TFs
A list of 2107 D. melanogaster candidate TFs encoded by 754 genes (the 754 genes encode 2107 splice isoforms) was extracted from Ensembl (release version 71; http://ensembl.org/), based on the protocol described in FlyTF (11,22). Specifically, a TF is chosen based on either the presence of a canonical DNA-binding domain predicted with the DBD database (16) or based on direct experimental evidence. The list of TFs is composed of 1970 proteins that possess canonical DNA binding domains and 137 that do not. TFs were classified based on the domains they possess that are defined in Interpro in a hierarchical fashion. For example, an Interpro entry might represent a subclass of a broad class of domains that share structure and/or function. On this basis of the 113 different Interpro entries represented in Drosophila, the TFs were grouped into 18 sets of DNA-binding domains that each include at least 10 TFs (OnTheFly Domain Name; see Supplementary Table S2). A 19th category, ‘Other’, contains Interpro entries with <10 TFs. We used Interpro (17) for classification because it integrates domain annotations based on 12 different methods including those used in DBD (16). We found 120 additional DNA-binding domains in Interpro that do not appear in DBD (see Supplementary Table S3 for examples).
TF structures
OnTheFly provides either experimentally derived structures or homology models for most (74%) of the TFs in the database. Experimental structures were obtained by querying the PDB using Protein KnowledgeBase (UniProtKB) accession numbers. Protein structures or protein–DNA complexes (X-Ray or NMR) were found for 65 of the D. melanogaster TFs; these structures were linked to OnTheFly. In cases where a TF was included in more than one structure, all relevant links to the PDB were included. For TFs for which experimental structures were not available, a search for homology models was conducted using the Modbase database (23), which was queried with UniProt accession numbers. Homology models were found in Modbase for 1171 of the D. melanogaster TFs and stored in OnTheFly.
Homology models in Modbase all have e-values < 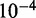 . To expand our structural coverage to TFs not in Modbase, homology models were constructed with the PUDGE homology modelling pipeline (24) using HHPRED 1.5 (25) for template selection (homology models were built only where e-values for template selection were <
. To expand our structural coverage to TFs not in Modbase, homology models were constructed with the PUDGE homology modelling pipeline (24) using HHPRED 1.5 (25) for template selection (homology models were built only where e-values for template selection were < 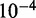 ), MODELLER for model building (26) and the pG score derived from PROSA-II (27,28) for model evaluation. Homology models were stored in OnTheFly only when the pG score was > 0.5. Using PUDGE, 318 homology models with an e-value <
), MODELLER for model building (26) and the pG score derived from PROSA-II (27,28) for model evaluation. Homology models were stored in OnTheFly only when the pG score was > 0.5. Using PUDGE, 318 homology models with an e-value < 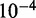 and a pG score > 0.5 were added to OnTheFly.
and a pG score > 0.5 were added to OnTheFly.
DNA shape parameters
When experimentally derived structural information (X-ray or NMR) on protein–DNA complexes was available, minor groove width, roll, propeller twist and helix twist were measured along the DNA sequence using CURVES 5.1 (29) and stored in the database (see example in Supplementary Figure S2). In addition, for all cases where Position Weighted Matrices (PWMs) were available, DNA shape parameters are provided via a link to a web server that predicts DNA structural features using a high-throughput (HT) method based on Monte Carlo simulations (30). Currently, the database represents the predicted DNA shape parameters for all DNA sequences that contributed to the PWM.
DATABASE CONTENT
OnTheFly annotates 2107 proteins derived from 754 genes. TF structures were obtained from the PDB (65 TFs) and homology models (1489 TFs, 1171 from Modbase and 318 using the PUDGE homology modelling pipeline). Inferred motifs of TFBSs are presented in the database using a PWM, and were obtained from several sources: 87 PWMs based on DNaseI footprint data were extracted from FlyReg (13); 327 PWMs based on B1H were extracted from FlyFactorSurvey (14); 22 PWMs based on SELEX data were extracted from a study of Hox proteins (6), from BDNTP (15) and from JASPAR (1). Taken together, OnTheFly houses DNA recognition motifs for >387 different genes encoding TFs (>50% of the genes), comprising the largest collection of TFBS recognition motifs currently available for D. melanogaster. The DNA recognition motifs in OnTheFly are organized by TF although in several cases where a PWM was connected with a gene and not with a TF, all gene isoforms are linked to the same PWM.
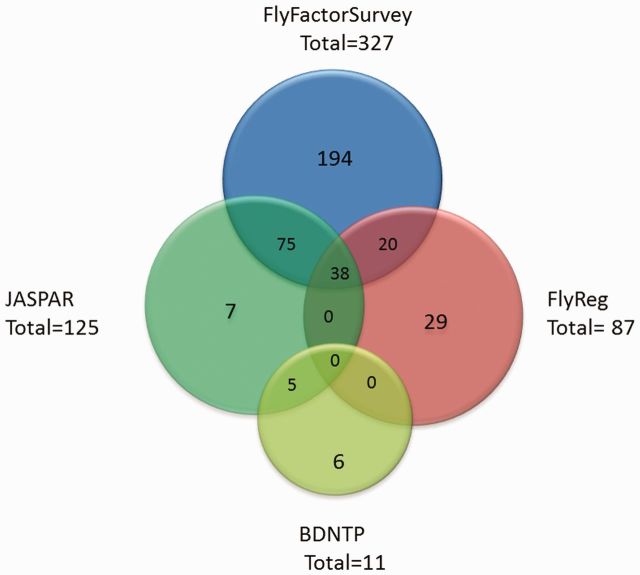
Figure 1 displays a Venn diagram reporting the contribution of the different databases to the PWMs collected in OnTheFly. As is evident, the largest contribution is from B1H data stored in FlyFactorSurvey (327 genes; 43% of all Drosophila TF genes), with smaller contributions coming from JASPAR, FlyReg and BDNTP. Combining the PWM motifs from all databases, OnTheFly includes PWMs for 387 genes; 51% of all Drosophila TF genes.
Figure 1.
The contribution of previous databases to the PWMs appearing in OnTheFly.
The distribution of TFs among different structural families is shown in Supplementary Figure S1A. TFs with multiple DNA-binding domains are classified by each of their respective families, whereas TF families with <10 members are classified as ‘other’. The Classical Zinc Finger (C2H2 and C2CH) family contains ∼700 TFs, about a third of all D. melanogaster TFs, and ∼300 TFs possess a homeodomain (encoded by 436 and 138 genes, respectively). As shown in Supplementary Figure S1B, the majority of D. melanogaster TFs possess a single DNA-binding domain, whereas 8% of all TFs possess two DNA-binding domains from different structural families. TFs possessing DNA-binding domains from three or more different structural families were not found. The combinations of DBD pairs are shown in Supplementary Figure S1C. Supplementary Figure S1D describes the number of TFs and genes encoding TFs from each of the DNA binding domain families for which a PWM is known. As shown in Supplementary Figure S1D, the homeodomain family has the largest number of known PWMs.
WEB INTERFACE
Database organization
All the information in OnTheFly is stored with MySQL, a free database management system widely used in bioinformatics.
Data searching
OnTheFly provides three different approaches for data searching: by TF, by DNA sequence and by DBD. Figure 2 shows a schematic workflow for a sample TF search. Movie S1 shows the search process by DNA sequence. PWMs are linked to 18 sets of Interpro DNA-binding domains to allow users to find PWMs for specific DNA-binding domains (see Supplementary Figure S2).
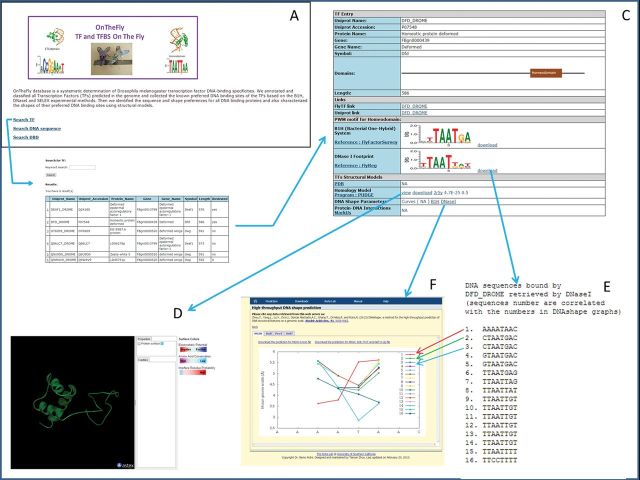
Figure 2.
TF search workflow in OnTheFly. This figure describes a search for a sequence-specific transcription factor, Homeotic protein Deformed (DFD). (A) In the Entry Screen, we choose TF search. (B) A search for the term Deformed retrieves six TFs. (C) Choosing the second, DFD_DROME, leads to a detailed TF screen. This screen shows that DFD possesses a homeodomain and has two known TFBS represented by a PWM, one based on B1H data and the other based on DNase I data. (D) A homology model for this protein shows three alpha helices comprising the homeodomain shown using the MarkUs viewer. (E) The DNA sequences retrieved by DNase I are sorted according to their putative binding affinity to this protein. (F) Opening the DNaseI or B1H links shows the results of the DNA shape server (30). Each line in the graph represents the minor groove width along a different DNA sequence, which was entered as input. The graph shows that most of the sequences possess a minimum in width (narrower minor groove width in the AT part of the DNA sequence motif).
MarkUs function-annotation server
The MarkUs server (31) integrates various sequence- and structure-based analysis tools to characterize the biochemical and biophysical properties of a protein structure and identifies structural neighbors as a basis of function annotation. The interface enables the selection and display of functional information associated with structural neighbours of the query protein. Overall annotations of a protein (GO term, EC class) and annotations associated with individual residues (UniProt sequence features, ligand interactions) can be displayed and used to filter structural neighbours to create subsets of functionally related proteins. Functional properties of a structural neighbour can also be visualized in the query structure itself using the AstexViewer 2.01. MarkUs allows the user to examine the query protein for properties such as electrostatic potentials, solvent accessible cavities, interfacial residues, domain information and amino acid conservation.
Protein structures, protein–DNA complexes and DNA structures can be visualized with MarkUS. Two types of representations are available for the display of DNA structures using either line representations or the molecular surfaces with convex regions coloured in green and concave regions coloured in gray. This type of curvature representation provides users a clear picture of major and minor groove shapes.
CONCLUSIONS
D. melanogaster is an important model organism, and its genome encodes numerous members of all known families of DNA-binding proteins. In the OnTheFly database, PWM motifs of DNA-binding sites are available for >50% of the genes encoding TFs in this organism, a relatively high percentage compared with other TF databases or known PWM datasets for other species [e.g. human (5) and mouse (32)]. OnTheFly is designed to annotate all DNA-binding TFs and their binding specificities and to assemble available sequence and structural information for all TFs encoded in the D. melanogaster genome, as well as their binding sites. OnTheFly can thus be of use for various applications such as studying interactions between TFs and DNA, predicting the most likely specific DNA sequence recognized by a novel TF or predicting the potential interactions between a TF and a specific DNA sequence, based on various DNA structural parameters.
OnTheFly will continue to be regularly updated as new structural and PWM data become available. In the coming year, the database will also be expanded to include PWMs for orthologs of Drosophila TFs (human, mouse and yeast) that are retrieved by PBM, B1H or SELEX methods. Whenever available, OnTheFly will also be expanded to increase the structural coverage of TFs and new information about DNA structure derived from improved simulations.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Funding for open access charge: National Institutes of Health [U54-CA121852 and RO1-GM054510].
Conflict of interest statement. None declared.
REFERENCES
- 1.Portales-Casamar E, Thongjuea S, Kwon AT, Arenillas D, Zhao X, Valen E, Yusuf D, Lenhard B, Wasserman WW, Sandelin A. JASPAR 2010: the greatly expanded open-access database of transcription factor binding profiles. Nucleic Acids Res. 2010;38:D105–D110. doi: 10.1093/nar/gkp950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A, Reuter I, Chekmenev D, Krull M, Hornischer K, et al. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 2006;34:D108–D110. doi: 10.1093/nar/gkj143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolma A, Taipale J. Methods for analysis of transcription factor DNA-binding specificity in vitro. Subcell Biochem. 2011;52:155–173. doi: 10.1007/978-90-481-9069-0_7. [DOI] [PubMed] [Google Scholar]
- 4.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 5.Jolma A, Kivioja T, Toivonen J, Cheng L, Wei G, Enge M, Taipale M, Vaquerizas JM, Yan J, Sillanpaa MJ, et al. Multiplexed massively parallel SELEX for characterization of human transcription factor binding specificities. Genome Res. 2010;20:861–873. doi: 10.1101/gr.100552.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slattery M, Riley T, Liu P, Abe N, Gomez-Alcala P, Dror I, Zhou T, Rohs R, Honig B, Bussemaker HJ, et al. Cofactor binding evokes latent differences in DNA binding specificity between Hox proteins. Cell. 2011;147:1270–1282. doi: 10.1016/j.cell.2011.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger MF, Bulyk ML. Universal protein-binding microarrays for the comprehensive characterization of the DNA-binding specificities of transcription factors. Nat. Protoc. 2009;4:393–411. doi: 10.1038/nprot.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng X, Brodsky MH, Wolfe SA. A bacterial one-hybrid system for determining the DNA-binding specificity of transcription factors. Nat. Biotechnol. 2005;23:988–994. doi: 10.1038/nbt1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robasky K, Bulyk ML. UniPROBE, update 2011: expanded content and search tools in the online database of protein-binding microarray data on protein-DNA interactions. Nucleic Acids Res. 2011;39:D124–D128. doi: 10.1093/nar/gkq992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marygold SJ, Leyland PC, Seal RL, Goodman JL, Thurmond J, Strelets VB, Wilson RJ. FlyBase: improvements to the bibliography. Nucleic Acids Res. 2013;41:D751–D757. doi: 10.1093/nar/gks1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfreundt U, James DP, Tweedie S, Wilson D, Teichmann SA, Adryan B. FlyTF: improved annotation and enhanced functionality of the Drosophila transcription factor database. Nucleic Acids Res. 2010;38:D443–D447. doi: 10.1093/nar/gkp910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo SM, Gerrard DT, Miner D, Simich M, Des Soye B, Bergman CM, Halfon MS. REDfly v3.0: toward a comprehensive database of transcriptional regulatory elements in Drosophila. Nucleic Acids Res. 2011;39:D118–D123. doi: 10.1093/nar/gkq999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergman CM, Carlson JW, Celniker SE. Drosophila DNase I footprint database: a systematic genome annotation of transcription factor binding sites in the fruitfly, Drosophila melanogaster. Bioinformatics. 2005;21:1747–1749. doi: 10.1093/bioinformatics/bti173. [DOI] [PubMed] [Google Scholar]
- 14.Zhu LJ, Christensen RG, Kazemian M, Hull CJ, Enuameh MS, Basciotta MD, Brasefield JA, Zhu C, Asriyan Y, Lapointe DS, et al. FlyFactorSurvey: a database of Drosophila transcription factor binding specificities determined using the bacterial one-hybrid system. Nucleic Acids Res. 2011;39:D111–D117. doi: 10.1093/nar/gkq858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, Misra S, Rubin GM. The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson D, Charoensawan V, Kummerfeld SK, Teichmann SA. DBD–taxonomically broad transcription factor predictions: new content and functionality. Nucleic Acids Res. 2008;36:D88–D92. doi: 10.1093/nar/gkm964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter S, Apweiler R, Attwood TK, Bairoch A, Bateman A, Binns D, Bork P, Das U, Daugherty L, Duquenne L, et al. InterPro: the integrative protein signature database. Nucleic Acids Res. 2009;37:D211–D215. doi: 10.1093/nar/gkn785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi R, Passner JM, Rohs R, Jain R, Sosinsky A, Crickmore MA, Jacob V, Aggarwal AK, Honig B, Mann RS. Functional specificity of a Hox protein mediated by the recognition of minor groove structure. Cell. 2007;131:530–543. doi: 10.1016/j.cell.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker SC, Hansen L, Abaan HO, Tullius TD, Margulies EH. Local DNA topography correlates with functional noncoding regions of the human genome. Science. 2009;324:389–392. doi: 10.1126/science.1169050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohs R, West SM, Sosinsky A, Liu P, Mann RS, Honig B. The role of DNA shape in protein-DNA recognition. Nature. 2009;461:1248–1253. doi: 10.1038/nature08473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dror I, Zhou T, Mandel-Gutfreund Y, Rohs R. Covariation between homeodomain transcription factors and the shape of their DNA binding sites. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adryan B, Teichmann SA. FlyTF: a systematic review of site-specific transcription factors in the fruit fly Drosophila melanogaster. Bioinformatics. 2006;22:1532–1533. doi: 10.1093/bioinformatics/btl143. [DOI] [PubMed] [Google Scholar]
- 23.Pieper U, Webb BM, Barkan DT, Schneidman-Duhovny D, Schlessinger A, Braberg H, Yang Z, Meng EC, Pettersen EF, Huang CC, et al. ModBase, a database of annotated comparative protein structure models, and associated resources. Nucleic Acids Res. 2011;39:D465–D474. doi: 10.1093/nar/gkq1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norel R, Petrey D, Honig B. PUDGE: a flexible, interactive server for protein structure prediction. Nucleic Acids Res. 2010;38:W550–W554. doi: 10.1093/nar/gkq475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33:W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eswar N, Eramian D, Webb B, Shen MY, Sali A. Protein structure modeling with MODELLER. Methods Mol. Biol. 2008;426:145–159. doi: 10.1007/978-1-60327-058-8_8. [DOI] [PubMed] [Google Scholar]
- 27.Sippl MJ. Recognition of errors in three-dimensional structures of proteins. Proteins. 1993;17:355–362. doi: 10.1002/prot.340170404. [DOI] [PubMed] [Google Scholar]
- 28.Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavery R, Sklenar H. The definition of generalized helicoidal parameters and of axis curvature for irregular nucleic acids. J. Biomol. Struct. Dyn. 1988;6:63–91. doi: 10.1080/07391102.1988.10506483. [DOI] [PubMed] [Google Scholar]
- 30.Zhou T, Yang L, Lu Y, Dror I, Dantas Machado AC, Ghane T, Di Felice R, Rohs R. DNAshape: a method for the high-throughput prediction of DNA structural features on a genomic scale. Nucleic Acids Res. 2013;41:W56–W62. doi: 10.1093/nar/gkt437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrey D, Fischer M, Honig B. Structural relationships among proteins with different global topologies and their implications for function annotation strategies. Proc. Natl Acad. Sci. USA. 2009;106:17377–17382. doi: 10.1073/pnas.0907971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei GH, Badis G, Berger MF, Kivioja T, Palin K, Enge M, Bonke M, Jolma A, Varjosalo M, Gehrke AR, et al. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 2010;29:2147–2160. doi: 10.1038/emboj.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]