Abstract
Electrical stimulation of the brain was one of the first experimental methods applied to understanding brain organization and function and it continues as a highly useful method both in research and clinical applications. Intracortical microstimulation (ICMS) involves applying electrical stimuli through a microelectrode suitable for recording the action potentials of single neurons. ICMS can be categorized into single pulse stimulation, high frequency, short duration stimulation and high frequency, long duration stimulation. For clinical and experimental reasons, considerable interest focuses on the mechanism of neural activation by electrical stimuli. In this paper, we discuss recent results suggesting that action potentials evoked in cortical neurons by high frequency electrical stimulation do not sum with the natural, behaviorally related background activity; rather, high frequency stimulation eliminates and replaces natural activity. We refer to this as neural hijacking. We propose that a major component of the mechanism underlying neural hijacking is excitation of axons by ICMS and elimination of natural spikes by antidromic collision with stimulus driven spikes evoked at high frequency. Evidence also supports neural hijacking as an important mechanism underlying the action of deep brain stimulation (DBS) in the subthalamic nucleus and its therapeutic effect in treating Parkinson’s disease.
Keywords: neural hijacking, electrical stimulation, motor cortex, EMG, ICMS
Electrical stimulation has a long history dating back to the 17th century and has contributed enormously to our understanding of localization of function in the brain. While many of the very earliest methods of brain stimulation were relatively crude, over the years the methods have become more refined with the capability to deliver more localized stimulation and activation of neurons. An important advance in brain electrical stimulation came in the late 1960’s when Asanuma and colleagues (Asanuma and Sakata 1967, Asanuma and others 1968, Stoney and others, 1968) introduced intracortical microstimulation (ICMS) as a method of activating neurons near the tip of a microelectrode designed to record the action potential activity of single cells. Microstimulation has grown into a very important tool for the investigation of neural circuit organization and function throughout the brain. Microstimulation may also become an important component of brain machine interface devices particularly for restoring function in damaged sensory systems. Despite its importance as a research tool, our understanding of the actions of microstimulation on neural circuits is incomplete and represents an important and continuing challenge. This paper will focus on recent work that has revealed a fundamental aspect of the way high frequency microstimulation interacts with cortical circuits. We have termed this phenomenon “neural hijacking” (Griffin and others 2011).
Forms of microstimulation
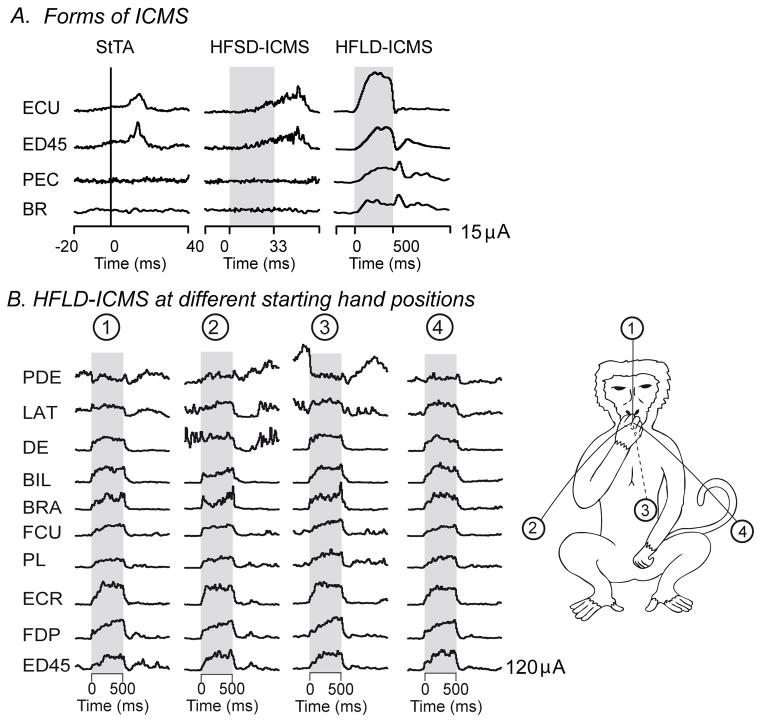
As implemented by Asanuma and colleagues (Asanuma and Sakata 1967, Asanuma and others 1968, Asanuma and Rosen 1972), ICMS is applied as a short train of high frequency pulses (for example, 11 pulses at 400 Hz, 25 ms train duration) capable of producing twitch-like movements when applied to motor areas of cerebral cortex. We refer to this form of microstimulation as high frequency, short duration ICMS (HFSD-ICMS). This form of ICMS elicits muscle twitches and has been used extensively for mapping motor areas of the brain (Cheney 2002; Dancause and others 2008; Preuss and others 1996). While HFSD-ICMS remains a highly useful approach for many purposes, new variations of ICMS have been introduced (Figure 1A). One major variation is termed single pulse microstimulation where stimuli are delivered at a rate low enough to preclude significant temporal summation of EPSPs and IPSPs (20 Hz or less). Because it limits physiological spread of the stimulus by avoiding summation of EPSPs, this method yields the most localized activation of neurons. A subtype of single pulse stimulation introduced by Cheney and Fetz (1985) and used to investigate the organization of output from motor areas of the brain is termed stimulus-triggered averaging of electromyographic (EMG) activity (StTA). This is a sub-threshold form of stimulation since there is no observable motor response corresponding to the individual stimuli. Nevertheless, each stimulus activates neural elements at the site of cortical stimulation, including neurons with synaptic linkages to motoneurons. The resulting EPSPs, although sub-threshold, will nevertheless raise the firing probability of synaptically linked motoneurons. This transient increase in firing probability is time locked to the stimulus and, although weak, can be reliably detected using signal averaging. EMG segments associated with a large number of individual stimuli are full-wave rectified and averaged. The EMG record represents the summation of action potential trains from many individual motor units. Averaging EMG segments associated with a large number of individual stimuli will reveal the transient increase in firing probability of motor units associated with the underlying EPSPs in motoneurons. This method has been used extensively to quantify and compare the properties of motor output from primary and secondary cortical motor areas as well as brainstem motor nuclei (Cheney and others 1991; Davidson and Buford 2004; Boudrias and others 2009, 2010; Widener and Cheney 1997). Although physiological spread of stimulus produced excitation with this method should be more limited than high frequency stimulation methods, Histed and others (2009) showed that even single low intensity stimuli (10 μA) can activate neurons up to 4 mm from the site of stimulation.
Figure 1.
Figure 1A. Forms of intracranial microstimulation (ICMS). Stimulus triggered averaging of EMG activity (StTA), high frequency, short duration ICMS (HFSD-ICMS), high frequency, long duration ICMS (HFLD-ICMS). Note the difference in time scales for each type of ICMS. Stimulus evoked responses in EMG activity obtained with each type of ICMS at one cortical site. StTA is a subthreshold method in that no movements are evoked. Stimuli are delivered at a low rate (20 Hz or less) to avoid temporal summation of excitatory postsynaptic potentials (EPSPs). Averages are aligned with stimuli occurring at zero time. With the two high frequency methods, stimuli are typically applied at 100–400 Hz for either 500–1000 ms (HFLD-ICMS) or as a train of 10–15 pulses (HFSD-ICMS) indicated by the shading. B. HFLD-ICMS applied at one cortical site with the hand at different starting positions indicated by the monkey drawing at the right. The circled numbers indicate four different starting positions of the hand when stimulation was applied. In all cases, stimulation drove the hand to a common end-point at the mouth. Stimulus intensity was 120μA for all records. For each muscle, note the similarity in the EMG level achieved during stimulation across all initial starting positions.
Another major variation of the original microstimulation method involves applying a high frequency stimulus train, like the original method, but extending the duration to 500–1000 ms to more closely match the duration of natural voluntary movements (Graziano and others 2002). A unique characteristic of this form of microstimulation is that, when delivered to a single cortical site, it produces movements to a common end-point position regardless of the starting position of the limb (Graziano and others 2002 and Figure 2C). We refer to this form of microstimulation as high frequency, long duration ICMS (HFLD-ICMS).
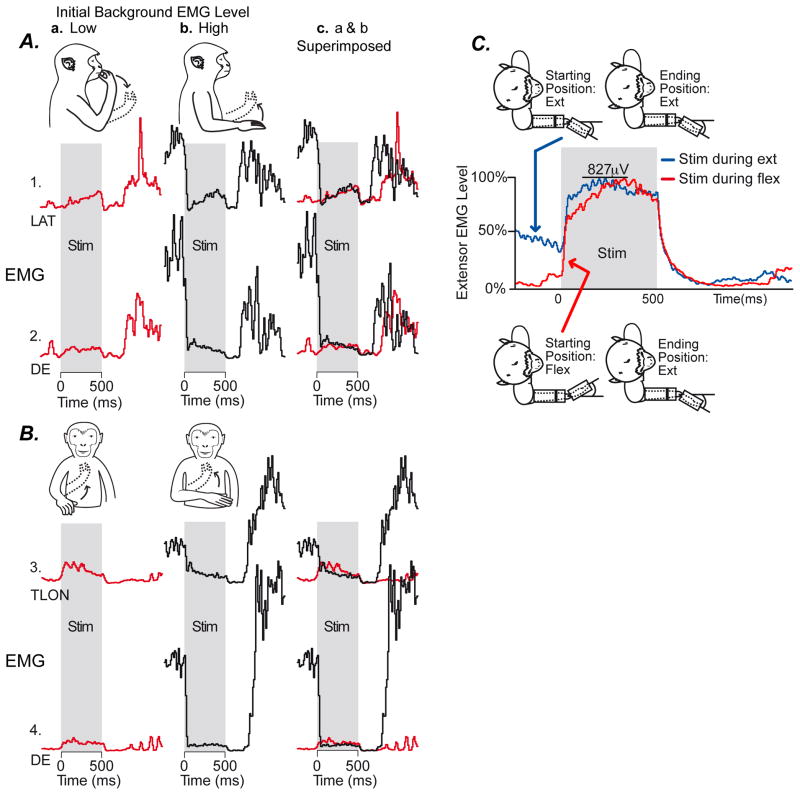
Figure 2.
Examples demonstrating hijacking of cortical output associated with HFLD-ICMS of motor cortex. Panels A and B. Data from two different cortical sites in which stimulation at one initial arm posture (column a, monkey drawing, solid line) increased EMG activity whereas stimulation at a different starting arm position (column b) produced a robust decrease in EMG activity. Column c superimposes the records from columns a and b. Note that stimulation (shading) drives EMG activity to the same final level regardless of the natural, voluntary movement related level of activity before stimulation. Arrows in the monkey drawings indicate the direction of movement evoked by stimulation. LAT (latissimus dorsi), DE (dorsoepitrochlearis), TLON (triceps long head). Panel C. Example in which stimulation produced an increase in EMG activity at two different starting wrist positions. Average of forelimb extensor muscles (EDC (extensor digitorum communis), ED2&3 (extensor digitorum 2,3), ED&5 (extensor digitorum 4,5), ECR (extensor carpi radialis), ECU (extensor carpi ulnaris)) is shown for stimulation during wrist flexion (red) and extension (blue). Again the EMG activity level produced by stimulation was the same regardless of the voluntary movement related pre-stimulus level. This example further illustrates the lack of summation of stimulus evoked EMG activity with natural voluntary movement related activity in a case where stimulation only produced increases in activity. (Modified from Griffin and others, 2011)
What is neural hijacking?
Hijacking is defined as the act of taking over or seizing something and forcing it to go a different direction or using it for a different purpose. Recent work has suggested that this term appropriately describes the interaction between high frequency electrical stimulation and at least some neural circuits. Consider the possible ways in which activity evoked by electrical stimulation might interact with natural, behaviorally related ongoing neural activity. A natural assumption might be that activity evoked by electrical stimulation would sum with ongoing, natural activity. However, our recent work suggests that summation is not the rule (Griffin and others 2011). Rather, high frequency electrical stimulation seems to eliminate and replace ongoing natural activity. We refer to this as “neural hijacking”.
Evidence for Neural Hijacking from microstimulation of motor cortex
Analysis of EMG records associated with high frequency microstimulation (HFLD-ICMS) of motor cortex in rhesus monkeys provides evidence in support of neural hijacking (Griffin and others 2011). In these experiments, EMG recordings are obtained from numerous forelimb muscles while HFLD-ICMS is applied to layer V sites in primary motor cortex (Figure 1B). The concurrently recorded EMG activity can be used as a surrogate for the activity of cortical output neurons. The existence of abundant monosynaptic connections from corticospinal neurons to motoneurons in old world primates adds credence to this approach (Porter and Lemon 1993). The results demonstrate that HFLD-ICMS evoked EMG activity, which reflects stimulus-evoked changes in the underlying level of cortical output activity, does not sum with the background movement related activity. Instead, it eliminates and replaces the natural background activity with activity that is purely stimulus driven. In these experiments, the monkey performs a task requiring reaching to specific locations in the work space as illustrated by the drawings in Figure 1B and Figure 2A and B. The EMG records show the level of activity associated with stimulation (shading) as well as the voluntary EMG level both before and after stimulation. The results for two cortical sites are detailed in Figure 2A and B. The solid lines in the monkey drawings indicate the starting position of the right arm and the dotted lines indicate the final HFLD-ICMS driven end-point position. Note that, preceding stimulation, a subset of starting hand positions (column a) were associated with a low level of voluntary EMG activity while a second subset of starting positions (column b) were associated with a relatively high level of EMG activity. At both cortical sites, HFLD-ICMS produced a small increase in muscles with a low level of EMG activity (column a). These two cortical sites thus appear to have a weak excitatory effect on motoneurons of the muscles illustrated. In contrast, at the starting positions where initial voluntary EMG activity was high (column b), the same stimulation produces a sharp decrease in EMG activity in the same muscles. Although this appears to be a potent inhibitory effect, and might suggest that the stimulation switches from excitatory to inhibitory depending on the initial posture of the limb, superimposing records from columns a and b reveals that, while stimulation increases activity in one case and decreases it in the other, the level of stimulus driven EMG activity attained in both cases is nearly identical (column c). This result suggests that it is incorrect to interpret the increases and decreases in EMG activity as excitatory and inhibitory respectively. Rather, high frequency stimulation drives EMG activity to a specific stimulus dependent level, independent of the initial limb posture and background level of EMG activity. Examination of 41 antagonistic sites of this type showed the same result (Griffin and others 2011). HFLD-ICMS drove EMG activity to a specific level regardless of the initial level of activity. Clearly, in cases like this, stimulus driven activity does not sum with the existing voluntary movement related activity.
In addition to the examples of opposing responses illustrated in Figures 2A and 2B, there were many more cortical site-muscle pairs (557) where stimulation produced either an increase or a decrease in EMG activity at all initial arm postures. However, even at these sites, the same basic principle explains the results. Stimulus-evoked activity did not sum with ongoing background activity; rather, it eliminated and replaced the background activity. An example is given in Figure 2C. In this case the monkey is performing a simple wrist movement task requiring movement between two target zones; one in flexion and one in extension. Stimulation was applied during either wrist flexion (red record) or extension (blue record) as indicated by the monkey drawings. EMGs from individual wrist extensor muscles (EDC, ED2,3, ED4,5, ECR and ECU) have been summed together into a composite record although individual muscles showed the same result. Note that whether stimulation is initiated with the wrist in flexion or extension, EMG activity achieves the same level during stimulation (827μV) despite a large difference in the pre-stimulus level of activity. Again, stimulus driven EMG activity does not sum with the level of EMG activity driven by the internal motor program for voluntary movement. If summation had occurred, the EMG level attained when stimulation was applied during wrist extension should have been the sum of the change in EMG activity with the wrist in flexion (~745μV) and the pre-stimulus level with the wrist in extension (~276μV). The sum of these is 1,021μV but instead the maximum EMG level achieved was 827μV – the same whether the wrist was in extension (high initial EMG level) or flexion (low initial EMG level).
Figure 1B provides further examples of HFLD-ICMS driving EMG activity of different muscles to the same level regardless of the starting posture of the arm. Noteworthy is DE which shows increases with stimulation for positions 1, 3 and 4. However, when stimulation was applied at starting position 2, where background at the onset of stimulation was equal to the stimulus driven level, no change in EMG level occurred.
Could the stimulus-evoked changes in EMG activity be, at least in part, a voluntary response to the stimulus? This possibility can be rejected because the changes have an onset that in most cases is well under 100 ms and much shorter than a voluntary reaction time (Griffin and others 2011). It is also noteworthy that at no time during stimulation did the EMG level drop an amount equal to the voluntary level that existed prior to stimulation - a result that would have suggested initial summation of stimulus driven activity with the existing voluntary activity followed by the monkey “letting go” during stimulation. Yet at the end of stimulation EMG activity quickly fell to zero indicating that somewhere during stimulation the voluntary internal motor program had, in fact, been terminated. The fact that no corresponding drop in EMG activity occurred during stimulation is further evidence that neural output had been hijacked and stimulus driven activity had completely replaced natural voluntary activity.
Mechanism of Neural Hijacking with Electrical Stimulation
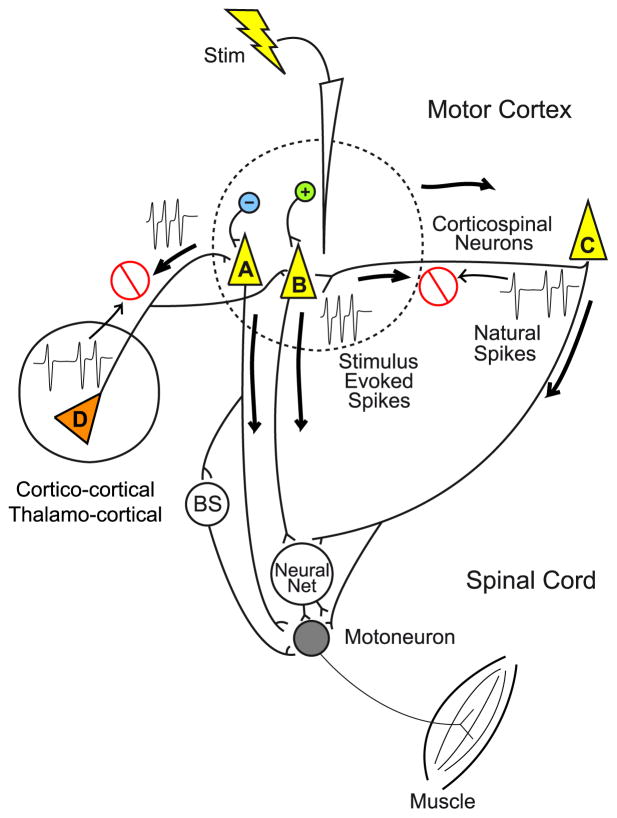
Our findings suggest that high frequency ICMS hijacks natural cortical output activity and replaces it with stimulus-evoked activity. Summation of natural activity with stimulus driven activity does not occur. Possible mechanisms that might explain these results are illustrated in Figure 3. The mechanism we favor has also been heavily implicated in explaining the effects of stimulating the subthalamic nucleus with DBS electrodes (Garcia and others 2005a,b). Extensive evidence supports the contention that axons have a lower threshold for activation by electrical stimulation than cell bodies (Nowak and Bullier 1998a; 1998b; Tehovnik and others 2006). Locations of direct excitation would most likely be axonal including initial segments and nodes of Ranvier with axonal nodes of Ranvier dominating (Nowak and Bullier 1998b). This is consistent with a large body of evidence supporting the conclusion that corticospinal output neurons are activated indirectly (synaptically) by ICMS rather than directly (Jankowska and others 1975). However, at the relatively high intensities of stimulation used for HFLD-ICMS in this study (100–200 μA), direct activation of both axons and cell bodies within a sphere surrounding the electrode tip would be expected (dotted line in Figure 3) (Jankowska and others 1975).
Figure 3.
Proposed mechanism of neural hijacking associated with HFLD-ICMS of motor cortex. High frequency stimulation activates axon terminals in the vicinity of stimulation (dotted line) as well as some corticospinal output cells directly (neurons A & B). Stimulation of axon terminals results in antidromic activation (neuron C) in which the stimulus evoked spikes collide with and eliminate the background naturally occurring spikes. This mechanism of elimination by collision potentially occurs with all afferent inputs to motor cortex including those from secondary cortical motor areas as well as thalamic inputs (neuron D). The stimulus driven spikes elicited in axon terminals also conduct orthodromically to activate output cells (A&B). At the higher stimulus strengths generally used with HFLD-ICMS, some corticospinal output cells are probably also activated directly. In either case, natural activity has been eliminated and replaced with spikes that are entirely stimulus driven. Neural Net refers to the spinal cord network mediating non-monosynaptic input to motoneurons from cortex and other sources. Brainstem (BS) pathways include reticulospinal and rubrospinal. (Modified from Griffin and others, 2011)
The neural elements activated and the mode of activation must explain why there is little or no summation of natural activity with stimulus-evoked activity. The lack of summation must result from a mechanism that eliminates natural synaptic input to corticospinal output neurons. The complete elimination of natural synaptic input amounts to what is essentially a reversible, localized cortical de-afferentation. We propose that an important component of the mechanism underlying this phenomenon is direct activation of axons, including the terminations of afferent inputs to corticospinal output neurons. The stimulus-evoked spikes then propagate both antidromically and orthodromically from the site of activation. Antidromic spikes will collide with naturally occurring movement related spikes as illustrated by neurons C and D in Figure 3. What happens to the orthodromically conducted spikes? These spikes will propagate to synaptic terminations, some of which will be on corticospinal output neurons resulting in EPSPs, and in the case of GABA inhibitory axons - IPSPs. The important point is that, even though it is synaptic activation of cortical output neurons, the afferent input is derived entirely from stimulus driven spikes, rather than naturally occurring spikes. As noted above, stimulation is also likely to produce direct activation of corticospinal output neurons through excitation at the neuron’s initial segment. Spikes generated at the initial segment will conduct orthodromically to the neuron’s post-synaptic targets as well as back into the cell body. If the rate of stimulation is high relative to the firing rate generated by synaptic input, the stimulus generated spikes are likely to dominate the activity of the cell. However, as discussed above, even the synaptic inputs to the cell are likely to be largely stimulus driven. It is unknown whether action potential generation in the cell follows 1-for-1 with each stimulus of the high frequency train or if it degrades to a frequency less than the stimulus rate but still a harmonic of the stimulus rate (Garcia and others 2005a).
What other mechanisms could occur with stimulation of layer V of motor cortex? The existence of a potent GABA inhibitory network in motor cortex is well known (Hendry and Jones 1981). In addition to activation of excitatory axons, activation of the axons of GABA inhibitory neurons (Figure 3, blue neuron) is also likely, especially with the higher intensities we have used for HFLD-ICMS. This activity will produce IPSPs in target neurons and further reduce the potential for any non-stimulus driven spike activity.
What parameters of HFLD-ICMS are effective in producing hijacking? We investigated this in two monkeys by applying ICMS at different frequencies, durations and magnitudes to determine parameters that would evoke complete and reproducible movements to a common, stable end-point position regardless of starting position. These stimulus-evoked movements are characteristic of neural hijacking. The effective parameters were frequencies ranging from 90–150 Hz and intensities ranging from 90–150 μA. Although hijacking occurs with much shorter train durations, movement to a stable end-point position was optimal with trains one second in duration (Van Acker and others 2010). An important characteristic of HFLD-ICMS, consistent with neural hijacking, is that movements at the affected joints are entirely stimulus driven and muscles at these joints seem to become inaccessible to voluntary control. At some sites this involves only muscles of distal joints while at other sites it only involves muscles at proximal joints. The joints affected by HFLD-ICMS appear locked in a stable position by stimulation while the monkey retains voluntary control of other joints. For example, the shoulder and elbow joints might be locked in a particular posture by stimulation but the monkey retains use of its wrist and digits and vice versa. This result has implications for the extent of spread of stimulus driven activity through motor circuits of the brain. It is clear that the monkey can still initiate and execute movements at joints beyond those directly represented at the site of stimulation. Therefore, stimulation does not produce a generalized “freezing” or paralyzing effect where execution of all voluntary movements is blocked. The ability to conceive of and program movements remains intact as does the ability to execute movements at joints not directly affected by stimulation.
Although neural hijacking seems to remain confined to somatotopic boundaries of the site stimulated, how far does it spread in the cortex and elsewhere within the brain? Physical spread of excitation from currents in the range of 90–150 μA is small relative to the total area of the M1 forelimb representation. However, physiological spread could greatly extend the range of neural hijacking to potentially include the entire distal or proximal forelimb cortical representation (Griffin and others 2011). In addition, high frequency antidromic activation of axons terminating in motor cortex from other cortical areas and from thalamus would also be subject to neural hijacking (Figure 3, Montgomery and Gale 2008). Again, it is interesting to note that even though neural hijacking is likely to spread to remote structures such as the thalamus, the effects on movement remained confined to joints represented at the M1 site stimulated. Therefore, neural hijacking seems to obey major somatotopic boundaries. A similar conclusion was emphasized by Stepniewska and others (2011) based on optical imaging of cortical areas activated by HFLD-ICMS in anesthetized galagos.
Finally, there is considerable evidence that neural hijacking is not a phenomenon limited to the cerebral cortex. Garcia and others (2003), using slice preparations, showed that high frequency stimulation forces subthalamic neurons to discharge spikes time-locked to the stimulation. The mechanism described to explain their results is very similar to the neural hijacking mechanism described above. For instance, they state that high frequency stimulation “is expected to activate subsets of both afferent and efferent axons, leading to antidromic spikes that collide with ongoing spontaneous ones and orthodromic spikes that evoke synaptic responses in target neurons” (Hammond and others 2008). In a related paper, Garcia and others (2005) state: “HFS not only suppresses pathological STN activity but also imposes a new activity on STN neurons. This is not simply excitation (spikes evoked among spontaneous ones) but rather total replacement of the pathological activity of STN neurons [associated with Parkinson’s disease] by a new HFS-driven pattern”.
In conclusion, high frequency electrical stimulation does not sum with the natural, behaviorally related background activity; rather, HFLD-ICMS eliminates and replaces natural activity. We refer to this as neural hijacking. Neural hijacking can produce outcomes in which it appears that stimulation of a site in motor cortex is excitatory under some conditions and inhibitory in others, but, in both cases, stimulation has simply eliminated natural activity and replaced it with activity that is solely stimulus driven. We propose that a major component of the mechanism underlying neural hijacking is excitation of axons by ICMS and elimination of natural spikes by antidromic collision with stimulus driven spikes evoked at high frequency.
Acknowledgments
The authors thank Ian Edwards for technical assistance.
This work was supported by NIH Grants NS051825 (PDC), NIH NS064054 (PDC), NIH Center Grant HD02528 (PDC) and KUMC Biomedical Research Training Grant (DMG and GMAV).
References
- Asanuma H, Sakata H. Functional organization of a cortical efferent system examined with focal depth stimulation in cats. J Neurophysiol. 1967;30:35–54. [Google Scholar]
- Asanuma H, Stoney SD, Abzug C. Relationship between afferent input and motor outflow in cat motorsensory cortex. J Neurophysiol. 1968;31:670–681. doi: 10.1152/jn.1968.31.5.670. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Rosen I. Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res. 1972;14:243–256. doi: 10.1007/BF00816161. [DOI] [PubMed] [Google Scholar]
- Boudrias MH, Lee SP, Svojanovsky S, Cheney PD. Forelimb muscle representations and output properties of the motor areas in the mesial wall of rhesus macaques. Cerebral Cortex. 2010;20:704–719. doi: 10.1093/cercor/bhp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudrias MH, McPherson RL, Frost SB, Cheney PD. Output properties and organization of the forelimb representation of motor areas on the lateral aspect of the hemisphere in rhesus macaques. Cerebral Cortex. 2009;20:169–186. doi: 10.1093/cercor/bhp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol. 1985;53:786–804. doi: 10.1152/jn.1985.53.3.786. [DOI] [PubMed] [Google Scholar]
- Cheney PD. Electrophysiological Methods for Mapping Brain Motor Circuits. In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Methods. 2. New York, NY: Academic Press; 2002. [Google Scholar]
- Cheney PD, Mewes K, Widener G. Effects on wrist and digit muscle activity from microstimuli applied at the sites of rubromotoneuronal cells in primates. J Neurophysiol. 1991;66:1978–1992. doi: 10.1152/jn.1991.66.6.1978. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Mahnken JD, Nudo RJ. Interhemispheric connections of the ventral premotor cortex in a new world primate. J Comp Neurol. 2008;505:701–715. doi: 10.1002/cne.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed with stimulus-triggered averaging. J Neurophysiol. 2004;92:83–95. doi: 10.1152/jn.00083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L, Audin J, D’Alessandro D, Bioulac B, Hammond C. Dual effect of high-frequency stimulation on subthalamic neuron activity. J Neurosci. 2003;23:8743–8751. doi: 10.1523/JNEUROSCI.23-25-08743.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L, D’Alessandro G, Bioulac B, Hammond C. High-frequency stimulation in Parkinson’s disease: more or less? Trends in Neurosci. 2005a;28:209–216. doi: 10.1016/j.tins.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Garcia L, D’Alessandro G, Fernagut P, Bioulac B, Hammond C. Impact of high frequency stimulation parameters on the pattern of discharge of subthalamic neurons. J Neurophysiol. 2005b;94:3662–3669. doi: 10.1152/jn.00496.2005. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Griffin DM, Hudson HM, Belhaj-Saif A, Cheney PD. Hijacking cortical motor output with repetitive microstimulation. J Neurosci. 2011;31:13088–13096. doi: 10.1523/JNEUROSCI.6322-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Ammari R, Bioulac B, Garcia L. Latest View on the mechanism of action of deep brain stimulation. Movement Disorders. 2008;15:2111–2121. doi: 10.1002/mds.22120. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. Sizes and distributions of intrinsic neurons incorporating tritiated GABA in monkey sensory-motor cortex. J Neurosci. 1981;1:390–408. doi: 10.1523/JNEUROSCI.01-04-00390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 2009;63:508–522. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol. 1975;249:617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery EB, Gale JT. Mechanisms of action of deep brain stimulation (DBS) Neurosci and BioBehav Rev. 2008;32:388–407. doi: 10.1016/j.neubiorev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. I. Evidence from chronaxie measurements. Exp Brain Res. 1998a;118:477–488. doi: 10.1007/s002210050304. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. I. Evidence from selective inactivation of cell bodies and axon initial segments. Exp Brain Res. 1998b;118:489–500. doi: 10.1007/s002210050305. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Clarendon Press; Oxford: 1993. [Google Scholar]
- Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J Comp Neurol. 1996;371:649–676. doi: 10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Stoney SD, Jr, Thompson WD, Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol. 1968;31:659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Nat Acad Sci. 2011;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehovnik EJ, Tolias AS, Sultan F, Slocum WM, Logothetis NK. Direct and indirect activation of cortical neurons by electrical stimulation. J Neurophysiol. 2006;96:512–521. doi: 10.1152/jn.00126.2006. [DOI] [PubMed] [Google Scholar]
- Van Acker GM, III, Amundsen SL, Messamore W, Hudson HM, Zhang HY, Luchies CW, et al. Optimal stimulus parameters for mapping muscle synergies and associated movements evoked from M1 cortex with repetitive ICMS. Soc for Neurosci 2010 On-line Abstract. [Google Scholar]
- Widener GW, Cheney PD. Effects on muscle activity from microstimuli applied to somatosensory (SI) and motor cortex (MI) during voluntary movement in the monkey. J Neurophysiol. 1997;77:2446–2465. doi: 10.1152/jn.1997.77.5.2446. [DOI] [PubMed] [Google Scholar]