Abstract
The report ‘I saw the stimulus’ operationally defines visual consciousness, but where does the ‘I’ come from? To account for the subjective dimension of perceptual experience, we introduce the concept of the neural subjective frame. The neural subjective frame would be based on the constantly updated neural maps of the internal state of the body and constitute a neural referential from which first person experience can be created. We propose to root the neural subjective frame in the neural representation of visceral information which is transmitted through multiple anatomical pathways to a number of target sites, including posterior insula, ventral anterior cingulate cortex, amygdala and somatosensory cortex. We review existing experimental evidence showing that the processing of external stimuli can interact with visceral function. The neural subjective frame is a low-level building block of subjective experience which is not explicitly experienced by itself which is necessary but not sufficient for perceptual experience. It could also underlie other types of subjective experiences such as self-consciousness and emotional feelings. Because the neural subjective frame is tightly linked to homeostatic regulations involved in vigilance, it could also make a link between state and content consciousness.
Keywords: subjective experience, brain–body interactions, perceptual awareness, self-consciousness, emotion, feeling
1. Introduction
‘What do you see?’ asks the neuroscientist interested in visual consciousness. ‘Well, right now, I see an article page, words and sentences, some titles highlighted in bold or italics …’ would answer the reader of this paper. In laboratory experiments, a standard way to measure visual consciousness is simply to ask to subjects whether or not they saw the stimulus. So far, studies aiming at understanding the neural basis of perceptual consciousness have mostly concentrated on the mechanisms bringing a specific content to the forefront of the mind [1–3]: why words rather than paper or screen texture are reported, or why a stimulus is seen or missed. In other words, the research programme on perceptual consciousness focused on the sensory and cognitive steps triggered by the stimulus itself which contribute to the report ‘I saw the stimulus’ that operationally defines visual consciousness. We argue here that to understand fully how the basic statement ‘I saw the stimulus’ can be produced, one has to consider the nature and biological implementation of the ‘I’ as well.
We introduce here the idea of a neural subjective frame, corresponding to neural mechanisms defining the organism as a unified entity to tag biologically your conscious experience as belonging to you. The existence of such a mechanism is necessary for perceptual consciousness, to be able to report ‘I see something’ or ‘I hear something’. It is indeed a hallmark of conscious percepts that they belong to the observer, and the observer never fails to perceive them as his or her own experience. A comprehensive model of perceptual consciousness should therefore include a biological implementation for this subjective frame. Note that to have your own visual conscious experience, you do not need to be reflectively and/or explicitly aware of the subjective frame. Implicit, pre-reflective, subjective aspects of perceptual experience have been largely underestimated in neural theories of consciousness so far, although philosophers of phenomenology [4–8] and some neuroscientists [9–14] acknowledged their existence and importance. We explore those issues in §2, which is devoted to the definition and properties of the subjective frame.
We further propose that the implicit neural subjective frame is based on the neural representation of afferent bodily signals. Bodily signals can arise from two distinct systems. One system corresponds to sensory–motor interactions, involved for instance, when reaching and grasping for a glass of wine. It seems unlikely that this type of bodily afferences is a core constituent of conscious perceptual experiences, because locked-in patients, who are fully paralysed and whose brain does not receive any feedback on action performance, nevertheless consciously experience the world around them [2]. It therefore seems more promising to turn to another type of brain–body interactions that involves vital internal organs such as the heart or the gut that are constantly monitored and regulated by the central nervous system. In §§3 and 4, we describe the pathways of visceral integration, and examine existing experimental evidence suggesting a link between perceptual experience and cardiac information.
In this study, we propose the existence of an implicit neural subjective frame based on afferent visceral information and inherent to conscious perceptual experience. This proposal has two important implications. First, it constitutes an important step towards explaining the subjective nature of perceptual experience, because it offers a biological mechanism for the first-person perspective inherent in subjective perceptual reports. Second, it creates a link between different aspects of subjective experience: perceptual experience, but also self-consciousness and emotional feelings. Indeed, emotions, although rarely discussed in the framework of consciousness studies (but see [15,16]), have an important subjective component.
2. Definition and properties of the subjective frame
(a). Introducing the concept of subjective frame
Conscious perceptual experiences are by essence private and subjective [17]: when I see a bunch of flowers, it triggers a specific feeling that is vivid yet difficult to describe, and that cannot be directly compared with yours. The terms to describe this constitutive aspect of conscious perceptual experience range from sensation [18], to phenomenal consciousness [19] or qualia [20]. In intuitive terms, a conscious perceptual experience refers to the way external information appears to us via our senses. Transposed to laboratory experiments, detecting a stimulus at threshold is subjective: there is no true or false experience of a stimulus at threshold. What the subject reports may not correspond to what was presented on the screen, but the subject knows what he or she has experienced, and this subjective aspect has to be accounted for.
The private and subjective dimension of perceptual consciousness has often been considered as a major difficulty for empirical studies of perceptual consciousness. As stated in the influential paper by Crick & Koch [21, p. 264], ‘there is also the problem of qualia. Some argue that certain aspects of consciousness […], being essentially private, cannot in principle be addressed in any objective, scientific study. We feel that this difficult issue is, for the moment, best left on one side’. This advice was indeed followed: theories of perceptual consciousness concentrate on the cascade of events triggered by stimulus processing, be it availability in a global frontoparietal workspace [3,22], integration/segregation [23] or recurrent processing [24]. The field remains either mute or elusive on the issue of the subjective dimension of experience, although new behavioural measures based on first-person methodologies are being developed to try to specifically capture this fundamental property [13,25]. The existing neural theories of consciousness sometimes seem to imply that the first-person perspective inherent to conscious perceptual experience would arise somehow from externally triggered signals. However, the first-person perspective does exist even in the absence of sensory stimulation, and should pre-exist perceptual experience: there cannot be a conscious experience from no one.
In a similar manner, most experiments on perceptual consciousness have concentrated so far on stimulus-related neural events and left aside the issue of subjectivity. For instance, at threshold for consciousness, a subject being presented with the same physical sensory information at each trial would sometimes report a hit, corresponding to the statement ‘I saw (or heard, or felt) the stimulus’ and sometimes a miss, corresponding to ‘I did not see (or hear, or feel) the stimulus’. Such fluctuations are typically attributed to fluctuations in the sensory and/or cognitive processing of the stimulus. However, the statement ‘I saw the stimulus’ contains two parts: one related to the stimulus, and the other, most often overlooked, related to the ‘I’. Fluctuations in the ‘I’, or in the strength of the connection between stimulus processing and the ‘I’ could also account for hits and misses.
We define the neural subjective frame as the basic biological mechanisms defining the subject as a biological entity, as an anchoring point from which the first-person statements characteristics of consciousness (I see a flower, I hear a construction site, etc.) can be expressed. Indeed, for a sensory experience to take place, perceptual processing has to be referred to the subject to become consciously experienced: when I report seeing a square rather than a diamond, or a face rather than a vase, this is my visual experience, my own perspective, and it is because of this first-person perspective that I have a subjective conscious sensory experience. As detailed in §§3 and 4, we argue that the neural subjective frame is a low-level building block of subjective experience which is not explicitly experienced by itself that is necessary but not sufficient for perceptual experience, and that could underlie not only perceptual experience, but also self-consciousness and subjective emotional feelings.
(b). Properties of the subjective frame
(i). The subjective frame is not explicitly experienced by itself
It is important to underline here that the subjective frame is not experienced in itself: one does not have to be thinking explicitly about oneself as experiencing a flower to experience the flower and report ‘I see a flower’. Another way to express this notion is that the presence of an active subjective frame linked to perceptual information does not imply explicit self-consciousness. Indeed, we insist that the subjective frame is not a reduced version of the explicit self, and in this sense, our proposal differs from the previous concept of the ‘minimal self’ developed in the literature.
Indeed, the concept of subjective frame presented here is reminiscent of the concept of ‘minimal self’ developed by a number of authors. For instance, Gallagher [6] proposed the concept of the ‘minimal self’ which is defined as ‘a consciousness of oneself as an immediate subject of experience’ and includes aspects such as sense of body ownership and agency. Similarly, ‘pre-reflective (bodily) self-consciousness’ means ‘the most primitive form of self-consciousness corresponds to the subjective dimension of experience’ [5]. Recently, Blanke & Metzinger [12] proposed a similar concept called ‘minimal phenomenal selfhood’ whose central defining features are global body ownership, self-location and first-person perspective. According to Vogeley [26], taking a first-person perspective which is necessary for human self-consciousness entails operating in an egocentric reference frame. Finally, Damasio [14] proposed a concept close to the neural subjective frame called the ‘proto-self’, defined as ‘a coherent collection of neural patterns which map, moment by moment, the state of the physical structure of the organism in its many dimensions’. Those proposals share with ours the idea of a basic building block to enable first-person experience. However, the terms ‘minimal self’, ‘minimal selfhood’ or even ‘proto-self’ tend to be misleading, because they suggest a strong bias towards explicit self-representation and reflective self-consciousness.
(ii). The subjective frame is necessary, but not sufficient, for subjective experience
The neural subjective frame would bring in an important element: it would enable the ‘I’ by referring sensory information to the subject. The subjective frame is therefore necessary for any percept to be subjectively experienced. However, the subjective frame is not by itself sufficient to create a conscious perceptual experience (figure 1): without any activity in sensory-related areas, for instance, there should be no percept, at least in normal subjects. Unconscious information processing, such as in implicit priming for instance, would correspond to sensory processing that failed at connecting with the neural subjective frame. Similarly, during sleep, the subjective frame does not necessarily have to be mute, it is sufficient to disconnect it from sensory processing.
Figure 1.
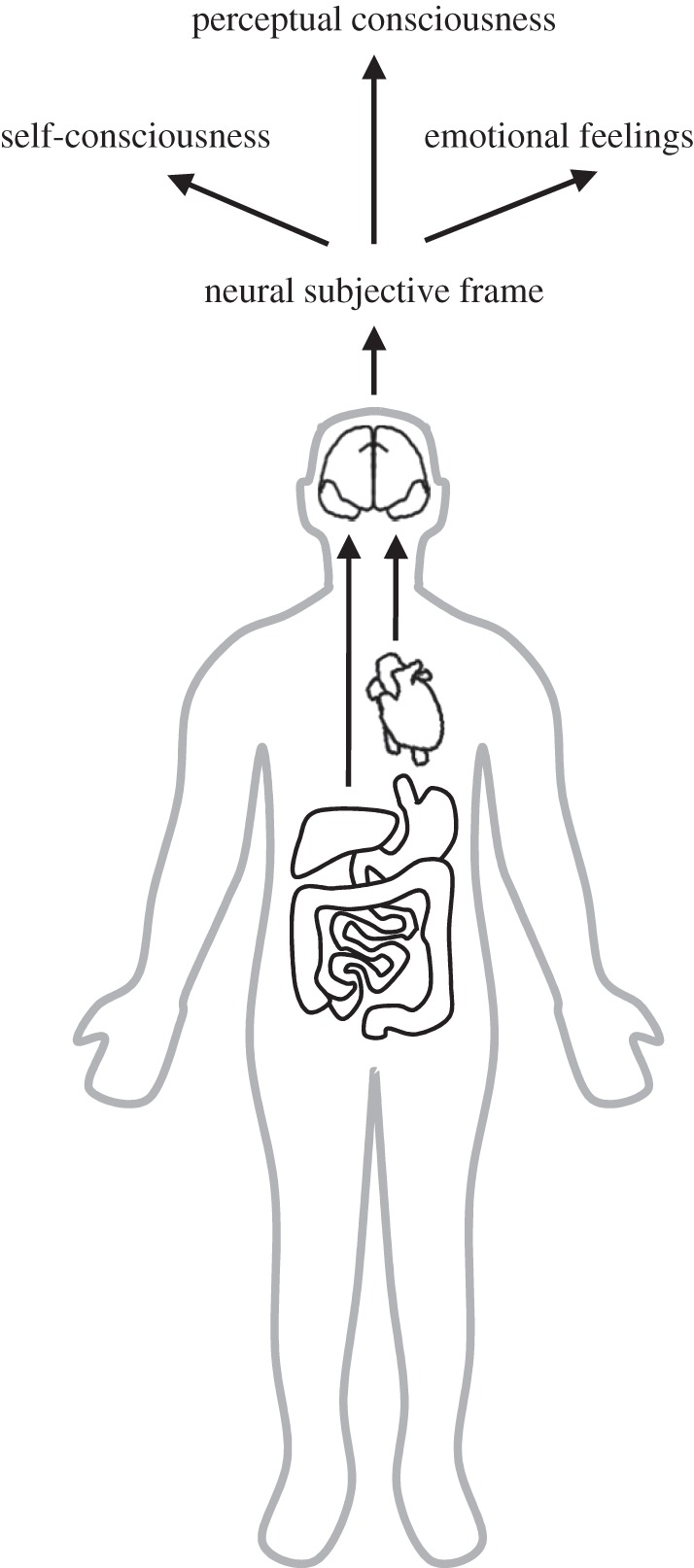
The neural subjective frame. Ascending visceral information from the heart and the gut targets cortical sites, including posterior insula, ventral anterior cingulate, amygdala and somatosensory cortex. We propose that this neural representation of visceral information creates the neural subjective frame that interacts with sensory processing to create the first-person perspective inherent to subjective perceptual consciousness. The neural subjective frame would be also be necessary to other types of subjective experiences, self-consciousness and emotional feelings.
Until now, we have proposed that the neural subjective frame is necessary for perceptual consciousness. We would like to go one step further and argue that this subjective frame is one of the most fundamental and intrinsic building block of every subjective experience. Subjectivity is a fundamental characteristic of perceptual consciousness, but this fundamental property of subjectivity is inherent to other concepts, namely self-consciousness and emotional feelings. It follows that the role of the subjective frame should be extended to those other concepts (figure 1). Self-consciousness refers to the ability to identify one's own thoughts and feelings, as well as one's own personality traits, etc.: it refers explicitly to the self. The subjective frame would therefore also be necessary in this case, as already underlined by a number of authors [5,6,12,14,26]. Emotions can be considered as being composed of two components: one being tightly related to the production of behavioural responses necessary for survival or maintenance of well-being [27], the other being the subjective feeling associated with an emotional stimulus or an emotional situation [15]. Emotional feelings, similar to perceptual experiences, are by essence subjective and private. Just as there is no true or false visual experience when being presented with a stimulus at threshold, there is no true or false emotional feeling triggered by a piece of art: only the subject experiencing an emotional feeling knows what it is like to experience this feeling.
(c). Rooting the subjective frame in the neural representation of visceral signals
We are attempting here to identify a biological mechanism that could enable subjective experiences—be they perceptual, reflective or emotional. There is a general consensus that brain–body interactions matter for self-consciousness [12,14,28,29] or emotions [9,30]. We argue here that brain–body interactions are also a critical component of conscious perceptual experience.
Can we be more specific about the type of brain–body interactions involved? Sensory–motor integration has been proposed to underlie the sense of agency/body ownership [6] or more generally self-specifying processes [5] as well as perceptual consciousness [31]. We discarded sensory–motor interactions, because sensory–motor theories cannot account for consciousness in the locked-in patients [2]. Following Damasio's [14] proposal of the proto-self, we argue here that a likely candidate for the subjective frame is brain–viscera interactions.
The central nervous system continuously monitors the state of internal organs such as the heart or the gut, to refine the homeostatic regulation of multiple biological essential parameters, from heart rate and blood pressure to stomach contraction. This monitoring remains at least partially functioning in locked-in patients [32]. Visceral monitoring is anchored in multiple neural systems, as detailed in §3a. It can operate at multiple time-scales, signalling short-lived events such as a heartbeat or longer-lasting states such as digestion. This multiplicity of time-scales makes it ideally suited to constitute the biological basis for the subjective frame, because it provides both a sense of continuity across long time-scales as well as the possibility to react quickly to external events. Because we are interested in biological mechanisms that can operate at the fast time-scale of conscious perception, we focus more on heart–brain interactions in §§3 and 4.
3. The biological basis of the subjective frame
Numerous pathways connect internal organs such as the heart or the gut to the neocortex in the multiple loops that are important for homeostatic regulation [32–34]. Here, we concentrate first on the nature of the information that is being relayed, and then on ascending pathways and cortical structures receiving signals from internal organs, mainly posterior insula, ventral anterior cingulate cortex, amygdala and somatosensory cortex.
(a). Internal bodily signals map onto multiple areas through multiple anatomical pathways
Signals from internal organs such as the heart or the gut arise from mechanosensory and chemosensory neurons transmitting information about the internal state of the body. Those neurons can be sensitive to physiological parameters having slow time constants, such as peptide concentration in the gut [33], or can reflect much faster events, such as mechanosensory neurons transiently emitting spikes at specific phases of the cardiac cycle [35]. The information relayed by visceral neurons can therefore inform the central nervous system on bodily state at multiple time-scales.
Ascending visceral afferents can enter the brain either through cranial nerve pathways or through spinal relays. The vagus nerve pathway targets the nucleus of the solitary tract in the brainstem and from there the parabrachial nucleus, before projecting to the ventromedial basal nucleus of the thalamus [32]. The spinal pathway originates from dorsal horn sensory neurons responding to visceral sensory afferents. Spinal visceral afferents can target, directly or indirectly, the posterior ventromedial nucleus of the thalamus [28,32]. From the thalamus, visceral afferents target the posterior insula [9,28]. In addition, the nucleus of the solitary tract projects to the ventral anterior cingulate cortex [36]. The amygdala also receives afferent visceral information directly from the parabrachial nucleus [37] and the nucleus of the solitary tract [36]. Last but not least, spinal visceral afferents can also converge with musculoskeletal and cutaneous afferents to reach the somatosensory cortex through spinothalamic and spinoreticular tracts [32].
Visceral afferents therefore target multiple cortical sites—posterior insula, ventral anterior cingulate cortex, amygdala, somatosensory cortex—through numerous pathways. These two properties—wide cortical availability and redundant pathways—are an important prerequisite for the point we want to make, namely that visceral afferents constitute a neural basis for the subjective frame. First, because information about the internal state of the body is represented in many different and well-connected structures, most other cortical sites are likely to receive, through one or two relays, information about bodily state. The neural subjective frame is therefore easily available and can potentially interact with most sensory processes to refer perceptual experience to the subject. Second, the redundancy of anatomical pathways and target sites of afferent visceral information implies that only massive, probably lethal, lesions could suppress or at least markedly alter the representation of internal bodily information. Indeed, disorders affecting all three types of subjective experience (self-experience, conscious perception and emotional feelings) are not reported. Besides, locked-in patients who suffer from major spinal cord injury, although having problems at homeostatic regulation, nevertheless still receive information on bodily states through the cranial nerve route [32].
(b). The brain registers each heartbeat occurrence
As mentioned in §3a, visceral state can vary at multiple time-scales. However, because we are interested in biological mechanisms that can operate at the fast time-scale of conscious perception, we focus on heart–brain interactions in the rest of the paper.
The heart acts as a spontaneously occurring stimulus and sends information to the central nervous system at each heartbeat. Animal studies revealed that mechanoreceptors in the heart wall transiently discharge a small burst of spikes at specific phases of heart contraction [35], information that can be relayed to the cortex through the pathways described in §3a. In humans, the cortical processing associated with the afferent signals from the cardiovascular system can be studied by measuring the heart-evoked response (HER). The HER is an evoked potential to heartbeats, obtained by averaging electroencephalogram (EEG) activity locked to the main peak of the electrocardiogram (ECG; [38]; for review, see [39]). The HER is characterized by a broad frontal and frontocentral distribution. HER amplitude correlates with the mechanical strength of myocardial action measured by a stroke volume per time unit in normal participants [39] and with stress-induced cardiac responses in cardiovascular patients [40]. Bearing in mind the intrinsic limitations of EEG dipole modelling, HER sources were found in the ventral anterior cingulate, the right mid-posterior insula, right inferior parietal regions and somatosensory cortices [41]. Recently, direct evidence for a robust HER in the human primary somatosensory cortex was obtained from electrocorticographic (ECoG) data recorded in epileptic patients [42].
A more indirect way to look at the links between brain and heart is to search for brain regions whose activity covaries with heart-rate variability. Indeed, normal heart activity is characterized by beat-to-beat variability over both short and long time-scales [43]. A meta-analysis of task-induced changes in heart-rate variability [44] reveals a consistent correlation of ventral anterior cingulate and amygdala with heart-rate variability, independently of the nature of the task (emotional or motor). The implication of these two structures is confirmed at rest [45,46]. It is, however, difficult to infer from such studies whether the neural correlates of heart-rate variability represent neural responses to afferent cardiac information relevant to the subjective frame, or simply efferent neural activity contributing to heart-rate regulation.
The HER constitutes an interesting potential building block of the neural subjective frame: although heartbeats are most often not explicitly consciously experienced, information about heartbeats is registered in the brain. Explicit conscious perception of heartbeats is not necessary for the existence of a HER, as clearly demonstrated in ECoG data [42]. Moreover, HER amplitude fluctuations in the absence of explicit heartbeat perception do have functional correlates: HER amplitude correlates with subjective pain ratings [47], and HER amplitude is enhanced when subjects rate the emotional expression of a face as compared with when they judge the symmetry of a face [48].
(c). Listening to one's heart: explicit interoceptive tasks
Brain responses to cardiac events occur automatically, but they are enhanced when cardiac events become task-relevant, such as when subjects have to count their own heartbeats. Although we argue that the neural subjective frame corresponds to the implicit processing of internal bodily information, it is worth briefly reviewing the literature on explicit interoceptive perception.
The amplitude of the HER is modulated by heartbeats’ task-relevance [49], and the modulation is all the more pronounced when subjects have been trained to perceive cardiac information [50] and are motivated [39]. HER amplitude in the heartbeat perception task is a good predictor of subjects’ explicit interoceptive abilities: HER amplitude is larger in good heartbeat perceivers compared with bad heartbeat perceivers [51], and HER amplitude is decreased in depressed patients who showed less accurate heartbeat perception than normal controls [52].
Critchley et al. [53] showed in an fMRI study that explicit cardiac perception increased activity in multiple brain regions, including the posterior and anterior insula, dorsal anterior cingulate, somatomotor cortices, etc. Among those numerous regions, grey matter volume of the right anterior insula correlated with both explicit interoceptive abilities and anxiety scores. The multiplicity of both visceral anatomical pathways and cortical regions linked to visceral information processing makes it likely that interoceptive abilities should be resistant to lesions. Indeed, a patient with massive bilateral insula and anterior cingulate damage performed well at the heartbeat detection task. His cardiac sensation was impaired only when the somatosensory pathway was disrupted as well, by applying an anaesthetic on the skin [54]. Despite those results, the anterior insula has been proposed to play a unique role in the emergence of the ‘I feel’ (cold, hungry,…), based on neuroimaging studies showing that several different kinds of feeling states are uniquely associated with anterior insula [28,29,55–57]. The specific role of the anterior insula remains controversial, and we will not review it exhaustively here [58]; however, it is worth mentioning the recent proposal that the anterior insula could play a specific role in computing an interoceptive prediction error to implement subjective states [16].
4. The processing of external stimuli and the cardiac cycle
Heartbeat information is systematically transmitted to multiple brain regions through multiple pathways, even when one does not pay attention to it. If the neural information related to cardiac monitoring represents, as we argue, a building block of the neural subjective frame necessary for perceptual consciousness, then the neural information related to cardiac monitoring should interact somehow with the processing of external stimuli. This hypothesis has not been directly tested yet (but see endnote1). However, a number of studies investigated the link between the processing of external stimuli and cardiac activity itself (rather than with the neural monitoring of cardiac activity). Those studies, reviewed below, reveal that simple perceptual tasks involving emotionally neutral stimuli can both impact and depend on cardiac activity itself: perceiving a stimulus can transiently alter the heart rate, and both behavioural performance and neural sensory processing can sometimes be modulated depending on when the stimulus is presented in the cardiac cycle.
(a). Transient heart-rate changes during perceptual decision-making
Transient changes in heart rate can be triggered by external stimuli and cognitive events, resulting in a transient lengthening or shortening of a given inter-beat interval. In a simple forewarned perceptual reaction-time task, the heart follows a typical pattern of deceleration followed by an acceleration. The initial findings were obtained by Lacey & Lacey back in the 1960s (for review, see [59,60]): in response to a warning stimulus, indicating the beginning of a trial, the heart decelerates. This effect has been associated with anticipatory preparation involving sustained attention to the environment, and called the ‘bradycardia of attention’ by Lacey & Lacey. The amplitude of the deceleration depends on task-relevance and time uncertainty [61]. Once the stimulus is presented and subjects respond, the heart accelerates again. This effect is all the more pronounced when the detected stimulus occurs early in the heart cycle [60]. Those results have since been reproduced by a number of groups [61,62].
Those results imply that a hidden but robust covariate in simple forewarned reaction-time task is heart deceleration/acceleration, a fact seldom taken into account in imaging studies [63], although differential activation between two conditions attributed to differences in cognitive processing could actually correspond to differential activity of brain regions either receiving heart information or controlling heart rate.
(b). Modulation of behavioural performance throughout the cardiac cycle
The cardiac cycle is characterized by a series of contraction and relaxation of the atria and ventricles, reflected by the peaks of the ECG. The most prominent event in the ECG is the R peak corresponding to ventricular depolarization, followed about 250 ms later by the T peak. Both ventricular and aortic pressures are maximal (systole) between the R and T peaks.
In a simple reaction-time auditory task, Birren et al. [64] initially reported a modest but robust reaction-time lengthening when the stimulus is presented around the R peak of the ECG. Despite some early failures at replicating those findings [62,65,66], an increase of about 10 ms in reaction times around R peak in simple reaction-time tasks with suprathreshold auditory [67,68], visual [67,69–71] or tactile [68] stimuli has consistently been observed.
Another series of studies attempted to test whether sensitivity to stimuli at threshold varied depending on the timing of the stimulus with respect to the cardiac cycle, with limited success. Timing with respect to the cardiac cycle had no influence on visual [72] and auditory [73,74] sensitivity. Evidence is less clear-cut in the case of cutaneous stimuli: sensitivity to cutaneous stimuli either at threshold for perception [75] or around pain threshold [76] was found to increase when the stimulus was presented about 300 ms after the R peak of the ECG. However, cardiac phase had no effect on pain ratings of nociceptive cutaneous stimuli above pain threshold [77].
Beyond detection, a few studies have investigated the influence of the phase of the cardiac cycle on behaviour in more complex tasks pertaining either to emotional feelings or to explicit self-representation. There is so far only limited evidence that emotional processes are influenced by cardiac phase. In an attentional blink paradigm, emotional words were equally detected on systole and diastole and with equal confidence, although a later subsequent memory test showed a reduced recall of words initially presented at systole [78]. Emotional appraisal of briefly presented faces did not differ between systole and diastole, although a direct test on each emotion separately (happiness, sadness, disgust and neutral) revealed that the expression of disgust was judged more intense when the face was presented on systole [79]. More strikingly, illuminating a virtual body in synchrony with the subject's heartbeats increased self-identification with and self-location towards the virtual body [80]. This experiment does not reveal whether heartbeats alone can alter self-representation, but it demonstrates that the temporal congruency of internal (heartbeats) and external (virtual body illumination) stimuli alters self-related experience.
(c). The neural processing of sensory stimuli depends on the cardiac phase
As reviewed above in §4b, the timing of the stimulus in the cardiac cycle can affect at least some aspects of behavioural performance. It follows that neural responses to external stimuli could also depend on the phase of the cardiac cycle. Indeed, early markers of sensory processing are sensitive to cardiac timing: both the auditory N1 [81] and visual P1 [82] components of sensory-evoked potentials are larger when arterial pressure is low. Similarly, response evoked by nociceptive stimuli is enhanced during diastole [83], especially when nociceptive stimuli are expected [77]. Depending on cardiac phase, somatosensory stimuli evoked different changes in blood pressure and differential blood oxygen level-dependent responses in right amygdala, left anterior insula and brainstem nuclei [84].
However, the link between fluctuations in neural response amplitude and behaviour was either not tested [81,82] or absent [77,83]. Whether amplitude fluctuations of neural sensory responses along the cardiac cycle directly translate in behavioural performance therefore remains an open issue.
(d). What those studies do and do not tell us about subjective experience
The experimental studies reviewed in §4a–c provide solid evidence that cardiac phase affects an objective aspect of perception (speed of response to suprathreshold stimuli). However, more subjective aspects of perception, such as the facilitation of the detection of stimuli at threshold, do not appear to be affected. As detailed below, those findings actually neither refute nor confirm the subjective frame hypothesis, because they tap onto a different issue.
With a few exceptions [80], the above-listed studies were inspired by the baroreceptor hypothesis developed by Lacey & Lacey [59,60]. Briefly, baroreceptors located in arterial walls register changes in blood pressure at each heart cycle as well as changes in mean blood pressure, and trigger the so-called baroreflex to maintain a stable blood pressure. Lacey & Lacey proposed that baroreceptor activation during systole inhibits sensory–motor functions. During diastole, baroreceptors are silent, and sensory function would be facilitated. Discussing in detail the validity of this hypothesis is beyond the scope this paper [34,61]. What is important to underline here is that all those studies tested whether cardiac information itself impacted perception.
The hypothesis of the neural subjective frame is different. In our view, subjective aspects of perceptual experience depend on how the brain registers information on the heart. The precise value of cardiac or vascular parameters is not necessarily informative. What matters is how the brain registers cardiac or vascular parameters in multiple neural representations of the internal bodily state to create the basis for the subjective framework. In other words, the subjective aspects of conscious perception, self-experience or emotional feelings should not be directly modulated by the state of the heart itself, but by how the brain responds to the heart.1
5. Conclusion
We argue here of the necessity to introduce a neural mechanism to account for the subjective dimension of perceptual experience. This mechanism would be based on the constantly updated neural maps of the internal state of the body and create a neural subjective frame from which first-person experience can be reported. It is important to underline that the neural subjective frame does not have to generate a conscious feeling of bodily state to tag the subject's conscious experience as belonging to the subject. Visceral information, as reviewed in this paper, but also potentially proprioceptive information [12,85], may fuel the neural subjective frame.
So far, direct evidence for our hypothesis is missing. However, we reviewed indirect arguments in favour of our model. First, the multiple anatomical pathways and cortical target sites of visceral information show that visceral information is redundantly represented in the brain and hence widely available to most cortical structures through short pathways. It follows that integrating the neural subjective frame with sensory information is biologically feasible. Second, heart-rate changes, traditionally associated with emotional processing, are also triggered by perceptual, emotionally neutral events. Third, behavioural performance and sensory processing can, sometimes, be altered depending on cardiac phase. However, to directly probe for the existence of the neural subjective frame, one would need to measure not cardiac activity itself, but the neural response to heartbeats for instance, in relation to perceptual experience, self-consciousness and emotional feelings.
Our hypothesis implies that at some point, neural responses to visceral inputs should be integrated with neural responses to stimuli from the external world. This issue pertains to the very general question of integration of information encoded in distributed regions that can potentially rely on two distinct types of mechanism. One possibility is that neural responses to internal and external inputs converge onto multisensory regions in a hierarchical architecture. While such multisensory convergence regions may exist, it seems unlikely that one single region ultimately combines all information from all sources of external and internal inputs into a single, unified conscious experience. The other possibility is that those regions that respond to visceral input coordinate their activity with those areas that respond to external stimuli, through inter-area communication. Inter-area communication would be facilitated by the wide cortical availability of neural responses to visceral inputs: because information about bodily state is redundantly represented in well-connected cortical regions, inter-area communication with either the visual, auditory or somatosensory systems should relatively easy to establish through short communication pathways. The advantage of this view is that it does not require a single region to act as a central controller [86]. Inter-area communication could be established through coordination mechanisms such as oscillatory synchrony [86]. More recently, predictive coding and the free-energy principle have been proposed as rules governing inter-area communication [87]. A major difference between those two mechanisms is that predictive coding requires the existence of a hierarchical architecture, which potentially reintroduces the need for convergence areas at the top of this hierarchy. Besides, predictive coding involving interoception has been proposed to account for explicit self-processing and bodily illusions such as the rubber hand [16,88]. In the case of the implicit subjective frame we are discussing here, it is not clear what the predictions of the system could be.
Our proposal has an important unifying power. First, as developed throughout the paper, it offers a common biological source, the neural subjective frame, to different types of subjective experiences that have been most often considered separately: self-consciousness, perceptual experience and emotional feelings. Second, it could also reconcile two aspects of consciousness that have so far been most often studied separately: state consciousness, related to vigilance states such as being awake or asleep, and transitive or content consciousness, that refers to being conscious of the external world or of oneself. State and content consciousness have been proposed as main targets for the neural correlates of consciousness programme [2,3], but have not been considered in the same comprehensive framework. We developed here the idea that the neural subjective frame is necessary for content consciousness. Because the neural subjective frame is tightly linked to homeostatic regulations involved in vigilance, it is also potentially relevant for state consciousness.
Funding statement
This work was supported by ANR-BLAN-12-BSH2-0002-01 to C.T.B., by ANR-11-0001-02 PSL* and ANR-10-LABX-0087.
Endnotes
Experimental evidence supporting the hypothesis of the neural subjective frame has recently been obtained [89].
References
- 1.Koch C. 2004. The quest for consciousness: a neurobiological approach. Reading, PA: Roberts; Bloxham, UK: Scion. [Google Scholar]
- 2.Tononi G, Koch C. 2008. The neural correlates of consciousness: an update. Ann. NY Acad. Sci. 1124, 239–261. ( 10.1196/annals.1440.004) [DOI] [PubMed] [Google Scholar]
- 3.Dehaene S, Changeux JP. 2011. Experimental and theoretical approaches to conscious processing. Neuron 70, 200–227. ( 10.1016/j.neuron.2011.03.018) [DOI] [PubMed] [Google Scholar]
- 4.Husserl E. 1984. Einleitung in die Logik und Erkenntnistheorie Vorlesungen 1906/07. Leiden, The Netherlands: Martinus Nijhoff.
- 5.Legrand D. 2007. Pre-reflective self-as-subject from experiential and empirical perspectives. Conscious. Cogn. 16, 583–599. ( 10.1016/j.concog.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 6.Gallagher II. 2000. Philosophical conceptions of the self: implications for cognitive science. Trends Cogn. Sci. 4, 14–21. ( 10.1016/S1364-6613(99)01417-5) [DOI] [PubMed] [Google Scholar]
- 7.Thompson E. 2007. Mind in life: biology, phenomenology, and the sciences of mind. Cambridge, MA: Belknap Press. [Google Scholar]
- 8.Zahavi D. 2005. Subjectivity and selfhood: investigating the first-person perspective. Cambridge, MA: MIT Press. [Google Scholar]
- 9.Damasio A. 2003. Feelings of emotion and the self. Ann. NY Acad. Sci. 1001, 253–261. ( 10.1196/annals.1279.014) [DOI] [PubMed] [Google Scholar]
- 10.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. 2006. Self-referential processing in our brain: a meta-analysis of imaging studies on the self. Neuroimage 31, 440–457. ( 10.1016/j.neuroimage.2005.12.002) [DOI] [PubMed] [Google Scholar]
- 11.Christoff K, Cosmelli D, Legrand D, Thompson E. 2011. Specifying the self for cognitive neuroscience. Trends Cogn. Sci. 15, 104–112. ( 10.1016/j.tics.2011.01.001) [DOI] [PubMed] [Google Scholar]
- 12.Blanke O, Metzinger T. 2009. Full-body illusions and minimal phenomenal selfhood. Trends Cogn. Sci. 13, 7–13. ( 10.1016/j.tics.2008.10.003) [DOI] [PubMed] [Google Scholar]
- 13.Varela FJ, Shear J. 1999. The view from within: first-person approaches to the study of consciousness. Thorverton, UK: Academic Press. [Google Scholar]
- 14.Damasio AR. 2000. The feeling of what happens: body and emotion in the making of consciousness. London, UK: W. Heinemann. [Google Scholar]
- 15.Tsuchiya N, Adolphs R. 2007. Emotion and consciousness. Trends Cogn. Sci. 11, 158–167. ( 10.1016/j.tics.2007.01.005) [DOI] [PubMed] [Google Scholar]
- 16.Seth AK. 2013. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 17, 565–573. ( 10.1016/j.tics.2013.09.007) [DOI] [PubMed] [Google Scholar]
- 17.Searle JR. 2000. Consciousness. Annu. Rev. Neurosci. 23, 557–578. ( 10.1146/annurev.neuro.23.1.557) [DOI] [PubMed] [Google Scholar]
- 18.Jackson F. 1982. Epiphenomenal qualia. Phil. Q. 32, 127–136. ( 10.2307/2960077) [DOI] [Google Scholar]
- 19.Block N. 2005. Two neural correlates of consciousness. Trends Cogn. Sci. 9, 46–52. ( 10.1016/j.tics.2004.12.006) [DOI] [PubMed] [Google Scholar]
- 20.Kanai R, Tsuchiya N. 2012. Qualia. Curr. Biol. 22, 392–396. ( 10.1016/j.cub.2012.03.033) [DOI] [PubMed] [Google Scholar]
- 21.Crick F, Koch C. 1990. Towards a neurobiological theory of consciousness. Semin. Neurosci. 2, 263–275. [Google Scholar]
- 22.Baars BJ. 2002. The conscious access hypothesis: origins and recent evidence. Trends Cogn. Sci. 6, 47–52. ( 10.1016/S1364-6613(00)01819-2) [DOI] [PubMed] [Google Scholar]
- 23.Tononi G. 2004. An information integration theory of consciousness. BMC Neurosci. 5, 42 ( 10.1186/1471-2202-5-42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamme VAF, Roelfsema PR. 2000. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 23, 571–579. ( 10.1016/S0166-2236(00)01657-X) [DOI] [PubMed] [Google Scholar]
- 25.Overgaard M, Gallagher S, Ramsoy TZ. 2008. An integration of first-person methodologies in cognitive science. J. Conscious. Stud. 15, 100–120. [Google Scholar]
- 26.Vogeley K, Fink GR. 2003. Neural correlates of the first-person-perspective. Trends Cogn. Sci. 7, 38–42. ( 10.1016/S1364-6613(02)00003-7) [DOI] [PubMed] [Google Scholar]
- 27.LeDoux J. 2012. Rethinking the emotional brain. Neuron 73, 653–676. ( 10.1016/j.neuron.2012.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig AD. 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. ( 10.1038/nrn894) [DOI] [PubMed] [Google Scholar]
- 29.Craig AD. 2009. How do you feel: now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. ( 10.1038/nrn2555) [DOI] [PubMed] [Google Scholar]
- 30.James W. 1894. The physical basis of emotion. Psychol. Rev. 1, 516–529. ( 10.1037/h0065078) [DOI] [PubMed] [Google Scholar]
- 31.O'Regan JK, Noe A. 2001. A sensorimotor account of vision and visual consciousness. Behav. Brain Sci. 24, 939–973; discussion 973–1031 ( 10.1017/S0140525X01000115) [DOI] [PubMed] [Google Scholar]
- 32.Saper CB. 2002. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 25, 433–469. ( 10.1146/annurev.neuro.25.032502.111311) [DOI] [PubMed] [Google Scholar]
- 33.Mayer EA. 2011. Gut feelings: the emerging biology of gut–brain communication. Nat. Rev. Neurosci. 12, 453–466. ( 10.1038/Nrn3071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Critchley HD, Harrison NA. 2013. Visceral influences on brain and behavior. Neuron 77, 624–638. ( 10.1016/j.neuron.2013.02.008) [DOI] [PubMed] [Google Scholar]
- 35.Armour JA, Ardell JL. 2004. Basic and clinical neurocardiology. Oxford, UK: Oxford University Press. [Google Scholar]
- 36.Vogt BA, Derbyshire SWG. 2009. Visceral circuits and cingulate-mediated autonomic functions. In Cingulate neurobiology and disease (ed. Vogt BA.), pp. 219–236. Oxford, UK: Oxford University Press. [Google Scholar]
- 37.Cechetto DF, Calaresu FR. 1985. Central pathways relaying cardiovascular afferent information to amygdala. Am. J. Physiol. 248, 38–45. [DOI] [PubMed] [Google Scholar]
- 38.Schandry R, Sparrer B, Weitkunat R. 1986. From the heart to the brain: a study of heartbeat contingent scalp potentials. Int. J. Neurosci. 30, 261–275. ( 10.3109/00207458608985677) [DOI] [PubMed] [Google Scholar]
- 39.Schandry R, Montoya P. 1996. Event-related brain potentials and the processing of cardiac activity. Biol. Psychol. 42, 75–85. ( 10.1016/0301-0511(95)05147-3) [DOI] [PubMed] [Google Scholar]
- 40.Gray MA, Taggart P, Sutton PM, Groves D, Holdright DR, Bradbury D, Brull D, Critchley HD. 2007. A cortical potential reflecting cardiac function. Proc. Natl Acad. Sci. USA 104, 6818–6823. ( 10.1073/pnas.0609509104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollatos O, Kirsch W, Schandry R. 2005. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum. Brain Mapp. 26, 54–64. ( 10.1002/hbm.20121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kern M, Aertsen A, Schulze-Bonhage A, Ball T. 2013. Heart cycle-related effects on event-related potentials, spectral power changes, and connectivity patterns in the human ECoG. Neuroimage 81C, 178–190. ( 10.1016/j.neuroimage.2013.05.042) [DOI] [PubMed] [Google Scholar]
- 43.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. 1981. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213, 220–222. ( 10.1126/science.6166045) [DOI] [PubMed] [Google Scholar]
- 44.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. 2012. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 36, 747–756. ( 10.1016/j.neubiorev.2011.11.009) [DOI] [PubMed] [Google Scholar]
- 45.Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. 2013. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68, 93–104. ( 10.1016/j.neuroimage.2012.11.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziegler G, Dahnke R, Yeragani VK, Bar KJ. 2009. The relation of ventromedial prefrontal cortex activity and heart rate fluctuations at rest. Eur. J. Neurosci. 30, 2205–2210. ( 10.1111/j.1460-9568.2009.07008.x) [DOI] [PubMed] [Google Scholar]
- 47.Shao S, Shen K, Wilder-Smith EP, Li X. 2011. Effect of pain perception on the heartbeat evoked potential. Clin. Neurophysiol. 122, 1838–1845. ( 10.1016/j.clinph.2011.02.014) [DOI] [PubMed] [Google Scholar]
- 48.Fukushima H, Terasawa Y, Umeda S. 2011. Association between interoception and empathy: evidence from heartbeat-evoked brain potential. Int. J. Psychophysiol. 79, 259–265. ( 10.1016/j.ijpsycho.2010.10.015) [DOI] [PubMed] [Google Scholar]
- 49.Montoya P, Schandry R, Muller A. 1993. Heartbeat evoked-potentials (HEP): topography and influence of cardiac awareness and focus of attention. Electroencephalogr. Clin. Neurophysiol. 88, 163–172. ( 10.1016/0168-5597(93)90001-6) [DOI] [PubMed] [Google Scholar]
- 50.Schandry R, Weitkunat R. 1990. Enhancement of heartbeat-related brain potentials through cardiac awareness training. Int. J. Neurosci. 53, 243–253. ( 10.3109/00207459008986611) [DOI] [PubMed] [Google Scholar]
- 51.Pollatos O, Schandry R. 2004. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology 41, 476–482. ( 10.111/1469-8986.2004.00170.x) [DOI] [PubMed] [Google Scholar]
- 52.Terhaar J, Viola FC, Bar KJ, Debener S. 2012. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin. Neurophysiol. 123, 1950–1957. ( 10.1016/j.clinph.2012.02.086) [DOI] [PubMed] [Google Scholar]
- 53.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. 2004. Neural systems supporting interoceptive awareness. Nat. Neurosci. 7, 189–195. ( 10.1038/nn1176) [DOI] [PubMed] [Google Scholar]
- 54.Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. 2009. The pathways of interoceptive awareness. Nat. Neurosci. 12, 1494–1496. ( 10.1038/Nn.2411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Craig AD, Chen K, Bandy D, Reiman EM. 2000. Thermosensory activation of insular cortex. Nat. Neurosci. 3, 184–190. ( 10.1038/72131) [DOI] [PubMed] [Google Scholar]
- 56.Craig AD. 2011. Significance of the insula for the evolution of human awareness of feelings from the body. Ann. NY Acad. Sci. 1225, 72–82. ( 10.1111/j.1749-6632.2011.05990.x) [DOI] [PubMed] [Google Scholar]
- 57.Craig AD. 2003. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 13, 500–505. ( 10.1016/S0959-4388(03)00090-4) [DOI] [PubMed] [Google Scholar]
- 58.Damasio A, Damasio H, Tranel D. 2013. Persistence of feelings and sentience after bilateral damage of the insula. Cereb. Cortex 23, 833–846. ( 10.1093/cercor/bhs077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lacey BC, Lacey JI. 1980. Presidential address, 1979. Cognitive modulation of time-dependent primary bradycardia. Psychophysiology 17, 209–221. ( 10.1111/j.1469-8986.1980.tb00137.x) [DOI] [PubMed] [Google Scholar]
- 60.Lacey BC, Lacey JI. 1978. Two-way communication between the heart and the brain. Significance of time within the cardiac cycle. Am. Psychol. 33, 99–113. ( 10.1037/0003-066X.33.2.99) [DOI] [PubMed] [Google Scholar]
- 61.Somsen RJ, Jennings JR, Van der Molen MW. 2004. The cardiac cycle time effect revisited: temporal dynamics of the central-vagal modulation of heart rate in human reaction time tasks. Psychophysiology 41, 941–953. ( 10.1111/j.1469-8986.2004.00241.x) [DOI] [PubMed] [Google Scholar]
- 62.Jennings JR, Wood CC. 1977. Cardiac cycle time effects on performance, phasic cardiac responses, and their intercorrelation in choice reaction time. Psychophysiology 14, 297–307. ( 10.1111/j.1469-8986.1977.tb01179.x) [DOI] [PubMed] [Google Scholar]
- 63.Gray MA, Minati L, Harrison NA, Gianaros PJ, Napadow V, Critchley HD. 2009. Physiological recordings: basic concepts and implementation during functional magnetic resonance imaging. Neuroimage 47, 1105–1115. ( 10.1016/j.neuroimage.2009.05.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birren JE, Phillips SL, Cardon PV. 1963. Reaction time as a function of cardiac cycle in young adults. Science 140, 195–196. ( 10.1126/science.140.3563.195-196) [DOI] [PubMed] [Google Scholar]
- 65.Salzman LF, Jaques N. 1976. Heart-rate and cardiac cycle effects on reaction-time. Percept. Motor Skill 42, 1315–1321. ( 10.2466/pms.1976.42.3c.1315) [DOI] [Google Scholar]
- 66.Thompson LW, Botwinic J. 1970. Stimulation in different phases of cardiac cycle and reaction time. Psychophysiology 7, 57–65. ( 10.1111/j.1469-8986.1970.tb02276.x) [DOI] [PubMed] [Google Scholar]
- 67.Saari MJ, Pappas BA. 1976. Cardiac cycle phase and movement and reaction-times. Percept. Motor Skill 42, 767–770. ( 10.2466/pms.1976.42.3.767) [DOI] [PubMed] [Google Scholar]
- 68.Edwards L, Ring C, McIntyre D, Carroll D, Martin U. 2007. Psychomotor speed in hypertension: effects of reaction time components, stimulus modality, and phase of the cardiac cycle. Psychophysiology 44, 459–468. ( 10.1111/j.1469-8986.2007.00521.x) [DOI] [PubMed] [Google Scholar]
- 69.Callaway E, Layne RS. 1964. Interaction between the visual evoked response and two spontaneous biological rhythms: the EEG alpha cycle and the cardiac arousal cycle. Ann. NY Acad. Sci. 112, 421–431. ( 10.1111/j.1749-6632.1964.tb26762.x) [DOI] [PubMed] [Google Scholar]
- 70.McIntyre D, Ring C, Hamer M, Carroll D. 2007. Effects of arterial and cardiopulmonary baroreceptor activation on simple and choice reaction times. Psychophysiology 44, 874–879. ( 10.1111/j.1469-8986.2007.00547.x) [DOI] [PubMed] [Google Scholar]
- 71.Stewart JC, France CR, Suhr JA. 2006. The effect of cardiac cycle phase on reaction time among individuals at varying risk for hypertension. J. Psychophysiol. 20, 1–8. ( 10.1027/0269-8803.20.1.1) [DOI] [Google Scholar]
- 72.Elliott R, Graf V. 1972. Visual sensitivity as a function of phase of cardiac cycle. Psychophysiology 9, 357–361. ( 10.1111/j.1469-8986.1972.tb03219.x) [DOI] [PubMed] [Google Scholar]
- 73.Velden M, Juris M. 1975. Perceptual performance as a function of intra-cycle cardiac activity. Psychophysiology 12, 685–692. ( 10.1111/j.1469-8986.1975.tb00075.x) [DOI] [PubMed] [Google Scholar]
- 74.Delfini LF, Campos JJ. 1972. Signal detection and the ‘cardiac arousal cycle’. Psychophysiology 9, 484–491. ( 10.1111/j.1469-8986.1972.tb01801.x) [DOI] [PubMed] [Google Scholar]
- 75.Edwards L, Ring C, McIntyre D, Winer JB, Martin U. 2009. Sensory detection thresholds are modulated across the cardiac cycle: evidence that cutaneous sensibility is greatest for systolic stimulation. Psychophysiology 46, 252–256. ( 10.1111/j.1469-8986.2008.00769.x) [DOI] [PubMed] [Google Scholar]
- 76.Martins AQ, Ring C, McIntyre D, Edwards L, Martin U. 2009. Effects of unpredictable stimulation on pain and nociception across the cardiac cycle. Pain 147, 84–90. ( 10.1016/j.pain.2009.08.016) [DOI] [PubMed] [Google Scholar]
- 77.Gray MA, Minati L, Paoletti G, Critchley HD. 2010. Baroreceptor activation attenuates attentional effects on pain-evoked potentials. Pain 151, 853–861. ( 10.1016/j.pain.2010.09.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garfinkel SN, Barrett AB, Minati L, Dolan RJ, Seth AK, Critchley HD. 2013. What the heart forgets: Cardiac timing influences memory for words and is modulated by metacognition and interoceptive sensitivity. Psychophysiology 50, 505–512. ( 10.1111/Psyp.12039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gray MA, Beacher FD, Minati L, Nagai Y, Kemp AH, Harrison NA, Critchley HD. 2012. Emotional appraisal is influenced by cardiac afferent information. Emotion 12, 180–191. ( 10.1037/A0025083) [DOI] [PubMed] [Google Scholar]
- 80.Aspell JE, Heydrich L, Marillier G, Lavanchy T, Herbelin B, Blanke O. 2013. Turning body and self inside out: visualized heartbeats alter bodily self-consciousness and tactile perception. Psych. Sci. 24, 2445–2453. ( 10.1177/0956797613498395) [DOI] [PubMed] [Google Scholar]
- 81.Sandman CA. 1984. Augmentation of the auditory event related potentials of the brain during diastole. Int. J. Psychophysiol. 2, 111–119. ( 10.1016/0167-8760(84)90004-7) [DOI] [PubMed] [Google Scholar]
- 82.Walker BB, Sandman CA. 1982. Visual evoked potentials change as heart rate and carotid pressure change. Psychophysiology 19, 520–527. ( 10.1111/j.1469-8986.1982.tb02579.x) [DOI] [PubMed] [Google Scholar]
- 83.Edwards L, Inui K, Ring C, Wang XH, Kakigi R. 2008. Pain-related evoked potentials are modulated across the cardiac cycle. Pain 137, 488–494. ( 10.1016/j.pain.2007.10.010) [DOI] [PubMed] [Google Scholar]
- 84.Gray MA, Rylander K, Harrison NA, Wallin BG, Critchley HD. 2009. Following one's heart: cardiac rhythms gate central initiation of sympathetic reflexes. J. Neurosci. 29, 1817–1825. ( 10.1523/Jneurosci.3363-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blanke O. 2012. Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci. 13, 556–571. ( 10.1038/nrn3292) [DOI] [PubMed] [Google Scholar]
- 86.Singer W, Gray CM. 1995. Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci. 18, 555–586. ( 10.1146/annurev.ne.18.030195.003011) [DOI] [PubMed] [Google Scholar]
- 87.Friston K. 2010. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 11, 127–138. ( 10.1038/nrn2787) [DOI] [PubMed] [Google Scholar]
- 88.Apps MA, Tsakiris M. In press. The free-energy self: a predictive coding account of self-recognition. Neurosci. Biobehav. Rev. ( 10.1016/j.neubiorev.2013.01.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park HD, Correia S, Ducorps A, Tallon-Baudry C. In press. Spontaneous fluctuations in neural responses to heartbeats predict visual detection. Nat. Neurosci. [DOI] [PubMed] [Google Scholar]


