Abstract
The dorsal and ventral cortical pathways, driven predominantly by magnocellular (M) and parvocellular (P) inputs, respectively, assume leading roles in models of visual information processing. Although in prior proposals, the dorsal and ventral pathways support non-conscious and conscious vision, respectively, recent modelling and empirical developments indicate that each pathway plays important roles in both non-conscious and conscious vision. In these models, the ventral P-pathway consists of one subpathway processing an object's contour features, e.g. curvature, the other processing its surface attributes, e.g. colour. Masked priming studies have shown that feed-forward activity in the ventral P-pathway on its own supports non-conscious processing of contour and surface features. The dorsal M-pathway activity contributes directly to conscious vision of motion and indirectly to object vision by projecting to prefrontal cortex, which in turn injects top-down neural activity into the ventral P-pathway and there ‘ignites’ feed-forward–re-entrant loops deemed necessary for conscious vision. Moreover, an object's shape or contour remains invisible without the prior conscious registration of its surface properties, which for that reason are taken to comprise fundamental visual qualia. Besides suggesting avenues for future research, these developments bear on several recent and past philosophical issues.
Keywords: magnocellular/parvocellular pathways, visual masking, conscious/non-conscious vision, feed-forward/re-entrant processing, surface/contour processing
1. Introduction
Along with Uttal [1], I define perception as the registration of sensory information in consciousness. Accordingly, vision deserves a much broader definition by accommodating not only conscious visual processing, i.e. visual perception, but also non-conscious and pre-conscious visual processing. After all, flies have vision, but it is highly unlikely that their vision is conscious [2]. By contrast, when we humans look around, we perceive a visual world composed of richly varied objects and events that can be characterized by a relatively small set of distinct perceptual dimensions or attributes. The subjectively experienced rich variations, particularly those of colour and brightness, correspond to Pollen's [3] primary visual percepts, also referred to as qualia. We also note that all parts of a visual scene can be characterized by the contours that define their shape or form and by their surface features. For example, as I look through my office window all components of the external scene, the sidewalks crisscrossing the grassy areas, the grassy areas themselves, the tree trunks, buildings adjacent to mine, and so on, have shape and surface characteristics. For that reason, without making metaphysical assumptions as to what truly constitutes an object, I will define a visual object very generally as any bounded segment of the visual field which is distinguishable from another bounded segment by virtue of its contour and surface properties.
Given such scenes, it is reasonable to expect that the functional design of an information gathering system like the visual brain includes distinct subsystems, each processing variations along only one or a few stimulus dimensions. These subsystems thus comprise a set of parallel processing channels. In recent decades, the magno- (M) and parvocellular (P) streams of processing in the monkey visual system [4,5] have assumed leading status for models of parallel processing in human vision. Cortically, M and P activities project primarily, but not exclusively, along the dorsal and ventral pathways, respectively [6,7]. A prevalent view of the dorsal M-dominant and the ventral P-dominant pathways is that the former supports vision for action, whereas the latter supports vision for perception [8,9]. Although associating the two pathways with their respective types of vision is a point of ongoing debate [8–11], the link between the dorsal and ventral pathways with non-conscious and conscious vision, respectively, [12,13] can by and large—with exceptions noted in §3—be maintained.
Here, as elsewhere [14], a central thesis is that the surface features of visual objects assume a critical role in conscious vision, in that sense rendering visual consciousness ‘superficial’. To support this thesis, a detailed look at object processing in the P-dominated ventral pathway will reveal that perceived attributes, such as colour or lightness, classic examples of surface qualia, are processed separately from the perceived shape attributes. These, as are characterized by spatial extent and configuration, will henceforth be referred to as geometric qualia. The gist of the proposal is that, insofar as the perception of geometric qualia depends on the prior conscious registration of surface qualia, without surface qualia there is no conscious object vision.
Not all visual processing results in perception. For example, in healthy observers one can experimentally induce transient stimulus blindness without affecting the non-conscious processing of geometric attributes, such as the shape or location [15,16]. Besides such instances of non-conscious vision in normal observers, studies of blindsight patients with damage to primary visual cortex have shown that the location, motion and wavelength of stimuli presented to the affected field can be discriminated without conscious registration of qualia [17]. Milner & Goodale [9] also studied a visual form agnosic patient who retains access to geometric attributes. For instance, this patient while failing to report the conscious registration of objects varying in width, can, when reaching for them, adjust her grip apertures to their widths. Hence, some visuo-cognitive functions, particularly those underlying the ‘online’ control of skeletomotor actions can proceed ‘beneath the dashboard’. By contrast, access to perceived information, in turn accessing information stored in long- and short-term visual memories, is important in situations requiring the ‘offline’ monitoring and deliberative resolving of skeletomotor plans competing for conscious control of action [18,19].
The starting point of the ideas developed here, that the conscious registration of surface qualia, for example the colour, of visual stimuli is necessary for their perception as visual objects, has been intuited and expressed by philosophers and cognitive scientists alike. For instance, regarding colour, the philosopher of art John Hyman [20], in his book The Objective Eye, states that ‘ … there is an intrinsic tie between color and sentience [perceptual awareness], as there is between smell or taste and sentience, which does not exist between sentience and shape.’ (p. 17; emphases added). Shortly thereafter he elaborates that ‘ … [one] cannot see the shape of a banana except by seeing its spatial boundaries, however fleeting and uncertain this experience may be. And [one] cannot see its spatial boundaries except by seeing the differences of color that make it visibly distinct from its surroundings’. (p. 18; emphasis added) Related views on the importance of surface features, for example colours, to our understanding of visual consciousness are expressed explicitly by Stephen Grossberg's model-driven claim that ‘surfaces are for seeing’ [21, p. 19]. As standard definitions of ‘sentience’ and ‘seeing’ refer to conscious awareness, Hyman's intuition and Grossberg's model assert that our visual awareness of shape depends foremost on awareness of surface properties, for example colour. Below, this thesis is elaborated within a neurocognitive framework that is consistent with existing psychophysical, neuroanatomical, neuropsychological and neural modelling approaches to visual cognition.
2. The roles of visual processing in the ventral pathway
(a). Neural network approaches to object vision
Biologically realistic models of vision [22,23] incorporate separate processing modules for specifying boundaries or contours of objects and for completing the surface properties within the boundaries of an object. The evolving versions of Grossberg's model of visual processing [23,24] provide particularly apt illustrations of these processes. The model incorporates a boundary contour system (BCS) and a feature contour system (FCS) both of which are associated with the P-dominant ventral pathway [23]. The BCS specifies the existence and location of object boundaries that delineate the outline of an object. The FCS specifies the surface features that fill the area delimited by the BCS. The perceived object is rendered as a composite of its form attributes (the geometrical qualia of orientation, width, curvature, etc.) and its surface attributes (the sensory qualia of colour, lightness, etc.). Thus we can perceptually distinguish two photo images of, say, an Anjou pear and a Bartlett pear by colour; of others, such as a green clover leaf and a green dandelion leaf by shape; and of still others, such as a banana and a pomegranate by colour and shape. A schematic depiction of the contributions of the form-processing BCS and the surface-processing FCS to object perception is illustrated in figure 1 for two objects, a black square and a white diamond.
Figure 1.
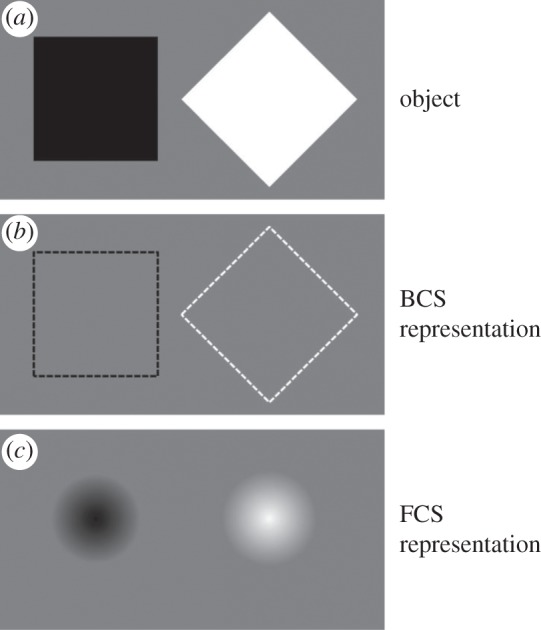
(a) Two visual objects, a black square and a white diamond. (b) Output of the BCS, schematized by dashed lines, renders the invisible BCS representation. (c) Output of the FCS that completes the surface between the BCS contour representations.
An important aspect of the BCS, indicated by the dashed lines in figure 1 delineating the contour outline of the objects, is that it processes form implicitly, i.e. at the non- or pre-conscious levels. Grossberg expresses the interactive roles of the BCS and the FCS in object perception as follows:
A boundary that is completed within the segmentation system (denoted BCS) does not generate visible contrasts within the BCS. In this sense, all boundaries are invisible [original emphasis]. Visibility is a property of the surface filling-in system (denoted FCS). The completed BCS boundary can directly activate the object recognition system (ORS) whether or not it is visible within the FCS [emphasis added]. In summary, a boundary may be completed within the BCS, and thereby improve pattern recognition by the ORS, without necessarily generating a visible brightness or color difference within the FCS.
[23, p. 59]
These properties of the form-processing and the surface-processing systems (i) resonate with a number of findings showing that an object's contours are processed separately from its surface [25–27] and (ii) have important implications for our understanding not only of object recognition [22] but also of conscious and non-conscious visual processing. Further implications will be explored after describing some neurobiological properties of vision that point to distinct form- and surface-processing subsystems.
(i). Neurobiological substrate for form-processing and surface-processing systems in primate cortex
Almost two and a half decades ago Livingstone & Hubel [28] proposed separate cortical channels for the processing of form, colour, movement and depth of visual stimuli. According to this proposal, form and colour are processed in the cortical P pathways while depth and movement are processed by the cortical M pathways. Along with the sharp distinction between M- and P-pathways, the strict subdivision of the cortical P-pathway into separate cortical P channels for colour and form, arising from the anatomically distinct blob and interblob areas in primary visual cortex (V1), respectively, is controversial [29,30] (figure 2). A significant number of orientation-selective neurons are also selective for wavelength [29,31]. Despite the lack of a strict segregation of form and colour processing systems, accumulating evidence indicates that there are anatomically identifiable pathways and areas in the early and intermediate cortical object-processing systems that process primarily the surface properties of colour and luminance, on the one hand, and the form properties of contour and edge orientation, on the other hand [30–35]. For instance, based on Felleman et al.'s [31] work, V4, like V1 and V2, has separate neural compartments for shape and surface processing. Supporting this scheme, Girard et al. [36] showed that reversible deactivation of V4 in macaque monkey can impair shape discrimination while leaving hue discrimination intact.
Figure 2.
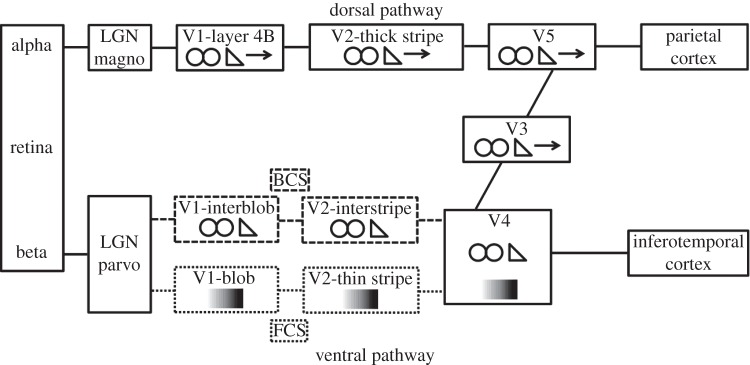
A schematic of cortical dorsal and ventral pathways. The areas of V1 and V2 included in the dashed lines comprise parts of the cortical BCS that processes contours defined by luminance contrast or by chromatic wavelength contrast ( symbol). Similarly, the areas of V1 and V2 included in the dotted lines comprise the cortical surface-processing system that processes either variations of achromatic luminance contrast or variations of chromatic wavelength contrast (
symbol). Similarly, the areas of V1 and V2 included in the dotted lines comprise the cortical surface-processing system that processes either variations of achromatic luminance contrast or variations of chromatic wavelength contrast ( symbol). Binocular processing (
symbol). Binocular processing ( symbol) occurs in both pathways. Adapted from DeYoe & Van Essen [4].
symbol) occurs in both pathways. Adapted from DeYoe & Van Essen [4].
Neurons tuned to both colour and orientation may be especially well suited for processing isoluminant contours defined only by wavelength differences. At isoluminance, such neurons can contribute to the form-processing system, while neurons tuned only to wavelengths contribute to the surface-processing system. Of course, luminance-defined borders and achromatic surface properties are also processed by the separate form- and surface-processing systems.
In a study of cortical chromatic processing, Xiao et al. [34] showed that the thin stripes in V2 contain functional wavelength-specific subregions in each of which variations of stimulus wavelength are systematically mapped onto varying locations. Moreover, Xiao et al [35] showed that cortical wavelength maps exist as early as in the V1 blob areas. These provide input to the spatially more extensive V2 thin-stripe wavelength maps [34,35]. Wang et al. [33] additionally found that adjacent to the wavelength maps within the V2 thin stripes are neurons responding differentially to positive (light-on-dark) and negative (dark-on-light) luminance contrast. As suggested by Wang et al. [33], the thin stripes of V2 thus comprise neural modules for processing surface properties of visual stimuli, whereas neurons found in the juxtaposed V2 interstripes respond selectively to contour or edge orientation of stimuli and receive input from V1 interblob areas (see [33], fig. 9).
(ii). Evidence for separate but interactive form-processing and surface-processing systems in human vision
Studies of neurologically impaired patients also reveal that shape and surface properties of visual objects are processed by dissociable cortical systems. Barbur et al. [37] and Kentridge et al. [38] have shown that, despite the loss of phenomenal hue perception of surfaces, achromatopsic (cortically colour-blind) patients can detect isoluminant chromatic edges or contours. Moreover, an achromatopsic patient investigated by Heywood et al. [39], while grossly impaired in discriminating isoluminant hues, was able to discriminate different achromatic greys. This is consistent with Wang et al.'s [33] findings in monkey indicating the existence of separate luminance and colour processing areas within V2 thin-stripe surface-processing modules. In addition, recalling that selective loss of shape discrimination with intact hue discrimination was reported by Girard et al. [36] when V4 was reversibly deactivated in macaque monkey, Zeki et al. [40] similarly showed that a patient, though all but form blind, was able to name objects’ colours.
In order to construct a veridical object representation, the BCS and FCS must be able to communicate or interact appropriately with each other. Disturbances in these systems or in their interaction should lead to distorted perceptions of object properties. Such distortions also are found in some neurological patients. In his review of deficits of colour perception, Critchley [41] reports patients who perceive the colour of an object irradiating outward beyond its boundaries, sometimes at great distances from the boundaries of the object, and who often report very fuzzy or extensively blurred object boundaries. In other cases, the colour is perceived as not adhering to the object's surface, but instead as free-floating in space, in a plane distinct from that of the object and usually phenomenally located somewhere between it and the patient. Using inter-ocular continuous flash suppression, Hong & Blake [42] recently reported related phenomena in a study of healthy observers. Rapidly changing achromatic (grey) Mondrian patterns were flashed to one eye while a stationary chromatic bar was presented to the other eye. Although, owing to inter-ocular suppression, observers failed to see the contours or shape of the bar, they did report its colour in a free-floating, cloud-like constellation. Thus, the phenomenal reports of neurological patients as well as healthy observers indicate that the form-processing BCS either does not provide the necessary spatial constraints for the filling-in process of the colour-FCS system or that such constraints are not communicated to the FCS system.
(b). Spatio-temporal dynamics of form and surface processing
(i). Non-conscious level
Here, the discussion of the spatio-temporal dynamics of form and surface processing takes as a starting point the generally agreed upon the claim that, at non-conscious levels, the processing of form precedes that of surface features. Grossberg [21,23] notes that the processing of surface features, such as colour or luminance contrast, requires computations that compensate for variable intensities and wavelength compositions of the illuminant. Such discounting of the illuminant, though far from perfect [43,44], results in the two perceptual invariances realized in lightness and colour constancy [45]. Neural processes underlying these invariances may be more time consuming than those used to detect the contours delimiting an object's form. Thus before surface features can render a stimulus visible by completing its surfaces, its boundary as well as its surface properties must be processed at non-conscious levels.
Without an intact primary visual cortex, V1, there are few, if any, qualia-rich contents of visual consciousness [46]. Although V1 neural activity is necessary for conscious vision, there are cogent theoretical and empirical reasons for believing that it is not sufficient [47–49]. Although the existence of double-opponent (chromatically and spatially opponent) mechanisms in V1 provide the beginning stages of lightness and colour constancy computations [29], these computations are not fully realized until at least the level of extrastriate area V4 [38,40,50,51]. Hence, without further processing, neural activity in V1 cannot qualify as the sufficient basis of conscious object vision; and if, following Grossberg's [21] claim, surfaces are for seeing and the FCS fills the area bounded by the contours specified by the BCS only after the FCS has established lightness and colour constancy, then the neural correlates of conscious vision must occur at or after the stage at which these constancy computations are completed. This comports with Bar & Biederman's [52] proposal that visual awareness of object identity is associated with activity at or beyond the late, anterior region (area TE) of inferotemporal (IT) cortex and is supported by human brain-imaging studies indicating that conscious report of stimuli fails to occur without sufficient activation of higher levels of cortical processing [53,54]. Activity at these higher levels may play a crucial role in conscious vision by re-entering lower levels via top-down projections [55,56].
A majority of neurons at early cortical sites, such as V1, tend to respond to a stimulus presented in their receptive fields regardless of whether or not it is perceived, and such responses are defined as stimulus dependent; by contrast, a majority of neurons at higher levels in the ventral pathway, such as V4, tend to respond to a stimulus only when it is perceived, and these responses are defined as percept dependent [49]. A relevant example of the distinction between stimulus-dependent and percept-dependent processing is found in the psychophysical results reported by Breitmeyer et al. [57]. Further exploring the results of priming with non-consciously processed masked colour primes reported by Schmidt [58], Breitmeyer et al. [57] demonstrated that a masked white stimulus produces a priming effect the magnitude of which more closely approaches that produced by a masked green than to that by a masked blue stimulus. This result in itself is not surprising because the medium-wavelength (green) phosphor in the RGB display used in their experiment contributes a lot more of the luminous energy to the white prime than the short-wavelength (blue) phosphor. However, in conjunction with additional experimental results showing that white and blue visible primes were perceptually much more confusable than were white and green visible primes, the priming results are surprising. In combination, what the two findings indicate is that the priming produced by masked white prime depended on its physical wavelength properties, rather than perceptual colour quality. For, had the priming been percept dependent, then, based on the obtained perceived colour confusions, one would expect the masked white prime to act more like a masked blue than a masked green prime.
At these early, stimulus-dependent levels, the processing of contour precedes the processing of surface properties. As noted above, electrophysiological recordings from V1 neurons in macaque reveal separate processing of contour and surface properties of stimuli, with neural responses corresponding to surface properties lagging behind those corresponding to contour properties by about 30 ms [26]. Related cortically evoked potential studies of humans also indicate slower surface than contour processing [59]. Additionally consistent with slower non-conscious processing of surface relative to contour properties are results of several psychophysical studies [60,61].
(ii). Conscious level
Surface completion: filling-in, filling-out or both?
To explain an object's surface completion, most prior studies have relied exclusively on filling-in processes (see [61]). For example, Grossberg's models [21,23] assume a surface completion process which begins at the contours of an object and diffuses inward. Based on both phenomenology and experimental findings [60,62], one can make a strong case for the reality of an object's surface completion by filling-in. However, in addition to a filling-in process, Breitmeyer & Jacob [63], employing the metacontrast masking technique, recently showed that the completion of surface properties also proceeds from the centre of an object outward toward its edges. Figure 3 depicts how the perceptual filling-out progresses from a barely visible central region of a rectangular target at a target-to-mask onset asynchrony (SOA) of 80 ms, to its fully filled out perceptual registration at an SOA of 240 ms. Such a filling-out process comports with the aforementioned reports by Critchley [41] of visually impaired neurological patients’ perception of colour appearing to irradiate outward, beyond an object's (perceptually blurred or indistinct) boundaries. Moreover, given the well-known finding that processing of visual information proceeds from coarse (low spatial frequencies) to fine (high spatial frequencies) [64–68], a sequential spatial frequency-dependent frame-and-fill-in process can give rise to the filling-out reported by Breitmeyer & Jacob [63].
Figure 3.
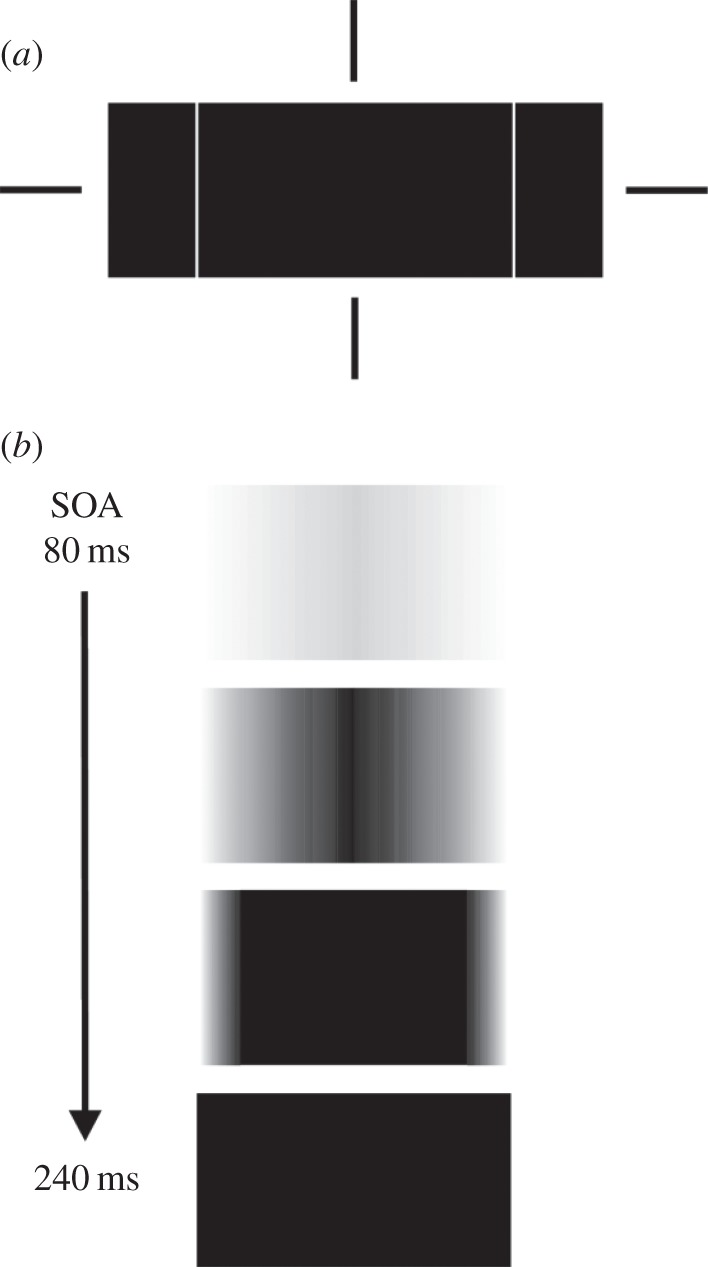
(a) Schematic of target rectangle and the two flanking mask rectangles centred at the notional fixation cross depicted by the vertically and horizontally collinear bars. (b) The appearance of the target at target-mask onset asynchronies (SOAs) increasing from 80 to 240 ms. Adapted from Breitmeyer & Jacob [63].
A reversal of temporal asynchrony
Despite some contrary findings and methodological critiques [69–71], numerous studies (for extensive citing, see [72]) report that changes of a stimulus's colour are perceived several tens of milliseconds earlier than the abrupt changes of its motion or form. Regarding form, this indicates that the temporal priority of the contour processing relative to surface processing at non-conscious cortical levels [61] appears to reverse at conscious levels [73–75]. Consistent with this finding, in the aforementioned study of inter-ocular continuous flash suppression reported by Hong & Blake [42], the colour of the chromatic bar presented to the (temporarily) suppressed eye nearly always emerged into dominance (consciousness) before the orientation (form) of the bar was perceived. Rather than revealing a contradiction or inconsistency, we take these results actually to point out a key feature of the transition from non-conscious, pre-perceptual phase to a conscious, perceptual phase of form processing. Given (i) that the conscious percept of an object's form requires the conscious percept of its surface [21,23] and (ii) that perceptual surface filling is not instantaneous but, once started, requires a (brief) interval of time to complete [63], it follows that contours, and therefore forms, are perceived only after the filling of surface features, for example colour, commences. Consequently, the lag of surface processing at the non-conscious level reverses, becoming a lead of surface processing at the conscious level.
3. The roles of visual processing in the dorsal pathway
I mentioned previously the theoretical positions holding that the dorsal M-dominant pathway does not support conscious vision. Contrasting these positions is evidence for its role in conscious vision. First, the dorsal pathway supports perception of motion and structure/form-from-motion [76,77]. Moreover, besides contributing to unconscious processing of manipulable, tool-like objects [78–80], recent evidence [81] indicates that the dorsal pathway also directly supports their conscious processing. Moreover, besides these direct contributions to motion and object perception, recent approaches to visual object recognition [82–86] indicate that the dorsal M-pathway plays an important role in conscious vision by modulating the processing of activity in the early (V1–V4) stages of cortical processing. Without detailing the intricacies of the proposal [84,85], the gist of the proposal—a specific version of a ‘frame-and-fill’ approach to scene and object perception [83] and here modified from Fenske et al. [84]—is illustrated in figure 4. A visual object, e.g. an umbrella, is processed in V1 and from there fast M-neuron responses project a coarse, low spatial frequency (LSF) neural image of the object along the dorsal pathway to the prefrontal cortex (PFC). This coarse image activates a space of possible objects—a lamp, an umbrella, a mushroom and so on—stored in visual long-term memory that might correspond to it. The possible image space serves as the set of ‘hypotheses’ the visual cognitive system entertains about what the stimulus might be. The information in PFC projects in a top-down manner to the IT cortex. There the best match between (i) the detailed information about the retinal input arriving in a bottom-up manner along the slow ventral pathway and (ii) the possible top-down object representations is assessed. The object representation yielding the best match (smallest error) registers in consciousness as the percept of the object. This model dovetails nicely with recent proposals for interactive roles of top-down and bottom-up hierarchies in vision [55]. Top-down influences, besides playing major roles in attentional modulation of lower level visual processing [56], thus can also play a major role in the formation of conscious percepts.
Figure 4.
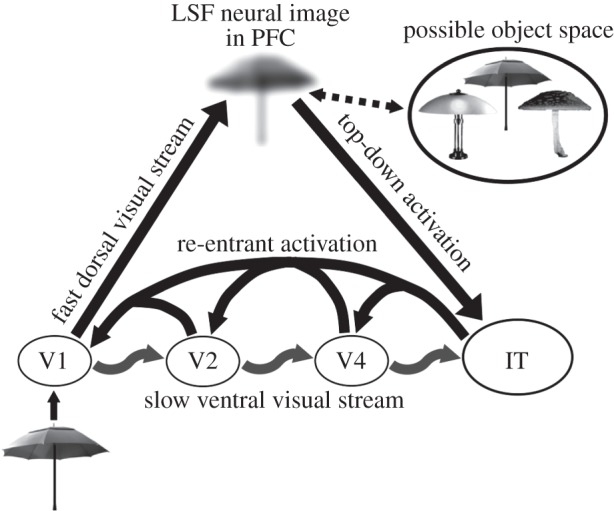
Schematic of visual input projecting from V1 via the fast dorsal stream to the PFC where an LSF representation activates a set of possible objects corresponding to it. This information projects in a top-down manner to IT cortex, which tests the possible objects against the higher resolution, bottom-up information projecting along the slower ventral stream. Adapted from Fenske et al. [84].
We [87] modified the model proposed by Fenske et al. [84] by adding cascading re-entrant activations from the higher areas of the ventral cortical stream of processing to its lower ones, for the following reasons. First, reciprocal anatomical (feed-forward and feedback) connections exist between successive areas in the cortical streams of visual processing [88,89]. Second, such reciprocal connections constitute an important component in formal top-down predictive-coding models [90,91]. Third, on the basis of theoretical and empirical considerations, re-entry is regarded as necessary for conscious vision [92–94]. And fourth, as shown in figure 5, Tapia & Breitmeyer's [87] results showed (i) that the contrast-response function1 of M neurons provided the best fit to the increase in the priming effect of consciously processed (unmasked) primes when their contrast was increased, and conversely (ii) that the contrast-response function of P neurons provided the best fit to the increase in the priming effect of non-consciously processed (masked) primes. While the latter results could be explained by invoking only feed-forward projections along the ventral P-pathway (the re-entrant signals being suppressed by the mask [96]), the former cannot. They can be explained, however, as a consequence of PFC M-activity projecting in top-down fashion and potentiating neural activity in IT cortex that in turn ‘ignites’ the cascading re-entrant–feed-forward neural loops in the ventral stream [97]. The level of the potentiation and consequently the strength of the re-entrant signals would be directly proportional to the response amplitude of M-neurons, which in turn is governed by their contrast response function. As re-entrant activity serves not only to select but also to amplify the responses at the lower levels [98], top-down M-generated activity from the PFC to the ventral object-recognition areas would potentiate the level of the re-entrant signals generated there. Over several iterations of the re-entrant–feed-forward loop, the selected and amplified signals at lower levels of the ventral pathway in turn would increase the specificity of neural responses not only at the lower levels [99] but also at successively higher levels [100]. Re-entrant activity could thus contribute to the neural substrate of the fill component of a frame-and-fill process starting with a coarse object frame that becomes filled with progressively finer information until not only the best matched but also the most completed object representation is extracted [83,86,101].
Figure 5.
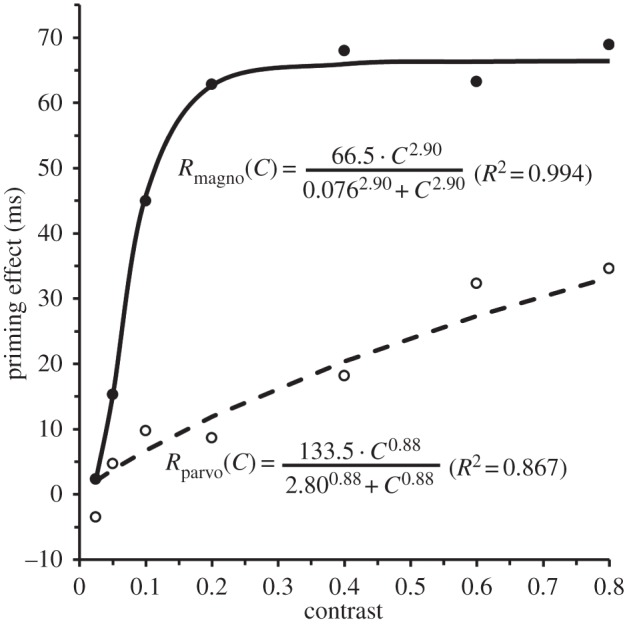
Priming effects as a function of prime contrast in the unmasked (visible)-prime condition (solid line, filled circles) and the masked (invisible) condition (dashed line, open circles), along with the best-fitting Michaelis–Menten equations. Adapted from Tapia & Breitmeyer [87].
4. Implications for visual cognition and neuroscience and for cognitive science
(a). A proposed relationship between surface processing and conscious processing
The aforementioned proposal that cortical feed-forward and re-entrant feedback activities provide a neural distinction between non-conscious and conscious processing, respectively, has recently been made in relation to backward masking by Breitmeyer [96] and VanRullen [102], and are consistent with Grossberg's [21,23] and Rees's [103] assertion that much of the cortical object recognition system can be activated at non-conscious levels of visual processing. What is made explicit here is that processing at these levels occurs in the cortical feed-forward sweep of the activity. In support of this claim, Boehler et al.'s [104], Fahrenfort et al.'s [105] and recent studies showed that the suppression of visibility of a target by an aftercoming mask correlates not with the reduction in early feed-forward activation in human visual cortex but rather with the reduction in the later re-entrant and recurrent activation. Thus, the intact feed-forward activity can on its own support non-conscious but not conscious vision. Regarding conscious vision, as cortical re-entrant activation is its strong correlate, and because, as noted in §2a, surface completion is the sine qua non for the conscious registration of visual stimuli, it follows that the cortical re-entrant activation that correlates with conscious registration of visual stimuli should also correlate with cortical surface completion processes. This inference comports with the following: (i) Lamme et al.'s [26] evidence for the crucial role of re-entrant activation in surface processing, (ii) Ramachandran's [106] argument that surface completion provides the foundation of visual qualia, and (iii) Orpwood's [107] model of iterative feed-forward–re-entrant loops in local cortical networks that underlies the emergence of qualia.
(b). Are temporal order and micro-consciousness related?
Given the asynchronies in the perceptual registration of stimulus attributes such as form, colour and motion, Zeki & Bartels [108] and Motoussis & Zeki [74] proposed the existence of separate modular micro-consciousness, one for each stimulus attribute. In contrast to this proposal, the present approach instead argues that there cannot be a consciousness of form separate from that of chromatic or achromatic surface properties. This assertion does not exclude the possibility of the failed binding of chromatic or achromatic surface features and form features [35,109,110]. Even when, say, a colour and form are misbound and yield an illusory conjunction, the claim being made is that there will be no conscious percept of the form of a visual object without a prior filling-in of, in these cases, the wrong or inappropriate colour information. On the other hand, as also noted above, conscious spatially diffuse registration of colour can exist separately from, i.e. without, conscious registration of form [41,42].
(c). Feature integration and object recognition
Neurophysiologically plausible models of visual object recognition assume that the earliest cortical form-selective representation of a visual object is in terms of line or edge orientation, whereas conjunctions of these or other feature primitives, such as curvature, size, colour and luminance contrast, occur at subsequent processing levels [111,112]. Such models are consistent with evidence showing that later stages of the ventral cortical processing stream are selective for patterns of input consisting of progressively more complex conjunctions of simple features [113–115]. In §1 we noted that one can experimentally induce transient stimulus blindness without affecting the processing of geometric qualia, such as the form, location or motion of a stimulus at a non-conscious level [15,16]. Using a masked priming paradigm, Breitmeyer et al. [116] showed that non-conscious processing of shape can occur at as late a level at which conjunctions of simple contour features (e.g. vertices) and even holistic shapes are processed. This confirms Grossberg's [21] proposal that the holistic processing of shapes occurs already at a non-conscious level of processing.
(d). Some specific psycho-philosophical considerations
Experimental findings have played a major role in philosophical discussions about the mind–brain relationship and specifically the nature of the visual conscious and unconscious. A well-known example is the relevance of Sperling's [117] investigations of visual iconic memory to the controversial distinctions between phenomenal and access consciousness [118–121]. How might the theoretical and empirical research covered above bear on other, more or less weighty, philosophical matters? Here, I briefly outline only four such implications.
(1) There are contrasting and controversial theoretical and philosophical views [122,123] on whether perceptual filling-in of, for example, a blind spot or scotoma in the visual field is accompanied by an isomorphic neural filling-in process or by a more symbolic process by which the brain simply labels or tags the to-be-filled-in region of visual space with the information available in the region surrounding the scotama. Relevant evidence relating to either perspective is discussed in Pessoa & de Weerd [61] (see especially their chapters 1, 6 and 9). A theory based on isomorphic filling-in processes would be supported by evidence of corresponding neural filling-in at relatively low cortical levels that retain retinotopic information [124]. Regarding the low-level cortical processes, I use the example of apparent motion, which phenomenally renders a sense of spatio-temporal filling-in. Here, one can note two brain-imaging studies conducted by Muckli et al. [125] and Sterzer et al. [126]. Combined they show that, in addition to the activation of the motion-sensitive area V5 (which is known to respond strongly to apparent-motion stimuli [127]) and activation of the retinotopic areas in V1 of human cortex that respond to the two spatially and temporally separated stimuli, V1 retinotopic areas that are not directly stimulated by the two static stimuli but that fall in their apparent-motion path are also significantly activated. A direct animal analogue of these human findings has been reported by Ahmed et al. [128] in their single-cell study of the ferret visual cortex. Extrapolating these findings to other cases of perceptual completion, one would expect similar low-level cortical isomorphisms for perceptual contour and surface filling-in in humans.
Nonetheless, in one sense Dennett [122] correctly invokes high-level, symbolic processes to explain perceptual filling-in. Whatever neural process occurs in the human motion-selective area (V5) clearly is of high level relative to the processes in V1. However, it would require some stretch to regard human V5 a high-level ‘symbolic’ area; moreover, it would not readily explain the concurrent neural activation of V1 regions intermediate to those activated by the two sequentially presented stimuli giving rise to the perception of motion.2
(2) Ramachandran [106] noted the intimate relationship between perceptual filling-in/completion of surfaces and qualia. In much philosophical speculation, colour is invoked as a prototypical visual quale. As (i) colour is a surface property of visual objects, and (ii) surface properties must be perceptually completed or filled, such a relationship makes evident sense. In this regard, it is interesting, and perhaps more than coincidental, that Dennett is a critic of filling-in and of qualia [122,129]. Nonetheless, qualia—those immediately apprehended, intrapersonal, private and hard to describe residuals intrinsic to phenomenal experience—continue to plague fully reductionist and functionalist accounts of consciousness [130]. Thus if, say, the experienced surface attribute red (i) is intimately tied to filling-in, and (ii) a filling-in process is, based on phenomenal and neural evidence, real, then it seems quite appropriate for cognitive scientists, neuroscientists and some philosophers [131] to regard redness as a genuine and real quale.
(3) Dealing with spatio-temporal visual phenomena, such as apparent motion and metacontrast masking, Dennett [122], after raising the contrast between Orwellian and Stalinesque scenarios, concludes that neither can account for the phenomenology of metacontrast masking but that his multiple-drafts scenario can. According to the Orwellian scheme, the target in a metacontrast masking paradigm briefly enters the privileged state of consciousness but its memory is obliterated or overwritten by the aftercoming mask. The Stalinesque scheme assumes that the second, mask stimulus intervenes and prevents the first, target stimulus from ever registering in consciousness. I will not describe the multiple-drafts scenario but refer the interested reader to Dennett [122, p. 142]. The point I wish to raise, however, is that the Stalinesque scenario does a fine, more than adequate, job—clearly better than the Orwellian and, in my opinion, better than the multiple-drafts scenario—of explaining the effects of a metacontrast mask on target processing. Recall that Breitmeyer et al.'s [57] findings (see §2b(i)) indicate that the effect of a colour prime whose visibility is suppressed by a metacontrast mask is best explained by the processing of wavelength-dependent, i.e. stimulus-dependent, characteristics of the prime than by processing its percept-dependent aspects. This, as noted, constitutes strong empirical evidence that the masked prime's colour did not register in consciousness—hence the inadequacy of the Orwellian scheme—but exerted its chromatic priming effect at the preconscious wavelength-dependent level of processing. It follows that the chromatic information of an unmasked prime (or any other stimulus) which eventually registers as colour in consciousness, would first be processed at a preconsciously wavelength-dependent level (one could regard this, adapting from Dennett [122], a preliminary draft) and then would need to be revised or converted to, in Stalinesque fashion, qualitatively different information at a percept-dependent level (the final draft) as it transits from the preconscious, wavelength-specific level to the conscious, colour-specific level.
(4) Finally, I venture some implications for the relationship between what historically have been defined as primary and secondary qualities of objects. Ontologically, primary qualities were taken to be mind-independent, inhering in real, i.e. physical, entities. The list of properties which have been deemed by various philosophers to qualify as inhering in objects has included shape, size, weight (mass), location and motion [132]. Note that the existence of these properties is objective in the sense that, especially for scientific purposes, they can be defined operationally by applying standard physical measurements. Properties of objects such as their colour have been deemed as secondary because they are observer dependent either because (i) they are merely dispositions of physical bodies that can give rise to subjective sensations or (ii) they exist solely as mental properties. However, how do the very notions of primary and secondary qualities arise? Our knowledge of primary qualities is not based on a priori concepts. The notion of primary qualities is abstracted from our percepts, i.e. our empirical observations, of objects and events in the world. As argued above, without surface qualia, for example colour, there is no percept of objects. Hence, our a posteriori concepts of primary qualities, such as their shapes and sizes, depend on prior registrations of secondary qualities, for example their colours, in visual awareness. In this epistemological sense, surface qualia are primary and geometric qualia are secondary.
Endnotes
The contrast-response functions were versions of the Michaelis–Menten equations also used by Kaplan & Shapley [95] to numerically describe the response of M and P neurons to variations of stimulus contrast.
References
- 1.Uttal WR. 1981. A taxonomy of visual processes. Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- 2.Mashour GA, Alkire MT. 2013. Evolution of consciousness: phylogeny, ontongeny, and emergence from general anesthesia. Proc. Natl Acad. Sci. USA 110, 10 357–10 364. ( 10.1073/pnas.1301188110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollen DA. 2008. Fundamental requirements for primary visual perception. Cereb. Cortex 18, 1991–1998. ( 10.1093/cercor/bhm226) [DOI] [PubMed] [Google Scholar]
- 4.DeYoe EA, Van Essen DC. 1988. Concurrent processing streams in monkey visual cortex. Trends Neurosci. 11, 219–226. ( 10.1016/0166-2236(88)90130-0) [DOI] [PubMed] [Google Scholar]
- 5.Schiller PH. 2010. Parallel information processing channels created in the retina. Proc. Natl Acad. Sci. USA 107, 17 087–17 094. ( 10.1073/pnas.1011782107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merigan WH, Maunsell JHR. 1993. How parallel are the primate pathways? Ann. Rev. Neurosci. 16, 369–402. ( 10.1146/annurev.ne.16.030193.002101) [DOI] [PubMed] [Google Scholar]
- 7.Sawatari A, Callaway EM. 1996. Convergence of magno- and parvocellular pathways in layer 4B of macaque primary visual cortex. Nature 380, 442–446. ( 10.1038/380442a0) [DOI] [PubMed] [Google Scholar]
- 8.Goodale MA, Milner AD. 1992. Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25. ( 10.1016/0166-2236(92)90344-8) [DOI] [PubMed] [Google Scholar]
- 9.Milner AD, Goodale MA. 1995. The visual brain in action. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.de Haan EHF, Cowey A. 2011. On the usefulness of ‘what’ and ‘where’ pathways in vison. Trends Cogn. Sci. 15, 460–466. ( 10.1016/j/tics.2011.08.005) [DOI] [PubMed] [Google Scholar]
- 11.Schenk T, McIntosh RD. 2010. Do we have independent visual streams for perception and action? Cogn. Neurosci. 1, 52–78. ( 10.1080/17588920903388950) [DOI] [PubMed] [Google Scholar]
- 12.Goodale MA. 2010. Transforming vision into action. Vis. Res. 51, 1567–1587. ( 10.1016/j.visres.2010.07.027) [DOI] [PubMed] [Google Scholar]
- 13.Milner AD, Goodale MA. 2008. Two visual systems re-viewed. Neuropsychologia 46, 774–785. ( 10.1016/j.neuropsychologia.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 14.Breitmeyer BG, Tapia E. 2011. Roles of contour and surface processing in microgenesis of object perception and visual consciousness. Adv. Cogn. Psychol. 7, 68–81. ( 10.2478/v10053-008-0088-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klotz W, Wolff P. 1995. The effect of a masked stimulus on the response to the masking stimulus. Psychol. Res. 58, 92–101. ( 10.1007/BF00571098) [DOI] [PubMed] [Google Scholar]
- 16.Öğmen H, Breitmeyer BG, Melvin R. 2003. The what and where in visual masking. Vis. Res. 43, 1337–1350. ( 10.1016/S0042-6989(03)00138-X) [DOI] [PubMed] [Google Scholar]
- 17.Weiskrantz L. 1997. Consciousness lost and found: a neuropsychological exploration. Oxford, UK: Oxford University Press. [Google Scholar]
- 18.Bridgeman B. 2011. Functions of consciousness. Cogn. Neurosci. 2, 115–116. ( 10.1080/17588928.2011.585228) [DOI] [PubMed] [Google Scholar]
- 19.Morsella E. 2005. The function of phenomenal states: supramodular interaction theory. Psychol. Rev. 112, 1000–1021. ( 10.1037/0033-295X.112.4.1000) [DOI] [PubMed] [Google Scholar]
- 20.Hyman J. 2006. The objective eye: color, form, and reality in the theory of art. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Grossberg S. 2003. Filling-in the forms: surface and boundary interactions in visual cortex. In Filling-in: from perceptual completion to cortical reorganization (eds Pessoa L, De Weerd P.), pp. 123–150. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Biederman I, Ju G. 1988. Surface versus edge-based determinants of visual recognition. Cogn. Psychol. 20, 38–64. ( 10.1016/0010-0285(88)90024-2) [DOI] [PubMed] [Google Scholar]
- 23.Grossberg S. 1994. 3-D vision and figure–ground separation by visual cortex. Percept. Psychophys. 55, 48–120. ( 10.3758/BF03206880) [DOI] [PubMed] [Google Scholar]
- 24.Grossberg S, Yazdanbakhsh A. 2005. Laminar cortical dynamics of 3D surface perception: stratification, transparency, and neon color spreading. Vis. Res. 45, 1725–1743. ( 10.1016/j.visres.2005.01.006) [DOI] [PubMed] [Google Scholar]
- 25.Breitmeyer BG, Kafalıgönül H, Öğmen H, Mardon L, Todd S, Ziegler R. 2006. Meta- and paracontrast reveal differences between contour- and brightness-processing mechanisms. Vis. Res. 46, 2645–2658. ( 10.1016/j.visres.2005.10.020) [DOI] [PubMed] [Google Scholar]
- 26.Lamme VA, Rodriguez-Rodriguez V, Spekreijse H. 1999. Separate processing dynamics for texture elements, boundaries and surfaces in primary visual cortex of the macaque monkey. Cereb. Cortex 9, 406–413. ( 10.1093/cercor/9.4.406) [DOI] [PubMed] [Google Scholar]
- 27.Scholte HS, Jolij J, Fahrenfort JJ, Lamme VAF. 2008. Feedforward and recurrent processing in scene segmentation: electroencephalography and functional magnetic resonance imaging. J. Cogn. Neurosci. 20, 2097–2109. ( 10.1162/jocn.2008.20134) [DOI] [PubMed] [Google Scholar]
- 28.Livingstone M, Hubel D. 1988. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science 240, 740–749. ( 10.1126/science.3283936) [DOI] [PubMed] [Google Scholar]
- 29.Gegenfurter KR. 2003. Cortical mechanisms of color vision. Nat. Rev. Neurosci. 4, 563–572. ( 10.1038/nrn1138) [DOI] [PubMed] [Google Scholar]
- 30.Sincich LC, Horton JC. 2005. The circuitry of V1 and V2: integration of color, form, and motion. Annu. Rev. Neurosci. 28, 303–326. ( 10.1146/annurev.neuro.28.061604.135731) [DOI] [PubMed] [Google Scholar]
- 31.Felleman DJ, Xiao Y, McClendon E. 1997. Modular organization of occipito-temporal pathways: cortical connections between visual area 4 and visual area 2 and posterior inferotemporal ventral area in macaque monkeys. J. Neurosci. 17, 3185–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sincich LC, Horton JC. 2005. Input to V2 thin stripes arises from V1 cytochrome oxidase patches. J. Neurosci. 25, 10 087–10 093. ( 10.1523/jneurosci.3313-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Xiao Y, Felleman DJ. 2007. V2 thin stripes contain spatially organized representations of achromatic luminance change. Cereb. Cortex 17, 116–129. ( 10.1093/cercor/bhj131) [DOI] [PubMed] [Google Scholar]
- 34.Xiao Y, Wang Y, Felleman DJ. 2003. A spatially organized representation of colour in macaque cortical area V2. Nature 421, 535–539. ( 10.1038/nature01372) [DOI] [PubMed] [Google Scholar]
- 35.Xiao Y, Casti A, Xiao J, Kaplan E. 2007. Hue maps in primate striate cortex. NeuroImage 35, 771–786. ( 10.1016/j.neuroimage.2006.11.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girard P, Lomber SG, Bullier J. 2002. Shape discrimination deficits during reversible deactivation of area V4 in the macaque monkey. Cereb. Cortex 12, 1146–1156. ( 10.1093/cercor/12.11.1146) [DOI] [PubMed] [Google Scholar]
- 37.Barbur JL, Harlow AJ, Plant G. 1994. Insights into the different exploits of colour in the visual cortex. Proc. R. Soc. Lond. B 258, 327–334. ( 10.1098/rspb.1994.0181) [DOI] [PubMed] [Google Scholar]
- 38.Kentridge RW, Heywood CA, Cowey A. 2004. Chromatic edges, surfaces, and constancies in cerebral achromatopsia. Neuropsychologia 42, 821–830. ( 10.1016/j.neuropsychologia.2003.11.002) [DOI] [PubMed] [Google Scholar]
- 39.Heywood CA, Wilson B, Cowey A. 1987. A case study of cortical colour ‘blindness’ with relatively intact achromatic discrimination. J. Neurol. Neurosurg. Psychiat. 50, 22–29. ( 10.1136/jnnp.50.1.220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeki S, Aglioti S, McKeefry D, Berlucchi G. 1999. The neurological basis of conscious color perception in a blind patient. Proc. Natl Acad. Sci. USA 96, 14 124–14 129. ( 10.1073/pnas.96.24.14124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Critchley M. 1965. Acquired anomalies of colour perception of central origin. Brain 88, 711–724. ( 10.1093/brain/88.4.711) [DOI] [PubMed] [Google Scholar]
- 42.Hong SW, Blake R. 2009. Interocular suppression differentially affects achromatic and chromatic mechanisms. Att. Percept. Psychophys. 71, 403–411. ( 10.3758/APP.71.2.403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adelson EH. 2001. On seeing stuff: the perception of materials by humans and machines. In Proc. SPIE 4299, human vision and electronic imaging VI, vol. 4299 (eds Rogowitz BE, Pappas TN.), pp. 1–12. Bellingham, WA: International Society for Optical Engineering. [Google Scholar]
- 44.McCann JJ. 2005. Do humans discount the illuminant? In Proc. SPIE 5666, human vision and electronic imaging X, vol. 5666 (eds Rogowitz BE, Pappas TN, Daly SJ.), pp. 1–16. Bellingham, WA: International Society for Optical Engineering. [Google Scholar]
- 45.Zeki S, Marini L. 1998. Three cortical stages of colour processing in the human brain. Brain 121, 1669–1685. ( 10.1093/brain/121.9.1669) [DOI] [PubMed] [Google Scholar]
- 46.Stoerig P. 1996. Varieties of vision: from blind responses to conscious recognition. Trends Neurosci. 19, 401–406. ( 10.1016/S0166-2236(96)10051-5) [DOI] [PubMed] [Google Scholar]
- 47.Crick F, Koch C. 2003. A framework for consciousness. Nat. Neurosci. 6, 119–126. ( 10.1038/nn0203-119) [DOI] [PubMed] [Google Scholar]
- 48.Crick F, Koch C. 1995. Are we aware of neural activity in primary visual cortex? Nature 375, 121–123. ( 10.1038/375121a0) [DOI] [PubMed] [Google Scholar]
- 49.Scheinberg DL, Logothetis NK. 1997. The role of temporal visual areas in perceptual organization. Proc. Natl Acad. Sci. USA 94, 3408–3413. ( 10.1073/pnas.94.7.3408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heywood CA, Gadotti A, Cowey A. 1992. Cortical area V4 and its role in the perception of color. J. Neurosci. 12, 4056–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh V, Carden D, Butler SR, Kulikowski JJ. 1993. The effects of V4 lesions on the visual abilities of macaques: hue discrimination and color constancy. Behav. Brain Res. 53, 51–62. ( 10.1016/S0166-4328(05)80265-7) [DOI] [PubMed] [Google Scholar]
- 52.Bar M, Biederman I. 1999. Localizing the cortical region mediating visual awareness of object identity. Proc. Natl Acad. Sci. USA 96, 1790–1793. ( 10.1073/pnas.96.4.1790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beck DM, Rees G, Frith CD, Lavie N. 2001. Neural correlates of change detection and change blindness. Nat. Neurosci. 4, 645–650. ( 10.1038/88477) [DOI] [PubMed] [Google Scholar]
- 54.Dehaene S, Naccache L, Cohen L, Le Bihan D, Mangin J-F, Poline J-P, Rivière D. 2001. Cerebral mechanisms of word masking and unconscious repetition priming. Nat. Neurosci. 4, 752–758. ( 10.1038/89551) [DOI] [PubMed] [Google Scholar]
- 55.Hochstein S, Ahissar M. 2002. View from the top: hierarchies and reverse hierarchies in the visual system. Neuron 36, 791–804. ( 10.1016/S0896-6273(02)01091-7) [DOI] [PubMed] [Google Scholar]
- 56.Posner MI. 2012. Attentional networks and consciousness. Front. Psychol. 3, 64 ( 10.3389/fpsyg.2012.00064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Breitmeyer BG, Ro T, Singhal N. 2004. Unconscious priming with chromatic stimuli occurs at stimulus- not percept-dependent levels of visual processing. Psychol. Sci. 15, 198–202. ( 10.1111/j.0956-7976.2004.01503009.x) [DOI] [PubMed] [Google Scholar]
- 58.Schmidt T. 2002. The finger in flight: real-time motor control by visually masked color stimuli. Psychol. Sci. 13, 112–118. ( 10.1111/1467-9280.00422) [DOI] [PubMed] [Google Scholar]
- 59.Caputo G, Romani A, Callieco R, Gaspari D, Cosi V. 1999. Amodal completion in texture visual evoked potentials. Vis. Res. 39, 31–38. ( 10.1016/S0042-6989(98)00015-7) [DOI] [PubMed] [Google Scholar]
- 60.Rossi AF, Paradiso MA. 2003. Surface completion: psychophysical and neurophysiological studies of brightness interpolation. In Filling-in: from perceptual completion to cortical reorganization (eds Pessoa L, De Weerd P.), pp. 59–80. Oxford, UK: Oxford University Press. [Google Scholar]
- 61.Pessoa L, De Weerd P. 2003. Filling-in: from perceptual completion to cortical reorganization. Oxford, UK: Oxford University Press. [Google Scholar]
- 62.Paradiso MA, Nakayama K. 1991. Brightness perception and filling-in. Vis. Res. 31, 1221–1236. ( 10.1016/0042-6989(91)90047-9) [DOI] [PubMed] [Google Scholar]
- 63.Breitmeyer BG, Jacob J. 2012. Microgenesis of surface completion in visual objects: evidence for filling-out. Vis. Res. 55, 11–18. ( 10.1016/j.visres.2011.12.010) [DOI] [PubMed] [Google Scholar]
- 64.Breitmeyer BG. 1975. Simple reaction time as a measure of the temporal response properties of transient and sustained channels. Vis. Res. 15, 1411–1412. ( 10.1016/0042-6989(75)90200-X) [DOI] [PubMed] [Google Scholar]
- 65.Hughes HC, Nozawa G, Kitterle F. 1996. Global precedence, spatial frequency channels, and the statistics of natural images. J. Cogn. Neurosci. 8, 197–230. ( 10.1162/jocn.1996.8.3.197) [DOI] [PubMed] [Google Scholar]
- 66.Lupp U, Hauske G, Wolf W. 1976. Perceptual latencies to sinusoidal gratings. Vis. Res. 16, 969–972. ( 10.1016/0042-6989(76)90228-5) [DOI] [PubMed] [Google Scholar]
- 67.Schyns PG, Oliva A. 1994. From blobs to boundary edges: evidence for time and spatial-scale-dependent scene recognition. Psychol. Sci. 5, 195–200. ( 10.1111/j.1467-9280.1994.tb00500.x) [DOI] [Google Scholar]
- 68.Vassilev A, Mitov D. 1976. Perception time and spatial frequency. Vis. Res. 16, 89–92. ( 10.1016/0042-6989(76)90081-X) [DOI] [PubMed] [Google Scholar]
- 69.Holcombe AO, Cavanagh P. 2008. Independent, synchronous access to color and motion features. Cognition 107, 552–580. ( 10.1016/j.cognition.2007.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holcombe AO. 2009. Temporal binding favours the early phase of colour changes, but not of motion changes, yielding the colour–motion asynchrony illusion. Vis. Cogn. 17, 232–253. ( 10.1080/13506280802340653) [DOI] [Google Scholar]
- 71.Nishida S, Johnston A. 2002. Marker location not processing latency determines temporal binding of visual attributes. Curr. Biol. 12, 359–368. ( 10.1016/S0960-9822(02)00698-X) [DOI] [PubMed] [Google Scholar]
- 72.Moutoussis K. 2012. Asynchrony in visual consciousness and the possible involvement of attention. Front. Psychol. 3, 1–12. ( 10.3389/fpsyg.2012.00314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clifford CWG, Arnold DH, Pearson J. 2003. A paradox of temporal perception revealed by a stimulus oscillating in colour and orientation. Vis. Res. 43, 2245–2253. ( 10.1016/S0042-6989(03)00120-2) [DOI] [PubMed] [Google Scholar]
- 74.Moutoussis K, Zeki S. 1997. Functional segregation and temporal hierarchy of the visual perceptive systems. Proc. R. Soc. Lond. B 264, 1407–1414. ( 10.1098/rspb.1997.0196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Viviani P, Aymoz C. 2001. Colour, form, and movement are not perceived simultaneously. Vis. Res. 41, 2909–2918. ( 10.1016/S0042-6989(01)00160-2) [DOI] [PubMed] [Google Scholar]
- 76.Andersen RA, Bradley DC. 1998. Perception of three-dimensional structure from motion. Trends Cogn. Sci. 2, 222–228. ( 10.1016/S1364-6613(98)01181-4) [DOI] [PubMed] [Google Scholar]
- 77.Kourtzi Z, Krekelberg B, van Wezel RJA. 2008. Linking form and motion in the primate brain. Trends Cogn. Sci. 12, 230–236. ( 10.1016/j.tics.2008.02.013) [DOI] [PubMed] [Google Scholar]
- 78.Almeida J, Mahon BZ, Nakayama K, Caramazza A. 2008. Unconscious processing dissociates along categorical lines. Proc. Natl Acad. Sci. USA 105, 15 214–15 218. ( 10.1073/pnas.0805867105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang F, He S. 2005. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat. Neurosci. 8, 1380–1385. ( 10.1038/nn1537) [DOI] [PubMed] [Google Scholar]
- 80.Sakuraba S, Sakai S, Yamanaka M, Yokosawa K, Hirayama K. 2012. Does the human dorsal stream really process a category for tools? J. Neurosci. 32, 3949–3953. ( 10.1523/jneurosci.3973-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hesselmann G, Malach R. 2011. The link between fMRI-BOLD activation and perceptual awareness is ‘stream-invariant’ in the human visual system. Cereb. Cort. 21, 2829–2837. ( 10.1093/cercor/bhr085) [DOI] [PubMed] [Google Scholar]
- 82.Bar M, et al. 2006. Top-down facilitation of visual recognition. Proc. Natl Acad. Sci. USA 103, 449–454. ( 10.1073/pnas.0507062103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen C-M, Lakatos PS, Shah AS, Mehta AD, Givre SJ, Javitt DC, Schroeder CE. 2007. Functional anatomy and interaction of fast and slow visual pathways in macaque monkeys. Cereb. Cortex 17, 1561–1569. ( 10.1093/cercor/bhl067) [DOI] [PubMed] [Google Scholar]
- 84.Fenske MJ, Aminoff E, Gronau N, Bar M. 2006. Top-down facilitation of visual object recognition: object-based and context-based contributions. Prog. Brain Res. 155, 3–21. ( 10.1016/S0079-6123(06)55001-0) [DOI] [PubMed] [Google Scholar]
- 85.Kveraga K, Boshyan J, Bar M. 2007. Magnocellular projections as the trigger of top-down facilitation in recognition. J. Neurosci. 27, 13 232–13 240. ( 10.1523/jneurosci.3481-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peyrin C, Michel CM, Schwartz S, Thut G, Seghier M, Landis T, Marendaz C, Vuilleumier P. 2010. The neural substrates and timing of top-down processes during coarse-to-fine categorization of visual scenes: a combined fMRI and ERP study. J. Cogn. Neurosci. 22, 2768–2780. ( 10.1162/jocn.2010.21424) [DOI] [PubMed] [Google Scholar]
- 87.Tapia E, Breitmeyer BG. 2011. Visual consciousness revisited: magnocellular and parvocellular contributions to conscious and nonconscious vision. Psychol. Sci. 22, 934–942. ( 10.1177/0956797611413471) [DOI] [PubMed] [Google Scholar]
- 88.Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47. ( 10.1093/cercor/1.1.1) [DOI] [PubMed] [Google Scholar]
- 89.Girard P, Hupé JM, Bullier J. 2001. Feedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocities. J. Neurophysiol. 85, 1328–1331. [DOI] [PubMed] [Google Scholar]
- 90.Friston K. 2005. A theory of cortical responses. Phil. Trans. R. Soc. B 360, 815–836. ( 10.1098/rstb.2005.1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rao RPN, Ballard DH. 1999. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2, 79–87. ( 10.1038/4580) [DOI] [PubMed] [Google Scholar]
- 92.Boly M, et al. 2011. Preserved feedforward but impaired top-down processes in the vegetative state. Science 332, 858–862. ( 10.1126/science.1202043) [DOI] [PubMed] [Google Scholar]
- 93.Bullier J. 2001. Feedback connections and conscious vision. Trends Cogn. Sci. 5, 369–370. ( 10.1016/S1364-6613(00)01730-7) [DOI] [PubMed] [Google Scholar]
- 94.Lamme VAF. 2010. How neuroscience will change our view of consciousness. Cogn. Neurosci. 1, 204–240. ( 10.1080/17588921003731586) [DOI] [PubMed] [Google Scholar]
- 95.Kaplan E, Shapley RM. 1986. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc. Natl Acad. Sci. USA 83, 2755–2757. ( 10.1073/pnas.83.8.2755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Breitmeyer BG. 2007. Visual masking: past accomplishments, present status, future developments. Adv. Cogn. Psychol. 3, 9–20. ( 10.2478/v10053-008-0010-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fisch L, et al. 2009. Neural ‘ignition’: enhanced activation linked to perceptual awareness in human ventral stream visual cortex. Neuron 64, 562–574. ( 10.1016/j.neuron.2009.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hupé JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J. 1998. Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature 394, 784–787. ( 10.1038/29537) [DOI] [PubMed] [Google Scholar]
- 99.Kok P, Jehee JFM, de Lange FP. 2012. Less is more: expectation sharpens representations in the primary visual cortex. Neuron 75, 265–270. ( 10.1016/j.neuron.2012.04.034) [DOI] [PubMed] [Google Scholar]
- 100.Jehee JFM, Roelfsema PR, Deco G, Murre JMJ, Lamme VAF. 2007. Interactions between higher and lower visual areas improve shape selectivity of higher level neurons: explaining crowding phenomena. Brain Res. 1157, 167–178. ( 10.1016/j.brainres.2007.03.090) [DOI] [PubMed] [Google Scholar]
- 101.Hegdé J. 2008. Time course of visual perception: coarse-to-fine processing and beyond. Prog. Neurobiol. 84, 405–439. ( 10.1016/j.pneurobio.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 102.VanRullen R. 2007. The power of the feed-forward sweep. Adv. Cogn. Psychol. 3, 167–176. ( 10.2478/v10053-008-0022-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rees G. 2007. Neural correlates of the contents of visual awareness in humans. Phil. Trans. R. Soc. B 362, 877–886. ( 10.1098/rstb.2007.2094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boehler CN, Schoenfeld MA, Heinze H-J, Hopf J-M. 2008. Rapid recurrent processing gates awareness in primary visual cortex. Proc. Natl Acad. Sci. USA 105, 8742–8747. ( 10.1073/pnas.0801999105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fahrenfort JJ, Scholte HS, Lamme VAF. 2007. Masking disrupts reentrant processing in human visual cortex. J. Cogn. Neurosci. 19, 1488–1497. ( 10.1162/jocn.2007.19.9.1488) [DOI] [PubMed] [Google Scholar]
- 106.Ramachandran VS. 2003. Foreword. In Filling-in: from perceptual completion to cortical reorganisation (eds Pessoa L, DeWeerd P.), pp. xi–xxii. New York, NY: Oxford University Press. [Google Scholar]
- 107.Orpwood R. 2013. Qualia could arise from information processing in local cortical networks. Front. Psychol. 4, 121 ( 10.3389/fpsyg.2013.00121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeki S, Bartels A. 1998. The asynchrony of consciousness. Proc. R. Soc. Lond. B 265, 1583–1585. ( 10.1098/rspb.1998.0475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Friedman-Hill SR, Robertson LC, Treisman A. 1995. Parietal contributions to visual feature binding: evidence from a patient with bilateral lesions. Science 269, 853–855. ( 10.1126/science.7638604) [DOI] [PubMed] [Google Scholar]
- 110.Hong SW, Shevell SK. 2006. Resolution of binocular rivalry: perceptual misbinding of color. Vis. Neurosci. 23, 561–566. ( 10.1017/S0952523806233145) [DOI] [PubMed] [Google Scholar]
- 111.Hummel JE, Biederman I. 1992. Dynamic binding in a neural network for shape recognition. Psychol. Rev. 99, 480–517. ( 10.1037/0033-295X.99.3.480) [DOI] [PubMed] [Google Scholar]
- 112.Treisman A. 1998. Feature binding, attention and object perception. Phil. Trans. R. Soc. Lond. B 353, 1295–1306. ( 10.1098/rstb.1998.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pasupathy A, Connor CE. 2002. Population coding of shape in area V4. Nat. Neurosci. 5, 1332–1338. ( 10.1038/972) [DOI] [PubMed] [Google Scholar]
- 114.Tanaka K, Saito H, Fukuda Y, Moriya M. 1991. Coding visual images of objects in the inferotemporal cortex. J. Neurophysiol. 66, 170–189. [DOI] [PubMed] [Google Scholar]
- 115.Tsunoda K, Yamane Y, Nishizaki M, Tanifuji M. 2001. Complex objects are represented in macaque inferotemporal cortex by the combination of feature columns. Nat. Neurosci. 4, 832–838. ( 10.1038/90547) [DOI] [PubMed] [Google Scholar]
- 116.Breitmeyer BG, Ogmen H, Ramon J, Chen J. 2005. Unconscious priming by forms and their parts. Visual Cogn. 12, 720–736. ( 10.1080/13506280444000472) [DOI] [Google Scholar]
- 117.Sperling G. 1960. The information available in a brief visual presentation. Psychol. Monogr. 74 (whole no. 498), 1–29. [Google Scholar]
- 118.Block N. 2005. Two neural correlates of consciousness. Trends Cogn. Sci. 9, 46–52. ( 10.1016/j.tics.2004.12.006) [DOI] [PubMed] [Google Scholar]
- 119.Phillips IB. 2011. Perception and iconic memory: what Sperling doesn't show. Mind Lang. 26, 381–411. ( 10.1111/j.1468-0017.2011.01422.x0) [DOI] [Google Scholar]
- 120.Raffone A, Pantani M. 2010. A global workspace model for phenomenal and access consciousness. Conscious. Cogn. 19, 580–596. ( 10.1016/j.concog.2010.03.013) [DOI] [PubMed] [Google Scholar]
- 121.Simione L, Raffone A, Wolters G, Salmas P, Nakatani C, Belardinelli MO, van Leeuwen C. 2012. ViSA: a neurodynamic model for visuo-spatial working memory, attentional blink, and conscious access. Psychol. Rev. 119, 745–769. ( 10.1037/a0029345) [DOI] [PubMed] [Google Scholar]
- 122.Dennett DC. 1991. Consciousness explained. Boston, MA: Little, Brown and Co. [Google Scholar]
- 123.Pessoa L, Thompson E, Noë A. 1998. Finding out about filling-in: a guide to perceptual completion for visual science and the philosophy of perception. Behav. Brain Sci. 21, 723–748. ( 10.1017/S0140525X98001757) [DOI] [PubMed] [Google Scholar]
- 124.Fiorani M, Jr, de Oliveira L, Volchan E, Pessoa L, Gattass R, Rocha-Miranda CE. 2003. Completion through a permanent scotoma: fast interpolation across the blind spot and the processing of occlusion. In Filling-in: from perceptual completion to cortical reorganisation (eds Pessoa L, DeWeerd P.), pp. 177–186. New York, NY: Oxford University Press. [Google Scholar]
- 125.Muckli L, Kohler A, Kriegeskorte N, Singer W. 2005. Primary visual cortex activity along the apparent-motion trace reflects illusory perception. PLoS Biol. 3, e265 ( 10.1371/journal.pbio.0030265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sterzer P, Haynes JD, Rees G. 2006. Primary visual cortex activation on the path of apparent motion is mediated by feedback from hMT+/V5. NeuroImage 32, 1308–1316. ( 10.1016/j.neuroimage.2006.05.029) [DOI] [PubMed] [Google Scholar]
- 127.Kaneoke Y, Bundou M, Koyama S, Suzuki H, Kakigi R. 1997. Human cortical area responding to stimuli in apparent motion. Neuroreport 8, 677–682. ( 10.1097/00001756-199702100-00020) [DOI] [PubMed] [Google Scholar]
- 128.Ahmed B, Hanazawa A, Undeman C, Eriksson D, Valentiniene S, Roland PE. 2008. Cortical dynamics subserving visual apparent motion. Cereb. Cortex 18, 2796–2810. ( 10.1093/cercor/bhn038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dennett D. 1994. Instead of qualia. In Consciousness in philosophy and cognitive neuroscience (eds Revonsuo A, Kamppinen M.), pp. 129–139. Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- 130.Harnad S. 2001. The mind/body problem is the feeling/function problem. See http://users.ecs.soton.ac.uk/harnad/Tp/dennett-chalmers.htm.
- 131.Wright E. 2008. The case for qualia. Cambridge, MA: MIT Press. [Google Scholar]
- 132.Nolan L. 2011. Primary and secondary qualities: the historic and ongoing debate. Oxford, UK: Oxford University Press. [Google Scholar]


