Abstract
During the past 15 years, the curriculum content for nonprescription medication and self-care therapeutics has expanded significantly. Self-care courses ranging from stand-alone, required courses to therapeutic content and skills laboratories, have evolved in colleges and schools of pharmacy to accommodate rapid changes related to nonprescription medications and to meet the needs of students. The design of and content delivery methods used in self-care courses vary among institutions. Teaching innovations such as team-based learning, role playing/vignettes, videos, and social media, as well as interdisciplinary learning have enhanced delivery of this content. Given that faculty members train future pharmacists, they should be familiar with the new paradigms of Nonprescription Safe Use Regulatory Expansion (NSURE) Initiative, nonprescription medications for chronic diseases, and the growing trends of health and wellness in advancing patient-care initiatives. This paper reviews the significant changes that may be impacting self-care curriculums in the United States.
Keywords: self-care, nonprescription medications, pharmacy education
INTRODUCTION
In 2012, the Nonprescription Medicines Academy (NMA) and the Self-Care Institute (SCI) celebrated their 15 and 10 year anniversaries, respectively. These anniversaries have encouraged reflection on the vast changes in self-care education, the growing role of nonprescription medicine in health care, and the value of faculty development programming to support faculty members who teach self-care in the pharmacy curriculum. During the past 15 years, the curriculum content for nonprescription medication and self-care therapeutics has expanded significantly. New nonprescription products, formulation modifications, safety concerns, and regulatory changes are impacting the evolving responsibilities of the pharmacist in self-care. With these changes, self-care courses also transform to include innovative teaching strategies as pharmacy students needs in this area become greater. This paper reviews the significant changes that may be impacting self-care curriculums in the United States.
MILESTONES IN SELF-CARE OVER THE PAST 15 YEARS
The nonprescription medication marketplace has grown extensively over the last 15 years. There are now more than 300,000 nonprescription medication products in 80 therapeutic classes marketed in the United States.1 This number has increased twofold since 2001 when just more than 100,000 nonprescription medication products were on the market.2 A contributing factor to this increase is the recent rise in prescription medications now being available in nonprescription formulation (prescription to nonprescription switches), allowing medications that once required a prescription to be purchased without one.
During this timeframe, more than 35 products have come to the market either directly as a nonprescription medication or as a prescription to nonprescription switch. Table 1 highlights major changes to the nonprescription market during this timeframe.3,4 In 2002, loratadine (Claritin) became the first second-generation antihistamine available without a prescription. This prescription to nonprescription switch revolutionized the treatment of seasonal and perennial allergies by providing the first non-sedating antihistamine option. It also changed managed-care pharmacy, as many pharmacy-benefit managers removed second-generation antihistamines from their formularies, forcing patients to use nonprescription medications as an initial therapeutic approach.
Table 1.
Approvals for Prescription Medications to be Available as Nonprescription Medications, 1997-2013
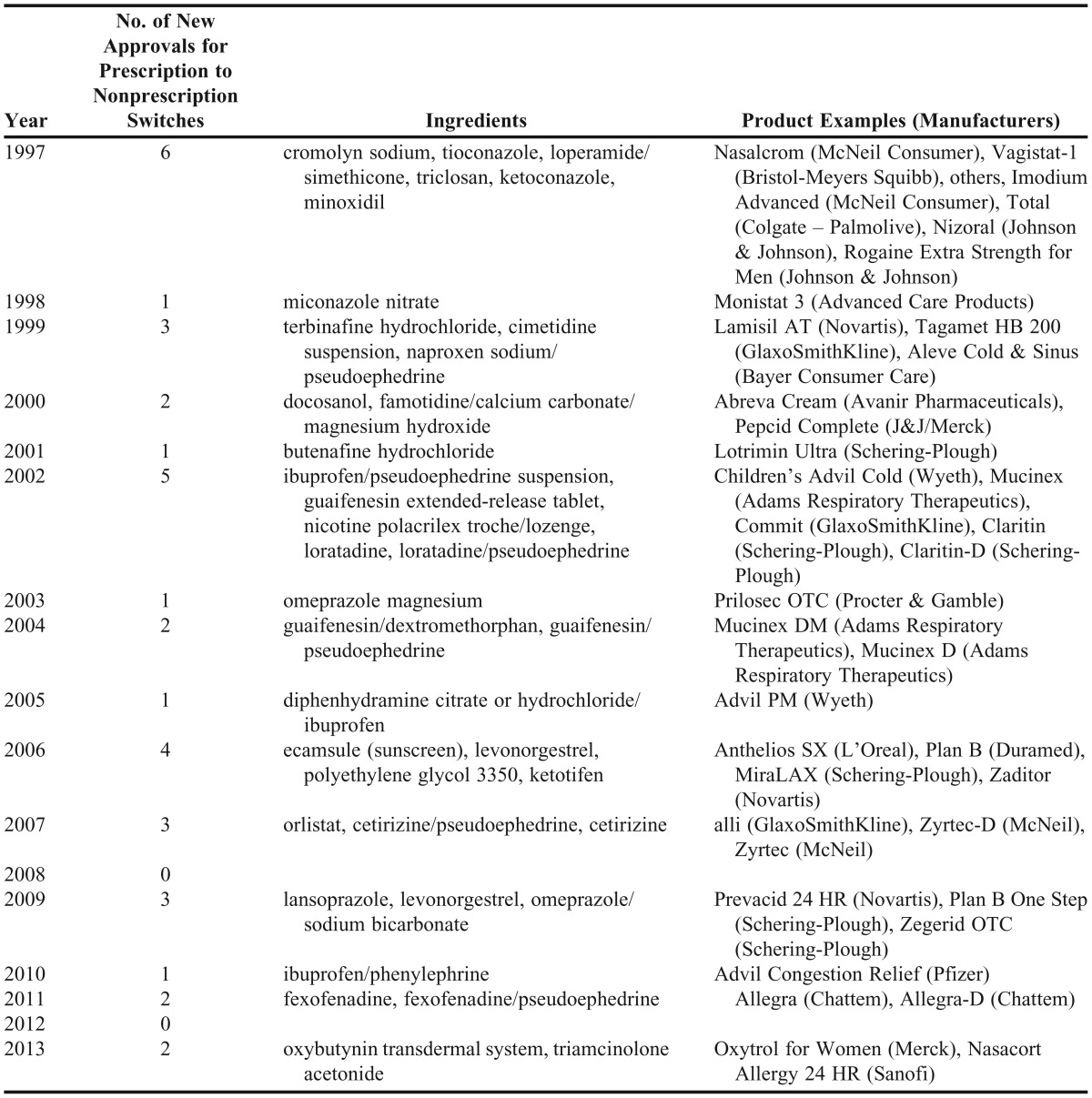
In 2003, omeprazole (Prilosec OTC) became the first proton-pump inhibitor (PPI) available without a prescription. This prescription to nonprescription switch transformed the treatment of frequent heartburn by providing patients an option beyond antacids and H2-antagonists. It also allowed pharmacy-benefit managers to implement step-therapy in their formularies, requiring patients to first use nonprescription omeprazole before trying prescription PPIs.
In 2006, levonorgestrel (Plan B) became the first dual-labeled product, ie, available as both a prescription and nonprescription, depending on patient age. Emergency contraception available without a prescription provides thousands of women increased access, which will be further increased now that the age limit has been lifted.5 In 2007, orlistat (alli) became the first nonprescription weight-loss medication to receive approval by the Food and Drug Administration (FDA). This prescription to nonprescription switch capitalized on the growing health and wellness trend, providing patients a weight-loss option that, when combined with diet and physical activity, is safer than stimulant products. This movement has expanded further to include chronic conditions with the recent 2013 switch of oxybutynin patch to treat overactive bladder and triamcinolone acetonide for nasal allergy symptoms.6,7
During the past 15 years, dozens of safety concerns have been raised and regulations have been instituted in the nonprescription medicine area. In 2005, the Combat Methamphetamine Epidemic Act was passed. This federal statute placed restrictions on pseudoephedrine sales and access and implemented reporting requirements.7 States have passed additional laws regarding restrictions to pseudoephedrine. Another regulatory issue occurred a year later with the passage of the Dietary Supplement and Nonprescription Drug Consumer Protection Act. This act required manufacturers, packers, distributers, and retailers to record and report adverse events.8 Regulations requiring current Good Manufacturing Practices for dietary supplements became law in 2006. This ruling ensures the quality of supplements by setting guidelines related to the manufacturing, labeling, and holding of these products; however, it does not provide additional safety, effectiveness, and dissolution evidence that is greatly needed.9 In 2007, the FDA rejected the prescription to nonprescription switch of a statin for the third time.10 Pediatric cough and cold products were recalled, relabeled, and are still being investigated for appropriateness in children 2-11 years old.11 Acetaminophen labeling and formulations for infants, children, and adults have also undergone changes to ensure safe usage.12 Historically, prescription to nonprescription changes have involved medications that treat acute problems (symptoms lasting less than 2 weeks). However, the most recent switches of 2013 are now dealing with more chronic issues and could increase the potential for further concerns in the future.
Significant changes in growing therapeutic categories as well as regulatory and safety concerns have impacted content delivered in self-care curricula. Both the SCI and NMA have been at the forefront of providing the most up-to-date information regarding these products to assist self-care faculty members with the integration of this information into both classroom and experiential teaching. The NMA Facebook page has been a resource highlighting these updates in nonprescription news, and editorial board members from SCI provide chapter updates through the American Pharmacists Association’s Handbook of Nonprescription Drugs.13,14
SELF-CARE EDUCATION
During this 15-year time period, there also have been great strides in self-care within pharmacy education. The blueprint for the North American Pharmacists Licensure Examination (NAPLEX) was revised in 2005 to recognize the importance of nonprescription medicines, eliminating any distinction of importance between prescription and nonprescription medicines.15,16 The Journal published a Nonprescription Medicines and Self-Care theme issue in 2006, which featured several articles reinforcing the importance of self-care instruction. Part of this supplement identifies the pharmacist as the “only health professional who receives in-depth formal education and skill development in nonprescription drug therapy.”17 Shortly after this publication, the 2007 Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree stated that education relating to nonprescription medicines and self-care should be incorporated throughout the pharmacy curriculum, including introductory and advanced pharmacy practice experiences (IPPEs and APPEs). Guidance regarding the incorporation of self-care and nonprescription medicines into the pharmacy curriculum can be found in Appendix B, C, and D of the accreditation standards.18 Establishment of the American Association of Colleges of Pharmacy Self-Care Therapeutics/Nonprescription Medicines Special Interest Group (SIG) soon followed, providing another opportunity for self-care faculty to network, learn, and promote the scholarship of self-care education.19 Practicing pharmacists are also enhancing their self-care knowledge through continuing education opportunities such as the National Alliance of State Pharmacy Associations partnership with NMA to conduct Student Pharmacists Self-Care Championships, which are held nationwide at annual state pharmacy conventions.20 These events are often led by local self-care faculty members as student teams compete in an event similar to a quiz bowl, during which they answer questions developed by NMA faculty members and which may involve continuing education credit.
Self-care instruction is incorporated into the pharmacy curriculum in many ways. Some colleges and schools require it to be a standalone course, while others offer it as an elective. Still others may integrate the curriculum within existing therapeutics courses, simulation laboratories, or in IPPEs and APPEs.21 Various methods have been used to assist with self-care instruction in the classroom. Although lecture is still the most common mode of delivery, active-learning strategies appear to be a growing trend.22-24 Use of active-learning strategies in the classroom to support and develop a student’s ability to critically think and problem solve has been well established. There are several active-learning strategies that could be incorporated into the self-care curriculum from think-pair-share, one-minute reflection, or the muddiest point exercises; to more complex activities, such as role-playing, problem-based learning, case-based learning, and team-based learning. Additionally, there are technology-based active-learning activities, such as audience-response systems, videos, and social media, that can be incorporated to engage students in their learning. Several strategies discussed herein have been incorporated into NMA and SCI programming to assist and encourage faculty members to use various methods to enhance the delivery of self-care instruction. Faculty members are regularly invited to present poster/platform presentations and roundtable discussions to share their innovations in teaching.25,26 Faculty resources, including complex cases, assessment questions, grading tools, syllabi, active-learning techniques and strategies are available on the NMA website. The SCI also makes available resources from the annual meetings and editors continually provide updates through the APhA Library and Pharmacy Today articles.
Two key learning components in the self-care curriculum are effective communication and patient assessment. The pharmacist must be able to effectively and efficiently ask the patient pertinent questions to determine the problem, the most bothersome symptoms, other medical conditions, and medications being used. Role-playing and vignettes are strategies that can be used to teach these skills. Scenarios can be created and the students can then act out what they would do for the patient. The faculty member can assess and evaluate the students not only on the process used (ie, communication skills and critical thinking) but also on their nonprescription medication knowledge. Role-playing activities wherein students engage in patient consultations also can be used to develop social emotional competences.27 There is a growing body of evidence to support the use of this type of active learning in the classroom to meet the educational needs of future pharmacists and ensure they develop necessary skills.28-35
Team-based learning (TBL) is a type of collaborative learning that shifts the focus of classroom time from a lecture format to the application of key concepts. This type of learning is sometimes called the “flipped classroom” because it requires students to acquire the initial content through readings, online presentations, or other resources prior to class. At the beginning of class, students are assessed on the preclass requirements by means of a Readiness Assurance Technique (RAT). Both individual assessments (I-RAT) and group assessments (G-RAT) are completed at the beginning of class. Having both RATs holds the students accountable for being prepared for class. Following the completion of both RATs, the remainder of the class is spent on application-type activities, such as case discussions, ethical dilemmas, and pro/con discussions. Use of TBL is emerging in the development and delivery of self-care therapeutics.36-39
Pharmacy school curricula have expanded to incorporate YouTube projects and Facebook campaigns in self-care and public health courses. This strategy is intended to expand the skills and abilities of pharmacy students in reaching the community at large with succinct self-care messages. The purpose of these messages is to stress the safe use of nonprescription medications and to promote the role of pharmacists in assisting and educating patients regarding self-care products.40 Example projects, course outcomes, and evidence of student impact have been presented at NMA, enabling other colleges and schools of pharmacy to consider adopting a similar approach in their courses. The use of YouTube for patient education is an area in which further research is needed to determine the impact that videos have on patients’ understanding of their medications, improving adherence, and reducing medications errors and adverse events. Social media content could be considered as labeling and advertisement of these products, which is under the jurisdiction of the FDA.41 FDA guidance and regulations regarding social media messaging will be an important statement for colleges and schools of pharmacy that are using media as an instructional tool to review and integrate into their curricula. 42
THE FUTURE OF SELF-CARE
Nonprescription Safe Use Regulatory Expansion (NSURE) Initiative
In 2012, the FDA announced it was looking at ways to approve the use of prescription medications by making them available as nonprescription through conditions of safe use. Under the FDA’s NSURE Initiative, the “conditions of safe use” could include pharmacist interventions or innovative technologies. Manufacturers can submit an application to the FDA for review and consideration. The FDA states that this paradigm could address the public health problem of under-treatment of common conditions.43,44 Other FDA considerations for this paradigm include: allowing some medication products that require an initial prescription to be available for refills without a prescription, with a condition of safe use for that purpose and permitting some drug products to be available by prescription and without a prescription simultaneously, with conditions of safe use.
This potential new paradigm is important to pharmacists, as it would serve as an opportunity for them to communicate and collaborate with the medical community to improve team-based care; reconnect and refer into the healthcare system those patients who may have undertreated chronic conditions or may need expanded access to life-saving rescue medications; and leverage patients’ access to pharmacists to safely increase the availability of certain medications, as pursued by manufacturers for nonprescription approval as developed within this initiative.45
Although the FDA is considering the NSURE Initiative, not all healthcare professions support this action. In June 2012, the American Medical Association House of Delegates adopted resolution 235, which was in opposition to the FDA’s Rx to OTC Paradigm Shift.46 The steering committee and editorial boards for NMA and SCI have incorporated regulatory issues such as NSURE into their programming when relevant so that self-care faculty members can share it with students in the classroom and with practicing pharmacists at the state level.
Health and Wellness Initiatives
As more Americans have become increasingly aware of their health, there is a growing trend toward disease prevention and maintaining healthy lifestyles. Although pharmacists have traditionally been available to offer advice and education on disease states, they are equally capable of offering advice and education on how to prevent disease through healthy lifestyles and information on nutritional supplementation, such as dietary supplements.
Over the past 3 to 4 years, most pharmacy retailers are encouraging pharmacists to offer wellness services to their patients.47-50 These include blood pressure and cholesterol screenings, blood glucose testing, risk assessments for chronic obstructive pulmonary disease and diabetes, immunizations, and weight-loss management. Some pharmacies even offer organic foods and fresh produce while nutrition experts educate patients on the benefits of healthy, nutritious foods.
Many insurance companies and employers are offering incentives for members to participate in wellness initiatives. The majority of services are offered free of charge to patients. Most of the financial benefits seen in pharmacies stem from increased sales of wellness products. Billing for pharmacy services not linked to a product, including wellness services, has traditionally been a challenge for pharmacists because they are not able to bill for cognitive services under their own National Provider Identification number but must collaborate with another provider or insurance company to gain reimbursement for services.
As Americans take control of their health through wellness initiatives, the pharmacist’s role becomes increasingly more important in the overall well-being of our patients. SCI and NMA continue to play a key role in providing self-care educators resources for preparing the next generation of pharmacists. Just as self-care has become more preventative, SCI and NMA have followed this trend, consistently incorporating topics related to health, wellness, and disease prevention and expanding on nonpharmacological measures, appropriate use of dietary supplements, and the growing role of home-based health-monitoring devices. Although current legislation does not allow for full reimbursement of services, we anticipate that future healthcare reform will give pharmacists the opportunity to be engaged in health and wellness initiatives.
CONCLUSION
Self-care has evolved and will continue to grow as patients become more empowered and medications that once were available only by prescription become more readily available without a prescription. Pharmacists have a vital role in triaging and assisting patients in the selection of the most appropriate treatment option. As self-care continues to expand, it is imperative that pharmacy colleges and schools meet the challenges of educating future pharmacists to effectively assist patients in this area. Although changes have been made in self-care curricula, there is a great need for outcomes research to evaluate how well this challenge is being met.
ACKOWLEDGMENT
The authors recognize Schwanda Flowers, PharmD, University of Arkansas for Medical Sciences College of Pharmacy, for her assistance with preparation of this manuscript.
REFERENCES
- 1.US Food and Drug Administration, US Department of Health and Human Services. Drug applications for over-the-counter (otc) drugs. http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/over-the-counterdrugs/default.htm. Accessed November 13, 2012.
- 2.Be Med Wise. Fact Sheet: The use of over-the-counter medications. http://www.bemedwise.org/press_room/may_2002_fact_otc.pdf. Accessed February11, 2014.
- 3.Consumer Healthcare Products Association. Ingredients and dosages transferred from Rx-to-OTC status (or new OTC approvals) by the Food and Drug Administration since 1975. http://www.chpa.org/SwitchList.aspx Accessed February 7, 2014.
- 4.U.S. Food and Drug Administration, US Department of Health and Human Services. Prescription to over-the-counter switch list, drug applications for over-the-counter (OTC) drugs. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106378.htm. Accessed November 9, 2012.
- 5.US Food and Drug Administration, US Department of Health and Human Services. FDA approves Plan B One-Step emergency contraceptive for use without a prescription for all women of child-bearing potential. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm358082. Accessed August 19, 2013.
- 6.US Food and Drug Administration, US Department of Health and Human Services. FDA approves over-the-counter Oxytrol for Women to treat overactive bladder. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm336815.htm. Accessed February 12, 2014.
- 7.US Food and Drug Administration, US Department of Health and Human Services. FDA approves over-the-counter Nasacort Allergy 24HR to treat hay fever and nasal allergies. http://www.fda.gov/drugs/newsevents/ucm370973.htm. Accessed February 13, 2014.
- 8.Combat Methamphetamine Epidemic Act 2005. Title VII of USA Patriot Improvement Reauthorization Act of 2005. Pub L No. 109-177. 109th Congress. March 9, 2006.
- 9.US Food and Drug Administration. Dietary Supplement and Nonprescription Drug Consumer Protection Act. Pub L No. 109 – 462. 109th Congress. December 22, 2006.
- 10.US Food and Drug Administration, US Department of Health and Human Services. Drug applications for over-the-counter (OTC) drugs. Current good manufacturing practice in manufacturing, packaging, labeling, or holding operations for dietary supplements. Final rule. Fed Regist. 2007;72(121):34751–34958. [PubMed] [Google Scholar]
- 11.Hooper CL. OTC Mevacor goes down a third time. Medical Progress Today Web site. http://www.medicalprogresstoday.com/spotlight/spotlight_indarchive.php?id=1742. Accessed November 26, 2012.
- 12.US Food and Drug Administration, US Department of Health and Human Services. Public health advisory: FDA recommends that over-the-counter (OTC) cough and cold products not be used for infants and children under 2 years of age, drug applications for over-the-counter (OTC) drugs. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051137. Accessed August 8, 2013.
- 13.US Food and Drug Administration, US Department of Health and Human Services. New steps aimed at cutting risks for acetaminophen. http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM239891.pdf. Accessed on November 11, 2012.
- 14.Nonprescription Medicines Academy. Facebook Web site. https://www.facebook.com/pages/Nonprescription-Medicines-Academy/106285896404. Accessed August 16, 2013.
- 15.APhA Pharmacy Library. Handbook of nonprescription drugs. http://pharmacylibrary.com/abstract/619023?type=update. Accessed August 16, 2013.
- 16.National Association of Boards of Pharmacy. NAPLEX Blueprint. http://www.nabp.net/programs/examination/naplex/naplex-blueprint/. Accessed November 11, 2012.
- 17.Lee MA. Nonprescription medicines and the North American Pharmacist Licensure Examination. Am J Pharm Educ. 2006;70(6):Article 138. doi: 10.5688/aj7006138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covington TR. Nonprescription therapy issues: issues and opportunities. Am J Pharm Educ. 2006;70(6):Article 137. doi: 10.5688/aj7006137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Accreditation Council for Pharmacy Education. Accreditation standards and guidelines for the professional program in pharmacy leading to the doctor of pharmacy degree. https://www.acpe-accredit.org/pdf/CPE_Standards_Final.pdf. Accessed August 15, 2013.
- 20.American Association of Colleges of Pharmacy. Self-care therapeutics/nonprescription medicines special interest group. http://www.aacp.org/governance/SIGS/selfcarenonprescriptionmedicine/Pages/default.aspx. Accessed November 11, 2012.
- 21.Nonprescription Medicines Academy. NASPA/NMA student pharmacist self-care championship. http://www.nmafaculty.org/pharmacy-resources/naspanma-student-pharmacist-self-care-championship. Accessed November 11, 2012.
- 22.Covington TR. National curriculum survey II: status of instruction in nonprescription drug therapy. Presented at: Annual Meeting of the American Association of Colleges of Pharmacy, San Diego, CA. July 2006.
- 23.Sulli MM, Pal S. National curriculum survey addressing nonprescription medicines in us schools/colleges of pharmacy. Am J Pharm Educ. 2009;74(4):Article 57. [Google Scholar]
- 24.Stoner SC, Fincham JE. Faculty role in classroom engagment and attendance. Am J Pharm Educ. 2012;76(5):Article 75. doi: 10.5688/ajpe76575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Pharmacists Association. APhA Self-Care Institute. http://www.pharmacist.com/apha-self-care-institute. Accessed August 16, 2013.
- 26.Nonprescription Medicines Academy. Faculty resources. http://www.nmafaculty.org/. Accessed August 16, 2013.
- 27.Galal S, Carr-Lopez S, Seal CR, Scott AN, Lopez C. Development and assessment of social and emotional competence through simulated patient consultations. Am J Pharm Educ. 2012;76(7):Article 132. doi: 10.5688/ajpe767132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buring SM, Kirby J, Conrad WF. A structured approach for teaching students to counsel self-care patients. Am J Pharm Educ. 2007;71(1):Article 8. doi: 10.5688/aj710108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hastings JK, Flowers SK, Pace AC, Spadaro D. An objective standardized clinical examination (OSCE) in an advanced nonprescription medicine course. Am J Pharm Educ. 2010;74(6):Article 98. doi: 10.5688/aj740698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao D. Skills developed using role-playing in a first-year pharmacy practice course. Am J Pharm Educ. 2011;75(5):Article 84. doi: 10.5688/ajpe75584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lupa AM, Stewart AL, O’Neil C. Comparison of active-learning strategies in motivational interviewing skills, knowledge, and confidence in first-year pharmacy students. Am J Pharm Educ. 2012;76(2):Article 28. doi: 10.5688/ajpe76228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers ER, King SR. The influence of a patient-counseling course on the communication apprehension, outcome expectations, and self-efficacy of first-year pharmacy students. Am J Pharm Educ. 2012;76(8):Article 152. doi: 10.5688/ajpe768152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orr KK. Integrating virtual patients into a self-care course. Am J Pharm Educ. 2007;71(2):Article 30. doi: 10.5688/aj710230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibbald D. Elective self-care course emphasizing critical reasoning principles. Am J Pharm Educ. 2011;75(9):Article 182. doi: 10.5688/ajpe759182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McFalls M. Integration of problem-based learning and innovative technology into a self-care course. Am J Pharm Educ. 2013;77(6):Article 127. doi: 10.5688/ajpe776127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson ME. Results of a team-based learning /traditional lecture model in a self-care therapeutics course. Am J Pharm Educ. 2011;75(5):Article 105. [Google Scholar]
- 37.Schneider SR, Ulbrick TR. Implementing team-based learning into an OTC/self-care course. Am J Pharm Educ. 2011;75(5):Article 105. [Google Scholar]
- 38.Ferreri SP, O’Connor SK. Redesign of a large lecture course into a small-group learning course. Am J Pharm Educ. 2013;77(1):Article 13. doi: 10.5688/ajpe77113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franks AS. Enhancing team-based active learning through hands-on experience with nicotine replacement therapy. Am J Pharm Educ. 2013;77(6):Article 128. doi: 10.5688/ajpe776128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haines SL, Van Amburgh JA. A vidcasting project to promote the pharmacist's role in bublic health. Am J Pharm Educ. 2010;74(6):Article 97. doi: 10.5688/aj740697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itskhoki A. Social media influence: foundational learning for pharmaceutical firms. http://www.knowledgenetworks.com/business-solutions/docs/DTC_SoMe_Itskhoki.pdf. Accessed November 26, 2012.
- 42.Wolff K. FDA’s policy on social media: why it matters for marketers of OTC drugs, food, dietary supplements and cosmetics. Update Magazine. March/April 2011. http://www.kelleydrye.com/publications/articles/1453/_res/id=Files/index=0/Wolff_FDA's%20Policy%20on%20Social%20Media_March%202011.pdf. Accessed November 26, 2012.
- 43.US Food and Drug Administration, US Department of Health and Human Services. FDA considers expanding definition of nonprescription drugs, drug applications for over-the-counter (OTC) drugs. http://www.fda.gov/Drugs/ResourcesForYou/SpecialFeatures/ucm297128.htm. Accessed November 26, 2012.
- 44.US Food and Drug Administration, US Department of Health and Human Services. Using innovative technologies and other conditions of safe use to expand which drug products can be considered nonprescription. Public hearing. Fed Regist. 2012;77(39):12059–12062. [Google Scholar]
- 45.American Pharmacists Association. Revisions to FDA drug paradigm. http://www.pharmacist.com/revisions-fda-drug-paradigm-otc. Accessed November 26, 2012.
- 46.American Pharmacists Association. APhA continues OTC + dialogue with AMA. September 1, 2012. http://www.pharmacist.com/apha-continues-otc-dialogue-ama-0. Accessed November 26, 2012.
- 47.Walgreens Pharmacy. Community health network and healthcare clinic at select Walgreens begin collaboration of healthcare services.
- 48. http://news.walgreens.com/article_display.cfm?article_id=5787. Accessed February 13, 2014. Walgreens. Walgreens Way to Well health tour with AARP. http://www.waytowelltour.com/. Accessed February 1, 2013.
- 49.Rite Aid Pharmacy. News room. http://www.riteaid.com/company/news/news_details.jsf?itemNumber=1623. Accessed February 1, 2013.
- 50.Humana. HumanaVitality – a wellness and rewards program for your well-being and healthy living. http://www.humana.com/vitality/. Accessed February 1, 2013.


