Abstract
This essay critically examines the extent to which binocular rivalry can provide important clues about the neural correlates of conscious visual perception. Our ideas are presented within the framework of four questions about the use of rivalry for this purpose: (i) what constitutes an adequate comparison condition for gauging rivalry's impact on awareness, (ii) how can one distinguish abolished awareness from inattention, (iii) when one obtains unequivocal evidence for a causal link between a fluctuating measure of neural activity and fluctuating perceptual states during rivalry, will it generalize to other stimulus conditions and perceptual phenomena and (iv) does such evidence necessarily indicate that this neural activity constitutes a neural correlate of consciousness? While arriving at sceptical answers to these four questions, the essay nonetheless offers some ideas about how a more nuanced utilization of binocular rivalry may still provide fundamental insights about neural dynamics, and glimpses of at least some of the ingredients comprising neural correlates of consciousness, including those involved in perceptual decision-making.
Keywords: binocular rivalry/visual awareness/neural correlates of consciousness, visual perception, neural dynamics, perceptual decision making
1. Introduction
The topic of this special issue—perceptual awareness and its neural basis—has intrigued philosophers for centuries, and in recent years it has become a core focus within cognitive neuroscience. Central to this topic is the notion ‘neural correlates of consciousness’ (NCsC) [1–6]. These are the brain events that underlie the conscious states experienced by sentient observers, the ‘minimal neuronal mechanisms jointly sufficient for one specific conscious percept’ [4]. In pursuit of clues for identifying NCsC, psychologists and neuroscientists have focused especially on a variety of compelling visual phenomena for which one's conscious percept changes over time even though the objects or events one is viewing remain unchanged [7,8]. These fluctuations in visual awareness occur because these objects or events are ambiguous with respect to their identity and, therefore, plausibly support conflicting interpretations. This combination of unchanging physical stimulation and varying awareness is valuable because it allows a comparison of neural events between moments that differ specifically with regard to conscious state, and not with regard to physical input.
Within the stable of visual phenomena that have been deployed to implement this strategy, binocular rivalry stands out as a real workhorse. In binocular rivalry, one eye views a given image while a different image is presented to the corresponding region of the other eye. This incongruence causes the observer to perceive only one of the images at a time, with perception alternating between images every few seconds. We can think of several possible reasons why scientists have been attracted to this particular form of perceptual bistability.
(i) Rivalry is compelling: a normally visible, potentially interesting and complex image (e.g. your own face) can appear and disappear from awareness for several seconds at a time.
(ii) Rivalry is very robust with regard to the content of the images: whether one is interested in perception of faces or perception of, say, colours or lines, rivalry is elicited by a broad range of images presented in interocular conflict.
(iii) Perceptual dominance fluctuates unpredictably over time during rivalry, implying the underlying involvement of dynamic neural processes.
(iv) Fluctuations in perception during rivalry can be influenced by manipulating simple stimulus variables such as contrast or contour density, and some evidence suggests that object-related properties of images (e.g. figural coherence) also can impact rivalry dynamics.
For these reasons, rivalry has become a paradigm phenomenon for pursuing the NCsC, at least as that term applies to visual awareness (e.g. [9]). Without a doubt, the fluctuations in visual awareness one experiences during rivalry are of particular interest to the neuroscience of consciousness, as they are endogenously mediated, being produced by variations in neural activity within visually activated regions of the brain. As such, these fluctuations are distinct from those experienced, say, when viewing two different stimuli that are physically turned on and off reciprocally over time, a sequence of events that will cause changes at many neural stages, including peripheral ones more closely associated with sensation than with perception (e.g. the photoreceptors in the retina).
While acknowledging this special position of rivalry in the search for NCsC, we here ask a more specific question: if one can identify the neural events driving perception during binocular rivalry, will one have identified NCsC? This is certainly what is explicitly or implicitly assumed by many studies on the topic, but our aim in this essay is to outline reasons that prompt scepticism. From the outset, we want to acknowledge that others have written cogent, critical essays on the potential pitfalls of drawing conclusions about consciousness from percept-related fluctuations in activity [10–12]. While our essay will touch on some of these same pitfalls, our focus is uniquely on binocular rivalry and what it can tell us about conscious visual perception. Our ideas are presented within the framework of four broad concerns about the use of rivalry for this purpose, one being methodological and the other three being conceptual. As the essay unfolds, we will offer some ideas about how a more nuanced utilization of binocular rivalry may still provide fundamental insights about neural dynamics and glimpses of at least some of the ingredients comprising NCsC. We start with our practical concern.
2. Concern 1: what constitutes an adequate comparison condition to rivalry?
Our first concern focuses on an experimental design issue when using binocular rivalry as an inferential tool for disrupting visual awareness. The concern, in a nutshell, centres on the selection of the appropriate comparison condition(s) with which to contrast observations made during binocular rivalry. Historically, this concern pertains to studies seeking to learn the extent to which a visual stimulus remains effective despite being suppressed from awareness [13], but it also arises when using rivalry as a tool for identifying NCsC. The most straightforward way to introduce this concern is by way of an example.
Decades ago, one of us (R.B.) co-authored a paper describing the influence of rivalry on the build-up of two well-known visual after-effects produced by prolonged adaptation to a grating pattern [14]. One after-effect is the temporary elevation in contrast threshold for subsequently viewed gratings identical to the adapting grating, and the other one is the brief misperception of the spatial frequency of gratings similar, but not identical, to the spatial frequency to the adapting grating. Knowing that these after-effects increased in strength with the duration of pattern adaptation, we asked what would happen if a monocularly viewed, high contrast adaptation grating was suppressed for a portion of the adaptation duration owing to its being engaged in rivalry with a dissimilar image presented to the other eye. To quote from that 1974 paper,
If the inducing stimulus remains effective while suppressed, the magnitude of the aftereffect will correspond to the duration of physical stimulation. But if the stimulus is rendered ineffective by suppression the aftereffect will be weakened, and its magnitude will correspond to the duration of phenomenal viewing [14, p. 488].
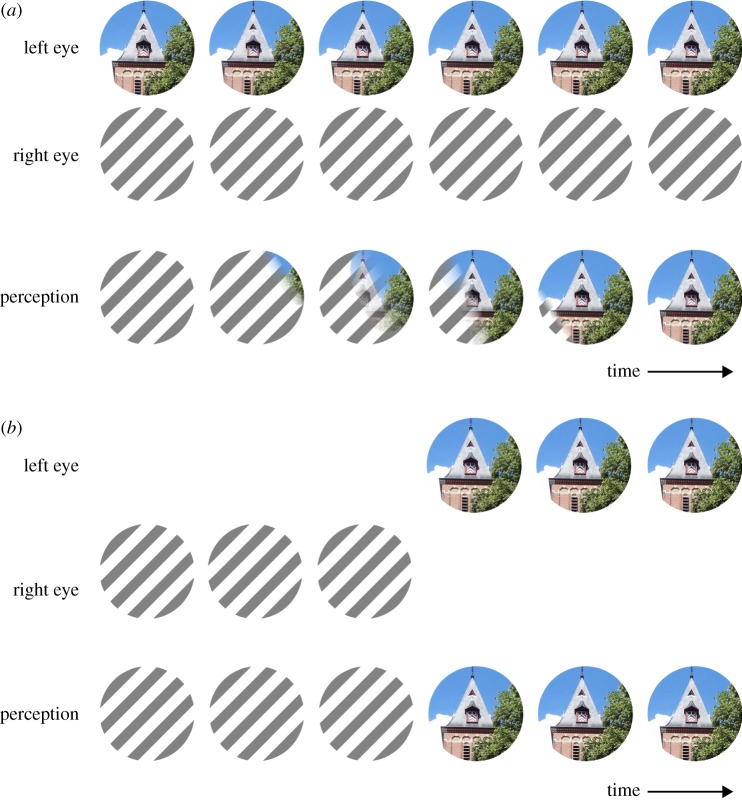
For the purposes of comparison that study included two monocular control conditions. The first involved presenting the high contrast adapting grating to one eye without pairing it with a rival grating in the other eye, thus providing an estimate of the after-effect strength expected if suppression had no effect on adaptation. The second condition, dubbed the rivalry mimic condition, entailed presenting and removing the monocular adaptation grating in a temporal pattern that followed the alternations of rivalry reported on a previous, genuine rivalry trial (figure 1). This latter condition, therefore, aimed to estimate the amount of reduction that would be expected if suppression did indeed render the adapting grating ‘ineffective’.
Figure 1.
Binocular rivalry and a rivalry mimic condition. (a) During binocular rivalry, each eye views a different image, and transitions in dominance from one image to the other often take the form of a wave whereby one image is swept from awareness by the other. (b) Many experimental designs also include a rivalry mimic condition, where the perceptual experience reported during rivalry is approximated using on-screen replay. In the implementation devised by Blake & Fox [14] illustrated here, two monocular images are alternately removed following a time series matching that actually reported on a previous trial of genuine rivalry alternations. Mimic conditions provide a baseline that approximates what would happen during rivalry if perceptual suppression completely eradicated neural processing of the suppressed image. A limitation of mimic conditions is that the perceptual match with real rivalry is only approximate. Notably, the dynamically changing perceptual experiences accompanying wave-like dominance transitions are not captured by standard mimic conditions. (Online version in colour.)
Versions of this experimental design, based on the same logic laid out in the 1974 paper, have been employed in many subsequent studies of rivalry suppression and visual adaptation [15–25], often with the aim of gauging the involvement of the adapting neural processes in conscious perception. Moreover, there are examples in the rivalry literature where measures of visual processing other than adaptation have been assessed under rivalry suppression and compared to values measured under non-rivalry, monocular conditions [26,27].
We can envision several potential problems associated with this strategy. First, the logic of the approach entails comparing measures obtained under monocular viewing without rivalry to measures obtained during rivalry. This comparison, however, introduces the possibility of confusing effects of invisibility with effects of the presence of a rivalling counterpart but not specific to periods of invisibility. After all, monocular stimulation is demonstrably different from binocular stimulation in other respects [28,29], and in several contemporary models of binocular vision those differences are embodied in contrast gain control mechanisms sensitive to the relative strengths of inputs to the two eyes [30,31]. In keeping with this notion, activity in visual cortex (as measured with functional magnetic resonance imaging, fMRI) evoked by a monocular image is reduced when a conflicting pattern is presented to the other eye, regardless of whether this conflicting pattern is strong enough to cause the original image to perceptually disappear [32].
In a related vein, the alternating monocular stimulation regime, illustrated schematically in figure 1b, was meant to mimic what an observer experiences during genuine binocular rivalry. For several reasons, we doubt whether this condition is sufficient for that purpose. First, neural events associated with suppression of a stimulus are certainly not equivalent to those associated with complete, intermittent removal of that stimulus—during suppression phases, evidence for traces of residual neural activity associated with the suppressed stimulus can found within early stages of visual processing as well as in higher tier visual areas [33–37]. At best, therefore, a rivalry mimic condition can reveal the upper limit on the extent to which rivalry suppression might squelch visual responses. This nuance becomes particularly important if the dependent variable being used to assess suppression's effect scales in a saturating fashion with stimulus strength (e.g. visual contrast), which is true for many variables used in this context, such as after-effect strength or fMRI response amplitude. In such cases, the mimic condition can be misleading. An illustration of this problem is the 1974 study we mentioned at the start of this section [14], which mistakenly inferred that suppression had no effect on the build-up of pattern adaptation produced by rival gratings. Subsequent work has since taught us that suppression can, in fact, weaken pattern adaptation but that this weakening only shows up for adapter gratings of moderate to low contrast, as a consequence of the saturating contrast dependence of pattern adaptation [38]. A more appropriate mimic condition for this form of adaptation, in other words, would be a monocular grating whose actual contrast was modulated over time between one value and another, with the lower value matched to the effective contrast reduction associated with suppression. Visual adaptation after-effects arising at higher visual stages [25] are more likely to require greater attenuation in stimulus strength to mimic an effect of suppression [39].
The second potential problem with the rivalry mimic condition pertains to studies that focus, not so much on the neural events that distinguish dominance from suppression states, but on neural events that co-occur with the alternations between states. This concern, too, can be illustrated by an example from the literature. In a highly influential paper, Lumer et al. [40] found that fronto-parietal areas of the human brain become temporarily active around the time an observer experiences a perceptual alternation during binocular rivalry, yet those same areas remained relatively inactive during conditions in which rivalry is mimicked by turning the left- and right-eye patterns on and off alternately over time (figure 1b). These differences in cortical activity during genuine and mimicked rivalry were taken as evidence that endogenous alternations in perception during rivalry were controlled by those fronto-parietal networks.
However, a different slant on this result was offered by Knapen et al. [41], who opined that the rivalry mimic condition, in fact, does not adequately recreate the potentially confusing perceptual judgement confronting observers during transitions in dominance from one rival stimulus to the other. Rather than dominance switching abruptly from one stimulus to the other, transitions in dominance are often dynamic and unpredictable: during a transition, one can experience mixed dominance where bits and pieces of both stimuli are simultaneously visible either superimposed or within different portions of the visual field, and the transition itself can resemble a wave whereby one image appears to be swept from awareness by the other (figure 1a). Consequently, an observer instructed to track switches in dominance (e.g. by pressing buttons) faces uncertainty about when or if to make a perceptual report (i.e. button press). The confusion and heightened attention prompted by these dynamic mixed perceptual states are not instigated by the conventional mimic condition (figure 1b). Indeed, when Knapen et al. [41] repeated Lumer et al.'s [40] procedure using more realistic mimic conditions that included mixture periods, they found that genuine rivalry and the mimic condition produced comparable activity in fronto-parietal networks. It remains to be learned just what role this activity has in rivalry, and that is going to hinge in part on when in time the activity arises relative to alternations [42]. But the methodological point remains indisputable: the oft-used mimic procedure is not perceptually equivalent to genuine rivalry alternations. In principle, this problem can be reduced by devising more realistic simulations of rivalry that include periods of mixed dominance using cross-faded blends as well as waves that originate at unpredictable locations and spread smoothly to produce a complete alternation [43–45]. However, nobody has yet devised a mimic condition so realistic that an informed observer might mistake it for rivalry.
How can these potential problems with monocular control conditions be avoided? With regard to work that aims to identify the neural and behavioural markers that distinguish two perceptual states, the most direct way is to compare measures obtained during dominance phases of a stimulus to those obtained during suppression phases of that same stimulus, so that the observer is viewing the same stimulus all along. This maximizes the likelihood that the stimulus conditions during periods of awareness of a given stimulus match the stimulus conditions during periods of unawareness of that stimulus. Comparing measures obtained during suppression to those obtained during dominance is the strategy very often employed in psychophysical studies using the so-called test probe technique [46], and the strategy can also be employed in concert with the adaptation procedure described above, by capitalizing on the fact that rivalry state durations vary considerably during the course of extended viewing (e.g. fig. 5 in [23]; fig. 3 in [38]). Moreover, it is the comparison often used in neurophysiological investigations of binocular rivalry [33,34], as well as in fMRI studies, in which the time course of fMRI response modulation is compared with the time course of perceptual dominance [35,47].
Next, we turn to the conceptual concerns that arise when using binocular rivalry to infer NCsC.
3. Concern 2: distinguishing abolished awareness from inattention
It is intuitively clear that there is a tight link between directing attention to an item and being aware of that item, prompting the question whether attention and awareness can, in fact, be studied as separate entities. The answer to this question remains a point of contention within the cognitive neuroscience literature (for reviews, see [48,49]). Side-stepping that debate, we want to discuss a more specific question: is it possible to distinguish a concomitant of stimulus invisibility during rivalry from inattention to that stimulus? When, for example, rivalry suppression reduces the effectiveness of an adaptation stimulus, is that reduction attributable to intermittent awareness of the stimulus or to intermittent withdrawal of attention from the stimulus? Or when the fMRI responses vary in strength over time in synchrony with alternations in perceptual dominance of a rival stimulus, is that variation in cortical activity attributable to neural suppression or to intermittent removal of attention?
It is plausible that part of the modulations that occur concomitantly with rivalry suppression arise from changes in attention. Some studies investigated the effectiveness of an adaptation stimulus that was suppressed during rivalry, while the observer was asked to direct his or her attention to either a feature (orientation; [50]) or the location [51] that was shared by this invisible stimulus. These studies found that the reduction in effectiveness of a suppressed stimulus can be partly recovered by this kind of attention instruction. Apparently, inattention contributes to the reduction in effectiveness that accompanies suppression in the absence of such an attention instruction. In a similar vein, Bahrami et al. [52] measured the fMRI responses in primary visual cortex (V1) to a stimulus suppressed in rivalry and reported that the smaller responses commonly found under suppression can be either compounded or partially counteracted, depending on the difficulty of a concurrent target detection task that presumably impacts the availability of residual attention that can be allocated to the suppressed stimulus.
In sum, several studies have now demonstrated that inattention is a likely contributor to modulations that occur during perceptual suppression. What has proved more difficult, however, is separating the effects of inattention from those of perceptual suppression per se. In this context, we would like to discuss two instances where such a separation has been claimed, but prematurely in our view. In one instance, observed changes in V1 fMRI response during perceptual suppression were reported to be entirely due to inattention, with no influence of perceptual suppression itself [6,53]. The second instance involves the opposite claim, namely that a particular suppression-related effect, in this case the modulation of negative afterimages, cannot be due to inattention, and must be due to perceptual suppression instead [23,54]. Interestingly, both these instances are associated with a very similar experimental approach, which we will discuss in more detail next.
The approach requires that an observer view a monocular stimulus that is either visible or suppressed in binocular rivalry, and at the same time the observer must attend either to the location of that stimulus or to another location elsewhere. The approach, in other words, entails a two-by-two design where attention and suppression are both varied, with the intention of separately assessing the effects of each factor. The approach was first developed by van Boxtel et al. [23] in a study that measured negative afterimages, a psychophysical marker of neuronal adaptation. This study demonstrated that afterimages were less persistent after viewing an adapter suppressed in binocular rivalry than after viewing a visible adapter (confirming [19,20]), yet more persistent after attending away from the adapter than after attending toward it (consistent with [54,55]). Thus, perceptual suppression reduced afterimage durations, whereas inattention enhanced them. These opposite effects would seemingly imply that reduced afterimage duration represents one effect of rivalry suppression that cannot be ascribed to inattention.
This implication would be premature, however, as revealed by a closer look at inattention's counterintuitive tendency to increase afterimage duration. This tendency is counterintuitive because inattention weakens neural responses and, presumably, the neural adaptation that underlies the formation of afterimages. A plausible resolution to this paradox is imaginable: there exists a second form of adaptation, pattern adaptation, that does not lead to afterimage formation but does impair sensitivity on visual detection tasks. This form of adaptation, too, is diminished when attention is diverted away from the adapter [55,56]. Asking an observer to report the duration of an afterimage after attending elsewhere than the location of the adapter, therefore, boils down to asking a more sensitive observer to report on a weaker stimulus, leaving room for a range of outcomes. Indeed, subsequent work has shown that inattention can either shorten or lengthen afterimage duration, depending on the exact stimulus conditions [24]. More importantly, this work demonstrated that inattention and suppression have qualitatively similar effects when the two contributing types of adaptation are assessed independently, thus leaving open the possibility that the effects of suppression on afterimages are, in part, due to inattention after all.
Using a design very similar to that of van Boxtel et al. [23], Watanabe et al. [53] also independently manipulated attention and stimulus awareness, now using fMRI to measure cortical activity in human V1. In this case, the design led to the conclusion that modulations of V1 activity that co-occur with absence of awareness are entirely due to inattention, not unawareness. This study found that directing attention toward the spatial location of a grating enhanced fMRI responses relative to attending elsewhere, and that this was true regardless of whether the grating was perceptually suppressed (replicating e.g. [52,57,58]). More surprisingly, the study also reported that perceptual suppression left V1 responses altogether unaffected, which contradicts earlier findings (see review by Tong et al. [59]). Watanabe et al. [53] concluded that those earlier findings must have reflected an artefact caused by a reduction of attention concurrent with rivalry suppression, and that rivalry suppression, in itself, does not modulate V1 activity. What is unsatisfying about this conclusion, however, is that Watanabe et al. failed to observe suppression-related modulation of V1 activity even in their condition requiring observers to attend to the grating's location, an instruction that exactly matches that of earlier studies that did find such modulation. Attributing the positive outcome of earlier studies to a temporary reduction of attention during suppression, as Watanabe et al. did, raises the question of why the matching condition in their experiment did not show the same result. A different study, co-authored by one of us (D.H.), reached the opposite conclusion as Watanabe et al. did, using substantially the same stimulus configuration and test procedure [60]. We did find reliable differences in V1 activity between conditions with and without suppression, with attention diverted away from the grating location. We suggested statistical power as one possible reason for the negative finding in the Watanabe et al. study.
Where do the above considerations leave us with regard to making a distinction between effects of suppression of awareness and effects of inattention? We have argued that inattention likely does contribute to modulations that co-occur with rivalry suppression, and that existing studies have not yet been entirely successful in teasing apart these two contributions. Also, we have pointed to designs that manipulate the amount of attention paid to a rival stimulus. With regard to this latter strategy, we would like to make the following additional note. Manipulations of attention are actually known to influence the dynamics of rivalry (for reviews, see [61,62]). In particular, rivalry alternations slow when attention is diverted to a concurrent task, with the degree of slowing directly related to the difficulty of the distracting task [63]. Even more remarkable, when attention is completely and unequivocally diverted for a few seconds away from the continued presence of rival stimuli, rivalry appears to cease altogether as evidenced by the state of rivalry when attention subsequently returns to the rival stimuli [64]. This latter finding dovetails with the finding that withdrawing attention from rival stimuli (i) abolishes the neural signature of changing states of rivalry, as measured with visually evoked responses recorded from human occipital cortex [65], and (ii) erases rivalry-related fMRI responses measured in human V2 and V3, but not V1, in response to exogenously triggered transitions in rivalry state [66]. These findings underscore the potential for confusion when trying to untangle the effects of inattention and stimulus invisibility by withdrawing attention from a rival stimulus.
4. Concern 3: do neural correlates of rivalry suppression generalize?
Studies of NCsC during rivalry typically assume that the results will generalize to other stimulus conditions and perceptual phenomena. The NCsC paradigm depends on such an assumption. If the NCsC differ for each and every stimulus condition, then there would be no way to get traction. But there is cause for concern.
Computational models posit a hierarchy of processing stages in binocular rivalry [67,68]. According to those models, component areas in the hierarchy may be differentially involved, depending on the nature of the stimuli used to instigate bistability and the manner in which those stimuli are presented. Some stimuli (for example, patterns comprising oriented contours) are optimally configured to evoke cooperative and competitive interactions among neurons in V1, because those neurons are functionally organized in a retinotopic map and within orientation and ocular dominance columns. Other stimuli, because of their more complex spatial configuration [39,69] or their dynamic properties [70,71], may evoke stronger neural competition in high-tier visual areas where neurons are selective for object categories [47,72] or for motion [73]. In other words, such models suggest that perceptual suppression may have different neural correlates, depending on the visual images and bistable phenomenon involved. Indeed, we will next discuss empirical observations that confirm this prediction.
As summarized above, binocular rivalry is accompanied by neural modulations within human primary visual cortex, which to the line of thinking about NCsC, could be suggestive evidence that V1 contains correlates of consciousness. Yet, other fMRI work that has measured V1 activity in observers experiencing motion-induced blindness, another compelling form of bistable perception in which conscious awareness fluctuates over time [74], found no hints of percept-dependent activity in V1 [75]. Instead, percept-related fluctuations in cortical activity were found only in higher tier areas of both dorsal and ventral streams. This disparity in results within V1 implies that the correlation between perception and V1 activity during rivalry may be a by-product of using rivalry as a tool.
Similar disparities have been observed, even within the binocular rivalry paradigm itself. Maier et al. [76] found that the firing rate of neurons in cortical area MT of macaque monkeys signalled perceptual dominance of these neurons’ preferred grating stimulus when this stimulus was engaged in rivalry with a particular conflicting image, but ceased to signify perception when the same preferred grating rivalled with a different image. Strikingly, the monkey's subjective experience during perception of the preferred grating is unlikely to depend much on what is perceptually suppressed at that moment, yet these findings indicate that the neural correlates of this experience do.
This brings us to our fourth and final concern, which is really a culmination of thoughts raised in the earlier parts of this essay together with broad concerns about the logic involved in identifying NCsC using any single phenomenon, for example rivalry.
5. Concern 4: are neural correlates of rivalry perception NCsC?
In developing their idea of NCsC, Crick & Koch [1,2] endorsed the use of binocular rivalry and other forms of bistable perception to separate neural populations into distinct categories, one consisting of neurons whose activity changes with perception and the other consisting of neurons whose activity remains invariant despite changes in perception, a division that builds on earlier notions in the psychology literature [77,78]. A widely cited example of this strategy in action is provided by the study by Leopold & Logothetis [34]. Recording single-unit activity from neurons in early visual cortex of alert, behaving monkeys, they categorized neurons in terms of whether or not the neurons' activity was modulated in synchrony with the perceptual reports made by the monkey (figure 2a). As indicated by the title of that paper—‘Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry’—some (but not all) neurons did indeed exhibit percept-related activity, with the proportion of percept-related neurons increasing in higher tier areas compared with V1. Brain imaging studies in humans using fMRI point to the same general findings, namely modulations in neural responses time-locked to fluctuations in perceptual dominance during rivalry (figure 2b). Those rivalry-related modulations in neural responses, like the single-unit responses, tend to be more pronounced within higher tier visual areas, particularly in the ventral stream pathway [47,72,79], but reliable rivalry modulations are also measurable in very early stages of the visual pathways including the thalamus [80,81] and primary visual cortex [35,36,81,82].
Figure 2.
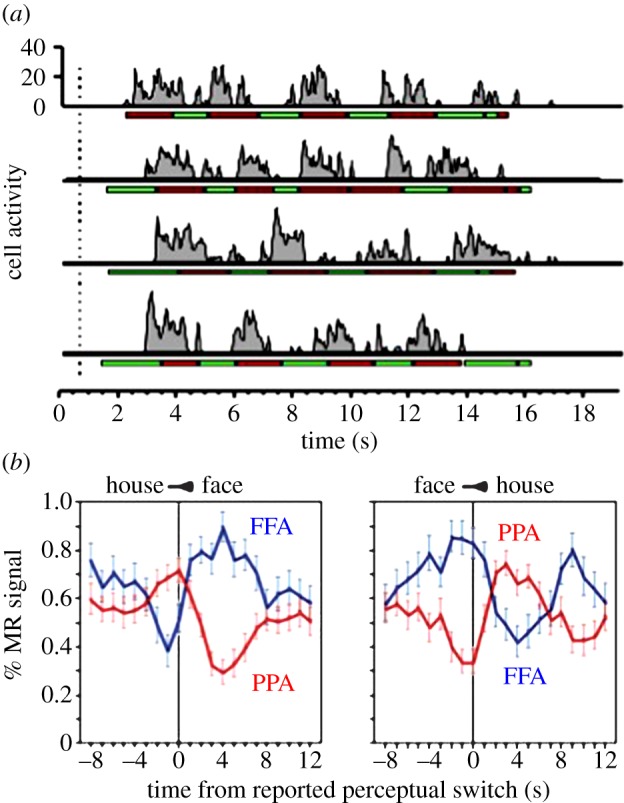
Two examples of modulations in neural responses coincident with fluctuations in perceptual state during binocular rivalry. (a) Neural spiking activity in a single cortical neuron recorded from an alert, behaving monkey trained to report fluctuations in perceptual dominance, which are denoted by the light and dark (red and green, respectively, online) horizontal lines below the spike train time series. Adapted from [7]. (b) Modulations in fMRI responses, timelocked to transitions in perceptual state during rivalry produced by dichoptic presentation of the image of a house and of a face. These modulations were measured in two ventral stream areas within the human brain, the fusiform face area (FFA) and the parahippocampal place area (PPA). Adapted from [47]. (Online version in colour.)
What conclusions can one draw from results like these? In particular, assuming that appropriate conditions are in place to rule out alternative accounts for such modulations, for example those raised by us in the earlier sections, and assuming that one thus obtained unequivocal evidence for a causal link between a fluctuating measure of neural activity and fluctuating perceptual states during rivalry, would this unequivocally show that this neural activity is part of the NCsC? We think not, as we will explain below.
Papers touting binocular rivalry as a powerful inferential tool for pursuing NCsC often include a statement to the effect that physical stimulation remains invariant while perception fluctuates during rivalry, the implication being that binocular rivalry dissects input-related neural events from percept-related neural events and, therefore, potentially reveals NCsC. But this characterization of rivalry and its associated implication we find questionable for two reasons.
First, the proximal stimulus, rather than remaining invariant, probably does change in subtle ways during rivalry, owing to tiny eye movements that arise consequent to rival stimulation [83] and to pupillary dilations preceding [84,85] and during [86] state changes in rivalry. Moreover, the neural representations of rivalling left- and right-eye inputs are impacted very early in visual processing, both in strength and in fidelity [87]. These consequences of rival stimulation, while percolating on to subsequent stages of visual processing, may have no bearing at all upon NCsC under non-rivalry conditions.
Second, the dichotomy drawn between input-related events and NCsC is probably oversimplified in the first place. This verdict of oversimplification has been voiced by others as well [10,11], and it follows from several of the issues discussed earlier in our essay. For instance, we now know that some neural events transpiring during rivalry turn out to correlate neither with the input nor with conscious perception per se, but instead with attention. Moreover, other neural events may correlate with perception when binocular rivalry is involved, but not when using a different phenomenon, for example motion-induced blindness to produce fluctuations in awareness. In other words, correlates of rivalry perception are not necessarily NCsC. What one ideally would want in the experimental search for NCsC is a procedure that not only leaves physical stimulation intact while perception fluctuates, but that also leaves all neural processing intact apart from that which is part of the NCsC for the particular perceptual states experienced by the observer. But the evidence indicates that binocular rivalry does not quite allow this idealized experimental approach.
6. Conclusion
The idea to use binocular rivalry to search for NCsC was never naive. As Crick and Koch developed the idea, spurred on by experimental results in monkeys undergoing rivalry [33,34], they noted that ‘just because a particular neuron follows a percept, it does not automatically imply that its firing is part of the NCC’ [1]. Since then the focus in the experimental literature has been on exploring the new territory in terms of its empirical characteristics (‘what correlates with rivalry dominance?’), but this exploration has unearthed new experimental hurdles, described in our first concern (§2), as well as problems of interpretation, described in the remaining concerns (§§2–5). Consequently, we have grown more sceptical about the utility of binocular rivalry as a tool for discovering the NCsC.
But in the process, we believe that there has been considerable progress toward a different goal of characterizing neural processing and neural dynamics. Binocular rivalry provides a unique window into neural dynamics. Indeed, a number of dynamic systems models have been proposed to explain the alternating periods of perceptual dominance for rival stimulation, including the periods of mixed percepts and travelling waves of changing dominance [31,88–92]. There is increasing evidence that the brain relies on a set of canonical neural computations, repeating them across brain regions and modalities to apply similar operations to different problems [93]. Consequently, the computational principle revealed by binocular rivalry may very well generalize and have widespread application, including shedding light on clinical conditions. Specifically, there is evidence that rivalry dynamics depend on the balance of excitation and inhibition in neural circuits [94], and an imbalance of excitation and inhibition has been hypothesized to underlie neural dysfunction in developmental disabilities, psychiatric illnesses and neurological diseases [95]. Such hypotheses can, therefore, be tested non-invasively through simple behavioural experiments with binocular rivalry [96].
Where, then, does this leave us with respect to the goal of characterizing the neural basis of perceptual awareness? Our guess is that fluctuations in perceptual awareness during rivalry and, for that matter, during other forms of multistable perception may depend critically on processes involved in perceptual decision-making. After all, when participating in a rivalry experiment, observers are instructed to press one of several buttons to indicate their changing perceptual state. Following that, instruction involves making an ongoing series of perceptual decisions (‘Do I see this image or that image?’), not unlike the perceptual detection and discrimination decisions that have been studied for decades using conventional psychophysical procedures with sequences of discrete trials. There is a large, growing literature on the neural circuits and computations underlying such perceptual decisions [97]. We are intrigued by the possibility that judgements about perceptual awareness (‘Do I see something, and if so, was it this image or that image?’) may, in fact, be embodied in the same neural machinery responsive for other forms of perceptual decisions.
Acknowledgements
We thank David Bloom for help with production of this paper.
Funding statement
R.B. was supported by grants from the National Research Foundation of Korea/Ministry of Education, Science and Technology (R31-10089) and from the National Institutes of Health (R21-EY022752). J.B. was supported by a Veni grant from the Netherlands Organisation for Scientific Research.
References
- 1.Crick F, Koch C. 1998. Consciousness and neuroscience. Cereb. Cortex 8, 97–107. ( 10.1093/cercor/8.2.97) [DOI] [PubMed] [Google Scholar]
- 2.Crick F, Koch C. 2003. A framework for consciousness. Nat. Neurosci. 6, 119–126. ( 10.1038/nn0203-119) [DOI] [PubMed] [Google Scholar]
- 3.Rees G, Kreiman G, Koch C. 2002. Neural correlates of consciousness in humans. Nat. Rev. Neurosci. 3, 261–270. ( 10.1038/nrn783) [DOI] [PubMed] [Google Scholar]
- 4.Koch C. 2004. The quest for consciousness: a neurobiological approach. Denver, CO: Roberts. [Google Scholar]
- 5.Lamme VAF. 2006. Towards a true neural stance on consciousness. Trends Cogn. Sci. 10, 494–501. ( 10.1016/j.tics.2006.09.001) [DOI] [PubMed] [Google Scholar]
- 6.Tonini G, Koch C. 2008. The neural correlates of consciousness: an update. Ann. NY Acad. Sci. 1124, 239–261. ( 10.1196/annals.1440.004) [DOI] [PubMed] [Google Scholar]
- 7.Leopold DA, Logothetis N. 1999. Multistable phenomena: changing views in perception. Trends Cogn. Sci. 3, 254–264. ( 10.1016/S1364-6613(99)01332-7) [DOI] [PubMed] [Google Scholar]
- 8.Kim C-Y, Blake R. 2005. Psychophysical magic: rendering the visible ‘invisible’. Trends Cogn. Sci. 9, 381–388. ( 10.1016/j.tics.2005.06.012) [DOI] [PubMed] [Google Scholar]
- 9.Maier A, Panagiotaropoulos T, Tsuchiya N, Keliris GA. 2012. Introduction to research topic. Binocular rivalry: a gateway to consciousness. Front. Hum. Neurosci. 6, 263 ( 10.3389/fnhum.2012.00263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aru J, Bachmann T, Singer W, Melloni L. 2012. Distilling the neural correlates of consciousness. Neurosci. Biobehav. Rev. 36, 737–746. ( 10.1016/j.neubiorev.2011.12.003) [DOI] [PubMed] [Google Scholar]
- 11.de Graaf TA, Hsieh P-J, Sack AT. 2012. The ‘correlates’ in neural correlates of consciousness. Neurosci. Biobehav. Rev. 36, 191–197. ( 10.1016/j.neubiorev.2011.05.012) [DOI] [PubMed] [Google Scholar]
- 12.Brascamp JW, Baker DH. 2013. Psychophysics of binocular rivalry. In The constitution of visual consciousness: lessons from binocular rivalry (ed. Miller SM.), pp. 109–140. Amsterdam, The Netherlands: John Benjamins. [Google Scholar]
- 13.Blake R. 1997. What can be ‘perceived’ in the absence of visual awareness? Curr. Dir. Psychol. Sci. 6, 157–162. ( 10.1111/1467-8721.ep10772935) [DOI] [Google Scholar]
- 14.Blake R, Fox R. 1974. Adaptation to invisible gratings and the site of binocular rivalry suppression. Nature 249, 488–490. ( 10.1038/249488a0) [DOI] [PubMed] [Google Scholar]
- 15.Lehmkuhle SW, Fox R. 1975. Effect of binocular rivalry suppression on the motion aftereffect. Vis. Res. 15, 855–859. ( 10.1016/0042-6989(75)90266-7) [DOI] [PubMed] [Google Scholar]
- 16.Wade NJ, Wenderoth P. 1978. The influence of colour and contour rivalry on the magnitude of the tilt after-effect. Vis. Res. 18, 827–836. ( 10.1016/0042-6989(78)90123-2) [DOI] [PubMed] [Google Scholar]
- 17.O'Shea RP, Crassini B. 1981. Interocular transfer of the motion after-effect is not reduced by binocular rivalry. Vis. Res. 21, 801–804. ( 10.1016/0042-6989(81)90177-2) [DOI] [PubMed] [Google Scholar]
- 18.Moradi F, Koch C, Shimojo S. 2005. Face adaptation depends on seeing the face. Neuron 45, 169–175. ( 10.1016/j.neuron.2004.12.018) [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya N, Koch C. 2005. Continuous flash suppression reduces negative afterimages. Nat. Neurosci. 8, 1096–1101. ( 10.1038/nn1500) [DOI] [PubMed] [Google Scholar]
- 20.Gilroy LA, Blake R. 2005. The interaction between binocular rivalry and negative afterimages. Curr. Biol. 15, 1740–1744. ( 10.1016/j.cub.2005.08.045) [DOI] [PubMed] [Google Scholar]
- 21.Maruya K, Watanabe H, Watanabe M. 2008. Adaptation to invisible motion results in low-level but not high-level aftereffects. J. Vis 8, 7 ( 10.1167/8.11.7) [DOI] [PubMed] [Google Scholar]
- 22.Yang E, Hong S-W, Blake R. 2010. Adaptation aftereffects to facial expressions suppressed from visual awareness. J. Vis. 10, 24 ( 10.1167/10.12.24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Boxtel JJA, Tsuchiya N, Koch C. 2010. Opposing effects of attention and consciousness on afterimages. Proc. Natl Acad. Sci. USA 107, 8883–8888. ( 10.1073/pnas.0913292107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brascamp JW, van Boxtel JJA, Knapen THJ, Blake R. 2010. A dissociation of attention and awareness in phase-sensitive but not phase-insensitive visual channels. J. Cogn. Neurosci. 22, 2326–2344. ( 10.1162/jocn.2009.21397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein T, Sterzer P. 2011. High-level face shape adaptation depends on visual awareness: evidence from continuous flash suppression. J. Vis. 11, 5 ( 10.1167/11.8.5) [DOI] [PubMed] [Google Scholar]
- 26.Stuit SM, Paffen CLE, van der Smagt MJ, Verstraten FAJ. 2011. Suppressed images selectively affect the dominant percept during binocular rivalry. J. Vis. 11, 7 ( 10.1167/11.10.7) [DOI] [PubMed] [Google Scholar]
- 27.Zadbood A, Lee S-H, Blake R. 2011. Stimulus fractionation by interocular suppression. Front. Hum. Neurosci. 5, 135 ( 10.3389/fnhum.2011.00135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legge GE. 1979. Spatial frequency masking in human vision: binocular interactions. J. Opt. Soc. Am. 69, 838–847. ( 10.1364/JOSA.69.000838) [DOI] [PubMed] [Google Scholar]
- 29.Blake R, Sloane M, Fox R. 1981. Further developments in binocular summation. Percept. Psychophys. 30, 266–276. ( 10.3758/BF03214282) [DOI] [PubMed] [Google Scholar]
- 30.Ding J, Sperling G. 2006. A gain-control theory of binocular combination. Proc. Natl Acad. Sci. USA 103, 1141–1146. ( 10.1073/pnas.0509629103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Said CP, Heeger DJ. 2013. A model of binocular rivalry and cross-orientation suppression. PLoS Comput. Biol. 9, e1002991 ( 10.1371/journal.pcbi.1002991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moradi F, Heeger DJ. 2009. Inter-ocular contrast normalization in human visual cortex. J. Vis. 9, 13 ( 10.1167/9.3.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logothetis NK, Schall JD. 1989. Neuronal correlates of subjective visual perception. Science 245, 761–763. ( 10.1126/science.2772635) [DOI] [PubMed] [Google Scholar]
- 34.Leopold DA, Logothetis NK. 1996. Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry. Nature 379, 549–553. ( 10.1038/379549a0) [DOI] [PubMed] [Google Scholar]
- 35.Polonsky A, Blake R, Braun J, Heeger D. 2000. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat. Neurosci. 3, 1153–1159. ( 10.1038/80676) [DOI] [PubMed] [Google Scholar]
- 36.Haynes J-D, Rees G. 2005. Predicting the stream of consciousness from activity in human visual cortex. Curr. Biol. 15, 1301–1307. ( 10.1016/j.cub.2005.06.026) [DOI] [PubMed] [Google Scholar]
- 37.Nguyen VA, Freeman AW, Wenderoth P. 2001. The depth and selectivity of suppression in binocular rivalry. Percept. Psychophys. 63, 348–360. ( 10.3758/BF03194475) [DOI] [PubMed] [Google Scholar]
- 38.Blake R, Tadin D, Sobel KV, Chong SC, Raissian TA. 2006. Strength of early visual adaptation depends on visual awareness. Proc. Natl Acad. Sci. USA 103, 4783–4788. ( 10.1073/pnas.0509634103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen VA, Freeman AW, Alais D. 2003. Increasing depth of binocular rivalry suppression along two visual pathways. Vis. Res. 43, 2003–2008. ( 10.1016/S0042-6989(03)00314-6) [DOI] [PubMed] [Google Scholar]
- 40.Lumer ED, Friston KJ, Rees G. 1998. Neural correlates of perceptual rivalry in the human brain. Science 280, 1930–1934. ( 10.1126/science.280.5371.1930) [DOI] [PubMed] [Google Scholar]
- 41.Knapen T, Brascamp J, Pearson J, van Ee R, Blake R. 2011. The role of frontal and parietal brain areas in bistable perception. J. Neurosci. 31, 10 293–10 301. ( 10.1523/JNEUROSCI.1727-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterzer P, Kleinschmidt A. 2007. A neural basis for inference in perceptual ambiguity. Proc. Natl Acad. Sci. USA 104, 323–328. ( 10.1073/pnas.0609006104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S-H, Blake R. 2004. A fresh look at interocular grouping during binocular rivalry. Vis. Res 44, 983–991. ( 10.1016/j.visres.2003.12.007) [DOI] [PubMed] [Google Scholar]
- 44.Baker DH, Graf EW. 2009. Natural images dominate in binocular rivalry. Proc. Natl Acad. Sci. USA 106, 5436–5441. ( 10.1073/pnas.0812860106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alais D, van Boxtel JJ, Parker A, van Ee R. 2010. Attending to auditory signals slows visual alternations in binocular rivalry. Vis. Res. 50, 929–935. ( 10.1016/j.visres.2010.03.010) [DOI] [PubMed] [Google Scholar]
- 46.Alais D, Cass J, O'Shea RP, Blake R. 2010. Visual sensitivity underlying changes in visual consciousness. Curr. Biol. 20, 1362–1367. ( 10.1016/j.cub.2010.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tong F, Nakayama K, Vaughan JT, Kanwisher N. 1998. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron 21, 753–759. ( 10.1016/S0896-6273(00)80592-9) [DOI] [PubMed] [Google Scholar]
- 48.van Boxtel JJA, Tsuchiya N, Koch C. 2010. Consciousness and attention: on sufficiency and necessity. Front. Psychol. 1, 217 ( 10.3389/fpsyg.2010.00217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen MA, Cavanaugh P, Chun MM, Nakayama K. 2012. The attentional requirements of consciousness. Trends Cogn. Sci. 16, 411–417. ( 10.1016/j.tics.2012.06.013) [DOI] [PubMed] [Google Scholar]
- 50.Kanai R, Tsuchiya N, Verstraten FAJ. 2006. The scope and limits of top-down attention in unconscious visual processing. Curr. Biol. 16, 2332–2336. ( 10.1016/j.cub.2006.10.001) [DOI] [PubMed] [Google Scholar]
- 51.Bahrami B, Carmel D, Walsh V, Rees G, Lavie N. 2008. Spatial attention can modulate unconscious orientation processing. Perception 37, 1520–1528. ( 10.1068/p5999) [DOI] [PubMed] [Google Scholar]
- 52.Bahrami B, Lavie N, Rees G. 2007. Attentional load modulates responses of human primary visual cortex to invisible stimuli. Curr. Biol. 17, 1–5. ( 10.1016/j.cub.2007.01.070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe M, Cheng K, Murayama Y, Ueno K, Asamizuya T, Tanaka K, Logothetis N. 2011. Attention but not awareness modulates the BOLD signal in the human V1 during binocular suppression. Science 334, 829–831. ( 10.1126/science.1203161) [DOI] [PubMed] [Google Scholar]
- 54.Koch C, Tsuchiya N. 2007. Attention and consciousness: two distinct brain processes. Trends Cogn. Sci. 11, 16–22. ( 10.1016/j.tics.2006.10.012) [DOI] [PubMed] [Google Scholar]
- 55.Suzuki S, Grabowecky M. 2003. Attention during adaptation weakens negative afterimages. J. Exp. Psychol. Hum. Percept. Perform. 29, 793–807. ( 10.1037/0096-1523.29.4.793) [DOI] [PubMed] [Google Scholar]
- 56.Wede J, Francis G. 2006. The time course of visual afterimages: data and theory. Perception 35, 1155–1170. ( 10.1068/p5521) [DOI] [PubMed] [Google Scholar]
- 57.Gandhi SP, Heeger DJ, Boynton GM. 1999. Spatial attention affects brain activity in human primary visual cortex. Proc. Natl Acad. Sci. USA 96, 3314–3319. ( 10.1073/pnas.96.6.3314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. 1999. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22, 751–761. ( 10.1016/S0896-6273(00)80734-5) [DOI] [PubMed] [Google Scholar]
- 59.Tong F, Meng M, Blake R. 2006. Neural bases of binocular rivalry. Trends Cogn. Sci. 10, 502–511. ( 10.1016/j.tics.2006.09.003) [DOI] [PubMed] [Google Scholar]
- 60.Yuval-Greenberg S, Heeger DJ. 2013. Continuous flash suppression modulates cortical activity in early visual cortex. J. Neurosci. 33, 9635–9643. ( 10.1523/JNEUROSCI.4612-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paffen CLE, Alais D. 2011. Attentional modulation of binocular rivalry. Front. Hum. Neurosci. 5, 105 ( 10.3389/fnhum.2011.00105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dieter KC, Tadin D. 2011. Understanding attentional modulation of binocular rivalry: a framework based on biased competition. Front. Hum. Neurosci. 5, 155 ( 10.3389/fnhum.2011.00155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paffen CLE, Alais D, Verstraten FAJ. 2006. Attention speeds binocular rivalry. Psychol. Sci. 17, 752–756. ( 10.1111/j.1467-9280.2006.01777.x) [DOI] [PubMed] [Google Scholar]
- 64.Brascamp J, Blake R. 2012. Inattention abolishes binocular rivalry: perceptual evidence. Psychol. Sci. 23, 1159–1167. ( 10.1177/0956797612440100) [DOI] [PubMed] [Google Scholar]
- 65.Zhang P, Jamison K, Engel S, He B, He S. 2011. Binocular rivalry requires visual attention. Neuron 71, 362–369. ( 10.1016/j.neuron.2011.05.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SH, Blake R, Heeger D. 2007. Hierarchy of cortical responses underlying binocular rivalry. Nat. Neurosci. 10, 1048–1054. ( 10.1038/nn1939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freeman AW. 2005. Multistage model for binocular rivalry. J. Neurophysiol. 94, 4412–4420. ( 10.1152/jn.00557.2005) [DOI] [PubMed] [Google Scholar]
- 68.Wilson HR. 2003. Computational evidence for a rivalry hierarchy in vision. Proc. Natl Acad. Sci. USA 100, 14 499–14 503. ( 10.1073/pnas.2333622100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alais D, Melcher D. 2007. Strength and coherence of binocular rivalry depends on shared stimulus complexity. Vis. Res. 47, 269–279. ( 10.1016/j.visres.2006.09.003) [DOI] [PubMed] [Google Scholar]
- 70.Alais D, Parker A. 2006. Independent binocular rivalry processes for motion and form. Neuron 52, 911–920. ( 10.1016/j.neuron.2006.10.027) [DOI] [PubMed] [Google Scholar]
- 71.Watson TL, Pearson J, Clifford CWG. 2004. Perceptual grouping of biological motion promotes binocular rivalry. Curr. Biol. 14, 1670–1674. ( 10.1016/j.cub.2004.08.064) [DOI] [PubMed] [Google Scholar]
- 72.Fang F, He S. 2005. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat. Neurosci. 8, 1380–1385. ( 10.1038/nn1537) [DOI] [PubMed] [Google Scholar]
- 73.Moutoussis K, Keliris G, Kourtzi Z, Logothetis N. 2005. A binocular rivalry study of motion perception in the human brain. Vis. Res. 45, 2231–2243. ( 10.1016/j.visres.2005.02.007) [DOI] [PubMed] [Google Scholar]
- 74.Bonneh YS, Cooperman A, Sagi D. 2001. Motion-induced blindness in normal observers. Nature 411, 798–801. ( 10.1038/35081073) [DOI] [PubMed] [Google Scholar]
- 75.Donner TH, Sagi D, Bonneh YS, Heeger DJ. 2008. Opposite neural signatures of motion-induced blindness in human dorsal and ventral visual cortex. J. Neurosci. 28, 10 298–10 310. ( 10.1523/JNEUROSCI.2371-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maier A, Logothetis NK, Leopold DA. 2007. Context-dependent perceptual modulation of single neurons in primate visual cortex. Proc. Natl Acad. Sci. USA 104, 5620–5625. ( 10.1073/pnas.0608489104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teller DY, Pugh EN., Jr 1983. Linking propositions in color vision. In Colour vision: physiology and psychophysics (eds Mollon JD, Sharpe LT.). London, UK: Academic Press. [Google Scholar]
- 78.Movshon JA. 2013. Three comments on Teller's ‘bridge locus’. Vis. Neurosci. 30, 219–222. ( 10.1017/S0952523813000527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sterzer P, Haynes J-D, Rees G. 2008. Fine-scale activity patterns in high-level visual areas encode the category of invisible objects. J. Vis. 8, 10 ( 10.1167/8.15.10) [DOI] [PubMed] [Google Scholar]
- 80.Wunderlich K, Schneider KA, Kastner S. 2005. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nat. Neurosci. 8, 1595–1602. ( 10.1038/nn1554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haynes J-D, Deichmann R, Rees G. 2005. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature 438, 496–499. ( 10.1038/nature04169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tong F, Engel SA. 2001. Interocular rivalry revealed in the human cortical blind-spot representation. Nature 411, 195–199. ( 10.1038/35075583) [DOI] [PubMed] [Google Scholar]
- 83.Sabrin HW, Kertesz AE. 1980. Microsaccadic eye movements and binocular rivalry. Percept. Psychophys. 28, 150–154. ( 10.3758/BF03204341) [DOI] [PubMed] [Google Scholar]
- 84.Einhäuser W, Stout J, Koch C, Carter O. 2008. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc. Natl Acad. Sci. USA 105, 1704–1709. ( 10.1073/pnas.0707727105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fahle MW, Stemmler T, Spang KM. 2011. How much of the ‘unconscious’ is just pre-threshold? Front. Hum. Neurosci. 5, 120 ( 10.3389/fnhum.2011.00120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naber M, Frässle S, Einhäuser W. 2011. Perceptual rivalry: reflexes reveal the gradual nature of visual awareness. PLoS ONE 6, e20910 ( 10.1371/journal.pone.0020910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ling S, Blake R. 2009. Suppression during binocular rivalry broadens orientation tuning. Psychol. Sci. 20, 1348–1357. ( 10.1167/8.6.246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lehky SR. 1988. An astable multivibrator model of binocular rivalry. Perception 17, 215–228. ( 10.1068/p170215) [DOI] [PubMed] [Google Scholar]
- 89.Bressloff PC, Webber MA. 2012. Neural field model of binocular rivalry waves. J. Comput. Neurosci. 32, 233–252. ( 10.1007/s10827-011-0351-y) [DOI] [PubMed] [Google Scholar]
- 90.Stollenwerk L, Bode M. 2003. Lateral neural model of binocular rivalry. Neural Comput. 15, 2863–2882. ( 10.1162/089976603322518777) [DOI] [PubMed] [Google Scholar]
- 91.Dayan P. 1998. A hierarchical model of binocular rivalry. Neural Comput. 10, 1119–1135. ( 10.1162/089976698300017377) [DOI] [PubMed] [Google Scholar]
- 92.Shpiro A, Moreno-Bote R, Rubin N, Rinzel J. 2009. Balance between noise and adaptation in competition models of perceptual bistability. J. Comput. Neurosci. 27, 37–54. ( 10.1007/s10827-008-0125-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carandini M, Heeger DJ. 2011. Normalization as a canonical neural computation. Nat. Rev. Neurosci. 13, 51–62. ( 10.1038/nrn3136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Loon AM, Knapen T, Scholte HS, St. John-Saaltink E, Donner TH, Lamme VAF. 2013. GABA shapes the dynamics of bistable perception. Curr. Biol. 23, 823–827. ( 10.1016/j.cub.2013.03.067) [DOI] [PubMed] [Google Scholar]
- 95.Rubenstein JL, Merzenich MM. 2003. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267. ( 10.1034/j.1601-183X.2003.00037.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Said CP, Egan RD, Minshew NJ, Behrmann M, Heeger DJ. 2013. Normal binocular rivalry in autism: implications for the excitation/inhibition imbalance hypothesis. Vis. Res. 77, 59–66. ( 10.1016/j.visres.2012.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gold JI, Shadlen MN. 2007. The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574. ( 10.1146/annurev.neuro.29.051605.113038) [DOI] [PubMed] [Google Scholar]