Abstract
Splenic metastasis of ovarian cancer appears to be more common in serous cystadenocarcinomas. Splenic metastasis usually occurs postoperatively and simultaneously with dissemination to the greater omentum and pelvic cavity. Compared with other ovarian cancer subtypes, ovarian clear cell adenocarcinoma (OCCA) is rare, accounting for <5% of all ovarian malignancies. OCCA has a distinct histological type with poor prognosis and resistance to platinum-based chemotherapy. In the present study, a case of isolated splenic metastasis of OCCA was reported. A 53-year-old female presented with a mass in the left upper quadrant without any other clinical manifestations. Subsequent abdominal and pelvic computed tomography scans revealed multiple mixed cystic-solid lesions, potentially predicting ovarian malignancy. Pathological tests following ovarian cytoreductive surgery revealed primary OCCA with metastases to the spleen. The current study also reviewed recently published literature on splenic metastasis of ovarian carcinoma and demonstrated that the reported case was a rare case of isolated splenic metastasis of OCCA.
Keywords: ovarian clear cell carcinoma, splenic metastasis, cytoreductive surgery, splenectomy
Introduction
Ovarian cancer is a common gynecological malignant tumor that presents at a late clinical stage in >80% of the total patient population (1). It is associated with a 5-year survival rate of 35% in advanced ovarian cancer patients. The incidence of ovarian cancer ranks third while the mortality rate is the highest of all gynecological malignant tumors. The National Comprehensive Cancer Network 2011 guidelines revealed that epithelial ovarian carcinoma (accounting for 80% of all malignant ovarian tumors) is the leading cause of mortality among gynecological cancers in the USA (2). In total, <40% of patients with epithelial ovarian carcinoma are completely cured (3,4). The occurrence of ovarian clear cell carcinoma (OCCA) is relatively infrequent, accounting for 5% of all ovarian malignancies and 3.7–12.1% of all epithelial ovarian carcinomas (5–7). As reported previously, OCCA has a distinct histological type with poor prognosis and resistance to platinum-based chemotherapy (7,14). The majority of patients with advanced or recurrent OCCA have a low benefit-to-failure ratio from palliative chemotherapy (7).
According to clinical data, splenic tumors metastasized from ovarian cancer are uncommon, accounting for 2–4% of all malignant tumors of the spleen with an incidence of 0.6% in autopsy studies and ~1.1% in splenectomies (8). In the present study, a rare case of splenic metastasis of OCCA was reported and an extensive review of the literature on splenic metastases of ovarian carcinomas published in the past decade was performed. The aim was to improve the diagnosis and treatment of splenic metastasis of ovarian cancer.
Case report
Patient history
A 53-year-old female was admitted to the First Affiliated Hospital of Xi’an Jiaotong University, (Xi’an, China) after presenting with a mass in the left upper quadrant which had been there for three months. The patient did not present with any other clinical manifestations. The study was conducted in accordance with the Declaration of Helsinki and was performed with approval from the Ethics Committee of Xi’an Jiaotong University. Written informed consent was provided by the participant.
Examination
An abdominal B-ultrasound, performed prior to hospitalization, revealed multiple mixed cystic-solid lesions in the left upper quadrant and the lower abdominal region. A needle biopsy of the mass revealed hyperplasia of fibrous granulation tissue, infiltration of chronic inflammatory cells, focal shape necrosis, active growth and tumor-like hyperplasia in sections of the cells. The histological features prompted the consideration of a mesenchymal tissue tumor. Following admission, the patient had a cancer antigen (CA)-125 level of up to 4,712 U/ml (normal <35 U/ml). Subsequent abdominal and pelvic computed tomography (CT) scans revealed multiple mixed cystic-solid lesions and abdominal and pelvic cavity effusion, presumably due to an ovarian malignancy and multiple metastatic intraperitoneal tumors (Fig. 1, prior to chemotherapy). Pathological results of the needle biopsy on the pelvic mass revealed a level II papillary adenocarcinoma accompanied by necrosis.
Figure 1.
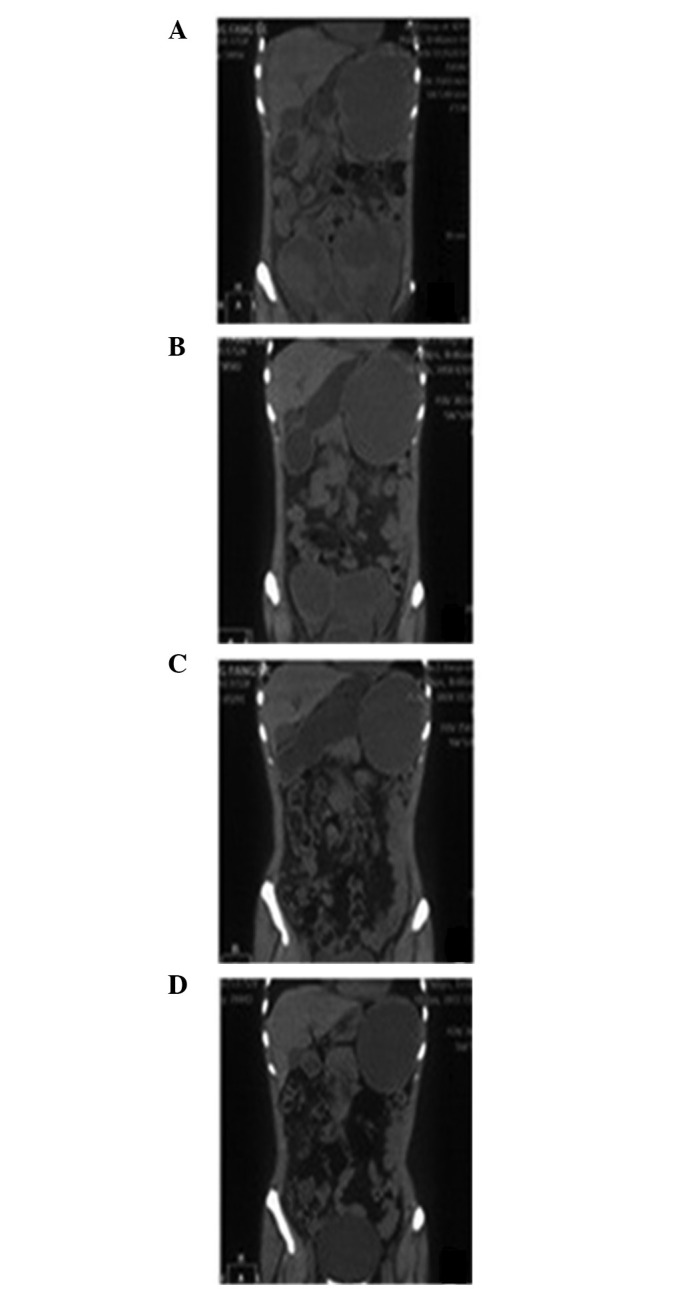
Primary pelvic and metastatic splenic tumors prior to and following chemotherapy. (A) Prior to chemotherapy, extensive lesions with vague boundaries were detected in the pelvic cavity and mixed cystic-solid masses were identified in the spleen. Following (B) two and (C) four cycles of chemotherapy, changes occurred in the lesions in the pelvic cavity and spleen. (D) Following six cycles of chemotherapy, the primary pelvic tumor was markedly degraded and the splenic mass gradually became cystic.
Treatment
The patient was diagnosed with ‘abdominal and pelvic cavity metastasis of ovarian carcinoma’ and administered six cycles of systemic chemotherapy (TP scheme). The patient received 100 mg docetaxel (day l) and 40 mg cisplatin (days 1–2) for 21 days in each cycle. During the course of therapy, the CA-125 level decreased from 4,712 U/ml to 39.42 U/ml. Follow-up CT reexamination demonstrated that the lesions had markedly diminished, thus, the therapeutic evaluation was partial remission (PR) (Fig 1). Subsequently the patient underwent ovarian cytoreductive surgery in addition to a splenectomy. The final pathology results revealed OCCA (oxyphil cell type) with massive necrosis and splenic metastasis of OCCA (oxyphil cell type) with massive necrosis, which were in accordance with the chemotherapeutic results. The immunohistochemical results were positive for CA-125, cytokeratin (CK)7, CK19 and epithelial membrane antigen, but negative for CK20 and CD68 (Fig. 2).
Figure 2.
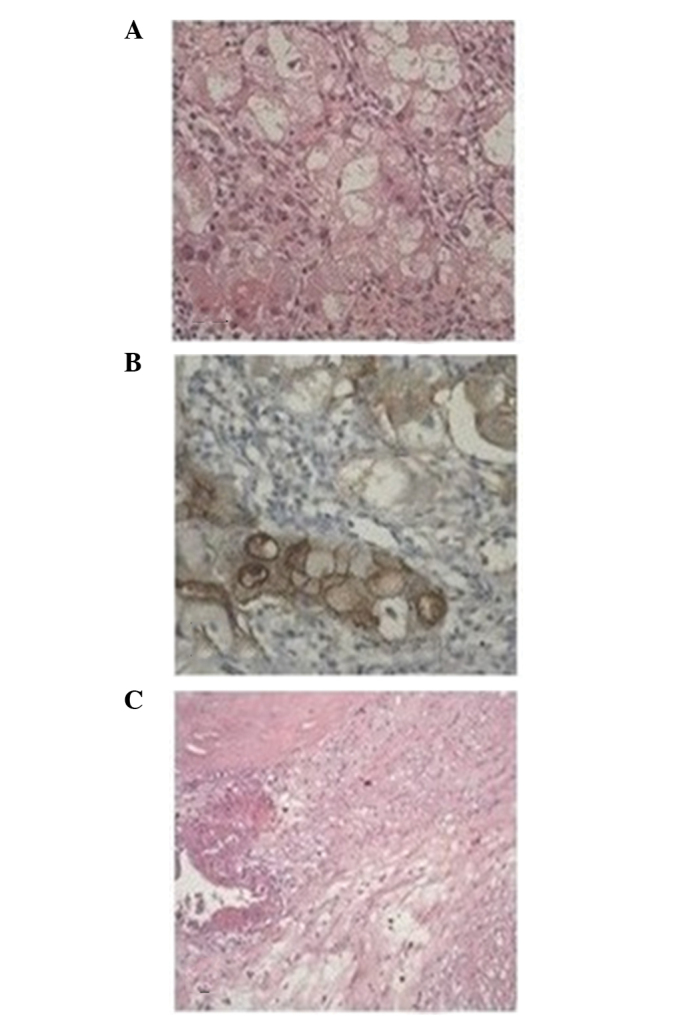
Postoperative pathological results of the lesions. (A) Ovarian pathology following cytoreductive surgery (H&E staining; magnification, ×400). (B) CA-125 expression in ovarian clear cell carcinoma (immunohistochemical staining; magnification, ×400). (C) Splenic pathology following splenectomy (H&E staining; magnification, ×200). H&E, hematoxylin and eosin; CA-125, cancer antigen-125.
Literature review
Case reports and literature reviews on splenic metastasis of ovarian carcinoma, which had been published in the past decade, were collected. Based on the search results, preliminary analysis of the clinical characteristics of splenic metastasis of ovarian carcinoma was conducted in 34 cases (Table I).
Table I.
Clinical characteristics of splenic metastasis of ovarian carcinoma in 34 cases published in the past decade.
| Case | Age (years) | Pathology | Time of postoperative splenic metastasis (years) | Characteristics of splenic metastasis (ref.) |
|---|---|---|---|---|
| 1 | 52 | Serous | 20 | Solitary (10) |
| 2 | 85 | Serous | Simultaneity | Solitary (11) |
| 3 | 38 | Serous | 3 | Solitary (12) |
| 4 | 29 | Serous | 1 | Solitary (13) |
| 5 | 61 | Serous | 3 (following subsequent surgery) | Isolated (14) |
| 6 | 51 | Serous | 1 | Bone metastatic (15) |
| 7 | 53 | Serous | 4 | Solitary (15) |
| 8 | 59 | Serous | 9 | Solitary (16) |
| 9 | 45 | Serous | 6 (following subsequent surgery) | Solitary (17) |
| 10 | 43 | Angiosarcoma | 2 | Solitary (18) |
| 11 | 59 | Serous | 6 | Solitary (19) |
| 12 | 72 | Carcinosarcoma | 4 | Solitary (20) |
| 13 | 57 | Serous | 12 | Solitary (21) |
| 14 | 55 | Serous | 2 | Solitary |
| 15 | 46 | Serous | 1 | Disseminated in the omentum majus and pelvic cavity |
| 16 | 80 | Serous | 8 | Solitary |
| 17 | 57 | Serous | 2 | Solitary |
| 18 | 59 | Serous | 1 | Solitary |
| 19 | 49 | Serous | 11 | Solitary |
| 20 | 52 | Serous | 3 | Solitary |
| 21 | 66 | Endometrial adenocarcinoma | 3 | Disseminated in the omentum majus and the abdominal cavity |
| 22 | 70 | Serous | 1 | Solitary, sporadic |
| 23 | 53 | Serous | 3 | a |
| 24 | 45 | Serous | 3 | a |
| 25 | 55 | Serous | Simultaneity | a |
| 26 | 43 | Serous | 4 | a |
| 27 | 64 | Serous | Simultaneity | a |
| 28 | 47 | Serous | Simultaneity | a |
| 29 | 53 | Serous | 2 | a |
| 30 | 55 | Serous | Simultaneity | a |
| 31 | 45 | Serous | 2 | a |
| 32 | 59 | Serous | 2 | a |
| 33 | 61 | Serous | 4 | a |
| 34 | 48 | Serous | 5 | a |
Information not provided; 1–13, data from case reports published in Western countries (references may be found on Pubmed); 14–34, data from case reports published in China (refer to the footnotes which cannot be found in Pubmed).
In total, 13 case reports were retrieved on splenic metastasis of ovarian cancer published in Western countries. Although each case had distinctive features, pathologically, the majority were serous papillary adenocarcinoma (9–16,18,20) with only one case of angiosarcoma (17) and one case of carcinosarcoma (19). No case reports regarding OCCA were found. Splenic metastasis occurred postoperatively with the exception of one case in which splenic metastasis occurred simultaneously with radical resectioning of ovarian cancer. Preliminary analysis was also conducted on 21 cases in ten case reports of splenic metastasis of ovarian cancer published in China over the past decade. The analytical results were largely consistent with the data from the case reports published in Western countries. Pathologically, these cases were serous papillary cystadenocarcinomas with two cases of mucinous papillary adenocarcinoma. Splenic metastasis primarily occurred years after surgery. Splenic metastasis was only detected in four cases when ovarian cancer was first diagnosed.
OCCA is infrequently reported in literature owing to its low incidence. Pathologically, the majority of cases in the reports on splenic metastasis of ovarian cancer belong to serous papillary cystadenocarcinoma and only individual cases are poorly differentiated transitional cell carcinomas. Based on the present analyses of the associated case reports and literature reviews, we hypothesize that splenic metastasis of ovarian carcinoma largely occurs postoperatively, following subsequent surgeries or years after radiotherapy and chemotherapy. Splenic metastasis is primarily accompanied by dissemination to the omentum majus and pelvic cavity. Isolated metastatic splenic lesions only occur in a few individual cases.
Discussion
OCCA is a malignant tumor found primarily among elderly females. It was formally defined as a special type of ovarian cancer in 1973 by the World Health Organization (21–23). OCCA originates from the Müllerian duct and is closely associated with endometriosis (24). Compared with other adenocarcinomas, OCCA has unique biological features, resulting in poor prognosis and high rates of recurrence and metastasis (25). The main therapeutic strategies focus on surgery and chemotherapy, although OCCA is relatively resistant to conventional platinum-based chemotherapy (7,26). In the present study, a rare case of splenic metastasis of OCCA was reported. No experience or rules concerning the treatment and prognosis of OCCA were available to follow. Thus, the aim was to provide new ideas for the treatment and prognosis of this rare disease.
The case reported in this study is valuable due to the unusual pathological type of OCCA. Dissemination to the spleen and pelvic cavity were detected at the initial diagnosis. The case was once misdiagnosed by needle biopsy, indicating that needle biopsy alone may easily lead to misdiagnosis for special pathological types of ovarian cancers, including OCCA, with splenic metastasis.
Compared with easily affected organs, including the liver and lung, the spleen is rarely affected (27) with an incidence of ~0.6% in autopsy studies and 1.1% in splenectomies (8). Primary tumors with splenic metastases, systematically reported in Western countries, are mainly melanomas and lymphomas, followed by breast, lung and ovarian cancers (28). It is generally recognized that among the routes of metastasis, hematogenous metastasis ranks first, followed by lymphatic and implantation metastases. Reasons for the rare occurrence of splenic metastases are as follows. Firstly, the sharp angle made by the splenic artery makes it difficult for tumor emboli to enter the spleen. Secondly, the rhythmic contractile nature of the spleen squeezes out the tumor embolus and prevents the tumor lodging in the spleen. Thirdly, the absence of afferent lymphatics that bring metastatic tumors to the spleen. Finally, antitumor activity due to the high concentration of lymphoid tissue in the spleen (29). It has been hypothesized that metastatic tumors rarely grow in the spleen since the spleen is a pharmacological and immunological sanctuary. Once a metastatic splenic tumor grows, it may indicate that monoclonal slow-growth is occurring (30).
There is no unified treatment and prognosis for late-stage ovarian cancer with splenic metastasis due to the low incidence. Therefore, more clinical studies are required. Bristow et al (31) confirmed that the thoroughness of cytoreductive surgery is key to prognosis. Thus, a more thoroughly performed surgery is likely to lead to a better prognosis. Destruction of 10% of the cancer cells enhances the median survival rate by 5.5%. Patients in whom >75% of the cancer cells have been eliminated have a median survival time of 33.9 months, while patients in whom <75% of the cancer cells have been eliminated have a median survival time of 22.7 months (P<0.05). However, in more than two-thirds of patients with ovarian cancer, the lesions have already spread prior to undergoing primary surgery. Thus, in order to excise all metastatic lesions, cytoreductive surgery involves the pelvic and abdominal cavities, including a splenectomy, diaphragmatic surgery and partial hepatectomy. Splenectomies, based on the accurate evaluation of a patient’s condition, may improve the patient’s median survival time and quality of life. However, the decision to perform surgery should be made cautiously. If satisfactory removal surgery cannot be achieved, priority should be given to palliative surgery and chemotherapy.
In the case of the present study, following six cycles of TP chemotherapy, the CA-125 levels decreased gradually to within a normal range. Imaging evaluation revealed that the curative effect reached PR. In addition, the patient underwent cytoreductive surgery and a splenectomy and the surgical results were satisfactory. In the following 8 months of follow-up, the patient was in a good condition without any sign of recurrence and metastasis. The patient continues to be followed-up and further observation is required for the long-term survival.
In conclusion, splenic metastasis of ovarian cancer may be diagnosed by a combination of clinical history, imaging information and histopathology. For space-occupying lesions of the spleen, CT is capable of demonstrating intraparenchymal and infiltrative splenic metastasis in patients with ovarian cancer, even in the absence of increased CA-125 levels (32,33). While aspiration cytology is not recommended, complete cytoreduction in primary or subsequent surgeries is an ideal treatment for space-occupying lesions of the spleen; even for recurrent carcinomas. Cytoreductive surgery is capable of prolonging progression-free survival times and improving quality of life (34–37). Due to the immunological function of the spleen, the formation of a metastatic splenic carcinoma usually indicates an advanced stage of the disease that has poor prognosis. In such cases, a splenectomy followed by a comprehensive therapy is the preferred course of treatment. Therapeutic treatments may be more accurately determined based on the consideration of the primary lesions, the general condition of the patient and whether multiple metastases have occurred in other organs.
References
- 1.Fleming GF, Ronnett BM, Seidman J. Epithelial ovarian cancer. In: Barakat RR, Markman M, Randall ME, editors. Principles and Practice of Gynecologic Oncology. 5th edition. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. pp. 763–836. [Google Scholar]
- 2.Chan JK, Cheung MK, Husain A, et al. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006;108:521–528. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Sugawa T, Umesaki N, Yajima A, et al. A group study on prognosis of ovarian cancer in Japan. Nihon Sanka Fukinka Gakki Zasshi. 1992;44:827–832. (In Japanese) [PubMed] [Google Scholar]
- 6.O’Brien ME, Schofield JB, Tan S, et al. Clear cell epithelial ovarian cancer (mesonephroid): bad prognosis only in early stages. Gynecol Oncol. 1993;49:250–254. doi: 10.1006/gyno.1993.1117. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama T, Kamura T, Kigawa J, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. [PubMed] [Google Scholar]
- 8.Lam KY, Tang V. Metastatic tumors to the spleen: a 25-year clinicopathologic study. Arch Pathol Lab Med. 2000;124:526–530. doi: 10.5858/2000-124-0526-MTTTS. [DOI] [PubMed] [Google Scholar]
- 9.Izuishi K, Sano T, Usuki H, et al. Isolated splenic metastasis of ovarian cancer 20 years after operation: a case report and literature review. Tumor. 2010;96:784–786. doi: 10.1177/030089161009600525. [DOI] [PubMed] [Google Scholar]
- 10.Ghani AA, Hashmi ZA, Chase DM, et al. Intraparenchymal metastases to the spleen from ovarian cancer: a case report. J Med Case Rep. 2010;4:30. doi: 10.1186/1752-1947-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yano H, Iwazawa T, Kinuta M, et al. Solitary splenic metastasis from ovarian cancer successfully treated by hand-assisted laparoscopic splenectomy: report of a case. Surg Today. 2002;32:750–752. doi: 10.1007/s005950200142. [DOI] [PubMed] [Google Scholar]
- 12.Koh YS, Kim JC, Cho CK. Splenectomy for solitary splenic metastasis of ovarian cancer. BMC Cancer. 2004;4:96. doi: 10.1186/1471-2407-4-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshioka R, Okabayashi T, Nishimori I, et al. A long-survived case with solitary splenic metastasis from ovarian carcinoma. Surg Technol Int. 2008;17:192–194. [PubMed] [Google Scholar]
- 14.Alloni R, Garberini A, Caputo D, Coppola R. Solitary splenic metastasis of ovarian carcinoma: report of two cases. Surg Today. 2008;38:1144–1147. doi: 10.1007/s00595-007-3747-6. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa N. Solitary splenic metastasis of ovarian cancer. Arch Gynecol Obstet. 2007;275:499–502. doi: 10.1007/s00404-006-0274-4. [DOI] [PubMed] [Google Scholar]
- 16.Ushijima K, Nishida T, Okura N, et al. Solitary splenic recurrence of ovarian cancer: case report and review of the literature. Arch Gynecol Obstet. 1999;263:79–81. doi: 10.1007/s004040050268. [DOI] [PubMed] [Google Scholar]
- 17.Valbuena JR, Levenback C, Mansfield P, Liu J. Angiosarcoma of the spleen clinically presenting as metastatic ovarian cancer. A case report and review of the literature. Ann Diagn Pathol. 2005;9:289–292. doi: 10.1016/j.anndiagpath.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Otrock ZK, Seoud MA, Khalifeh MJ, et al. Laparoscopic splenectomy for isolated parenchymal splenic metastasis of ovarian cancer. Int J Gynecol Cancer. 2006;16:1933–1935. doi: 10.1111/j.1525-1438.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- 19.Olsen AB, Parqman S, Gillespie T. Solitary splenic metastasis from ovarian carcinosarcoma: a case report. J Med Case Rep. 2011;5:56. doi: 10.1186/1752-1947-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa H, Naitoh H, Tsuchihashi H, et al. A case of solitary splenic metastasis from an ovarian cancer 12 years after primary resection. Gan To Kagaku Ryoho. 2010;37:1799–1803. (In Japanese) [PubMed] [Google Scholar]
- 21.Teilum G. Gonocytoma homologous ovarian and testicular tumours. Acta Pathol Microbiol Scand. 1946;23:242–251. [Google Scholar]
- 22.Scully RE. Recent progress in ovarian cancer. Hum Pathol. 1970;1:73–98. doi: 10.1016/s0046-8177(70)80005-3. [DOI] [PubMed] [Google Scholar]
- 23.Serov SF, Scully RE, Sobin LH. Histologic typing of ovarian tumours. 9. World Health Organization; Geneva: 1973. International histologic classification of tumours. [Google Scholar]
- 24.Tan DS, Kaye S. Ovarian clear cell adenocarcinoma: a continuing enigma. J Clin Pathol. 2007;60:355–360. doi: 10.1136/jcp.2006.040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto M, Takano M, Goto T, et al. Clear cell histology as a poor prognostic factor for advanced epithelial ovarian cancer: a single institutional case series through central pathologic review. J Gynecol Oncol. 2013;24:37–43. doi: 10.3802/jgo.2013.24.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takano M, Kikuchi Y, Yaegashi N, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–1374. doi: 10.1038/sj.bjc.6603116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgenstern L, Rosenberg J, Geller SA. Tumors of the spleen. World J Surg. 1985;9:468–476. doi: 10.1007/BF01655283. [DOI] [PubMed] [Google Scholar]
- 28.Siler J, Hunter TB, Weiss J, Haber K. Increased echogenicity of the spleen in benign and malignant disease. AJR Am J Roentgenol. 1980;134:1011–1014. doi: 10.2214/ajr.134.5.1011. [DOI] [PubMed] [Google Scholar]
- 29.Capizzi PJ, Allen KB, Amerson JR, Skandalakis JE. Isolated splenic metastasis from rectal carcinoma. South Med J. 1992;85:1003–1005. doi: 10.1097/00007611-199210000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Lauro S, Trasatti L, Capalbo C, et al. Solitary splenic recurrence of epithelial ovarian cancer:a case report and review. Anticancer Res. 2002;22:3643–3645. [PubMed] [Google Scholar]
- 31.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 32.Spencer NJ, Spencer JA, Perren TJ, Lane G. CT appearances and prognostic significance of splenic metastasis in ovarian cancer. Clin Radiol. 1998;53:417–421. doi: 10.1016/s0009-9260(98)80269-9. [DOI] [PubMed] [Google Scholar]
- 33.Senturk S, Karcaaltıncaba M, Akata D. CT diagnosis of intrasplenic metastasis from ovarian carcinoma. Eur J Radiol. 2012;81:1094–1099. doi: 10.1016/j.ejrad.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 34.Chen LM, Leuchter RS, Lagasse LD, Karlan BY. Splenectomy and surgical cytoreduction for ovarian cancer. Gynecol Oncol. 2000;77:362–368. doi: 10.1006/gyno.2000.5800. [DOI] [PubMed] [Google Scholar]
- 35.Eisenkop SM, Spirtos NM, Friedman RL, et al. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with ovarian cancer: a prospective study. Gynecol Oncol. 2003;90:390–396. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 36.Eisenkop SM, Spirtos NM, Lin WCM. Splenectomy in the context of primary cytoreductive operations for advanced epithelial ovarian cancer. Gynecol Oncol. 2006;100:344–348. doi: 10.1016/j.ygyno.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Magtibay PM, Adams PB, Silverman MB, et al. Splenectomy as part of cytoreductive surgery in ovarian cancer. Gynecol Oncol. 2006;100:369–374. doi: 10.1016/j.ygyno.2006.03.028. [DOI] [PubMed] [Google Scholar]


