Abstract
Tribolium castaneum Transformer (TcTra) is essential for female sex determination and maintenance through the regulation of sex-specific splicing of doublesex (dsx) pre-mRNA. In females, TcTra also regulates the sex-specific splicing of its own pre-mRNA to ensure continuous production of functional Tra protein. Transformer protein is absent in males and hence dsx pre-mRNA is spliced in a default mode. The mechanisms by which males inhibit the production of functional Tra protein are not known. Here, we report on functional characterization of transformer-2 (tra-2) gene (an ortholog of Drosophila transformer-2) in T. castaneum. RNA interference-mediated knockdown in the expression of gene coding for tra-2 in female pupae or adults resulted in the production of male-specific isoform of dsx and both female and male isoforms of tra suggesting that Tra-2 is essential for the female-specific splicing of tra and dsx pre-mRNAs. Interestingly, knockdown of tra-2 in males did not affect the splicing of dsx but resulted in the production of both female and male isoforms of tra suggesting that Tra-2 suppresses female-specific splicing of tra pre-mRNA in males. This dual regulation of sex-specific splicing of tra pre-mRNA ensures a tight regulation of sex determination and maintenance. These data suggest a critical role for Tra-2 in suppression of female sex determination cascade in males. In addition, RNAi studies showed that Tra-2 is also required for successful embryonic and larval development in both sexes.
Keywords: Sex determination, RNAi, Transformer, Doublesex, Alternative splicing
1. Introduction
Sex in insects is determined by a wide range of mechanisms (Gempe and Beye, 2011; Saccone et al., 2002; Sanchez, 2008; Shearman, 2002). This diversity comes from the presence of different upstream signals (Verhulst et al., 2010). In Drosophila melanogaster the X:A ratio (X:A = 1) or the X chromosome dose acts as a signal to initiate the female determination cascade in XX embryos (Erickson and Quintero, 2007; Penalva and Sanchez, 2003). As a result, sex-lethal (sxl) is turned on, which later acts as a memory device for the female determination cascade (Keyes et al., 1992; Penalva and Sanchez, 2003). Sxl, besides regulating the female-specific splicing of its own pre-mRNA, also regulates the female-specific splicing of the pre-mRNA of transformer (tra), resulting in the production of functional Tra protein only in females (Boggs et al., 1987; Inoue et al., 1990). Transformer-2 (Tra-2), a protein containing a RNA Recognition Motif (RRM) domain and SR (serine/arginine) rich regions heterodimerizes with Tra and binds to exonic splicing enhancer (ESE) sequences present in the 4th exon (female-specific exon) of doublesex (dsx) pre-mRNA (Hoshijima et al., 1991; Lynch and Maniatis, 1996; Tian and Maniatis, 1992, 1993). This results in the assembly of spliceosomal complex at the adjacent 3′ splice site and, in turn, inclusion of exon 4 in the dsxf transcript. Due to the absence of Tra protein in males, the pre-mRNA of dsx splices in a default male mode resulting in the production of male-specific Dsx protein. Sex specific Dsx proteins (a transcription factor) differ at their C-terminal ends, hence sex-specifically regulate target genes to control different aspect of somatic sexual differentiation (Burtis and Baker, 1989; Burtis et al., 1991; Coschigano and Wensink, 1993; Erdman and Burtis, 1993; Erdman et al., 1996; Luo et al., 2011).
Dsx has been found to be involved in the sex determination cascade in all the insect species studied to date (Graham et al., 2003; Salz, 2011; Shukla and Nagaraju, 2010). In all insects except silk moths, Tra forms a conserved central axis of sex determination (Verhulst et al., 2010); downstream to tra is dsx and upstream to tra are diverse sex-determining signals. Tra-2 has been characterized in several dipterans including D. melanogaster, Musca domestica (Burghardt et al., 2005), Bactrocera oleae (Lagos et al., 2005), Anastrepha obliqua (Sarno et al., 2010), Ceratitis capitata (Salvemini et al., 2009), Lucilia cuprina (Concha and Scott, 2009), and Sciara ocellaris (Martin et al., 2011). Tra-2 has been identified in a hymenopteran [Apis mellifera (Nissen et al., 2012)] and in a lepidopteran insect [Bombyx mori (Niu et al., 2005; Suzuki et al., 2012)]. In all these insects tra-2 is expressed in a non-sex specific manner, producing the same protein in both males and females. Involvement of Tra-2 in the female-specific splicing of dsx pre-mRNA has been found to be conserved in all the dipteran and hymenopteran insect species studied. In some of the dipteran insects, M. domestica, C. capitata, Anastrepha suspensa, A. obliqua, and A. mellifera Tra-2 has been found to be involved in the female-specific splicing of tra pre-mRNA (Burghardt et al., 2005; Concha and Scott, 2009; Martin et al., 2011; Nissen et al., 2012; Salvemini et al., 2009; Sarno et al., 2010). In B. mori Tra-2 is not involved in the sex-specific splicing of dsx pre-mRNA, but it plays an essential role in the testis development (Suzuki et al., 2012). Recent report showed that Tra-2 in A. mellifera is required for the sex-specific splicing of tra pre-mRNA in both male and female. The authors of this publication also proposed a role for Tra-2 in embryonic development (Nissen et al., 2012).
Red flour beetle, Tribolium castaneum is a coleopteran model insect which follows XX/XY sex determination system (XX female and XY male). Recently, we characterized dsx and tra homologs (Tcdsx and Tctra) from this insect (Shukla and Palli, 2012a,b). Functional TcTra protein is produced only in females, which besides regulating the female-specific splicing of Tcdsx, also regulates the female-specific splicing of its own pre-mRNA. TcTra lacks any RNA-binding domain indicating the involvement of a co-factor (with RNA binding properties) that might be working with TcTra in the splicing of Tcdsx and Tctra pre-mRNA (Shukla and Palli, 2012b). The studies presented here identified and characterized such a protein, Transformer-2 homolog (Tctra-2), which is essential for sex-specific splicing of both Tctra and Tcdsx pre-mRNAs. This is the first study on identification and characterization of tra-2 homolog in a coleopteran insect. Unlike in dipteran insects but similar to that in A. mellifera (Nissen et al., 2012), Tra-2 is required for female-specific splicing of Tcdsx and Tctra pre-mRNAs, male-specific splicing of Tctra pre-mRNA and embryonic development. TcTra-2 also plays an important role in regulation of larval development in T. castaneum.
2. Material and methods
2.1. Insect rearing, RNA isolation and RT-PCR
Strain GA-1 of T. castaneum was used in all the studies presented here. Beetles were reared on organic whole wheat flour supplemented with 10% yeast. Sexing of pupa and adults was done on the basis of the presence of sex-specific structures described previously (Shukla and Palli, 2012b). Trizol (Invitrogen Corporation, USA) was used to isolate total RNA. Total RNA was DNAse treated, denatured at 75 °C for 5 min and then immediately chilled on ice. First strand cDNA was synthesized using MMLV reverse transcriptase (Invitrogen, USA), using the mixture of 17-mers polyT and random primers. PCR conditions with initial denaturation at 94 °C for 2 min, 32 cycles of 94 °C for 30s, 58°C for 30s, 72 °C for 2 min, and final extension at 72 °C for 10 min were used for amplification.
2.2. Sequence analysis
Exons and introns, of Tctra-2 were identified by aligning sequences of RT-PCR products with their corresponding genomic DNA sequences obtained from the Beetlebase (http://beetlebase.org/) and the NCBI (http://www.ncbi.nlm.nih.gov/). Exon–intron boundaries were confirmed by aligning the sequences through the Spidey program (http://www.ncbi.nlm.nih.gov/spidey/).
2.3. Developmental expression of Tctra-2
Under rearing conditions used, embryonic development is completed in about 70 h, final instar larval stage and pupal stages are completed in approximately 7 and 6 days respectively. Eggs laid by mated females were collected and staged. Total RNA was isolated from eggs sampled at 0–1, 5–6, 10–11, 15–16, 20–25, 30–35, 40–45, and 60–65 h after laying and from the newly hatched larvae. RNA was also isolated from several individual day 0 larvae, sex-separated day 0 pupae, and day 0 adults, and the RNAs were converted to cDNAs and used as templates to perform reverse transcriptase PCR (RT-PCR) using primers specific to the common region of Tctra-2 (Fig.1). T. castaneumrp49 was used as an endogenous control. Sex of larvae was determined by performing RT-PCR using cDNA made from RNA isolated from 10 individual larvae, as the template and Tcdsx primers [Tcdsx is sex-specifically spliced to produce three female and one male-specific isoforms (Shukla and Palli, 2012b)].
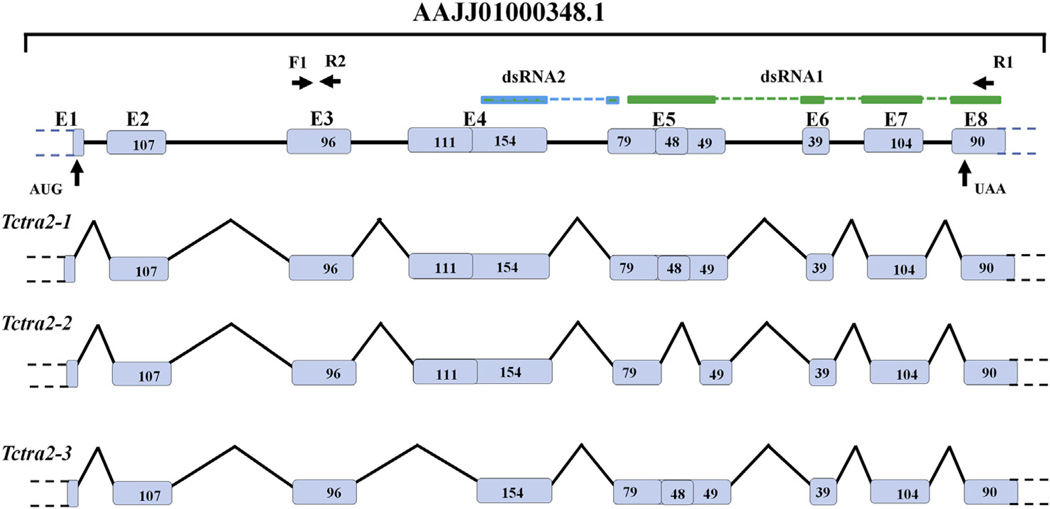
Fig. 1.
Schematic representation of the genomic organization of Tctra-2 gene showing exons (boxes-E1 to E8), introns (lines), and primer positions (horizontal arrows). Green and blue bars represent the regions used for the synthesis of dsRNA1 and dsRNA2, respectively. Vertical arrows represent start and stop codon positions. Numbers within the boxes represent the length of the exons, and the dashed line represents the gap. Primers F1 and R2 were used for qRT PCR, whereas F1 and R1 were used for semi-quantitative PCR. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.4. Double stranded RNA (dsRNA) synthesis and injections
Primers flanked with T7 promoter sequence at their 5′ ends were used to amplify two different regions (dsRNA1 and dsRNA2), common to all the Tctra-2 isoforms (Fig. 1). Purified PCR products were used as templates to synthesize dsRNA using the MEGAscript T7 kit (Ambion, Austin, Texas). Control dsRNA was prepared for a region corresponding to the Escherichia coli malE gene. dsRNAs were injected into newly ecdysed larvae, pupae, or adults as described previously (Shukla and Palli, 2012b). Knockdown efficiency of Tctra-2 expression in the RNAi insects was calculated as the ratio of Tctra-2 expression between insects injected with dsRNA specific to Tctra-2 or malE genes.
2.5. Quantitative real-time PCR
The SYBR Green kit (Roche, USA) was used to perform quantitative PCR according to the manufacturer’s instructions. Three independent biological replicates were analyzed for each treatment. Expression of rp49 gene of T. castaneum was used as an endogenous control for the normalization of the expression data. Gene expression levels were analyzed by 2−ΔΔCt method (Livak and Schmittgen, 2001).
2.6. Imaging and documentation
The gonads were dissected from the injected beetles on 6th day post-adult eclosion (PAE). Images of gonads were taken using an Olympus 1 × 71 Inverted Research Microscope. Megna Fire software (version 1.5) was used to control the microscope, image acquisition, and exportation of TIFF files. Adobe Photoshop Element 9 was used to assemble all micrographs.
2.7. Analysis of parental RNAi
To analyze the effect of depletion of Tctra-2 in early embryos, parental RNAi of Tctra-2 was performed as described previously (Shukla and Palli, 2012b).
3. Results
3.1. Genomic organization of T. castaneum transformer-2 gene
To identify tra-2 homolog (Tctra-2) in T. castaneum, Blast (tblastn) searches were performed in the Beetlebase (http://beetlebase.org/) and in the NCBI (http://www.ncbi.nlm.nih.gov/) using D. melanogaster Tra-2 protein sequence (NP_476764.1) as a query. Two ESTs (98999753 and 99004500) and two predicted Tctra-2 sequences (XM_963457.2 and GLEAN_12340) with overlapping regions were identified. Primers were designed at different locations of these sequences, and RT-PCR was performed using cDNAs prepared from RNA isolated from female and male beetles. Cloning, sequencing, and analysis of sequences identified the presence of three alternatively spliced isoforms of Tctra-2 (Fig. 1). RT-PCR amplified three fragments when cDNA made using RNA isolated either from male or female and primers F1 and R1 were used. All the three amplicons were gel purified, cloned into pGEMT easy vector, and sequenced. Alignment of these sequences and blastx analysis confirmed them to be alternatively spliced isoforms of Tctra-2. Sequences of all three isoforms of Tctra-2 were aligned with the Tctra-2 genomic sequence (AAJJ01000348.1) and exon intron boundaries were identified. Tctra-2 harbors at least 8 exons and 7 introns; Tctra-2-1 contains all the exons whereas TcTra-2-2 lacks a region of 48 bp from the middle of exon 5 and Tctra-2-3 lacks 111 bp sequence from the 5′ end of exon 4 (Fig. 1).
3.2. TcTra-2 protein is highly conserved within its RRM domain
The open reading frame (ORF) of all three Tctra-2 isoforms (Tctra-2-1, 813 bp; Tctra-2-2, 765 bp; and Tctra-2-3, 702 bp) shares common starts (AUG) and stop codons (UAA). The conceptual translation of ORFs of all three isoforms showed the presence of a single RRM domain (RBD-RNA binding domain) and two RS-rich regions on either sides of the RRM domain, a characteristic feature of Tra-2 proteins. The lengths of deduced amino acid sequences of Tctra-2-1, Tctra-2-2 and Tctra-2-3 are 270, 254, and 230 amino acids, respectively. TcTra-2-2 lacks most of the N-terminal SR-rich region, whereas TcTra-2-3 has a smaller RRM domain in its C-terminus (Fig. S1). The cDNA sequences of Tctra-2 isoforms have been submitted to GenBank (accession numbers for Tctra-2-1, Tctra-2-2 and Tctra-2-3 are KF235892, KF235893, and KF235894, respectively).
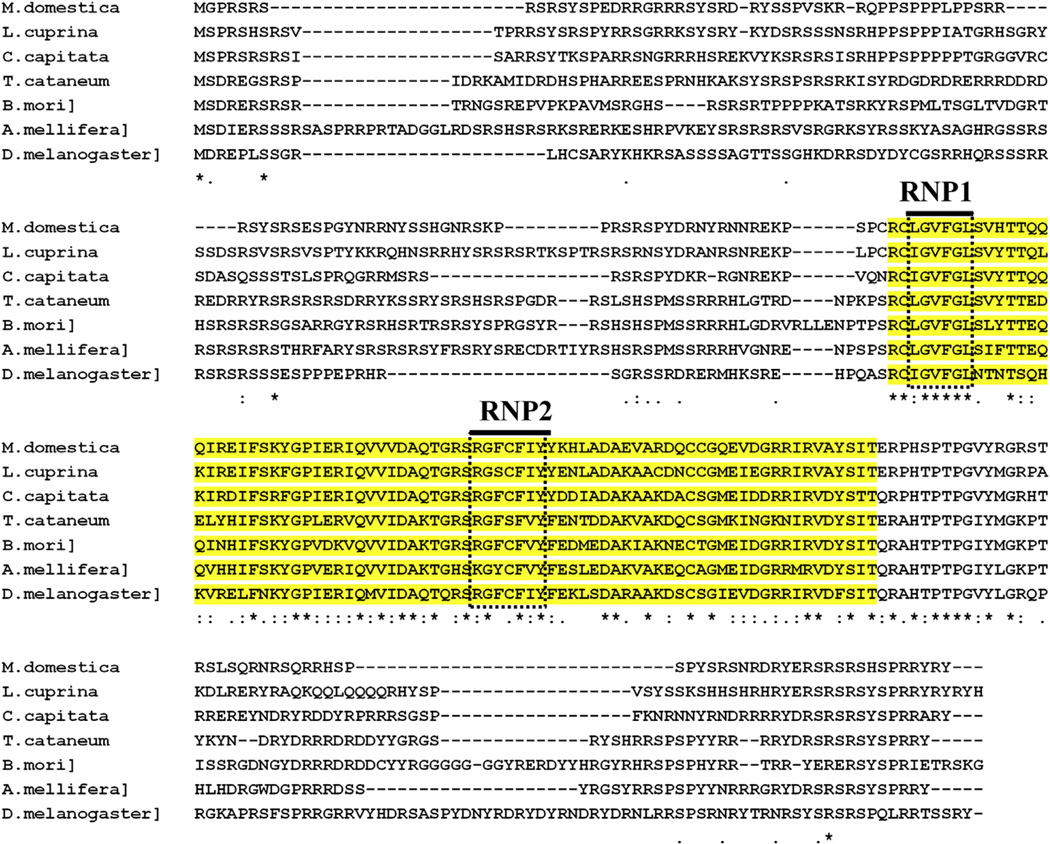
Multiple sequence alignment of TcTra-2 proteins with the Tra-2 proteins from other insects [M. domestica (AAW34233.1), L. cuprina (ACS34688.1), C. capitata (ACC68674.1), B. mori (NP_001119708.1), A. mellifera (AFJ15561.1), and D. melanogaster (NP_476764.1)] showed a high degree of sequence conservation within the RRM domain (Fig. 2). The amino acid sequence flanking the RRM domain is highly variable among different Tra-2 proteins.
Fig. 2.
Amino acid alignment of Tra-2 proteins of insect species from Diptera (D. melanogaster-NP_476764.1, M. domestica-AAW34233.1, C. capitata-ACC68674.1, and L. cuprina-ACS34688.1), Hymenoptera (A. mellifera-AFJ15561.1), Lepidoptera (B. mori-NP_001119708.1), and Coleoptera (T. castaneum). RRM (RBD) domain (yellow shaded) but not the flanking regions showed amino acid similarity among proteins compared. Conserved amino acid sequences (ribonucleoprotein 1, RNP1 and ribonucleoprotein 1, RNP2) shown in the boxes with dotted lines are the characteristic features of RRM domain. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Tctra-2 gene is expressed constitutively throughout the development in a non sex-specific manner
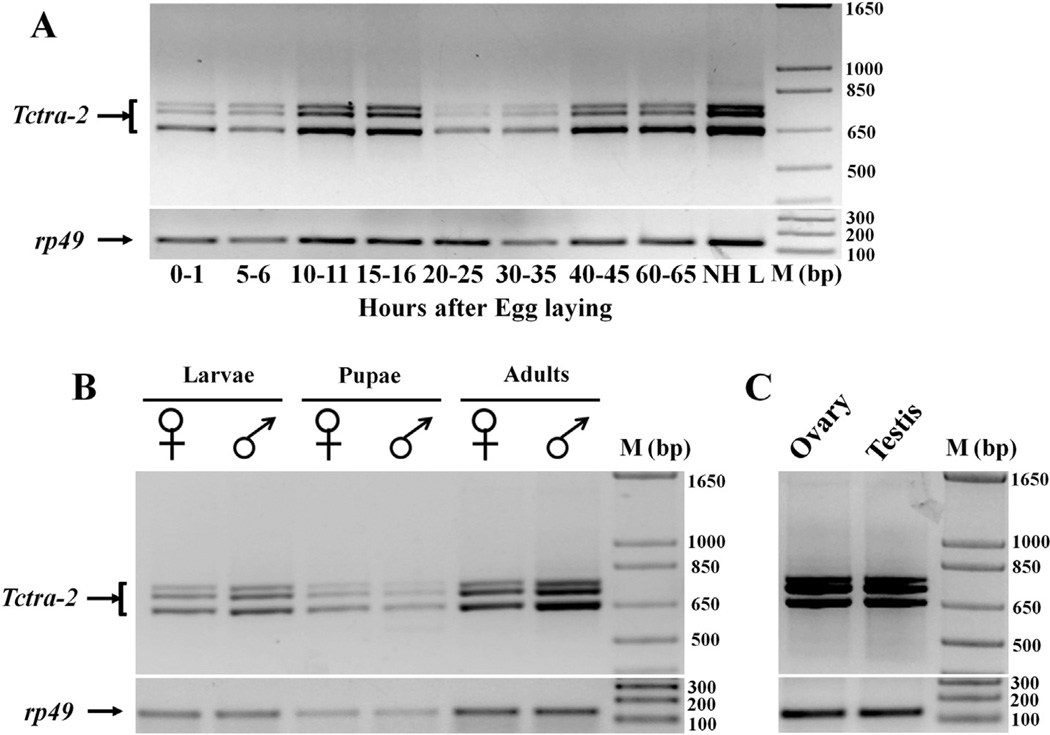
Relative Tctra-2 mRNA levels did not show significant differences during different developmental stages tested. All the three Tctra-2 mRNAs were detected during embryonic, larval, pupal, and adult stages (Fig. 3A and B). Expression of Tctra-2 during very early embryonic stage, i.e., during 0–1 h of embryonic growth suggests maternal transfer of Tctra-2 mRNAs. We also detected all three isoforms of Tctra-2 in testis and ovaries dissected from adults (Fig. 3C).
Fig. 3.
Semi-quantitative RT-PCR analysis of Tctra-2 mRNA levels. Primers F1 and R1 shown in Fig. 1 and cDNA copied from RNA isolated from staged embryos (A), larvae, pupae or adults (B) as well as ovary and testis (C) dissected from adults were used to amplify tra-2. Bottom panels in each Figure show rp49 amplified using the same cDNA samples. NHL, newly hatched first instar larvae.
3.4. Knockdown in the expression of gene coding for TcTra-2 affects the female-specific splicing of Tcdsx and both female- and male-specific splicing of Tctra
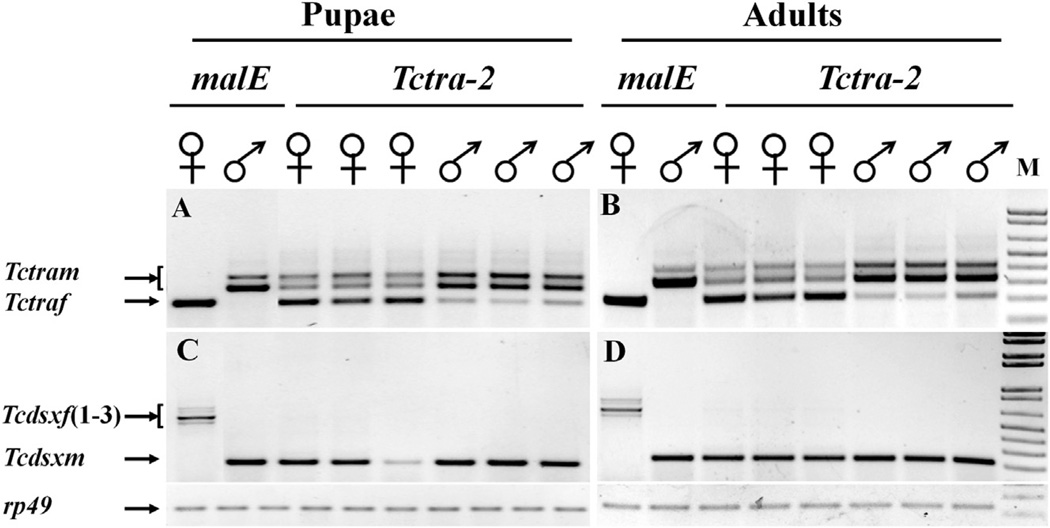
To confirm the predicted function of Tra-2 as a splice regulator of dsx pre-mRNA, dsRNA (dsRNA1) targeting the common region of Tctra-2 was injected into sex-separated newly ecdysed pupae and adults. Total RNA was isolated on the fifth day after injection of dsRNA and was used to analyze the splicing status of Tcdsx and Tctra pre-mRNAs. The pre-mRNA of Tcdsx is spliced to produce three female- and one male-specific isoforms, whereas the pre-mRNA of Tctra is spliced to produce one female and two male isoforms (Shukla and Palli, 2012a, b). Injection of Tctra-2 dsRNA1 caused an efficient knockdown in the expression of gene coding for TcTra-2 (Fig. S2). Injection of Tctra-2 dsRNA into newly ecdysed male pupae or male adults caused an appearance of both male and female isoforms of Tctra in male RNAi adults (Fig. 4A and B). Similarly, injection of Tctra-2 dsRNA into newly ecdysed female pupae or female adults caused an appearance of both female and male Tctra isoforms in female RNAi adults (Fig. 4A and B). Injection of Tctra-2 dsRNA into newly ecdysed male pupae or male adults did not affect Tcdsx isoform status as only male isoform was detected (Fig. 4C and D). In contrast, injection of Tctra-2 dsRNA into newly ecdysed female pupae or female adults caused an appearance of male but not female isoforms of Tcdsx in these female pupae and adults (Fig. 4C and D).
Fig. 4.
Sex-specific splicing of Tctra (A & B) and Tcdsx (C & D) in insects injected with malE or Tctra-2 dsRNA. malE or Tctra-2 dsRNAs were injected into newly ecdysed pupa (A & C) or newly emerged adults (B & D). Total RNA was isolated on 6th day after injection of dsRNA and cDNAs were synthesized. RT-PCR was done to detect the splicing status of Tctra and Tcdsx using the primers reported previously (Shukla and Palli, 2012b). Tctra and Tcdsx isoforms were detected by resolving RT-PCR products on agarose gels. Bottom panels in each Figure show rp49 amplified using the same cDNA samples.
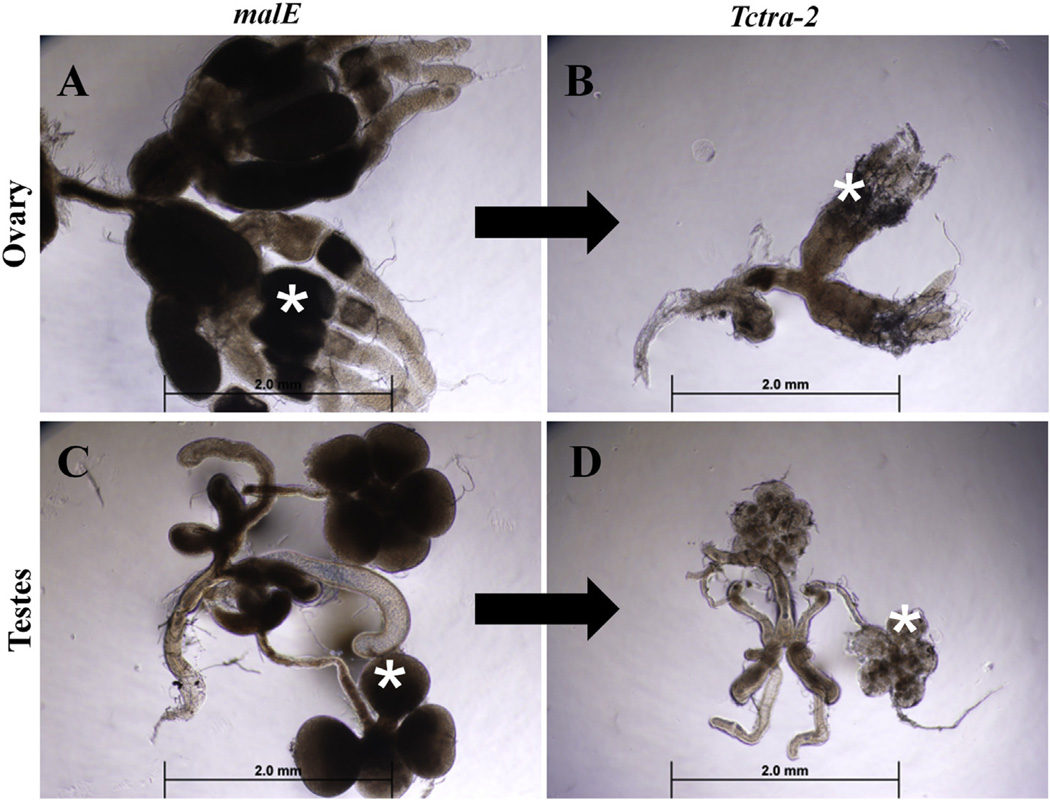
The ovary and testes dissected from adults developed from the pupae injected with Tctra-2 dsRNA were highly regressed compared to those in control adults developed from malE dsRNA injected pupae (Fig. 5). The ovaries (Fig. 5B) were affected more than the testes in RNAi insects (Fig. 5D). Consistent with this, Tctra-2 RNAi females did not produce even a single egg when they were mated with virgin control males developed from malE dsRNA injected pupae (Table 1A). On the other hand, virgin control females developed from malE dsRNA injected pupae laid reduced number of eggs when mated with Tctra-2 RNAi males. All the eggs laid successfully hatched, and the hatched larvae completed their life cycle (Table 1A). Similar results were obtained when dsRNA was injected into newly eclosed adults (Fig. S3 and Table 1B). These data suggest that TcTra-2 is required for sex-specific splicing of Tctra in both sexes and sex-specific splicing of Tcdsx in females but not in males. Also, TcTra-2 has an important role in the development of reproductive organs (either directly or through Tcdsx) in both male and female and, in turn, regulates fertility.
Fig. 5.
Effect of tra-2 knockdown on the development of gonads. Ovaries (A & B) or testes (C & D) dissected from insects injected with malE (A & C) or Tctra-2 (B & D) dsRNA. Tctra-2 or malE dsRNA were injected into sex-separated day 0 pupae, and gonads were dissected on 6th day PAE. Pictures were taken at 5× magnification. Asterisks are used to mark ovarioles and testis lobes.
Table 1.
| A Effect of Tctra-2 knockdown on the fecundity in the adults developed from Tctra-2 dsRNA injected pupae. | |||
|---|---|---|---|
| Control female × control male |
RNAi female × RNAi male |
RNAi female × control male |
Control female × RNAi male |
| 59 ± 2.4 | 0 | 0 | 18 ± 2.16 |
| B Effect of Tctra-2 knockdown on the fecundity in the adults injected with Tctra-2 dsRNA. | |||
|---|---|---|---|
| Control female × control male |
RNAi female × RNAi male |
RNAi female × control male |
Control female × RNAi male |
| 60 ± 2.9 | 0 | 0 | 25 ± 2.3 |
The values represent Mean ± S.D (n = 6).
3.5. Tctra-2 has a vital role during development
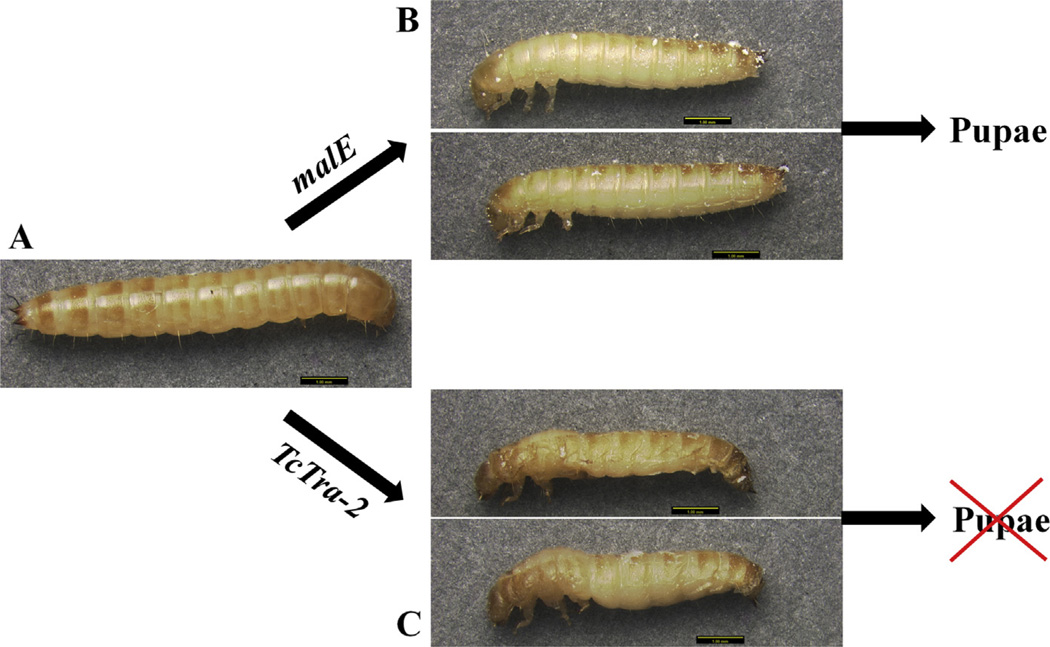
Knockdown in the expression of Tctra-2 in females, during pupal or adult stages exhibited the same splicing pattern of Tctra and Tcdsx as observed previously in Tctra RNAi beetles (Shukla and Palli, 2012b). Further, knockdown in the expression of Tctra during the larval stages (but not during pupal and adult stages) resulted in the development of male sexually dimorphic structures in genetic females (Shukla and Palli, 2012b). We performed injections of Tctra-2 dsRNA into final instar larvae expecting the same change in sexually dimorphic structures as in Tctra knockdown. Surprisingly, none of the Tctra-2 RNAi larvae developed to pupal stage, and all of them died on the fifth or sixth day after injection (Table 2 and Fig. 6). All the malE dsRNA injected larvae developed to become pupae within 5–6 days. These data suggest that TcTra-2 is required for development of both male and female larvae. To confirm that the larval deaths by Tctra-2 RNAi is not due to off target effects of TcTra-2 dsRNA, we designed and prepared dsRNA (dsRNA2) targeting a different region of Tctra-2 than the region targeted by dsRNA1 (Fig. 1). Injection of dsRNA2 into newly ecdysed final instar larvae showed the similar phenotype as seen in dsRNA1 injected larvae, suggesting that the larval developmental arrest phenotype observed in TcTra-2 RNAi larvae is specific to knockdown in the expression of TcTra-2.
Table 2.
Effect of Tctra-2 knockdown during the larval stage on larval development.
| Injection | No of larvae injected |
No of larvae metamorphosed to pupae and adult |
No of larvae died |
|---|---|---|---|
| malE | 20 | 20 | 0 |
| TcTra-2 | 20 | 0 | 20 |
The values represent Mean ± S.D (n = 3).
Fig. 6.
Effect of tra-2 knockdown on the development of larvae. Tctra-2 or malE dsRNA were injected into day 0 final instar larvae (A). Control larvae injected with malE dsRNA entered into quiescent stage and pupated in 5–6 days of injection of dsRNA (B). The development of Tra-2 dsRNA injected larvae was arrested and the larvae died within 5–6 days after injection of dsRNA (B).
Parental RNAi of Tctra causes production of all male progenies. To determine whether the parental RNAi of Tctra-2 also induces the same phenotype, 5 day-old female adults were injected with either Tctra-2 or malE dsRNA. After 24 h, five malE or five Tctra-2 dsRNA injected females were mated with an equal number of un-injected virgin males in separate cups. Eggs laid by malE or Tctra-2 RNAi females were counted from each cup on15th day after initiation of mating. Number of eggs laid by Tctra-2 RNAi females was low compared to the number of eggs laid by malE RNAi females (Table 3). Also, eggs laid by Tctra-2 RNAi females did not develop into larvae (Table 3). These data suggest that TcTra-2 is required for embryonic development.
Table 3.
Effect of parental RNAi of Tctra-2 on fecundity and hatching.
| dsRNA | Eggs laid | Eggs hatched |
|---|---|---|
| malE | 295 ± 12.9 | All |
| TcTra-2 | 12.5 ± 2.08 | None |
The values represent Mean ± S.D (n = 5).
4. Discussion
Comparative and evolutionary studies on sex-determination mechanisms in insects belonging to different orders suggest existence of a conserved central axis around which sex is determined. This central axis is formed by Tra protein (Verhulst et al., 2010). Tra, along with Tra-2 regulates splicing of dsx pre-mRNA in females. In males, dsx pre-mRNA splices in a default male mode in the absence of functional Tra. The domesticated silk moth, B. mori, presents an exception to this rule where tra homologs have not been identified in its genome (Mita et al., 2004), and BmPSI and BmIMP have been implicated in the splicing of Bmdsx pre-mRNA (Suzuki et al., 2008, 2010).
T. castaneum represents an excellent model insect for the order Coleoptera where RNAi has been demonstrated to work efficiently (Posnien et al., 2009). Recently, we reported on the characterization of the homologs of tra and dsx from T. castaneum (Shukla and Palli, 2012a,b). In this paper, we report on the identification and characterization of tra-2 homolog (Tctra-2) from T. castaneum and show its role in the regulation of sex determination and development. Data included in this paper showed the requirement of TcTra-2 in the sex-specific splicing of Tctra and Tcdsx pre-mRNA in females and also in the sex-specific splicing of Tctra in males. In addition, we propose a vital role for TcTra-2 during embryonic and larval development in T. castaneum.
Blast searches in the Tribolium genome and transcriptome databases led to the identification of Tctra-2, which codes for three isoforms. Alignment of TcTra-2 (isoform1) with Tra-2 protein sequences from other insects showed the similarity only within the RRM domain (Fig. 2), confirming suggestions in previous reports that the adjoining regions to the RRM evolve faster than RRM (Nissen et al., 2012). All three isoforms of Tctra-2 are expressed in both male and female beetles during all the stages tested (Fig. 3). Detection of Tctra-2 mRNA during early embryonic stages (0–1 h) prior to initiation of zygotic expression suggests the maternal transfer of Tctra-2. Similar predictions of maternal transfer of tra-2 mRNA have been suggested for M. domestica (Burghardt et al., 2005), C. capitata (Salvemini et al., 2009) and A. obliqua (Sarno et al., 2010). In many dipteran insects studied so far, tra-2 pre-mRNA is not spliced alternatively and only a single transcript has been detected (Burghardt et al., 2005; Concha and Scott, 2009; Sarno et al., 2010; Schetelig et al., 2012). However, alternative splicing and production of multiple tra-2 isoforms is not uncommon as multiple isoforms of tra-2 have been reported in S. ocellaris, Bradysia coprophila, B. mori, and A. mellifera (Martin et al., 2011; Nissen et al., 2012; Suzuki et al., 2012). All the three isoforms of TcTra-2 (TcTra-2-1, TcTra-2-2, and TcTra-2-3) contain an RRM domain flanked by RS rich regions (Fig. S1).
Knockdown in the expression of Tctra-2 during pupal and adult stages altered the splicing status of Tctra, in both female and male insects (Fig. 4A and B). The regulation of Tctra pre-mRNA splicing by Tctra-2 in beetles is different from that in dipteran insects where Tra-2 regulates only the female-specific splicing of tra pre-mRNA (Burghardt et al., 2005; Concha and Scott, 2009; Martin et al., 2011; Ruiz et al., 2007; Salvemini et al., 2009; Sarno et al., 2010). Regulation of sex-specific splicing of fem/tra pre-mRNA by tra-2 in both male and female has recently been reported in A. mellifera (Nissen et al., 2012). How does the same protein Tra-2 promote splicing in females and suppress splicing in males? Serine and arginine rich proteins are known to act as both splicing activators (Graveley and Maniatis, 1998; Shen et al., 2004) as well as splicing suppressors (Chandler et al., 2003; Qi et al., 2007). In D. melanogaster Tra-2 (a SR protein), together with Tra act as a splicing activator for splicing of dsx pre-mRNA into female mode (Nagoshi et al., 1988). Tra-2, together with Puf68, act as splicing repressor of male-specific exon of its own pre-mRNA in testis (Wang et al., 2013).
TcTra-2 also promotes the female-specific splicing of Tcdsx pre-mRNA as knockdown in the expression of Tctra-2 led to the detection of only male Tcdsx isoform in females (Fig. 4C and D). In spite of the presence of female isoform of Tctra in Tctra-2 RNAi males (Fig. 4A and B), we did not detect any female-specific isoform of Tcdsx in the RNAi males (Fig. 4C and D). This may be due to lower levels of female TcTra protein that may not have reached the threshold levels essential for the female-specific splicing of Tcdsx pre-mRNA resulting in the production of only male isoform of Tcdsx mRNA in Tctra-2 RNAi males. This hypothesis is also supported by the data that showed the presence of only male isoform of Tcdsx in Tctra-2 RNAi females (Fig. 4C and D) in spite of the presence of higher levels of female-specific isoform of Tctra compared to the levels of male-specific isoforms (Fig. 4A and B). Additionally, these RNAi females showed lower levels of female-specific isoforms of Tctra compared to control females (Fig. 4A and B).
In females, knockdown in the expression of Tctra-2 (present study) produced similar results at the molecular levels as that of knockdown of Tctra (Shukla and Palli, 2012b), i.e., presence of male-specific isoform of Tcdsx and both female and male isoforms of Tctra (Fig. 4). In contrast, we did not notice any testis-like lobes (Fig. 5B) seen in the ovaries of Tctra RNAi insects (Shukla and Palli, 2012b). This observation suggests the requirement of TcTra-2 for the development of gonads in both male and female insects. TcTra-2 is indeed required for testis development in T. castaneum, as evident by smaller testis and other male reproductive organs in adult males developed from Tctra-2 RNAi pupae (Fig. 5D).
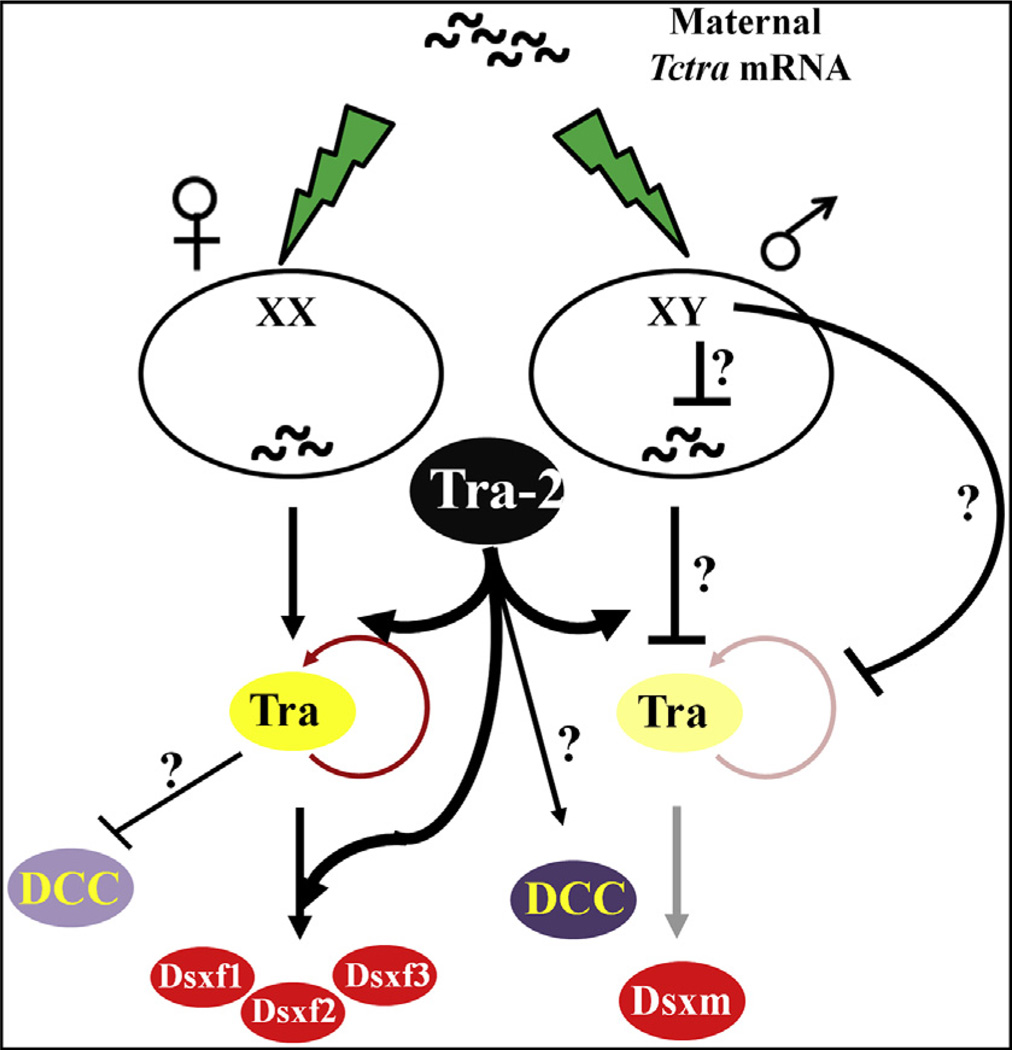
Injection of Tctra-2 dsRNA into embryos led to the transformation of XX female into pseudo males in C. capitata and Anastrepha species (Salvemini et al., 2009; Sarno et al., 2010). These pseudo males displayed gonad morphology that included male and female internal genital structures (Salvemini et al., 2009; Sarno et al., 2010). In M. domestica, genetic female embryos were converted to fertile males as a result of tra-2 dsRNA injection during embryonic stage (Burghardt et al., 2005). In contrast, knockdown of tra-2 during the early embryonic stage of A. mellifera resulted in the death of embryos after approximately 70 h of development (Nissen et al., 2012), leading to a suggestion of a vital developmental role for Tra-2 during embryonic development in this insect (Nissen et al., 2012). Parental RNAi of Tctra-2 led to the production of a few eggs which failed to hatch (Table 3), suggesting that TcTra-2 is required for embryonic development in T. castaneum as well. The mechanism of action of TcTra-2 in embryonic development is not known at this time. However, it is likely that the role of TcTra-2 during embryonic development may be related to regulation of Tra/Tra-2 target genes coding for proteins such as those involved in the formation of dosage compensation complex. Dosage compensation is a process by which the overall amount of X-linked gene products are equalized in females (2X) and males (1X), in D. melanogaster (Cline and Meyer, 1996; Lucchesi, 1973). Fig. 7 illustrates the model of sex determination in T. castaneum based on current understanding of data obtained in this and previous studies (Shukla and Palli, 2012a,b).
Fig. 7.
Model of the sex-determination cascade in T. castaneum [the model published recently (Shukla and Palli, 2012b) was modified to show the function of TcTra-2]. TcTra-2 is required for the female-specific splicing of Tctra and Tcdsx pre-mRNAs in females. TcTra is also required for the male-specific splicing of Tctra pre-mRNA in males. Both Tra and Tra-2 may be required to inhibit the formation of dosage compensation complex in females whereas in males Tra-2 alone is required for the formation of dosage compensation complex. This is based on the fact that Tctra knockdown leads to death of only females (Shukla and Palli, 2012b) whereas Tctra-2 knockdown leads to death of both female and male (present study).
RNAi experiments clearly demonstrated the function of Tra2 in sex determination and development in both sexes of T. castaneum (present study) and A. mellifera (Nissen et al., 2012). Hence, mis-regulation of Tctra/tra-2 target genes likely occurs in both males and females as a result of Tctra-2 knockdown. Future studies on global identification of TcTra and Tctra-2 target genes in T. castaneum should clarify the sex determination and development roles of these proteins.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (GM070559-09). The information reported in this paper (No.13-08-117) is part of a project of the Kentucky Agricultural Experiment Station and is published with the approval of the Director.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ibmb.2013.08.010.
References
- Boggs RT, Gregor P, Idriss S, Belote JM, McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987;50:739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- Burghardt G, Hediger M, Siegenthaler C, Moser M, Dubendorfer A, Bopp D. The transformer2 gene in Musca domestica is required for selecting and maintaining the female pathway of development. Dev. Genes Evol. 2005;215:165–176. doi: 10.1007/s00427-004-0464-7. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Coschigano KT, Baker BS, Wensink PC. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 1991;10:2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DS, Qi J, Mattox W. Direct repression of splicing by transformer-2. Mol. Cell Biol. 2003;23:5174–5185. doi: 10.1128/MCB.23.15.5174-5185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline TW, Meyer BJ. Vive la difference: males vs females in flies vs. worms. Annu. Rev. Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- Concha C, Scott MJ. Sexual development in Lucilia cuprina (Diptera, Calliphoridae) is controlled by the transformer gene. Genetics. 2009;182:785–798. doi: 10.1534/genetics.109.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 1993;7:42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- Erdman SE, Burtis KC. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J. 1993;12:527–535. doi: 10.1002/j.1460-2075.1993.tb05684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman SE, Chen HJ, Burtis KC. Functional and genetic characterization of the oligomerization and DNA binding properties of the Drosophila doublesex proteins. Genetics. 1996;144:1639–1652. doi: 10.1093/genetics/144.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JW, Quintero JJ. Indirect effects of ploidy suggest X chromosome dose, not the X: a ratio, signals sex in Drosophila. PLoS Biol. 2007;5:e332. doi: 10.1371/journal.pbio.0050332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gempe T, Beye M. Function and evolution of sex determination mechanisms, genes and pathways in insects. Bioessays. 2011;33:52–60. doi: 10.1002/bies.201000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham P, Penn JK, Schedl P. Masters change, slaves remain. Bioessays. 2003;25:1–4. doi: 10.1002/bies.10207. [DOI] [PubMed] [Google Scholar]
- Graveley BR, Maniatis T. Arginine/serine-rich domains of SR proteins can function as activators of pre-mRNA splicing. Mol. Cell. 1998;1:765–771. doi: 10.1016/s1097-2765(00)80076-3. [DOI] [PubMed] [Google Scholar]
- Hoshijima K, Inoue K, Higuchi I, Sakamoto H, Shimura Y. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science. 1991;252:833–836. doi: 10.1126/science.1902987. [DOI] [PubMed] [Google Scholar]
- Inoue K, Hoshijima K, Sakamoto H, Shimura Y. Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature. 1990;344:461–463. doi: 10.1038/344461a0. [DOI] [PubMed] [Google Scholar]
- Keyes LN, Cline TW, Schedl P. The primary sex determination signal of Drosophila acts at the level of transcription. Cell. 1992;68:933–943. doi: 10.1016/0092-8674(92)90036-c. [DOI] [PubMed] [Google Scholar]
- Lagos D, Ruiz MF, Sanchez L, Komitopoulou K. Isolation and characterization of the Bactrocera oleae genes orthologous to the sex determining Sex-lethal and doublesex genes of Drosophila melanogaster. Gene. 2005;348:111–121. doi: 10.1016/j.gene.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lucchesi JC. Dosage compensation in Drosophila. Annu. Rev. Genet. 1973;7:225–237. doi: 10.1146/annurev.ge.07.120173.001301. [DOI] [PubMed] [Google Scholar]
- Luo SD, Shi GW, Baker BS. Direct targets of the D. melanogaster DSXF protein and the evolution of sexual development. Development. 2011;138:2761–2771. doi: 10.1242/dev.065227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KW, Maniatis T. Assembly of specific SR protein complexes on distinct regulatory elements of the Drosophila doublesex splicing enhancer. Genes Dev. 1996;10:2089–2101. doi: 10.1101/gad.10.16.2089. [DOI] [PubMed] [Google Scholar]
- Martin I, Ruiz MF, Sanchez L. The gene transformer-2 of Sciara (Diptera, Nematocera) and its effect on Drosophila sexual development. BMC Dev. Biol. 2011;11:19. doi: 10.1186/1471-213X-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita K, Kasahara M, Sasaki S, Nagayasu Y, Yamada T, Kanamori H, Namiki N, Kitagawa M, Yamashita H, Yasukochi Y, Kadono-Okuda K, Yamamoto K, Ajimura M, Ravikumar G, Shimomura M, Nagamura Y, Shin IT, Abe H, Shimada T, Morishita S, Sasaki T. The genome sequence of silkworm, Bombyx mori. DNA Res. 2004;11:27–35. doi: 10.1093/dnares/11.1.27. [DOI] [PubMed] [Google Scholar]
- Nagoshi RN, McKeown M, Burtis KC, Belote JM, Baker BS. The control of alternative splicing at genes regulating sexual differentiation in D. melanogaster. Cell. 1988;53:229–236. doi: 10.1016/0092-8674(88)90384-4. [DOI] [PubMed] [Google Scholar]
- Nissen I, Muller M, Beye M. The Am-tra2 gene is an essential regulator of female splice regulation at two levels of the sex determination hierarchy of the honeybee. Genetics. 2012;192:1015–1026. doi: 10.1534/genetics.112.143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu BL, Meng ZQ, Tao YZ, Lu SL, Weng HB, He LH, Shen WF. Cloning and alternative splicing analysis of Bombyx mori transformer-2 gene using silkworm EST database. Acta Biochim. Biophys. Sin (Shanghai) 2005;37:728–736. doi: 10.1111/j.1745-7270.2005.00106.x. [DOI] [PubMed] [Google Scholar]
- Penalva LO, Sanchez L. RNA binding protein sex-lethal (Sxl) and control of Drosophila sex determination and dosage compensation. Microbiol. Mol. Biol. Rev. 2003;67:343–359. doi: 10.1128/MMBR.67.3.343-359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnien N, Schinko J, Grossmann D, Shippy T, Konopova B. RNAi in the red flour beetle (Tribolium) Cold Spring Harb. Protoc. 2009 doi: 10.1101/pdb.prot5256. pdb prot5256. [DOI] [PubMed] [Google Scholar]
- Qi J, Su S, Mattox W. The doublesex splicing enhancer components Tra2 and Rbp1 also repress splicing through an intronic silencer. Mol. Cell Biol. 2007;27:699–708. doi: 10.1128/MCB.01572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MF, Eirin-lopez M, Stefani RN, Perondini AL, Selivon D, et al. The gene doublesex of Anastrepha fruit flies (Diptera, Tephritidae) and its evolution in insects. Dev. Genes Evol. 2007;217:725–731. doi: 10.1007/s00427-007-0178-8. [DOI] [PubMed] [Google Scholar]
- Saccone G, Pane A, Polito LC. Sex determination in flies, fruitflies and butterflies. Genetica. 2002;116:15–23. doi: 10.1023/a:1020903523907. [DOI] [PubMed] [Google Scholar]
- Salvemini M, Robertson M, Aronson B, Atkinson P, Polito LC, Saccone G. Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. Int. J. Dev. Biol. 2009;53:109–120. doi: 10.1387/ijdb.082681ms. [DOI] [PubMed] [Google Scholar]
- Salz HK. Sex determination in insects: a binary decision based on alternative splicing. Curr. Opin. Genet. Dev. 2011;21:395–400. doi: 10.1016/j.gde.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez L. Sex-determining mechanisms in insects. Int. J. Dev. Biol. 2008;52:837–856. doi: 10.1387/ijdb.072396ls. [DOI] [PubMed] [Google Scholar]
- Sarno F, Ruiz MF, Eirin-Lopez JM, Perondini AL, Selivon D, Sanchez L. The gene transformer-2 of Anastrepha fruit flies (Diptera, Tephritidae) and its evolution in insects. BMC Evol. Biol. 2010;10:140. doi: 10.1186/1471-2148-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetelig MF, Milano A, Saccone G, Handler AM. Male only progeny in Anastrepha suspensa by RNAi-induced sex reversion of chromosomal females. Insect Biochem. Mol. Biol. 2012;42:51–57. doi: 10.1016/j.ibmb.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Shearman DC. The evolution of sex determination systems in dipteran insects other than Drosophila. Genetica. 2002;116:25–43. doi: 10.1023/a:1020955507978. [DOI] [PubMed] [Google Scholar]
- Shen H, Kan JL, Green MR. Arginine-serine-rich domains bound at splicing enhancers contact the branchpoint to promote prespliceosome assembly. Mol. Cell. 2004;13:367–376. doi: 10.1016/s1097-2765(04)00025-5. [DOI] [PubMed] [Google Scholar]
- Shukla JN, Nagaraju J. Doublesex: a conserved downstream gene controlled by diverse upstream regulators. J. Genet. 2010;89:341–356. doi: 10.1007/s12041-010-0046-6. [DOI] [PubMed] [Google Scholar]
- Shukla JN, Palli SR. Doublesex target genes in the red flour beetle, Tribolium castaneum. Sci. Rep. 2012a;2:948. doi: 10.1038/srep00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla JN, Palli SR. Sex determination in beetles: production of all male progeny by Parental RNAi knockdown of transformer. Sci. Rep. 2012b;2:602. doi: 10.1038/srep00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki MG, Imanishi S, Dohmae N, Asanuma M, Matsumoto S. Identification of a male-specific RNA binding protein that regulates sex-specific splicing of Bmdsx by increasing RNA binding activity of BmPSI. Mol. Cell Biol. 2010;30:5776–5786. doi: 10.1128/MCB.00444-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki MG, Imanishi S, Dohmae N, Nishimura T, Shimada T, Matsumoto S. Establishment of a novel in vivo sex-specific splicing assay system to identify a trans-acting factor that negatively regulates splicing of Bombyx mori dsx female exons. Mol. Cell Biol. 2008;28:333–343. doi: 10.1128/MCB.01528-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki MG, Suzuki K, Aoki F, Ajimura M. Effect of RNAi-mediated knockdown of the Bombyx mori transformer-2 gene on the sex-specific splicing of Bmdsx pre-mRNA. Int. J. Dev. Biol. 2012;56:693–699. doi: 10.1387/ijdb.120049ms. [DOI] [PubMed] [Google Scholar]
- Tian M, Maniatis T. Positive control of pre-mRNA splicing in vitro. Science. 1992;256:237–240. doi: 10.1126/science.1566072. [DOI] [PubMed] [Google Scholar]
- Tian M, Maniatis T. A splicing enhancer complex controls alternative splicing of doublesex pre-mRNA. Cell. 1993;74:105–114. doi: 10.1016/0092-8674(93)90298-5. [DOI] [PubMed] [Google Scholar]
- Verhulst EC, van de Zande L, Beukeboom LW. Insect sex determination: it all evolves around transformer. Curr. Opin. Genet. Dev. 2010;20:376–383. doi: 10.1016/j.gde.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Wang S, Wagner EJ, Mattox W. Half pint/Puf68 is required for negative regulation of splicing by the SR splicing factor Transformer2. RNA Biol. 2013;10(8) doi: 10.4161/rna.25645. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.