Abstract
Enzymatic activities of the cytidine 5′-diphosphate choline pathway for lecithin biosynthesis were demonstrated in homogenates of onion stem (Allium cepa). Choline kinase activity was present in the postmicrosomal supernatant, with less than 3% sedimenting with the particulate fractions. Phosphorylcholine-cytidyl transferase was distributed among all fractions, and phosphorylcholine-glyceride transferase was predominantly found in the particulate fraction.
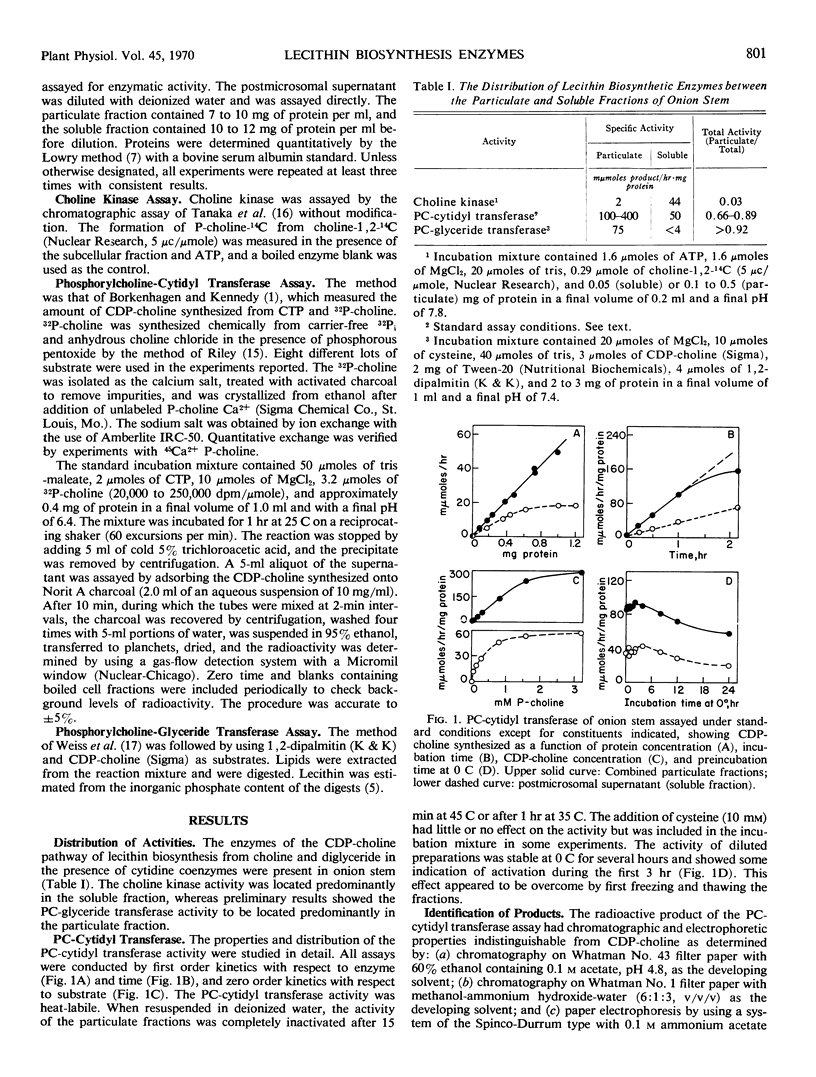
The phosphorylcholine-cytidyl transferase activity of onion stem required a divalent ion (Mg2+ or Mn2+) for activity, was inhibited by Ca2+, and was specific for cytidine triphosphate, with optimal activity in the range pH 6 to 7. To evaluate the distribution among cell fractions, conditions of pH, cofactors, substrate, and assay were optimized for each fraction. One-third of the transferase activity sedimented with the mitochondria-proplastids fraction, and one-third was in the microsomal supernatant. The dictyosome fraction contained about 10% of the total activity but showed a greater specific activity than the other fractions. Similar results were obtained with homogenates from rat liver, in that purified Golgi apparatus fractions contained the highest phosphorylcholine-cytidyl transferase activity on a protein basis when compared with other cell fractions at pH 7.2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORKENHAGEN L. F., KENNEDY E. P. The enzymatic synthesis of cytidine diphosphate choline. J Biol Chem. 1957 Aug;227(2):951–962. [PubMed] [Google Scholar]
- BREMER J., GREENBERG D. M. Biosynthesis of choline in vitro. Biochim Biophys Acta. 1960 Jan 1;37:173–175. doi: 10.1016/0006-3002(60)90104-9. [DOI] [PubMed] [Google Scholar]
- Cunningham W. P., Morré D. J., Mollenhauer H. H. Structure of isolated plant Golgi apparatus revealed by negative staining. J Cell Biol. 1966 Feb;28(2):169–179. doi: 10.1083/jcb.28.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H., CHAMBERS J. E. GLUTARALDEHYDE STABILIZATION AS AN AID TO GOLGI APPARATUS ISOLATION. Exp Cell Res. 1965 Jun;38:672–675. doi: 10.1016/0014-4827(65)90392-7. [DOI] [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H. ISOLATION OF THE GOLGI APPARATUS FROM PLANT CELLS. J Cell Biol. 1964 Nov;23:295–305. doi: 10.1083/jcb.23.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J. In vivo incorporation of radioactive metabolites by Golgi apparatus and other cell fractions of onion stem. Plant Physiol. 1970 Jun;45(6):791–799. doi: 10.1104/pp.45.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Tolbert N. E., Gohlke A. F. Choline kinase and phosphorylcholine phosphatase in plants. Plant Physiol. 1966 Feb;41(2):307–312. doi: 10.1104/pp.41.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS S. B., SMITH S. W., KENNEDY E. P. The enzymatic formation of lecithin from cytidine diphosphate choline and D-1,2-diglyceride. J Biol Chem. 1958 Mar;231(1):53–64. [PubMed] [Google Scholar]
- WILGRAM G. F., KENNEDY E. P. INTRACELLULAR DISTRIBUTION OF SOME ENZYMES CATALYZING REACTIONS IN THE BIOSYNTHESIS OF COMPLEX LIPIDS. J Biol Chem. 1963 Aug;238:2615–2619. [PubMed] [Google Scholar]
- WILSON J. D., GIBSON K. D., UDENFRIEND S. Studies on the precursors of the methyl groups of choline in rat liver. J Biol Chem. 1960 Nov;235:3213–3217. [PubMed] [Google Scholar]
- Yunghans W. N., Keenan T. W., Morré D. J. Isolation of golgi apparatus from rat liver. 3. Lipid and protein composition. Exp Mol Pathol. 1970 Feb;12(1):36–45. doi: 10.1016/0014-4800(70)90073-0. [DOI] [PubMed] [Google Scholar]


