Abstract
This study compared nicotine intake and changes in food intake and weight gain in naïve adolescent, naïve adult, and adult rats that were exposed to nicotine during adolescence. An extended intravenous self-administration (IVSA) model was used whereby rats had 23-hour access to saline or increasing doses of nicotine (0.03, 0.06, and 0.09 mg/kg/0.1 mL infusion) for 4-day intervals separated by 3-day periods of abstinence. Rats began IVSA as adolescents (PND 32–34) or adults (PND 75). A separate group of rats was exposed to nicotine via osmotic pumps (4.7 mg/kg) for 14 days during adolescence and then began nicotine IVSA as adults (PND 75). The rats that completed the nicotine IVSA regimen were also tested for nicotine-seeking behavior during extinction. The results revealed that nicotine intake was highest in adolescents followed by adults that were pre-exposed to nicotine during adolescence as compared to naïve adults. A similar pattern of nicotine-seeking behavior was observed during extinction. In contrast to nicotine intake, naïve adults displayed robust appetite and weight suppressant effects of nicotine, an effect that was absent in adolescents and adults that were pre-exposed to nicotine during adolescence. Our findings suggest that adolescence is a unique period of enhanced vulnerability to the reinforcing effects of nicotine. Although adolescents gain weight faster than adults, the food intake and weight suppressant effects of nicotine are reduced during adolescence. Importantly, our findings suggest that adolescent nicotine exposure produces long-lasting consequences that enhance nicotine reward and promote tolerance to the anorectic effects of this drug.
Keywords: Age, Adolescent, Pre-exposure, Intravenous, Weight, Food intake
1. Introduction
Adolescent tobacco use is a major heath and economic concern. Young persons display a strong propensity to experiment with tobacco products and are most likely to become long-term smokers [1–4]. In fact, a recent report by the Center for Disease Control (CDC) suggests that 90% of tobacco use begins before the age of 18 [5]. Also, while there has been a slight decline in tobacco use in the United States, the rates of smoking initiation among adolescents remains high in recent years. The CDC report also indicates that the high rates of nicotine dependence and smoking-related diseases are reflected economically in rising healthcare costs and losses in worker productivity [6]. Given that tobacco use leads to deleterious health consequences, adolescents are at heightened risk of developing tobacco-related diseases later in life.
Clinical studies have unveiled several factors that likely contribute to adolescent tobacco use. These include (but are not limited to) peer pressure, impulsivity, risk-taking, affective disorders, and stress management [7–10]. To our knowledge, clinical studies have not directly compared the rewarding effects of nicotine in adolescents versus adults. However, adult smokers who initiated tobacco use during adolescence generally reported more pleasant than unpleasant effects during their first smoking episode as compared to their naïve adult counterparts [11–14]. Therefore, the possibility exists that adults that were exposed to nicotine during adolescence may experience enhanced rewarding effects of nicotine as compared to adult starters.
Pre-clinical studies in rodents suggest that the rewarding effects of nicotine are enhanced during adolescence. For example, adolescent rats and mice display a more robust conditioned place preference (CPP) produced by nicotine as compared to adults, across a wide range of experimental protocols, nicotine doses, and routes of administration [15–19]. Consistent with these studies, work in our laboratory demonstrated that adolescent rats display enhanced nicotine-induced CPP relative to their adult male and female counterparts [20,21]. Studies using oral self-administration procedures have also shown that nicotine intake is higher in mice that were tested during the early (post-natal day [PND] 24–35) versus late (PND 37–60) phases of adolescence [22]. Similarly, studies using intravenous self-administration (IVSA) procedures have demonstrated that nicotine intake is about two-fold higher in adolescent versus adult rats [23].
Rodent studies have also shown that exposure to nicotine during adolescence enhances the rewarding effects of nicotine later in adulthood. This effect has been observed using both CPP [24] and IVSA [25] procedures. Also, adult mice that were pre-exposed to nicotine during adolescence display a more robust CPP produced by nicotine when they were exposed to nicotine during the early (PND 27–33) versus late (PND 50–56) phases of adolescence [26]. Taken together, these studies suggest that adolescence is a unique period of enhanced vulnerability to the rewarding effects of nicotine.
In addition to the rewarding effects of nicotine, clinical reports indicate that tobacco products are also used to control appetite and limit weight gain. Indeed, tobacco use has been strongly associated with a decrease in food intake and weight gain [27–29]. The anorectic effects of nicotine likely play a major contributing factor to adolescent tobacco use, given that adolescents are highly concerned with weight gain and often perceive themselves as overweight [30]. Given the increased probability to experiment with tobacco products during adolescence, an important question is whether adolescent nicotine exposure alters the long-term appetite and weight suppressant effects of this drug in adulthood. This issue is important to consider given recent findings showing that nicotine exposure produces long-term increases in abdominal fat, hepatic steatosis, glucose intolerance, and insulin resistance [27,31–33]. Thus, the interaction between tobacco use and food
| Group | Pump or sham surgery | Catheter surgery | Food training | IVSA (Cycle 1) | IVSA (Cycle 2) | Extinction |
|---|---|---|---|---|---|---|
| Adolescent–onset | N/A | 25–27 | 27–29 | 32–34 | 53–55 | 74–76 |
| Adult | 28–30 | 61–66 | 65–70 | 72–77 | 93–98 | 112–117 |
consumption may place adolescents at greater risk of developing metabolic disorders such as diabetes.
Pre-clinical studies have shown that nicotine exposure alters food intake and weight gain in a different manner during adolescence versus adulthood. As an example, Faraday et al. demonstrated that nicotine produced a robust decrease in food intake and weight gain in adult rats that was lower than adolescents [34]. Consistent with this, exposure to oral nicotine intake during adolescence does not alter weight gain [35,36]. With regard to the long-term effects of adolescent nicotine exposure, Slaweki et al. observed that adult rats that were exposed to nicotine during adolescence displayed fewer approaches to food pellets and showed lower food intake as compared to naïve adults [37]; however, there were no weight differences among these groups of rats.
Taken together, the literature suggests that adolescence is a period of development characterized by enhanced rewarding effects of nicotine and long-term vulnerability to tobacco use. Also, the clinical data suggest that adolescents may use tobacco to suppress appetite and manage their weight. In order to fully appreciate the effects of nicotine during adolescence, one must consider that nicotine has both short-term effects during adolescence, as well as long-term consequences following exposure to this drug during adolescence. To our knowledge, no study has directly compared the long-term reinforcing effects of nicotine in naïve adolescent, naïve adult, and adult rats that were pre-exposed to nicotine during adolescence. Moreover, there is a paucity of information regarding the short- and long-term consequences of adolescent nicotine exposure on the anorectic effects of nicotine. To our knowledge, this is the first study that directly compares nicotine intake, food intake, and weight gain in adolescents, adults and adults that were pre-exposed to nicotine during adolescence. This is an important distinction because previous work has focused on either adolescent versus adult rats or nicotine pre-exposed adults as compared to naïve adults. The study also presents novel data with regard to the long-term impact of adolescent nicotine exposure using nicotine pump procedures that produce dependence to this drug. We used an extended-access model of nicotine IVSA whereby rats were given 23-h access to increasing doses of nicotine separated by brief periods of drug abstinence. The advantage of this model is that concomitant measures of food and nicotine intake can be monitored across several weeks of operant testing. Using this procedure, nicotine IVSA, food intake, and weight changes were assessed in separate groups of naive adolescents, naïve adults, and adults that were pre-exposed to nicotine during adolescence. Nicotine-seeking behavior was also compared during extinction in these groups.
2. Materials and methods
2.1. Subjects
Male Wistar rats were tested using extended access to saline or nicotine IVSA. Our experimental groups consisted of naïve adolescent rats receiving access to saline (n = 6) or nicotine IVSA (n = 10), and naïve adults receiving access to saline (n = 6) or nicotine (n = 13) IVSA. A separate group of adult rats were pre-exposed to nicotine during adolescence and received access to nicotine IVSA (n = 9). The naïve adult group received a sham pump surgery during adolescence as a control procedure, so these rats could serve as controls for the pre-exposed adult group. The table below depicts the PND for the different groups of rats during all of the experimental procedures.
Rats were handled for 3–5 days prior to the start of experimentation and received ad libitum access to food and water. Rats were housed in groups of 2–3 per cage in a humidity- and temperature-controlled (20–22 °C) vivarium. Rats were bred from a fully out-bred stock from Harlan, Inc. (Indianapolis, IN). All procedures were approved by the UTEP Institutional Animal Care and Use Committee.
2.2. Operant chambers
All rats were tested in operant chambers from Med Associates (St. Albans, VT) that were kept on a regular light/dark cycle (lights on 6 AM–6 PM) inside sound-attenuated chambers with continuous white noise. The exit port of the catheter fittings was connected to polyethylene tubing contained inside a protective metal spring that was suspended into the chamber from a liquid swivel attached to a balance arm. Operant sessions were conducted using two retractable levers (active and inactive) that extended 2.5 cm into the chamber. Each response on the active lever resulted in the delivery of the nicotine solution via syringe pumps from Razel Scientific Research Instruments (St. Albans, VT) in a volume of 0.1 mL per second. A 28 V white cue light was illuminated above the active lever at the onset of the 1 s infusion and was terminated after a 20 s time-out period, during which responses on the active lever had no scheduled consequences and were not recorded. In contrast, responses on the inactive lever had no scheduled consequences and were recorded without a time-out period.
Each day during testing, the rats were removed from the operant chambers from 10–11 AM and were placed into their home cages (n = 2–3 per cage) so the chambers could be cleaned and the water and food could be replenished. During the 3-day abstinence periods, the rats were housed in pairs in their home cage in the same behavioral test room and food and water were available ad libitum.
2.3. Adolescent nicotine exposure
To examine the long-term effects of adolescent nicotine exposure, a group of adolescent rats were exposed to nicotine via osmotic pumps for 14 days during adolescence and began nicotine IVSA later during adulthood. The pump administration was used because it delivers a fixed amount of nicotine that has been previously employed in studies comparing age differences in the behavioral and neurochemical effects of nicotine withdrawal in rats [38,39]. Thus, our procedures allowed us to compare the immediate and long-term effects of nicotine on IVSA, food intake, and weight gain. The adolescents were first anesthetized with an isoflurane/oxygen vapor mixture (1–3%) and were surgically prepared with 14-day osmotic pumps (Alzet model 2ML2; 5.0 μL/h). The pumps were implanted subcutaneously on the back of the animal, parallel to the spine. The pumps were filled with a dose of nicotine (4.7 mg/kg/day; base) that was adjusted according to the weight of the rat at the time of the surgery. After 14 days of nicotine exposure, the pumps were surgically removed under isoflurane anesthesia. Naïve adult control rats received sham surgery without pump implantation. Rats were then returned to their home cage until they were given access to the IVSA procedures as adults.
2.4. Food and water training
All rats were first trained on a fixed ratio 1 (FR-1) schedule of reinforcement to obtain palatable chow pellets (45 mg dustless precision food pellets from Bio-Serv; Frenchtown, NJ) from a pellet dispenser with a swing door mounted between two levers on the front wall of the chamber. A nose-poke response was also required in a separate hole positioned on the back of the chamber for administration of 0.1 mL aliquots of water into an adjacent metal dipper cup. All rats reached stable levels of food and water responding within 3–5 days, and then were returned to their home cages for one day before catheter surgery.
2.5. Catheter implantation
Rats were anesthetized with an isoflurane/oxygen vapor mixture (1–3%) and were then prepared with catheters into the jugular vein. A 14 cm length of silastic tubing (0.94 mm o.d., Dow-Corning; Midland, MI) was inserted into the jugular vein approximately 2 cm for adolescents and 4 cm for adults. The free end of the catheter was tunneled subcutaneously to the incision on the back and attached to an acrylic-based pedestal with a small piece of surgical mesh that was mounted to a 21-gauge guide cannula that was bent at a right angle (Plastics One, Roanoke, VA). Following insertion of the catheter, the pedestal containing the exit port of the catheter was sutured subcutaneously on the animals’ back and the wound was closed around the fitting. The animals were allowed a period of 2–3 days to recover in their home cage. Catheters were flushed daily for the duration of the experiment with a 0.3–0.5 mL infusion of a 100 mg/mL antibiotic solution containing Timentin (Glaxco-SmithKline; Research Triangle Park, NC) that was diluted in sterile saline containing heparin (30 USP units/mL). To verify patency of the catheters, the rats received a 0.1 mL IV infusion of a 10 mg/mL solution of the ultra short-acting barbiturate anesthetic Brevital® sodium (1% methohexital sodium, Eli Lilly, Inc.). Patency tests were conducted prior to IVSA and at least once every two weeks during the IVSA period. This test was also performed when aberrant shifts in IVSA behavior were observed. Non-patent animals were excluded from the study.
2.6. Food and water re-establishment
Following recovery from surgery, all rats were re-introduced into the chambers by connecting the exit port of the catheter fittings to the metal spring that was attached to the swivel and balance arm. This procedure was done to establish baseline body weight, as well as food and water intake prior to the introduction of the levers in the next phase of the study. During this period, rats performed nose-poke responses for food and water on a FR-1 schedule of reinforcement in 23-h sessions for 4 days in the absence of any levers.
2.7. IVSA procedures
Following food and water re-establishment, the rats were presented with inactive and active levers. Responses on the active lever delivered saline or various doses of nicotine in 4-day increments beginning Monday at 11 AM until Friday at 10 AM. Rats had concomitant access to nose-poke operandum for food and water throughout IVSA testing. During each 4-day period, rats receiving nicotine IVSA were given access to an increasing unit dose of nicotine (expressed as base) in the following order: 0.03, 0.06, and 0.09 mg/kg/0.1 mL infusion. (–) Nicotine hydrogen tartrate (St. Louis, MO) was dissolved in 0.9% sterile saline and adjusted to a pH of 6.5–7.5. The nicotine solutions were prepared daily based on the animals’ weights from the previous day. Control rats received saline throughout the IVSA period. In between the 4-day periods of IVSA, the rats were given a brief period of forced abstinence during which they were returned to their home cages for 3 days. Thus, the first dose regimen (Cycle 1) was completed in 3 weeks. The entire dose regimen with the intermittent abstinence periods was then repeated (Cycle 2) to examine whether the group differences in IVSA behavior persisted for a second regimen of testing. Thus, the rats received 2 cycles of the nicotine IVSA dosing regimen.
2.8. Extinction procedures
Three days after the final IVSA session, the nicotine syringe was filled with saline and drug-seeking behavior was examined in the nicotine IVSA animals for 8 days. Rats had concomitant access to nose-poke for food and water throughout extinction testing. During the first 4 days of extinction, responding on the active lever resulted in infusions of saline and presentation of the drug-associated cues (i.e., IV infusion, pump noise, and cue light). During the last 4 days of extinction, responses on the active lever had no scheduled consequences and the drug-associated cues were turned off.
2.9. Data analysis
The dependent measures of this study were operant responses (nose pokes for food and active/inactive lever responses) and changes in body weight (expressed as % changes from baseline body weight in g). Operant responses and weight changes were assessed on a daily basis across different doses of nicotine in the same rats. Thus, the data in the top panels of Figs. 1–3 were analyzed using repeated measures analyses of variance (ANOVA) with group (naïve adolescent, naïve adult, and pre-exposed adults) as a between subject factor, and dose (saline or escalating doses of nicotine) and day as within subject factors. Significant group × dose × day interactions were then further analyzed to compare day-to-day differences in our dependent measures at individual doses of nicotine (Fisher's LSD tests; P ≤ 0.05). The data in the bottom panels of Figs. 1–3 were analyzed using separate one-way ANOVAs during weeks 1–3 of Cycle 1. This was done to examine group differences at a particular dose of nicotine. Only the data from Cycle 1 were included to compare group differences during a time when adolescents had not reached adulthood. Significant interactions were followed up with post hoc comparisons of individual experimental groups within a dose of nicotine (Fisher's LSD tests; P ≤ 0.05). Our approach to these data allowed for a comparison of experimental groups across time (top panels) as well as an examination of group differences at individual doses of nicotine (bottom panel).
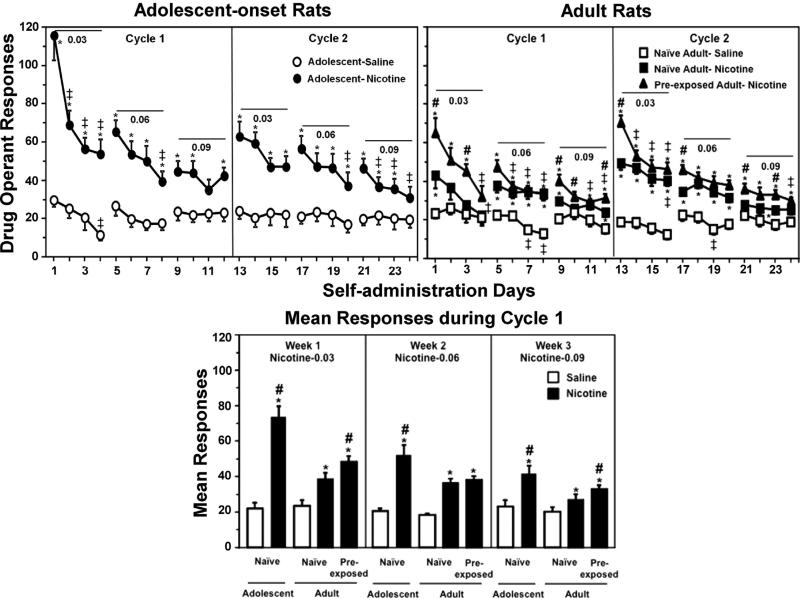
Fig. 1.
The top graphs reflect mean operant responses (±SEM) across IVSA days in adolescent-onset (n = 6–10), naïve adult (n = 6–13), and adult rats that were pre-exposed to nicotine during adolescence (n = 9). Rats were given 23-h access to perform operant responses for saline (open symbols) or escalating doses of nicotine (filled symbols; 0.03, 0.06, or 0.09 mg/kg/0.1 mL infusion) for 4-day intervals separated by 3-day periods of abstinence. The rats received 2 cycles of the IVSA regimen. The bottom graph reflects mean responses (±SEM) separated by the weekly dosing regimen of nicotine IVSA. Only Cycle 1 is presented in order to compare group differences in operant responses when the adolescent rats were still in the adolescent period. Asterisks (*) denote significant group differences from saline IVSA. Number signs (#) denote significant group differences from naïve adults. Double cross signs (‡) denote significant differences from the first day of IVSA at each of the 4-day intervals of testing (P ≤ 0.05).
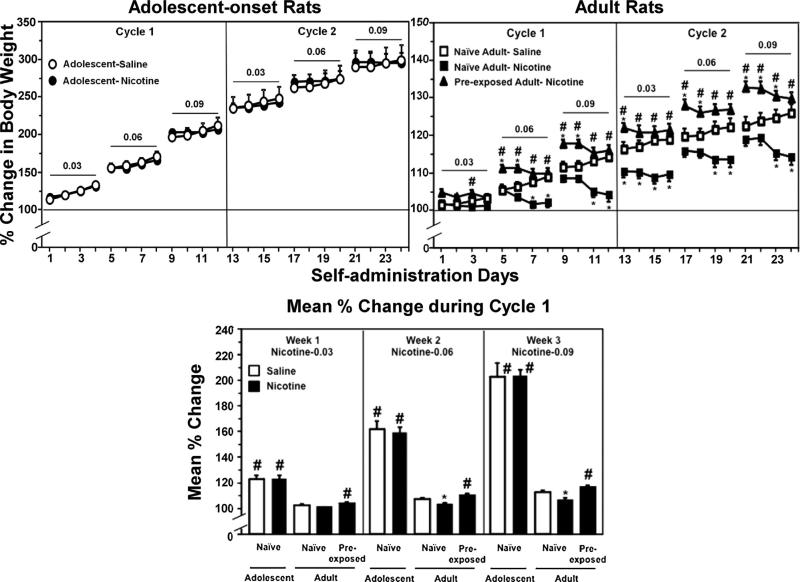
Fig. 3.
The top graphs reflect % change in body weight (±SEM) across IVSA days in adolescent-onset (n = 6–10), naïve adult (n = 6–13), and adult rats that were pre-exposed to nicotine during adolescence (n = 9). Rats were given 23-h access to perform operant responses for saline (open symbols) or escalating doses of nicotine (filled symbols; 0.03, 0.06, or 0.09 mg/kg/0.1 mL infusion) for 4-day intervals separated by 3-day periods of abstinence. The rats received 2 cycles of the IVSA regimen. The bottom graph reflects mean % change values (±SEM) separated by the weekly dosing regimen of nicotine IVSA. Only Cycle 1 is presented in order to compare group differences in operant responses when the adolescent rats were still in the adolescent period. Asterisks (*) denote significant group differences from saline IVSA. Number signs (#) denote significant group differences from naïve adults (P ≤ 0.05).
The extinction data in the left panel of Fig. 4 were analyzed using repeated measures ANOVA with group (naïve adolescent, naïve adult, and pre-exposed adults) as a between-subject factor and day and extinction phase (with and without drug cues) as within-subject factors. A significant interaction effect was followed up with post hoc comparisons of experimental groups within an individual day of testing (Fisher's LSD tests; P ≤ 0.05). The data in the right panel of Fig. 4 were analyzed using separate one-way ANOVAs at each extinction phase. This was done to examine group differences in the presence and absence of drug cues. The inactive lever data were also analyzed using separate one-way ANOVAs during weeks 1–3 of IVSA testing and then during extinction. Significant interactions were followed up with post hoc comparisons of individual experimental groups within a dose of nicotine (Fisher's LSD tests; P ≤ 0.05).
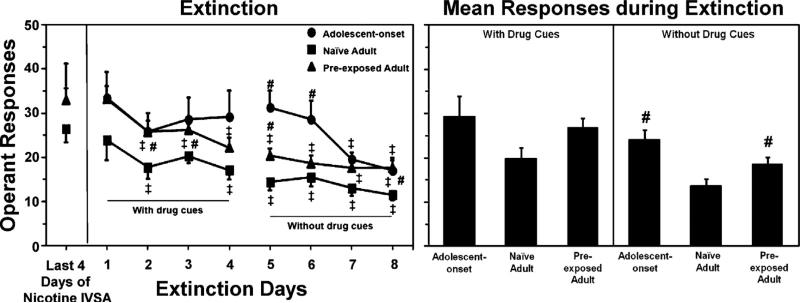
Fig. 4.
The left graph reflects operant responses (±SEM) prior to and then during extinction in adolescent-onset (n = 7), naïve adult (=10), and adult rats that were pre-exposed to nicotine during adolescence (n = 9). Extinction testing occurred first with and then without the presentation of drug cues. The right graph reflects mean responses (±SEM) separated by extinction testing in the presence and absence of drug cues. Number signs (#) denote significant group differences from naïve adults. Double cross signs (‡) denote significant differences from the average of the last 4 days of nicotine IVSA (P ≤ 0.05).
3. Results
3.1. IVSA behavior
Fig. 1 reflects operant responses on the active lever during IVSA procedures in naïve adolescent, naïve adult, and adult rats that were pre-exposed to nicotine during adolescence. The panel on the left is referred to as adolescent-onset since the adolescents reached adulthood by the start of Cycle 2. Overall, the results revealed that adolescents displayed the highest level of nicotine intake followed by pre-exposed adults that were higher than naïve adults.
Our overall analysis of the data in the top graphs revealed a significant 3-way interaction of group, day, and dose (F(60, 585) = 1.8, P ≤ 0.001). Post hoc analyses of the adolescent-onset rats revealed that nicotine intake was higher than their respective saline controls on days 1–23 (*P ≤ 0.05). An evaluation of operant responding as compared to the first day of IVSA revealed that nicotine intake decreased in adolescent-onset rats on days 2–4, 8, 20, and 22–24 (‡P ≤ 0.05), and saline intake decreased in controls on day 4 (‡P ≤ 0.05). Post hoc analyses of naïve adult rats revealed that nicotine intake was higher than their respective saline controls on days 1, 5–9, 11–20, and 23 (*P ≤ 0.05). An evaluation of operant responding as compared to the first day of IVSA revealed that nicotine intake decreased in naïve adult rats on days 4 and 16 (‡P ≤ 0.05), and saline intake decreased in controls on days 7, 8 and 19 (‡P ≤ 0.05). Post hoc analyses of the pre-exposed adults revealed that nicotine intake was higher than saline controls on days 1–3, 5–9, and 11–24 (*P ≤ 0.05), and higher than naïve adults receiving nicotine IVSA on days 1, 3, 9–10, 12, 13, 17, 21 and 23 (#P ≤ 0.05). The pre-exposed adults also displayed a decrease in nicotine intake relative to the first day of IVSA on days 4, 6–17, 11–12, 14–16, and 21–24 (‡P ≤ 0.05).
Our analysis of the data in the bottom panel of Fig. 1 revealed a main effect of group at the 0.03 (F(4, 39) = 21.7, P ≤ 0.001), 0.06 (F(4, 39) = 14.2, P ≤ 0.001), and 0.09 (F(4, 39) = 7.6, P ≤ 0.001) doses of nicotine. Post hoc analyses revealed that regardless of dose, all rats displayed higher nicotine intake as compared to their respective saline controls (*P ≤ 0.05). Adolescent-onset rats displayed higher intake at all nicotine doses relative to naïve adults (#P ≤ 0.05). Also, pre-exposed adults displayed higher nicotine intake as compared to naïve adults during IVSA of the 0.03 and 0.09 doses of nicotine (#P ≤ 0.05).
3.2. Food operant responses
Fig. 2 reflects operant responses for food during the final days of food training (BL) and then during IVSA procedures in naïve adolescent, naïve adult, and adult rats that were pre-exposed to nicotine during adolescence. An analysis of baseline food intake did not reveal an interaction (F(12, 117) = 0.6, P = NS) or any other group differences (F(4, 39) = 2.1, P = NS) prior to IVSA procedures. During IVSA, the results revealed that only naive adult rats that received nicotine IVSA displayed a decrease in food intake relative to saline controls.
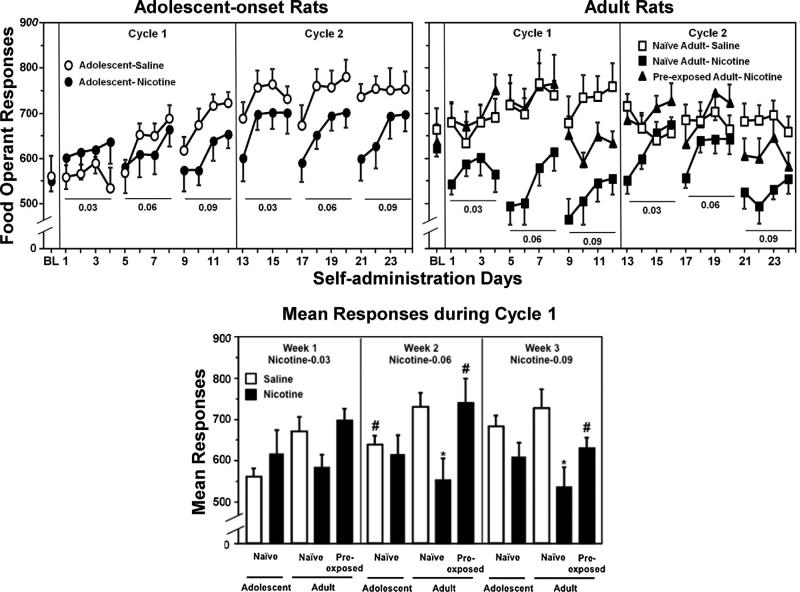
Fig. 2.
The top graphs reflect operant responses for food (±SEM) across IVSA days in adolescent-onset (n = 6–10), naïve adult (n = 6–13), and adult rats that were pre-exposed to nicotine during adolescence (n = 9). Rats were given 23-h access to perform operant responses for saline (open symbols) or escalating doses of nicotine (filled symbols; 0.03, 0.06, or 0.09 mg/kg/0.1 mL infusion) for 4-day intervals separated by 3-day periods of abstinence. The rats received 2 cycles of the IVSA regimen. The bottom graph reflects mean responses (±SEM) separated by the weekly dosing regimen of nicotine IVSA. Only Cycle 1 is presented in order to compare group differences in operant responses when the adolescent rats were still in the adolescent period. Asterisks (*) denote significant group differences from saline IVSA. Number signs (#) denote significant group differences from naïve adults (P ≤ 0.05).
Our overall analysis of the data in the top graphs revealed there was no significant 3-way interaction of group, day, and dose. However, a significant 2-way interaction emerged between group and dose (F(20, 195) = 3.7, P ≤ 0.001).
Our analysis of the data in the bottom panel of Fig. 2 revealed a main effect of group during IVSA of the 0.06 (F(4, 39) = 3.5, P ≤ 0.05) and 0.09 (F(4, 39) = 4.7, P ≤ 0.01) doses of nicotine. Post hoc analyses of the adolescent-onset rats revealed that adolescents that received saline IVSA displayed lower food responses versus their naïve adult counterparts during Week 2 of IVSA (#P ≤ 0.05). Post hoc analyses of naïve adult rats revealed that operant responses for food were lower during IVSA of the 0.06 and 0.09 nicotine doses as compared to their respective saline controls (*P ≤ 0.05). Also, pre-exposed adults displayed higher operant responses for food during IVSA of the 0.06 and 0.09 nicotine doses as compared to naïve adult rats (#P ≤ 0.05).
3.3. Change in body weight
Fig. 3 reflects % change in body weight during IVSA procedures in naïve adolescent, naïve adult, and adult rats that were pre-exposed to nicotine during adolescence. An analysis of baseline body weight revealed a significant interaction of group and day (F(12, 117) = 5.3, P ≤ 0.001). Post hoc analyses revealed that adolescents displayed lower baseline body weights relative to adults (P ≤ 0.05; data not shown). However, there were no differences in body weight within the respective age groups. During IVSA, the results revealed that only naïve adult rats that received nicotine IVSA displayed a decrease in body weight relative to saline controls.
Our overall analysis of the data in the top graphs revealed a significant interaction of group, day, and dose (F(60, 585) = 3.0, P ≤ 0.001). Post hoc analyses of the adolescent-onset rats revealed a similar increase in body weight across time as compared to their respective naïve adult counterparts (#P ≤ 0.05). In contrast, naïve adults receiving nicotine IVSA displayed a decrease in body weight relative to saline controls on days 7–8, 11–16, 19–20, and 23–24 (*P ≤ 0.05). Post hoc analyses of the pre-exposed adults revealed an increase in body weight relative to saline controls on days 5–6, 9–10, 13, 17–18, and 21–23 (*P ≤ 0.05). Also, the increase in body weight observed in pre-exposed adults was higher than naïve adults that received nicotine IVSA on days 3 and 5–23 (#P ≤ 0.05).
Our analysis of the data in the bottom panel of Fig. 3 revealed a main effect of group during IVSA of the 0.03 (F(4, 39) = 45.5, P ≤ 0.001), 0.06 (F(4, 39) = 108.4, P ≤ 0.001), and 0.09 (F(4, 39) = 161.1, P ≤ 0.001) doses of nicotine. Post hoc analyses of the adolescent-onset groups revealed that adolescents displayed a similar increase in weight gain throughout Cycle 1 testing as compared to their naïve adult counterparts (#P ≤ 0.05). Post hoc analyses of naïve adult rats revealed a decrease in body weight at the 0.06 and 0.09 nicotine doses as compared to their respective saline controls (*P ≤ 0.05). Also, pre-exposed adults displayed a larger increase in weight gain during IVSA of all nicotine doses relative to naïve adults (#P ≤ 0.05).
3.4. Comparisons of all data across cycles 1 and 2
Table 1 reflects IVSA behavior, food responses, and changes in body weight across Cycles 1 and 2.
Table 1.
IVSA behavior, food responses, and change in body weight across cycles.
| IVSA Group | Saline |
Nicotine |
||||
|---|---|---|---|---|---|---|
| Adolescent-onset | Adult-onset | Adolescent-onset | Adult-onset | Pre-exposed Adult-onset | ||
| IVSA Behavior | Cycle 1 | 21.7 ± 2.0 | 20.1 ± 1.9 | 55.9 ± 4.8*,# | 315 ± 15* | 40.4 ± 42.9*,# |
| Cycle 2 | 21.3 ± 4.4 | 18.5 ± 1.6 | 46.3 ± 4.0*,#,‡ | 353 ± 2.1* | 42.9 ± 1.6*,# | |
| Food Responses | Cycle 1 | 628.3 ± 21.0 | 709.4 ± 36.4 | 614.7 ± 42.5 | 550.4 ± 31.5* | 689.7 ± 30.7# |
| Cycle 2 | 742.4 ± 30.7‡ | 677.6 ± 31.8 | 663.4 ± 34.3‡ | 592.0 ± 183* | 667.3 ± 24 9# | |
| % Change In Body | Cycle 1 | 162.8 ± 4.6# | 107.3 ± 13 | 162.2 ± 3.2# | 103.7 ± 0.9* | 110.5 ± 0.9# |
| Weight | Cycle 2 | 267.3 ± 17.9#,‡ | 120.9 ± 2.1‡ | 268.7 ± 9.9#,‡ | 113.8 ± 1.7*,‡ | 126.5 ± 1.5*,#,‡ |
Denotes significant group differences from saline IVSA.
Denotes significant group differences from naïve adults.
Denotes significant differences from Cycle 1.
Our analysis of IVSA behavior revealed a significant interaction of group and cycle (F(4, 39) = 4.0; P ≤ 0.01). Post hoc analyses revealed higher nicotine versus saline IVSA across all groups of rats (*P ≤ 0.05). Both adolescent-onset rats and pre-exposed adult rats displayed more nicotine IVSA as compared to naive adults (#P ≤ 0.05). The post hoc analyses across time revealed that adolescent-onset rats displayed a decrease in nicotine IVSA from Cycle 1 to Cycle 2 (‡P ≤ 0.05). Our analysis of food responses revealed a significant interaction of group and cycle (F(4, 39) = 5.1; P ≤ 0.01). Post hoc analyses revealed a significant decrease in food responses in adult-onset rats that received nicotine versus saline IVSA in both cycles (*P ≤ 0.05). Pre-exposed adults displayed significantly higher food responding compared to naive adults (#P ≤ 0.05). The post hoc analyses across time revealed that adolescent-onset rats displayed an increase in food intake from Cycle 1 to Cycle 2 (‡P ≤ 0.05). Our analysis of body weight revealed a significant interaction of group and cycle (F(4, 39) = 81.6; P ≤ 0.001). Post hoc analyses revealed a significant decrease in weight gain in adult-onset rats that received nicotine versus saline IVSA in both cycles (*P ≤ 0.05). Both adolescent-onset rats and pre-exposed adult rats displayed a larger increase in weight gain as compared to naive adults (#P ≤ 0.05), regardless of nicotine or saline IVSA conditions. The post hoc analyses across time revealed that all rats displayed an increase in weight gain from Cycle 1 to Cycle 2 (‡P ≤ 0.05).
3.5. Extinction of nicotine IVSA behavior
Fig. 4 reflects operant responses on the active lever during extinction in naïve adolescent, naïve adult, and adult rats that were pre-exposed to nicotine during adolescence. Overall, adolescent-onset and pre-exposed adult rats displayed higher levels of nicotine-seeking behavior during extinction relative to naïve adults, an effect that was more pronounced during uncued extinction procedures.
Our overall analysis of the data in the left panel revealed a significant interaction of group, day, and extinction phase (F(6, 69) = 2.5, P ≤ 0.05). Post hoc analyses of the adolescent-onset rats revealed more operant responses on the active lever as compared to naïve adults on extinction days 5–6 (#P ≤ 0.05). A comparison of extinction responding as compared to the average of the last 4 days of nicotine IVSA revealed that adolescent-onset rats displayed a decrease in nicotine-seeking behavior on days 7–8 (‡P ≤ 0.05). Naïve adult rats displayed a decrease in nicotine-seeking behavior on days 2, 4, and 5–8 (‡P ≤ 0.05). Post hoc analyses of the pre-exposed adults revealed higher operant responses on the active lever as compared to naïve adults on extinction days 2, 3, 5, and 8 (#P ≤ 0.05). Also, pre-exposed adults displayed a decrease in nicotine-seeking behavior on days 2–8 (‡P ≤ 0.05).
Our analysis of the data in the right panel revealed a main effect of group during uncued extinction (F(2, 23) = 2.9; P ≤ 0.01). Post hoc analyses revealed that adolescent-onset rats and pre-exposed adults displayed more nicotine-seeking behavior during the uncued extinction phase versus naïve adults (#P ≤ 0.05).
3.6. Inactive lever responses
Table 2 reflects inactive lever responses during Weeks 1–3 of Cycle 1 and during extinction in naïve adolescent, naïve adult, and adult rats that were pre-exposed to nicotine during adolescence. Overall, the results revealed that adolescent-onset rats receiving nicotine IVSA displayed higher inactive responses during Cycle 1 relative to naïve adults. However, this age difference was no longer evident during extinction testing.
Table 2.
Inactive lever responses during Cycle I.
| JVSA Group | Saline |
Nicotine |
|||
|---|---|---|---|---|---|
| Adolescent-onset | Naive Adult-onset | Adolescent-onset | Naive Adult-onset | Pre-exposed Adult-onset | |
| Week 1 | 75.0 ± 15.1# | 21.6 ± 4.6 | 137.6 ± 30.2# | 20.8 ± 3.1 | 30.3 ± 4.6 |
| Week 2 | 39.4 ± 11.3#,‡ | 10.5 ± 2.1‡ | 119.9 ± 34.4*,# | 7.9 ± 1.7‡ | 7.7 ± 1.6‡ |
| Week 3 | 24.6 ± 6.6#,‡ | 10.0 ± 2.7‡ | 57.1 ± 15.3#,‡ | 8.1 ± 1.l‡ | 7.8 ± 1.5‡ |
| Extinction | N/A | N/A | 21.0 ± 7.0‡ | 10.3 ± 1.9‡ | 8.7 ± 2.6‡ |
Denotes significant group differences from saline IVSA.
Denotes significant group differences from naïve adults.
Denotes significant differences from Week 1.
Our analysis of inactive lever data revealed a main effect of group during Week 1 (F(4, 39) = 10.3, P ≤ 0.001), Week 2 (F(4, 39) = 8.0, P ≤ 0.001), and Week 3 (F(4, 39) = 7.5, P ≤ 0.001) of testing. Post hoc analyses revealed that adolescent-onset rats that received nicotine IVSA displayed higher inactive responses than their saline counterparts during Week 2 (*P ≤ 0.05). Regardless of nicotine or saline IVSA, adolescent-onset rats displayed more inactive responses as compared to naïve adults (#P ≤ 0.05). Post hoc analyses also revealed that all rats displayed a decrease in inactive lever responding by Week 2 of testing (‡P ≤ 0.05). However, the adolescent-onset rats that received nicotine IVSA did not display a decrease in inactive responses until Week 3. There were no group differences across experimental conditions during extinction (F(2, 23) = 1.1, P = NS).
4. Discussion
The main finding of this study is that nicotine intake is highest in adolescent rats followed by adults that were pre-exposed to nicotine during adolescence as compared to naïve adults. This pattern of nicotine intake was similar during extinction, suggesting that the group differences are related to the motivational properties of nicotine. In contrast to our findings with nicotine, the food intake and weight suppressant effects of this drug were largest in naïve adult rats. In fact, these effects were absent in both adolescents and in adults that were pre-exposed to nicotine during adolescence. Taken together, these findings suggest that adolescence is a unique period in development characterized by enhanced sensitivity to the rewarding effects of nicotine and insensitivity to the anorectic effects of nicotine. Furthermore, our findings suggest that there are long-term effects of adolescent nicotine exposure that confer enhanced sensitivity to the rewarding effects and tolerance to the anorectic effects of nicotine during adulthood.
With regard to the short-term effects of nicotine during adolescence, our findings revealed that adolescent rats displayed the highest level of nicotine intake relative to all other groups across several doses of nicotine. The high level of nicotine intake in adolescent rats was also observed following each of the forced abstinence periods from nicotine IVSA. This was evident as an increase in nicotine intake on the first day of IVSA as compared to subsequent days. Moreover, adolescent rats displayed the highest level of nicotine-seeking behavior during extinction relative to naïve adults. Interestingly, extinction responding was more pronounced during uncued versus cued extinction, suggesting that there may be age and treatment differences in the degree to which drug cues contribute to nicotine-seeking behavior. Taken together, these findings suggest that the motivational properties of nicotine are enhanced in adolescent rats. Our findings are consistent with other studies showing higher levels of nicotine IVSA in adolescent versus adult rats [40,23]. Oral nicotine intake is also higher in adolescent versus adult rats and mice [22,41–44]. Adolescent rats and mice also display enhanced place preference for a compartment paired with nicotine across a wide range of experimental protocols, doses, and routes of administration [15–21]. Taken together, these findings suggest that the rewarding effects of nicotine are enhanced during the adolescent period of development.
The present study also revealed that adolescent rats display high levels of inactive lever responding that are important to consider in the interpretation of our results. However, it is particularly imperative to consider that the inactive lever data may not be directly comparable to the data collected on the active lever. This is because there was no time-out period on the inactive lever as compared to the active lever – where responses were not recorded for 20 s after each drug delivery. Thus, responding on the inactive lever is artificially higher than that of the active lever. Despite this limitation, our data revealed that adolescent rats displayed higher levels of inactive responding relative to naïve adults. The data for inactive responses reveal important information regarding adolescent IVSA behavior, particularly for future studies comparing age differences in drug IVSA behavior. The higher inactive responses during adolescence may be related to a greater increase in activity produced by nicotine, as previously demonstrated [34,45–47]. Oral nicotine intake has also been shown to produce an increase in a variety of behaviors such as nose-poking, rearing, locomotion, and novelty-seeking in adolescent mice [48,49]. Alternatively, the high levels of inactive responding may reflect impulsive behavior, given that adolescent rats have been shown to display higher impulsivity in delay-discounting procedures as compared to adults [50]. The findings may also reflect impaired habituation to the drug-associated cues, especially given the persistence of high inactive responding in adolescents throughout Cycle 1 that eventually diminished during extinction. Lastly, the possibility exists that the high levels of inactive lever responding reflect enhanced nicotine-seeking behavior in adolescents. This interpretation would be consistent with the greater rewarding effects of nicotine as well as the higher levels of active lever responding during extinction in adolescent versus adult rats.
Regardless of age, all rats displayed a decrease in nicotine IVSA from Day 1 to 4. The decrease in nicotine intake across time has been reported in adult rats given intermittent 23-h access to nicotine IVSA [51,52]. The present study revealed that the decreases in nicotine intake were most evident in adolescent rats that displayed robust intake on the first day of access to nicotine following a forced abstinence period. These data suggest that there are age differences in the degree to which nicotine intake changes over time.
With regard to the long-term effects of adolescent nicotine exposure, another major finding of this report is that adult rats that were pre-exposed to nicotine during adolescence displayed increases in the reinforcing effects of nicotine in adulthood. Namely, nicotine intake was higher in pre-exposed adults across multiple doses of nicotine versus naïve adult rats. Relatedly, the adolescent-onset rats displayed persistently higher nicotine intake as they entered Cycle 2 of IVSA as adults. A similar pattern of group differences was observed following abstinence from nicotine and during extinction testing, suggesting that the effects of adolescent nicotine exposure are related to enhanced motivation for nicotine. Our findings are consistent with other studies showing that adult rats that were pre-exposed to nicotine during adolescence display an increase in nicotine IVSA later in adulthood [25,53]. Also, pre-exposed adults display enhanced place preference produced by nicotine relative to naïve adult controls [24,26]. However, we recognize previous studies that reported a reduction in nicotine intake as adolescent male rats transition into adulthood [23]. Also, nicotine intake and place preference produced by nicotine has been shown to be lower in adult rats that were pre-exposed to nicotine during adolescence relative to naïve adult controls [41,54]. The discrepancy in these reports as compared to the findings of the present study may be related to methodological differences including sex, strain, route of administration, and/or dose of nicotine.
Previous research has shown that chronic nicotine exposure decreases food intake and weight gain in adult rodents [52,55–57]. Consistent with this, the present study revealed that naïve adult rats that received nicotine IVSA displayed a reduction in food intake and weight gain relative to adults that were given access to saline IVSA. Intermittent abstinence periods also produced robust weight gain during ad libitum access to food, consistent with the effects that have been observed during nicotine withdrawal [56,58]. In contrast to our findings with naïve adults, adolescent rats displayed a robust increase in food intake and weight gain regardless of whether they were given access to nicotine or saline IVSA. This is consistent with the mild effects of chronic nicotine exposure on food intake and weight gain during adolescence [22,34,59–61]. A recent report also revealed that there is no correlation between the level of nicotine IVSA and food intake across multiple strains of adolescent rats [62]. These studies suggest that nicotine may not produce strong anorectic effects in adolescent rats. Moreover, the motivational constructs that mediate nicotine and food intake may be distinct during adolescence, and future studies are needed to examine this possibility. One important consideration is that food intake was measured concomitantly during 23-h access to nicotine or saline IVSA. This raises the possibility that the decreases in food intake observed in naïve adults are related to competing motivation for nicotine versus food, and not the anorexic effect of nicotine alone. Future studies are needed to examine whether nicotine changes the valence of reward-related processes in an age-dependent manner.
With regard to the long-term effects of adolescent nicotine exposure, the present study revealed that the food intake and weight suppressant effects of nicotine were absent in adult rats that were pre-exposed to nicotine during adolescence. In fact, pre-exposed adults displayed a significant increase in food intake and weight gain relative to naïve adults. Previous studies have reported that nicotine only produces mild anorectic effects in adult rats that were pre-exposed to nicotine during adolescence [37,41]. However, we recognize a recent report that demonstrated that exposure to nicotine during early development did not alter operant responding for food later in adulthood [63]. Taken together, the findings of the present study suggest that exposure to nicotine during adolescence produces long-term tolerance to the anorectic effects of this drug.
In naïve adult rats, nicotine served as a potent food suppressant. However, in adolescent rats (in Cycle 1) nicotine did not suppress food intake or body weight. As the adolescents became adults (in Cycle 2), nicotine began to suppress food intake. We suspect that this was likely due to maturation into adulthood at the onset of Cycle 2. Interestingly, in adult rats that were exposed to nicotine during adolescence, nicotine did not suppress food intake in either cycle. Based on the latter results, one might have expected that adolescent-onset rats may have displayed a decrease in the food suppressant effects of nicotine during Cycle 2. However, the differences between adolescent-onset rats in Cycle 2 versus pre-exposed adults may be related to differences in the amount of nicotine pre-exposure, the delay in nicotine exposure prior to testing, and/or differences in the age of these groups at the time of testing.
The present findings provide important clinical implications regarding the short- and long-term effects of adolescent nicotine exposure. The finding that adolescent nicotine exposure enhances the rewarding effects of this drug later in adulthood suggests that adolescents may be more vulnerable to long-term tobacco use. In support of this hypothesis, Pomerleau et al. observed that initial smoking experiences were rated as more pleasurable in adult smokers that began smoking during adolescence [13,14]. With regard to the anorectic effects of nicotine, the present findings suggest that tobacco is not an effective weight loss tool for young people. This is based on our finding that adolescence is a period of rapid weight gain that is not suppressed by nicotine. Moreover, the ability of nicotine to produce anorectic effects may be compromised in an adult that has been pre-exposed to nicotine during adolescence. In fact, given the repeated exposure to and withdrawal from nicotine, food intake and weight gain in pre-exposed adults may even exceed that of an adult-onset tobacco user. Thus, our findings are important for the development of educational programs for young persons, given that adolescents are more likely to use nicotine to manage their weight and are more likely to experience weight control issues later in life as a result of smoking [30,64,65]. Our findings also have important implications for interventions that target cholinergic mechanisms (such as nicotine replacement therapies) that may produce long-term effects on food intake and weight gain. Future studies are needed to further examine the underlying neurobiology that modulates the behavioral effects observed in the present study.
HIGHLIGHTS.
Adolescent rats display strong rewarding effects of nicotine.
Adolescent rats display diminished anorectic effects of nicotine.
Adolescent nicotine exposure enhances intake of this drug in adulthood.
Adolescent nicotine exposure reduces the anorectic effects of this drug in adulthood.
Acknowledgements
The authors thank Jesus A. Orona, Francisco Roman, and Hugo A. Tejeda for their technical assistance. This research was supported by the National Institute on Drug Abuse (R01-DA021274; LEO), the Diversity-promoting Institutions Drug Abuse Research Program (UTEP-R24-DA029989 and Drew-R24-DA017298), and the Research Experience for Undergraduates Training Program (R25-DA033613). This project was also supported by the American Diabetes Association (7-12-BS-135) and The National Institute of Minority Health Disparities (G12MD007592) as part of the UTEP Border Biomedical Research Center. Dr. Luis Natividad was supported by the NIH Ruth L. Kirschstein Fellowship Program (F31-DA021133) and the APA Diversity Program in Neuroscience (T32-MH018882).
References
- 1.Brook JS, Saar NS, Zhang C, Brook DW. Familial and non-familial smoking: effects on smoking and nicotine dependence. Drug Alcohol Dependence. 2008;101:62–8. doi: 10.1016/j.drugalcdep.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janson H. Longitudinal patterns of tobacco smoking from childhood to middle age. Addiction Behavior. 1999;24:239–49. doi: 10.1016/s0306-4603(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 3.Riggs NR, Chou CP, Li C, Pentz MA. Adolescent to emerging adulthood smoking trajectories: when do smoking trajectories diverge, and do they predict early adulthood nicotine dependence. Nicotine Tobacco Research. 2007;9:1147–54. doi: 10.1080/14622200701648359. [DOI] [PubMed] [Google Scholar]
- 4.Taioli E, Wynder EL. Effect of the age at which smoking begins on frequency of smoking in adulthood. New England Journal of Medicine. 1991;325:968–9. doi: 10.1056/NEJM199109263251318. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control Prevention Current tobacco use among middle and high school students—United States, 2011. Morbidity and Mortality Weekly Report. 2012;61:581–5. [PubMed] [Google Scholar]
- 6.Centers for Disease Control Prevention Current Cigarette Smoking Among Adults—United States, 2011. Morbidity and Mortality Weekly Report. 2012;61:889–901. [PubMed] [Google Scholar]
- 7.Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review of Psychology. 2004;55:463–91. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- 8.Corrigall WA, Zack M, Eissenberg T, Belsito L, Scher R. Acute subjective and physiological responses to smoking in adolescents. Addiction. 2001;96:1409–17. doi: 10.1046/j.1360-0443.2001.961014095.x. [DOI] [PubMed] [Google Scholar]
- 9.Hurt RD, Croghan GA, Beede SD, Wolter TD, Croghan IT, Patten CA. Nicotine patch therapy in 101 adolescent smokers: efficacy, withdrawal symptom relief, and carbon monoxide and plasma cotinine levels. Archives of Pediatrics and Adolescent Medicine. 2000;154:31–7. [PubMed] [Google Scholar]
- 10.McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics. 2000;96:671–7. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Curry SJ, Peterson AV, Mann SL. Investigation of first opportunities to use cigarettes and smokeless tobacco. Health Education Resource. 1989;4:27–34. [Google Scholar]
- 12.Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug and Alcohol Dependence. 2000;59:S41–60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 13.Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–9. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- 14.Pomerleau CS, Pomerleau OF, Namenek RJ, Marks JL. Initial exposure to nicotine in college-age women smokers and never-smokers: a replication and extension. Journal of Addictive Disorder. 1999;18:13–9. doi: 10.1300/J069v18n03_02. [DOI] [PubMed] [Google Scholar]
- 15.Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–95. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 16.Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. Journal of Pharmacology and Experimental Therapeutics. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- 17.Shram MJ, Funk D, Li Z, Lê AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186:201–8. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- 18.Shram MJ, Lê AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behavioral Brain Research. 2010;206:240–4. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology and Behavior. 2002;77:107–14. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 20.Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology. 2009;206:303–12. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres OV, Tejeda HA, Natividad LA, O'Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacology. Biochemistry and Behavior. 2008;90:658–63. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adriani W, Macrì S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–24. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 23.Levin ED, Slade S, Wells C, Cauley M, Petro A, Vendittelli A, et al. Threshold of adulthood for the onset of nicotine self-administration in male and female rats. Behavioral Brain Research. 2011;225:473–81. doi: 10.1016/j.bbr.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV. Pre-exposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology. 2006;184:382–90. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- 25.Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, et al. Evidence for enhanced neurobehavioral vulnerability to nicotine during peri-adolescence in rats. Journal of Neuroscience. 2003;23:4712–6. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kota D, Robinson SE, Damaj IM. Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochemical Pharmacology. 2009;78:873–9. doi: 10.1016/j.bcp.2009.06.099. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Saad S, Sandow SL, Bertrand PP. Cigarette smoking and brain regulation of energy homeostasis. Frontiers in Neuropharmacology. 2012;3:147. doi: 10.3389/fphar.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heishman SJ. Behavioral and cognitive effects of smoking: relationship to nicotine addiction. Nicotine and Tobacco Research. 1999;1:S143–7. doi: 10.1080/14622299050011971. [DOI] [PubMed] [Google Scholar]
- 29.Perkins KA. Effects of tobacco smoking on caloric intake. British Journal of Addiction. 1992;87:193–205. doi: 10.1111/j.1360-0443.1992.tb02693.x. [DOI] [PubMed] [Google Scholar]
- 30.Fulkerson JA, French SA. Cigarette smoking for weight loss or control among adolescents: gender and racial/ethnic differences. Journal of Adolescent Health. 2003;32:306–13. doi: 10.1016/s1054-139x(02)00566-9. [DOI] [PubMed] [Google Scholar]
- 31.Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, et al. Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obesity Research. 2005;13:1466–75. doi: 10.1038/oby.2005.177. [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Chen WQ, David SP, Tyndale RF, Wang H, Chen YM, et al. Interaction between heavy smoking and CYP2A6 genotypes on type 2 diabetes and its possible pathways. European Journal of Endocrinology. 2011;165:961–7. doi: 10.1530/EJE-11-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman TC, Sinha-Hikim I, Parveen M, Najjar SM, Liu Y, Mangubat M, et al. Additive effects of nicotine and high-fat diet on hepatic steatosis in male mice. Endocrinology. 2012;153:5809–20. doi: 10.1210/en.2012-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacology. Biochemistry and Behavior. 2001;70:475–89. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro-Carvalho A, Lima CS, Medeiros AH, Siqueira NR, Filgueiras CC, Manhães AC, et al. Combined exposure to nicotine and ethanol in adolescent mice: effects on the central cholinergic systems during short and long term withdrawal. Neuroscience. 2009;162:1174–86. doi: 10.1016/j.neuroscience.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro-Carvalho A, Lima CS, Nunes-Freitas AL, Filgueiras CC, Manhães AC, Abreu-Villaça Y. Exposure to nicotine and ethanol in adolescent mice: effects on depressive-like behavior during exposure and withdrawal. Behavioral Brain Research. 2011;221:282–9. doi: 10.1016/j.bbr.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Slawecki CJ, Gilder A, Roth J, Ehlers CL. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacology, Biochemistry, and Behavior. 2003;75:355–61. doi: 10.1016/s0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 38.O'Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, et al. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology. 2006;186:612–9. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- 39.Natividad LA, Tejeda HA, Torres OV, O'Dell LE. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64:136–45. doi: 10.1002/syn.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32:700–9. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- 41.Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61:189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maehler R, Dadmarz M, Vogel WH. Determinants of the voluntary consumption of nicotine by rats. Neuropsychobiology. 2000;41:200–4. doi: 10.1159/000026660. [DOI] [PubMed] [Google Scholar]
- 43.Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Annals of the New York Academy of Sciences. 2004;1021:462–4. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- 44.Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacology, Biochemistry, and Behavior. 2004;78:13–25. doi: 10.1016/j.pbb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Cao J, Belluzzi JD, Loughlin SE, Dao JM, Chen Y, Leslie FM. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacology, Biochemistry and Behavior. 2010;96:82–90. doi: 10.1016/j.pbb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Adolescent and adult female rats differ in sensitivity to nicotine's activity effects. Pharmacology, Biochemistry and Behavior. 2005;80:567–75. doi: 10.1016/j.pbb.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine's activity effects. Pharmacology, Biochemistry, and Behavior. 2003;74:917–31. doi: 10.1016/s0091-3057(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 48.Abreu-Villaça Y, Queiroz-Gomes Fdo E, Dal Monte AP, Filgueiras CC, Manhães AC. Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behavioral Brain Research. 2006;167:175–82. doi: 10.1016/j.bbr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Gyekis J, Foreman JE, Anthony K, Klein LC, Vandenbergh DJ. Activity-related behaviors in the hole-board predict nicotine consumption in C57B6 mice perinatally exposed to nicotine. Behavioral Brain Research. 2010;206:139–42. doi: 10.1016/j.bbr.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 50.Doremus-Fitzwater TL, Barreto M, Spear LP. Age-related differences in impulsivity among adolescent and adult Sprague-Dawley rats. Behavioral Neuroscience. 2012;126:735–41. doi: 10.1037/a0029697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRFCRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proceedings of the National Academy of Sciences of the USA. 2007;104:17198–203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Dell LE, Koob GF. ’Nicotine deprivation effect’ in rats with intermittent 23-h access to intravenous nicotine self-administration. Pharmacology, Biochemistry, and Behavior. 2007;86:346–53. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chistyakov V, Patkina N, Tammimäki A, Talka R, Salminen O, Belozertseva I, et al. Nicotine exposure throughout early development promotes nicotine self-administration in adolescent mice and induces long-lasting behavioural changes. European Journal of Pharmacology. 2010;640:87–93. doi: 10.1016/j.ejphar.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 54.Nesil T, Yararbas G, Mola G, Kanit L, Pogun S. Previous chronic exposure eliminates the conditioning effect of nicotine in rats. Brain Research Bulletin. 2011;85:339–45. doi: 10.1016/j.brainresbull.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Chen H, Vlahos R, Bozinovski S, Jones J, Anderson GP, Morris MJ. Effect of short-term cigarette smoke exposure on body weight, appetite and brain neuropeptide Y in mice. Neuropsychopharmacology. 2005;30:713–9. doi: 10.1038/sj.npp.1300597. [DOI] [PubMed] [Google Scholar]
- 56.Dandekar MP, Nakhate KT, Kokare DM, Subhedar NK. Effect of nicotine on feeding and body weight in rats: involvement of cocaine- and amphetamine-regulated transcript peptide. Behavioral Brain Research. 2011;219:31–8. doi: 10.1016/j.bbr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF. The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating and weight. Physiology and Behavior. 2011;104:143–8. doi: 10.1016/j.physbeh.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hur YN, Hong GH, Choi SH, Shin KH, Chun BG. High fat diet altered the mechanism of energy homeostasis induced by nicotine and withdrawal in C57BL/6 mice. Molecules and Cells. 2010;30:219–26. doi: 10.1007/s10059-010-0110-3. [DOI] [PubMed] [Google Scholar]
- 59.Lamota L, Bermudez-Silva FJ, Marco EM, Llorente R, Gallego A, Rodríguez de Fonseca F, et al. Effects of adolescent nicotine and SR 147778 (Surinabant) administration on food intake, somatic growth and metabolic parameters in rats. Neuropharmacology. 2008;54:194–205. doi: 10.1016/j.neuropharm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Nolley EP, Kelley BM. Adolescent reward system perseveration due to nicotine: studies with methylphenidate. Neurotoxicology and Teratology. 2007;29:47–56. doi: 10.1016/j.ntt.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 61.Wilmouth CE, Spear LP. Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacology, Biochemistry, and Behavior. 2006;85:648–57. doi: 10.1016/j.pbb.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen H, Hiler KA, Tolley EA, Matta SG, Sharp BM. Genetic factors control nicotine self-administration in isogenic adolescent rat strains. PLoS One. 2012;7:e44234. doi: 10.1371/journal.pone.0044234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitchell MR, Mendez IA, Vokes CM, Damborsky JC, Winzer-Serhan UH, Setlow B. Effects of developmental nicotine exposure in rats on decision-making in adulthood. Behavioral Pharmacology. 2012;23:34–42. doi: 10.1097/FBP.0b013e32834eb04a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saules KK, Levine MD, Marcus MD, Pomerleau CS. Differences in smoking patterns among women smokers with childhood versus later onset of weight problems. Eating Behavior. 2006;8:418–22. doi: 10.1016/j.eatbeh.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saules KK, Pomerleau CS, Snedecor SM, Brouwer RN, Rosenberg EE. Effects of disordered eating and obesity on weight, craving, and food intake during ad libitum smoking and abstinence. Eating Behavior. 2004;5:353–63. doi: 10.1016/j.eatbeh.2004.04.011. [DOI] [PubMed] [Google Scholar]