Abstract
Background
Netrin-1 was recently identified as an early diagnostic biomarker of chronic kidney disease (CKD) in an experimental animal model. However, its usefulness for early diagnosis of CKD in humans is unknown. The current study evaluated whether netrin-1 is increased in urine from human diabetic patients.
Methods
Spot urine samples from healthy volunteers, diabetes without microalbuminuria, diabetes with microalbuminuria and diabetes with macroalbuminuria were collected after receiving consent. Netrin-1 in urine was quantified by enzyme-linked immunosorbent assay (ELISA) and the data analyzed to determine whether urinary netrin-1 significantly correlates with disease progression
Results
Urinary netrin-1 levels were significantly increased in normoalbuminuric diabetic patients compared to healthy controls, and still further elevated in patients with microalbuminuria and overt nephropathy. Urinary netrin-1 was significantly associated with albuminuria and estimated glomerular filtration rate (eGFR), independently of age and sex.
Conclusion
Netrin-1 is detectable in urine from diabetic patients and may serve as a useful early diagnostic biomarker predicting the development of CKD in diabetes.
Keywords: Biomarker, Netrin-1, Diabetic nephropathy, Albuminuria
Introduction
Diabetic nephropathy is by far the most common cause of end-stage renal disease (ESRD) in industrial countries, representing about 45 % of all new ESRD cases in the United States. Approximately 25 % of type1 diabetic patients will develop overt diabetic nephropathy [1]. Although microalbuminuria is widely recognized as the main risk predictor of diabetic nephropathy, many patients have already developed established glomerular lesions by the time microalbuminuria is detected. Moreover, not all patients with microalbuminuria will progress to ESRD while many become normoalbuminuric on follow-up. The occurrence of microalbuminuria in type 1 diabetes can already be associated with diabetic nephropathy (DN) lesions comparable to the ones found in overt diabetic nephropathy. Therefore, identification and validation of biomarkers for early diagnosis of diabetic kidney disease (DKD), i.e. before microalbuminuria develops, may help develop an effective treatment for DKD. Although diabetic nephropathy is classically considered as a glomerular disease, recent studies suggest that tubulointerstitial injury may precede the appearance of glomerulopathy [2]. We have recently identified a novel tubular injury biomarker called netrin-1, which may be used to diagnose acute kidney injury much earlier than existing diagnostic tests. Recent studies from our laboratory in an animal model of DN determined that netrin-1 is significantly elevated early in the course of diabetic nephropathy. However, its utility as an early diagnostic biomarker in human diabetic nephropathy is unknown.
The diagnostic test currently used in clinical practice, i.e. increased albumin excretion rate, has significant limitations. Microalbuminuria is rarely found during the first 5 years of a patient's diabetes, suggesting that it is a sign of early glomerular damage rather than a marker for susceptibility to it. Furthermore, the presence of microalbuminuria by itself may not be an adequate indicator for disease progression in all diabetic subjects given that a number of type 1 diabetes patients revert to normoalbuminuria without treatment within a 5-year period [3]. Lastly, the occurrence of microalbuminuria in type 1 diabetes can already be associated with diabetic nephropathy (DN) lesions comparable to the ones found in overt diabetic nephropathy. Therefore, identification and validation of biomarkers for early diagnosis of DKD, i.e. before microalbuminuria develops, may help develop an effective treatment for DKD. Several recent studies suggest that a tubular defect is the cause of early microalbuminuria [4–6] suggesting that tubulointerstitial injury may precede apparent glomerulopathy [2]. This view is further supported by studies that show increased levels of tubular injury biomarkers such as kidney injury molecule-1 (KIM-1) and N-acetyl-β-D-glucosaminidase (NAG) in patients with type 1 diabetes [7, 8] and decreased levels of these biomarkers were associated with regression of microalbuminuria [8]. Unfortunately, currently available therapies are not effective in fully preventing progression of diabetic nephropathy. Early start of treatment may be more effective. However, there is a paucity of diagnostic markers that can predict the development of progressive kidney disease in diabetes. Therefore, there is an urgent need to identify early diagnostic biomarkers to enable early intervention and preventive therapies.
Netrin-1 is a laminin-related secreted protein highly induced after acute and chronic kidney injury and excreted in urine in both mice and humans [9, 10]. However, whether the netrin-1 expression is increased in DKD and its utility as a biomarker for early diagnosis of DKD in humans is unknown. The purpose of the present study was to determine whether tubular injury marker netrin-1 excretion in urine is increased early in the course of diabetes and correlates with disease progression in humans.
Materials & Methods
Patient Recruitment and Sample Collection
This is a post-hoc study using urine samples collected from a previously published study [7]. The current study was carried out on 87 of 92 diabetic samples published earlier [7]. Type 1 and type 2 diabetic patients who were visiting a diabetes specialty clinic were recruited between April and September 2009 at the University Medical Center of Groningen, The Netherlands. The study protocol was approved by the local ethics committee of the University Medical Center of Groningen and the Human Assurance Committee at the Georgia Health Sciences University, USA. Patients were stratified by the amount of albuminuria based on a first-morning urine sample. Of the 87 diabetic patients, 40 had normoalbuminuria, 38 had microalbuminuria, and 9 had macroalbuminuria. To ensure that macroalbuminuria was the consequence of diabetic nephropathy, patients with macroalbuminuria were required to have diabetic retinopathy. Patients who had cancer, infections or inflammatory conditions, and renal disease other than diabetic nephropathy, those who used nephrotoxic drugs, or had a renal transplant, or who were pregnant were excluded. Patients aged < 18 years were also excluded. As control group, we included 42 non-diabetic subjects without chronic kidney disease (ratio of non-diabetic to diabetic patients, 1:2). Subjects were excluded from the control group if they had a fasting glucose > 7.0 mmol/l, used glucose-lowering medication, had an estimated glomerular filtration rate (eGFR) < 60 ml/min per 1.73 m2, or had an albumin-to-creatinine ratio (ACR) > 30 mg/g.
Netrin-1 quantification in urine
Netrin-1 in urine was measured by enzyme-linked immunosorbent assay (ELISA) (USCNk Life Sciences, Wuhan, China) as described before [10]. All assays were performed in duplicate. Urinary netrin-1 excretion is expressed in picograms (pg) per mg of creatinine.
Analysis of netrin-1 by western blot
A volume of urine containing 15 µg creatinine for each human sample was used for western blot analysis of netrin-1. The urine sample was loaded into 4–12 % polyacrylamide gel, separated and then transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was probed with goat anti-netrin-1 antibody (cat # EB07007, Everest Biotech, Upper Heyford, Oxfordshire, UK). Proteins were detected using enhanced chemiluminescence detection reagents (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA).
Measurements
We instructed patients to collect their first-morning urine void on the day of their clinic visit. Blood pressure was assessed with a single measurement. Urinary albumin concentration was determined by nephelometry (BNII; Dade Behring Diagnostics, Marburg, Germany). Serum and urine creatinine were measured with an enzymatic creatinine assay (Roche, Mannheim, Germany). Glucose, glycated hemoglobin (HbA1c), and cholesterol levels were measured with standard laboratory testing.
Definitions
We defined history of cardiovascular disease as occurrence of myocardial infarction, stroke, cardiovascular surgery or endovascular treatment for coronary carotid or peripheral (legs, abdominal or aorta) artery disease. Hypertension was defined as systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg or use of blood pressure–lowering medication. Normoalbuminuria was defined as an ACR < 30 mg/g, microalbuminuria as ACR 30–299 mg/g, and macroalbuminuria as ACR ≥ 300 mg/g [11]. Serum creatinine values were used to calculate eGFR using the abbreviated Modification of Diet in Renal Disease (MDRD) formula.
Statistical Analysis
Data were assessed for normality and appropriate transformations were used when necessary. Median (min, max) values are reported for continuous non-normal data, mean ± standard deviation (SD) is reported otherwise. Chi-square tests were used to assess the relationship between control and albuminuria groups with categorical variables. One-way analysis of variation (ANOVA) was used to assess the relationship of control and albuminuria groups with continuous variables. Tukey’s multiple comparison tests were used to determine group differences for significant ANOVA findings. To investigate the association between albumin creatinine ratio (albuminuria), eGFR, or hypertension status and log netrin-1 we performed a linear regression using log-netrin-1 as the dependent variable and albuminuria, eGFR or hypertension as dependent variables. Various models were built to adjust for possible confounding. Model 1 assessed crude associations between albuminuria, eGFR or hypertension and log netrin-1 using simple linear regression. Model 2 included effects from model 1 plus age and sex adjustment in a multiple regression to assess albuminuria, eGFR or hypertension effects independent of age and sex differences in log netrin-1. Model diagnostics on residuals were performed for all analyses. Significance was determined at alpha = 0.05. SAS© 9.3 analytics software (SAS Institute, Inc., Cary, NC, USA) was used for all analyses.
Results
Characteristics of subjects
Table I presents the general characteristics, clinical parameters and their significance of the different study groups. A total of 87 patients with diabetes and 42 control subjects participated in this study. Patients in the macroalbuminuric group were more often male and smokers, more often had a history of cardiovascular disease, used more antihypertensive medications and had a lower eGFR when compared with normoalbuminuric diabetic subjects. We also analyzed the data based on type of diabetes. Supplementary tables I and II show details on the different clinical parameters and their significance. Type 2 diabetic patients with normoalbuminuria had significantly higher netrin-1 excretion as compared to control than type 1 diabetes with normoalbuminuria.
Table I.
Relationship of albumin creatinine categories based on ACR with variables of interest
| Dependent Variables | Albumin creatinine | p-value | |||
|---|---|---|---|---|---|
| Control | Normo | Micro | Macro | ||
| n=42 | n=40 | n=38 | n=9 | ||
| Sex, n (% Female) # | 20 (48) | 10 (25) | 13 (34) | 2 (22) | 0.15 |
| Age, y * | 53.3 ± 13.1 b | 59.0 ± 12.4 a,b | 64.0 ± 11.9 a | 62.3 ± 15.6 a,b | 0.0028 |
| Weight, kg * | 84.3 ± 18.3 | 91.3 ± 14.8 | 93.9 ± 18.0 | 92.4 ± 10.3 | 0.065 |
| Height, m * | 1.76 ± 0.08 | 1.75 ± 0.07 | 1.72 ± 0.09 | 1.73 ± 0.09 | 0.092 |
| BMI, kg/m2 * | 27.2 ± 6.3 b | 30.0 ± 5.3 a,b | 31.9 ± 6.4 a | 31.5 ± 5.2 a,b | 0.0055 |
| SBP, mmHg * | 131.9 ± 16.7 b | 138.7 ± 15.4 b | 141.8 ± 17.5 b | 155.5 ± 15.7 a | 0.0007 |
| DBP, mmHg * | 74.1 ± 9.1 | 77.7 ± 10.2 | 76.7 ± 9.5 | 80.2 ± 12.4 | 0.24 |
| Hypertension, n (% Yes) # | 14 (33) | 33 (85) | 35 (97) | 8 (100) | <0.0001 |
| Hypertension drug use, n (% Yes) # | 6 (14) | 27 (71) | 33 (94) | 8 (100) | <0.0001 |
| Smoking, n (% Yes) # | 9 (21) | 4 (10) | 11 (29) | 3 (33) | 0.16 |
| Cardiovascular history, n (% Yes) # | 0 (0) | 9 (23) | 16 (42) | 6 (67) | <0.0001 |
| Statin use, n (% Yes) # | 8 (19) | 17 (45) | 21 (60) | 6 (75) | 0.0006 |
| HbA1c, % mean ± SD * | 5.37 ± 0.33 b | 7.67 ± 1.06 a | 7.69 ± 1.33 a | 7.74 ± 0.76 a | <0.0001 |
| Serum creatinine, µ mol/l ** | 72 (54, 97) b | 74 (54, 155) b | 89 (55, 168) b | 107 (72, 312) a | <0.0001 |
| eGFR * | 86.3 ± 14.3 a | 85.3 ± 21.3 a | 72.7 ± 21.8 a | 51.8 ± 27.3 b | <0.0001 |
| Urinary creatinine, mmol/l** | 11.4 (3.7, 24) a | 8.5 (1.5, 30) a,b | 6.6 (2.6, 18) b | 5.7 (2.3, 14) b | <0.0001 |
| Urinary albumin, mg/l ** | 9.4 (2.2, 82) c | 5.5 (2.1, 21) c | 57 (16, 564) b | 515 (171, 1738) a | <0.0001 |
| Albumin creatinine, mg/mmol ** | 0.56 (0.29, 7.22) c | 0.70 (0.17, 3.33)c | 8.38 (3.40, 31.6) b | 115.4 (41.8, 262.1)a | <0.0001 |
| Urine netrin-1, pg/ml ** | 430 (229, 1087) b | 648 (111, 2636)a,b | 676 (242, 1644) a | 921 (305, 2555) a | 0.0001 |
ACR, albumin-creatinine ratio; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; eGFR, estimated glomerular filtration rate.
column %, p-value reflects result from chi-square test of Albumin creatinine group proportion equality
mean ± SD, p-value reflects result from one-way ANOVA test for albumin creatinine group mean equality
pairs of means with different letters are significantly different, p < 0.05, Tukey’s Honestly Significant Difference (HSD) test
log value used in analysis, median (min, max) represented
Damage-marker concentrations
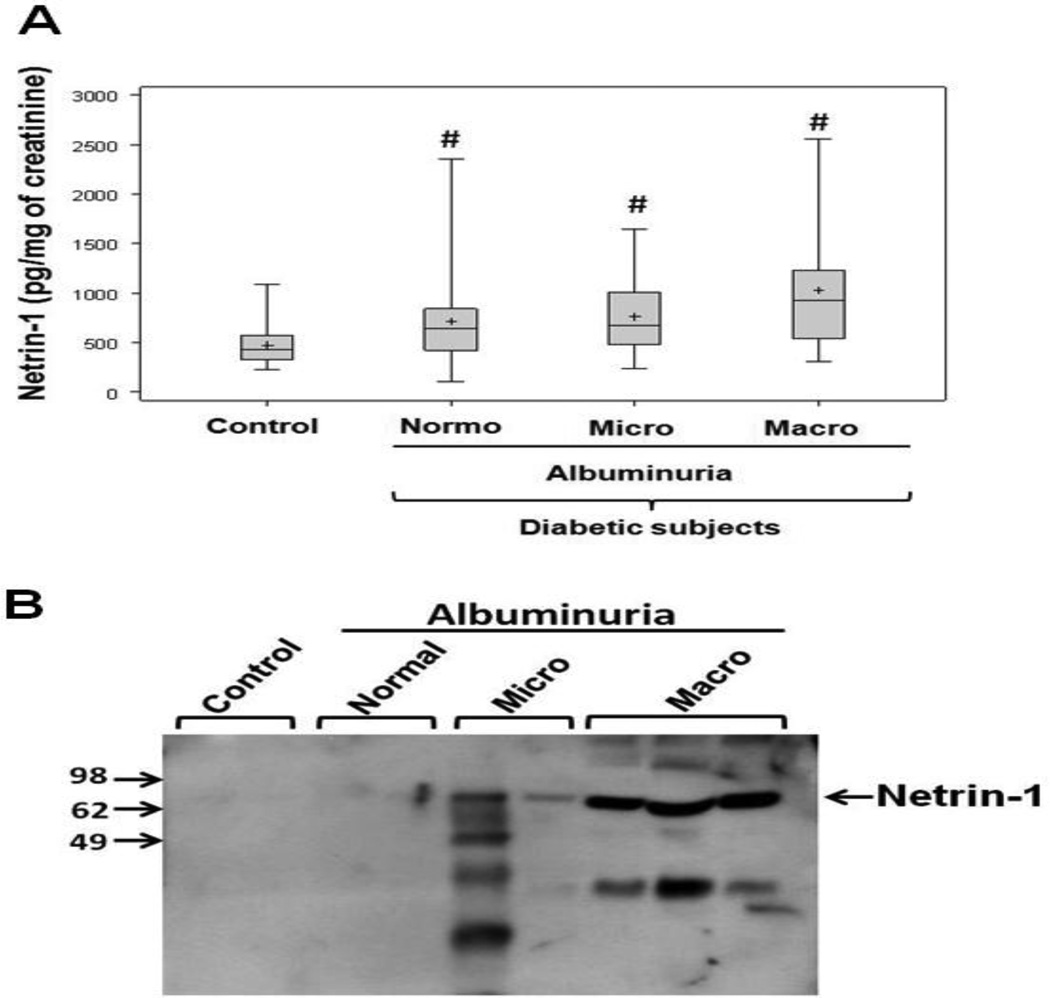
Netrin-1 concentrations in non-diabetic controls and in diabetic patients are shown in Figure 1A. Urinary netrin-1 concentrations were significantly elevated in diabetic patients with normoalbuminuria compared to controls, suggesting early tubular damage and the usefulness of netrin-1 as an early marker of nephropathy. Moreover, netrin-1 levels were elevated in later stages of nephropathy, including patients with microalbuminuria and macroalbuminuria as compared to controls. There was no significant difference in netrin-1 levels between the different categories of albuminuria. Consistent with ELISA-based quantification, western blot analysis also showed a similar pattern of increase (Fig. 1B).
Figure 1. Urinary netrin-1 in different groups.
A. Urinary netrin-1 was quantified by enzyme-linked immunosorbent assay (ELISA) as described in Materials and Methods. The central line represents the median, boxes span from the 25th to 75th percentiles, and the error bars extend from the 10th to 90th percentiles. #, p < 0.05 vs. control. B. Western blot analysis of netrin-1 in human urine from controls and different groups of diabetic patients. Netrin-1 excretion is increased with albuminuria.
Association of damage marker netrin-1 with albuminuria and eGFR in diabetic patients
Associations between netrin-1 and albuminuria were analyzed in all diabetic patients. Urinary netrin-1 was found to be significantly associated with albuminuria in the crude model (Table II) without significant change after adjusting for age and sex (model 2). Urinary netrin-1 was also significantly associated with eGFR in crude models (model 1), and after adjusting for age and sex the result did not change. In contrast, urinary netrin-1 was found to be significantly associated with hypertension in the crude model (Table II) but not when it was adjusted for age and sex. Subjects in the sample with hypertension were significantly older than those without hypertension (63 ± 12 vs. 49 ± 12, p < 0.0001) so that age and hypertension were confounded.
Table II.
Multivariable regression analysis of damage marker netrin-1 vs. albuminuria or eGFR or hypertension
| Log albumin creatinine | eGFR | Hypertension | ||||
|---|---|---|---|---|---|---|
| Standard β | p-value | Standard β | p-value | Standard β | p-value | |
| Model 1 | 0.37 | <0.0001 | −0.42 | <0.0001 | 0.25 | 0.0043 |
| Model 2 | 0.30 | 0.0002 | −0.27 | 0.0035 | 0.09 | 0.30 |
Bold print indicates associations between log netrin-1 and albuminuria or eGFR or hypertension that reach statistical significance. Model 1: crude; model 2: with adjustment for age and sex.
eGFR, estimated glomerular filtration rate.
In addition, a significant (p < 0.001) positive association was seen between urinary netrin-1 and the presence of cardiovascular history, ACR, serum creatinine, and HbA1c, and a negative correlation with urine creatinine at univariate analysis. Age and height were also significantly associated with urine netrin-1 levels (positively and negatively, respectively). It is interesting to note that, when data were analyzed based on type 1 and type 2 diabetes to determine whether albuminuria correlated with a reduction in eGFR, only type 1 diabetic patients showed a significant association, but not type 2 diabetic patients (Supplementary table III). However, the sample size in type 2 diabetes with macroalbuminuria is too small and the insignificance may be due to the sample size.
Discussion
Netrin-1 was recently identified as a biomarker of acute kidney injury [9, 10] and found to be elevated in an animal model of diabetic nephropathy [12]. Both animal model and human studies have demonstrated that netrin-1 level is elevated in urine in many forms of acute kidney injury. However, its usefulness for early diagnosis of chronic kidney disease (CKD) in humans is unknown. This is the first study to evaluate netrin-1 as an early diagnostic biomarker of CKD in diabetic patients. Our results demonstrate that netrin-1 excretion is significantly elevated early in patients without microalbuminuria and at all later stages of diabetic nephropathy as compared to controls. Despite the common views that diabetic nephropathy is primarily a glomerular disease, we found high levels of the tubular injury marker netrin-1 in urine in patients without microalbuminuria. These results suggest that tubular damage may precede glomerular injury in chronic kidney injury and warrant further study.
Our results are in line with a recent report that several proximal tubular epithelial markers are increased in DKD [7, 13]. Kidney injury molecule-1 and N-acetyl-β-D-glucosaminidase were elevated in urine of diabetic subjects without micro- or macroalbuminuria [7, 8]. Similarly, we found in the present study that netrin-1 is already increased in diabetic subjects with normoalbuminuria and normal eGFR when compared to non-diabetic control subjects, suggesting that an early tubular defect may precede glomerular defects in developing micro- and macroalbuminuria. This view is further supported by recent studies demonstrating that the severity of DKD depends not only on the severity of glomerular lesions but also on the degree of tubulointerstitial damage. In fact, a histological study [14] showed that proximal tubular basement membrane width is thickened in normoalbuminuric diabetic patients compared with healthy controls, and another study [15] in microalbuminuric diabetic patients with similar eGFR showed that only 29 % had typical histological glomerular features of diabetic nephropathy, whereas 42 % had severe tubulointerstitial lesions disproportionate to the mild glomerular involvement. Even urinary albumin excretion, long believed to be a consequence of glomerular damage, is now also recognized as a tubular injury biomarker secondary to nephrotoxins by the US Food and Drug Administration and the European Medicines Agency [16]. Therefore, it is important to recognize that albuminuria is a marker of tubular injury in addition to increase in glomerular permeability. The importance of tubular epithelial cells in developing increased levels of albuminuria is reinforced by the recent observation that a mutation in cubilin, a receptor for albumin to mediate its endocytosis, caused severe proteinuria in humans [17]. In addition, a proximal tubular epithelial cell-specific cubilin knockout mice also developed albuminuria [5].
Recent studies suggest a protective role of netrin-1 in diabetic nephropathy and other diabetic complications. Administration of recombinant netrin-1 or overexpression of netrin-1 improved conductance velocity of nerves and increased angiogenesis in diabetic mice [18]. More recently, diabetic retinopathy was associated with increased levels of netrin-1 in the vitreous fluid [19, 20]. Studies from our laboratory had shown that epithelial cell-derived netrin-1 may reduce albuminuria and interstitial fibrosis through suppression of inflammation and increased uptake of albumin [21]. A similar role was seen in an acute model of kidney injury [22–24]. The signal for the induction of netrin-1 in the tubular epithelial cells of the diabetic kidney is unknown. Our in vitro studies have determined that hyperglycemia down-regulates netrin-1 production, whereas high levels of albumin induce netrin-1 production from proximal tubular epithelial cells [25]. This protein-induced increase in netrin-1 production was mediated through the ERK and Akt kinase pathways by enhancing translation [25]. Western blot analysis of mouse urine shows that a large amount of albumin is excreted even under basal condition as well in diabetes suggesting that increased albumin traffic in tubules due to dysfunction of proximal tubules in early diabetes may trigger netrin-1 production as a compensatory mechanism. Netrin-1 is shown to increase albumin uptake in proximal tubular epithelial cells [21]. In addition, overexpression of netrin-1 in proximal tubular epithelial cells suppressed albuminuria [21]. Therefore, both tubular epithelial injury and increased albumin may induce netrin-1 expression in tubular epithelial cells to protect them from injury and to increase albumin uptake. Animal studies in acute and chronic models of kidney injury show that the source of netrin-1 are the proximal tubular epithelial cells [9] (Journal of Nephrology 2013, in press).
There are several limitations to the current study. First, our clinical data are from a single-center, cross-sectional study. A more controlled longitudinal study design with serial follow-up would be more valuable for understanding the time course of netrin-1 production and its usefulness as an early diagnostic biomarker of diabetic nephropathy in humans. Second, although our human data suggest a correlation between urinary netrin-1 levels and the development of nephropathy, there was no significant difference among the different albuminuria groups in our diabetic patients. This could be due to several reasons, such as the small sample size, random sample collection, unknown kinetics of netrin-1 secretion and the variability of tubular defects in these patients.
In conclusion, we have demonstrated for the first time that urinary excretion of the proximal tubular marker netrin-1 is elevated early during diabetes in humans and that netrin-1 may be a useful early biomarker predicting the development of renal injury in diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health grant R01 grant (7R01DK083379-02) to GR.
Footnotes
STATEMENT OF COMPETING FINANCIAL INTERESTS
Dr. Ramesh holds a patent for the discovery of netrin-1 as an early diagnostic biomarker of disease and injury. All other authors have disclosed no competing interest.
REFERENCES
- 1.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983;25:496–501. doi: 10.1007/BF00284458. [DOI] [PubMed] [Google Scholar]
- 2.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M. The Early Natural History of Nephropathy in Type 1 Diabetes. Diabetes. 2005;54:2164–2171. doi: 10.2337/diabetes.54.7.2164. [DOI] [PubMed] [Google Scholar]
- 4.Gekle M. Renal Proximal Tubular Albumin Reabsorption: Daily Prevention of Albuminuria. News Physiol Sci. 1998;13:5–11. doi: 10.1152/physiologyonline.1998.13.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R. Cubilin Is Essential for Albumin Reabsorption in the Renal Proximal Tubule. Journal of the American Society of Nephrology. 2010;21:1859–1867. doi: 10.1681/ASN.2010050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009;20:489–494. doi: 10.1681/ASN.2008050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nauta FL, Boertien WE, Bakker SJL, van Goor H, van Oeveren W, de Jong PE, Bilo H, Gansevoort RT. Glomerular and Tubular Damage Markers Are Elevated in Patients With Diabetes. Diabetes Care. 2011;34:975–981. doi: 10.2337/dc10-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, Krolewski AS, Bonventre JV. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-[beta]-D-glucosaminidase. Kidney Int. 2011;79:464–470. doi: 10.1038/ki.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reeves WB, Kwon O, Ramesh G. Netrin-1 and kidney injury. II. Netrin-1 is an early biomarker of acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F731–F738. doi: 10.1152/ajprenal.00507.2007. [DOI] [PubMed] [Google Scholar]
- 10.Ramesh G, Krawczeski CD, Woo JG, Wang Y, Devarajan P. Urinary netrin-1 is an early predictive biomarker of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2010;5:395–401. doi: 10.2215/CJN.05140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, Zeeuw Dd, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 12.White JJ, Mohamed R, Jayakumar C, Ramesh G. Tubular injury marker netrin-1 is elevated early in experimental diabetes. J Nephrol. 2013;0 doi: 10.5301/jn.5000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu WJ, Xiong SL, Fang YG, Wen S, Chen ML, Deng RT, Zheng L, Wang SB, Pen LF, Wang Q. Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: a cross-sectional study. Endocrine. 2011 doi: 10.1007/s12020-011-9509-7. [DOI] [PubMed] [Google Scholar]
- 14.Brito PL, Fioretto P, Drummond K, Kim Y, Steffes MW, Basgen JM, Sisson-Ross S, Mauer M. Proximal tubular basement membrane width in insulin-dependent diabetes mellitus. Kidney Int. 1998;53:754–761. doi: 10.1046/j.1523-1755.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 15.Fioretto P, Mauer M, Brocco E, Velussi M, Frigato F, Muollo B, Sambataro M, Abaterusso C, Baggio B, Crepaldi G, Nosadini R. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39:1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y, Jin H, Holder D, Ozer JS, Villarreal S, Shughrue P, Shi S, Figueroa DJ, Clouse H, Su M, Muniappa N, Troth SP, Bailey W, Seng J, Aslamkhan AG, Thudium D, Sistare FD, Gerhold DL. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotechnol. 2010;28:470–477. doi: 10.1038/nbt.1624. [DOI] [PubMed] [Google Scholar]
- 17.Ovunc B, Otto EA, Vega-Warner V, Saisawat P, Ashraf S, Ramaswami G, Fathy HM, Schoeb D, Chernin G, Lyons RH, Yilmaz E, Hildebrandt F. Exome sequencing reveals cubilin mutation as a single-gene cause of proteinuria. J Am Soc.Nephrol. 2011;22:1815–1820. doi: 10.1681/ASN.2011040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Xia X, Xiong S, Le Y, Xu H. Intravitreous high expression level of netrin-1 in patients with proliferative diabetic retinopathy. Yan.Ke.Xue.Bao. 2011;26:35–42. doi: 10.3969/j.issn.1000-4432.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian XF, Xia XB, Xiong SQ, Jiang J, Liu D, Liu JL. Netrin-1 overexpression in oxygen-induced retinopathy correlates with breakdown of the blood-retina barrier and retinal neovascularization. Ophthalmologica. 2011;226:37–44. doi: 10.1159/000324474. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed R, Jayakumar C, Ranganathan PV, Ganapathy V, Ramesh G. Kidney Proximal Tubular Epithelial-Specific Overexpression of Netrin-1 Suppresses Inflammation and Albuminuria through Suppression of COX-2-Mediated PGE2 Production in Streptozotocin-Induced Diabetic Mice. The American Journal of Pathology. 2012;181:1991–2002. doi: 10.1016/j.ajpath.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajasundari A, Pays L, Mehlen P, Ramesh G. Netrin-1 overexpression in kidney proximal tubular epithelium ameliorates cisplatin nephrotoxicity. Lab Invest. 2011;91:1717–1726. doi: 10.1038/labinvest.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Reeves WB, Pays L, Mehlen P, Ramesh G. Netrin-1 overexpression protects kidney from ischemia reperfusion injury by suppressing apoptosis. Am J Pathol. 2009;175:1010–1018. doi: 10.2353/ajpath.2009.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185:3750–3758. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- 25.Jayakumar C, Mohamed R, Ranganathan PV, Ramesh G. Intracellular Kinases Mediate Increased Translation and Secretion of Netrin-1 from Renal Tubular Epithelial Cells. PLoS ONE. 2011;6:e26776. doi: 10.1371/journal.pone.0026776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.