Summary
Background
Thrombosis is a marker of poor prognosis in individuals with solid tumors. The expression of tissue factor (TF) on the cell surface membrane of malignant cells is a pivotal molecular link between activation of coagulation, angiogenesis, metastasis, aggressive tumor behavior and poor survival. Interestingly, thrombosis is associated with shortened survival in solid, but not in lymphoid neoplasias.
Objectives
We sought to study whether the lack of impact of thrombosis on survival in lymphoid neoplasias could be due to a lack of tumor-derived TF expression.
Methods
We analyzed TF gene (F3) expression in lymphoid (N=114), myeloid (N=49) and solid tumor (N=856) cell lines using the publicly available dataset from the Broad-Novartis Cancer Cell Line Encyclopedia (http://www.broadinstitute.org/ccle/home), and in 90 patient-derived lymphoma samples. TF protein expression was studied by immunohistochemistry (IHC).
Results
In sharp contrast to wide F3 expression in solid tumors (74.2%), F3 was absent in all low and high grade T- and B-cell lymphomas, and in most myeloid tumors, except for select acute myeloid leukemias with monocytic component. IHC confirmed the absence of TF protein in all indolent and high-grade B-cell (0/90) and T-cell (0/20) lymphomas, and acute leukemias (0/11).
Conclusions
We show that TF in lymphomas does not derive from the malignant cells, since these do not express either F3 or TF protein. Therefore, it is unlikely that thrombosis in patients with lymphoid neoplasms is secondary to tumor-derived tissue factor.
Keywords: tissue factor, lymphomas, leukemias, cancer, hematological malignancies
Introduction
The occurrence of venous thromboembolism (VTE) in individuals with solid tumors is associated with more than a three-fold higher risk of short-term death, as compared to cancer patients without thrombosis [1,2]. The expression of tissue factor (TF) by cancer cells is one of the key underlying mechanisms linking thrombosis with aggressive tumor behavior [3]. Indeed, TF, a 47 kDa cell-surface trans-membrane glycoprotein that triggers the activation of coagulation by binding to activated factor VII [4], is expressed in variable degrees by most solid tumors, and has been shown to be involved in cell signaling, angiogenesis, inhibition of apoptosis, tumor growth, and metastasis [5,6]. Recent studies show that targeting TF is feasible and may both prevent thrombosis and down-modulate tumor aggressiveness [7,8]. Therefore, accurate information on the types of tumors that express TF, and distinction from those that do not, becomes relevant.
The pathophysiology of thrombosis in individuals with hematological malignancies may differ from that of patients with solid tumors. Interestingly, the occurrence of VTE does not have a negative impact on survival in individuals with multiple myeloma (MM), despite the high rate of thrombosis in the setting of chemotherapy [9,10]. Similarly, VTE in individuals with lymphoma is seen predominantly in association with chemotherapy and, as shown in a large prospective study, does not impact survival [11]. These findings suggest that activation of coagulation in lymphoid-cell-derived tumors may not be driven by TF expressed by neoplastic cells. Indeed, we recently described the absence of expression of the gene that encodes for TF (F3) in MM cell lines and in primary patient samples [12]. In order to determine whether other lymphoid neoplasms express TF, we analyzed F3 and protein expression levels in low- and high-grade lymphoid tumors, as well as in representative myeloid and solid tumors.
Materials and Methods
Gene expression levels were analyzed using the publicly available dataset of the Broad-Novartis Cancer Cell Line Encyclopedia (http://www.broadinstitute.org/ccle/home) comprising 847 solid-tumor cell lines and 114 lymphoid-tumor (84 lymphomas and lymphoid leukemias and 30 myelomas) and 49 acute and chronic myeloid leukemia cell lines. Expression data for each cell line was generated using the Affymetrix HG-U133_Plus_2 arrays. Raw intensity values were generated using Robust Multi-array Average (RMA) algorithm, inter- and intra-array normalized and log2 transformed. RMA log2 values were used to estimate gene expression as follows: cell lines with RMA log2 values below 6.5 were categorized as not expressing F3; cell lines with values between 6.5 and 7.5 were considered as having marginal expression; and cell lines with values above 7.5 were considered as expressing F3 in intermediate to high levels. Immunohistochemistry (IHC) staining for TF protein was performed as previously described [12], using standard procedures on tumor tissue microarrays (TMA) on representative solid-tumor biopsies and myeloid leukemias, including 1 acute promyelocytic leukemia, 1 myelo-monocytic leukemia and 9 leukemias without a monocytic component. In addition 129 lymphoid tumors including 10 precursor-cell acute lymphoblastic leukemias, 99 mature B-cell (9 low-grade and 90 high-grade), and T-cell (20) lymphomas were studied for TF expression. Briefly, slides were deparaffinized for 4 minutes at 72°C using xylene-free dewaxing reagent (EZprep, Ventana Medical Systems) and stained for IHC using a Bench Mark ULTRA automated slide stainer (Ventana). Following CC1 alkaline antigen retrieval (95°C, 8 min.), TMAs were incubated at 36°C for 4 minutes with primary rabbit polyclonal anti-human TF antibody (dilution 1:75,FL-295, Santa Cruz Biotechnology). Ventana ultraView Universal polymer-based diaminobenzidine (DAB) detection kit was used for visualization of antibody localization. TMAs were counterstained with Harris haematoxylin, and mounted with non-aqueous medium. Patient samples were obtained under informed consent at the Mayo Clinic (primary cell lines were prepared from tumor biopsies for the study of F3 expression from 90 high grade lymphomas), and at the Instituto Nacional de Ciencias Médicas y Nutrición (tissue microarrays were prepared from diagnostic lymph node or bone marrow biopsies for the study of TF protein expression by IHC). The study was conducted under IRB approval.
Results
In order to determine whether TF is relevant in lymphoid neoplasia biology, we studied TF gene (F3) and protein expression in these tumors. We found that F3 was absent in all precursor and mature lymphoid tumor cell lines including low- and high-grade lymphomas, acute lymphoblastic leukemias (except for a single cell line with low marginal F3 expression, which interestingly derives from a chronic myeloid leukemia in blast crisis) (Fig. 1). F3 expression was also absent in primary patient samples from 90 high-grade non-Hodgkin’s lymphomas, including diffuse large B-cell (DLBCL) and Burkitt´s lymphomas (data not shown). This contrasted sharply with the common expression levels of F3 in the solid tumors 74.2% (high in 526/61.5% and marginal in 109/12.7%) (Fig. 1). Notably, Hodgkin´s lymphomas (HL) showed frequent F3 expression (see supplemental table for GEP values for each tumor).
Figure 1. Gene expression analysis of TF gene (F3).
The number of cases of each tumor entity are indicated in parenthesis. F3 expression (RMA log2 expression values) range is depicted in red bars for solid tumors and in green bars for hematological (both lymphoid and myeloid) neoplasias. Estimative of expression levels are as follows: RMA log2 values below 6.5 were associated with lack of expression; values between 6.5 and 7.5 (gray box) as marginally expressed; and values above 7.5 were considered as intermediate to high expression. Results clearly show absence of F3 expression in cell lines established form all lymphoid neoplasias including progenitor cell-derived acute leukemias, and mature B and T-cell lymphomas. Myeloid derived acute leukemias are negative except for 25%, which show marginal F3 expression. Finally, a subset of Hodgkin´s lymphomas, which are predominantly (99%) composed of microenvironment, express F3. The findings in hematological neoplasms contrast with the significantly higher F3 expression levels found in most solid-tumor cell lines.
Abbreviations: Acute myeloid leukemia (AML), B-cell acute lymphoblastic leukemia (B-ALL), chronic myeloid leukemia (CML), T-cell acute lymphoblastic leukemia (T-ALL), mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), multiple myeloma (MM).
Gene expression findings were validated using IHC. TF protein was highly expressed in a variety of representative solid tumors, except for renal cell carcinoma (Fig. 2, panel A). In contrast, TF was not expressed in any of the lymphoid neoplasias studied of either B-cell or T-cell origin. These included all acute lymphoblastic leukemias, peripheral T-cell lymphomas, high-grade B-cell Burkitt´s and DLBCL (25 germinal center, and 64 non-germinal center phenotype), and low-grade lymphomas (extranodal marginal zone, follicular, mantle-cell lymphoma and small lymphocytic lymphoma) (Fig. 2, panel B).
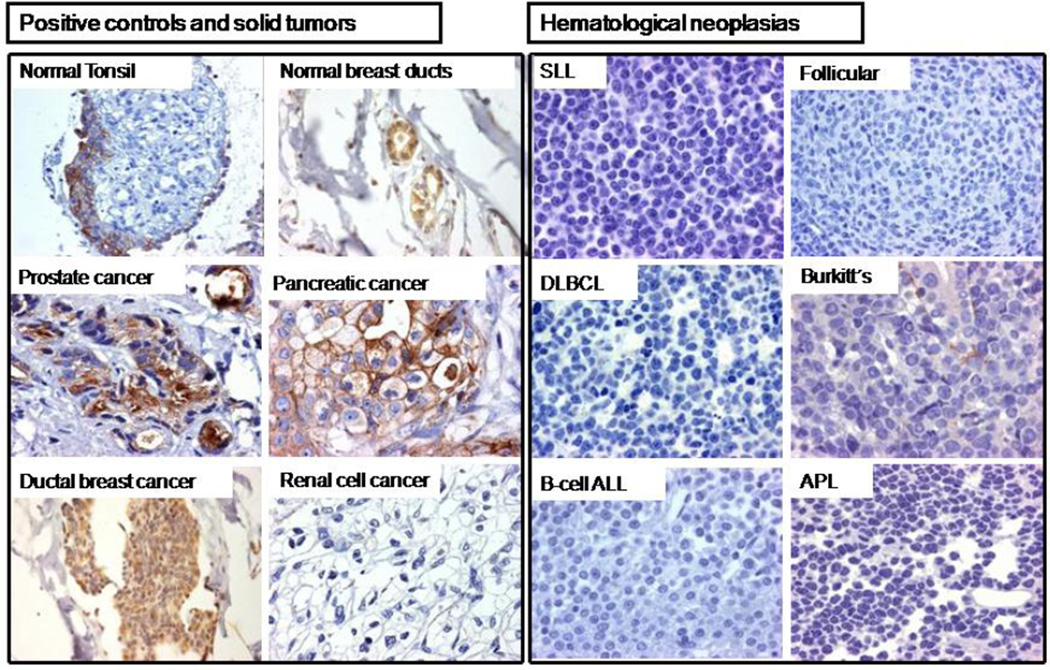
Figure 2. Tissue factor expression in representative tumors by immunohistochemistry (IHC).
(A) Tonsillar epithelium and normal breast ducts were used as positive controls for TF expression, while tonsillar subepithelial connective tissue was used as negative control. TF was expressed by solid tumors (prostate, pancreatic, and ductal breast adenocarcinoma), but not by clear cell renal cell carcinoma. (B) All 129 lymphoid neoplasias were negative for TF expression, including 89 high-grade diffuse large B-cell lymphoma (DLBCL) patient samples (25 with germinal center and 64 with non-germinal center phenotype), as well as 9 low-grade lymphomas including small lymphocytic (SLL) and follicular lymphomas, 10 acute lymphoblastic leukemias (ALL), and 20 peripheral T-cell lymphomas. In Burkitt’s lymphoma, the positive TF staining seen in the basement membrane of gastric glands contrasts with the absence of TF in neoplastic cells. TF was absent in this case of acute promyelocytic leukemia (APL).
Regarding myeloid-derived neoplasias, F3 expression was absent in chronic granulocytic leukemia with marginal to inter-median expression in a fourth of acute myeloid leukemias (positive leukemias included 8 with monocytic or myelomonocytic blasts, one acute eosinophilic leukemia, and one promyelocytic leukemia). None of the acute myeloid leukemias studied stained for TF by IHC.
Discussion
Activation of coagulation with or without the development of overt thrombosis is a frequent occurrence in individuals with cancer. Lower-extremity venous thrombosis and pulmonary embolism in individuals with solid tumors constitutes a clinical indicator of an underlying biological advantage to cancer growth and metastasis, as evidenced by worse outcomes in individuals with solid tumors who develop VTE [2]. Patients with hematological malignancies also develop thrombosis particularly in the setting of treatment [13,14]. However, prospective studies have shown that survival is not affected by the occurrence of thrombosis in individuals with lymphoid-cell-derived tumors, including myelomas and lymphomas [9,10]. A recent, large (N=686) prospective study of newly diagnosed Hodgkin´s and non-Hodgkin´s lymphoma reported VTE in 8% and 6.7% of patients respectively, with no VTE-related deaths, and no difference in overall survival in patients with or without VTE. This suggests that the hypercoagulable state in hematological malignancies is independent of neoplastic behavior, and that the mechanisms leading to thrombosis may differ from those in solid tumors.[11].
There are several molecular links between a prothrombotic state and an oncogenic phenotype [6,15]. Both in vitro and in vivo experiments point to the expression of TF by malignant cells as one of the key factors in both thrombosis and aggressive tumor behavior [5–8,16–18]. Furthermore, elevated circulating microparticle-associated TF activity has been shown to correlate with poorly differentiated and invasive tumors and poor survival [19].TF has a well characterized role in the inhibition of apoptosis, induction of tumor cell proliferation, angiogenesis, and metastasis, through both clotting-dependent and independent mechanisms [6,7,20]. As reported by others, we too found TF to be expressed at different levels by most solid tumors, with variation within distinct tumor types (Fig. 1). In addition, however, we show that neither F3 nor TF are found in precursor or mature B-or T-cell lineage lymphomas and leukemias. As background, Mohan Rao showed that TF protein was absent by IHC in a third of epitehlial neoplasias, and in most non-epithelial tumors including 4 lymphomas of unspecified subtype [21]. These findings are in line with the fact that under physiologic conditions, TF is not expressed by lymphocytes, although a subpopulation of lymphocytes of B-cell origin may express functional TF in vitro, in response to stimulation with phorbol myristate [22].
Interestingly, we found that Hodgkin´s lymphomas (HL) showed F3 expression in approximately a third of cases, which may be explained by the fact that malignant cells make up only 1% of the tumor bulk in HL [23]. TF expression likely corresponds to cells from the tumor microenvironment. In line with this hypothesis, the high expression of TF from peripheral blood monocytes of HL patients has been reported to be associated with VTE [24].
Regarding myeloid-cell-derived tumors, F3 was expressed by a fourth of acute myeloid leukemias (AML), most of which had a monocytic component. The only acute promyelocytic leukemia included, expressed high levels of TF, a finding previously reported by others, and thought to contribute to the procoagulant state in these malignancies [25]. Although TF protein was absent by IHC in all patient-derived bone marrow biopsies with AML, we believe that it is likely that including a larger number of samples would likely detect a subset of positive cases, since tumor-cell-derived TF has been reported in leukemias of monocytic or promyelocytic origin. Furthermore, TF has been shown to be present in the circulation as TF-bearing microparticles derived from myeloid blasts [26,27].
The main weakness of this study is that the data are descriptive, and based on current knowledge of the role of TF in cancer, yet no mechanistic or experimental data are provided. The other important limitation is that we do not evaluate malignant behavior or thrombosis in this study in relation to TF expression. Further work on the mechanisms of thrombosis and the cell of origin of TF in solid versus hematological tumors is warranted.
In conclusion, the absence of TF in hematological tumors contrasts sharply with the frequency of its expression in solid tumors. This fact is relevant, since targeting tumor-associated TF as is currently proposed for solid tumors (through the use of immuno-conjugates, anti-TF antibodies, TF pathway inhibitors, targeted photodynamic therapy, and microRNAs), may not be useful in lymphoid neoplasias [4,28,29]. This does not rule out however, a role for TF from non-neoplastic cell sources (activated endothelium, platelets and or monocytes) as a result of host response to cancer or its treatment, in the development of thrombosis or in the induction of malignant tumor behavior [30]. Indeed blood-borne microparticle-associated TF is increased across individuals with lymphomas and leukemias, and TF mRNA levels have been shown by others to be significantly increased in patients with lymphoma undergoing chemotherapy [31,32]. Our findings suggest that the source of this circulating TF is most likely not tumor-cell-derived. This opens the question as to whether it is mainly the TF produced by the neoplastic cells that drives malignant behavior in solid tumors. To our knowledge, this is the first report showing that F3 and TF are not expressed by the neoplastic cells in T- or B-cell lymphoid malignancies, or by most myeloid derived tumors, adding to the understanding of cancer–related thrombosis.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the Broad Institute, and the Novartis Institutes for Biomedical Research for making available the genomics data of The Cancer Cell Line Encyclopedia (CCLE) project. E.B. is a recipient of the Marriott Specialized Workforce Development Awards in Individualized Medicine, The Henry Predolin Foundation Career Development Award and the George Haub Family Career Development Award Fund in Cancer Research.
Sources disclosure: Rafael Fonseca is a Clinical Investigator of the Damon Runyon Cancer Research Fund. This work is supported by grants SPORE CA90297052, P01 CA62242, R01 CA83724, ECOG CA 21115T, Predolin Foundation, Mayo Clinic Cancer Center and the Mayo Foundation.
We thank Mrs. Lara Karchmar M.A., Ed for proofreading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions
GC-M, EB and RF: made substantial contributions to the concept and/or study design, performed research and drafted the manuscript.d
CL-M and ALG-L: performed research and contributed to study design.
Disclosure of Conflicts of Interest
No disclosures.
References
- 1.Levitan N, Dowlati A, Remick S, Tahsildar HI, Sivinski LD, Beyth R, Rimm AA. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine. 1999;78:285–291. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Sørensen HT, Mellemkjær L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 3.Van den Berg YW, Osanto S, Reitsma PH, Versteeg HH. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood. 2012;119:924–932. doi: 10.1182/blood-2011-06-317685. [DOI] [PubMed] [Google Scholar]
- 4.Bach R, Gentry R, Nemerson Y. Factor VII binding to tissue factor in reconstituted phospholipid vesicles: induction of cooperativity by phosphatidylserine. Biochemistry. 1986;25:4007–4020. doi: 10.1021/bi00362a005. [DOI] [PubMed] [Google Scholar]
- 5.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27:4834–4838. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boccaccio P, Comoglio PM. Genetic link between cancer and thrombosis. J Clin Oncol. 2009;27:4827–4833. doi: 10.1200/JCO.2009.22.7199. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira AS, Lima LG, Mariano-Oliveira A, Machado DE, Nasciutti LE, Andersen JF, Petersen LC, Francischetti IMB, Q Monteiro R. Inhibition of tissue factor by ixolaris reduces primary tumor growth and experimental metastasis in a murine model of melanoma. Thromb Res. 2012;130:e163–e170. doi: 10.1016/j.thromres.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole M, Bromberg M. Tissue factor as a novel target for treatment of breast cancer. Oncologist Tissue factor as a novel target for treatment of breast cancer. Oncologist. 2013;18:14–18. doi: 10.1634/theoncologist.2012-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zangari M, Barlogie B, Cavallo F, Bolejack V, Fink L, Tricot G. Effect on survival of treatment-associated venous thromboembolism in newly diagnosed multiple myeloma. Blood Coagul Fibrinol. 2007;18:595–598. doi: 10.1097/MBC.0b013e3281067fb2. [DOI] [PubMed] [Google Scholar]
- 10.Zangari M, Tricot G, Polavaram L, Zhan F, Finlayson A, Knight R, Fu T, Weber D, Dimopoulos MA, Niesvizky R, Fink L. Survival effect of venous thromboembolism in patients with multiple myeloma treated with lenalidomide and high-dose dexamethasone. J Clin Oncol. 2010;28:132–135. doi: 10.1200/JCO.2009.23.0169. [DOI] [PubMed] [Google Scholar]
- 11.Park LC, Woo SY, Kim S, Jeon H, Ko YH, Kim SJ, Kim WS. Incidence, risk factors and clinical features of venous thromboembolism in newly diagnosed lymphoma patients: Results from a prospective cohort study with Asian population. Thromb Res. 2012;130:e6–e12. doi: 10.1016/j.thromres.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Cesarman-Maus G, Braggio E, Maldonado H, Fonseca R. Absence of tissue factor expression by neoplastic plasma cells in multiple mieloma. Leukemia. 2012;26:1671–1674. doi: 10.1038/leu.2012.43. [DOI] [PubMed] [Google Scholar]
- 13.Falanga A, Marchetti M, Russo L. Venous thromboembolism in the hematologic malignancies. Curr Opin Oncol. 2012;24:702–710. doi: 10.1097/CCO.0b013e3283592331. [DOI] [PubMed] [Google Scholar]
- 14.Mateos MV. Management of treatment-related adverse events in patients with multiple myeloma. Cancer Treat Rev. 2010;36:S24–S32. doi: 10.1016/S0305-7372(10)70009-8. [DOI] [PubMed] [Google Scholar]
- 15.Garnier D, Magnus N, D'Asti E, Hashemi M, Meehan B, Milsom C, Rak J. Genetic pathways linking hemostasis and cancer. Thromb Res. 2012;129:S22–S29. doi: 10.1016/S0049-3848(12)70012-9. [DOI] [PubMed] [Google Scholar]
- 16.Abu Saadeh F, Norris L, O'Toole S, Mohamed BM, Langhe R, O'Leary J, Gleeson N. Tumour expresion of tissue factor and tissue factor pathway inhibitor in ovarian cancer- relationship with venous thrombosis risk. Thromb Res. 2013;132:627–634. doi: 10.1016/j.thromres.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Bharthuar A, Khorana AA, Hutson A, Wang JG, Key NS, Mackman N, Lyer RV. Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res. 2013;132:180–184. doi: 10.1016/j.thromres.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 18.Thaler J, Preusser M, Ay C, Kaider A, Marosi C, Zielinski C, Pabinger I, Hainfellner JA. Intratumoral tissue factor expression and risk of venous thromboembolism in brain tumor patients. Thromb Res. 2013 Feb;131(2):162–165. doi: 10.1016/j.thromres.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Thaler J, Ay C, Mackman N, Metz-Schimmerl S, Stift J, Kaider A, Müllauer L, Gnant M, Scheithauer W, Pabinger I. Microparticle-associated tissue factor activity in patients with pancreatic cancer: correlation with clinicopathological features. Eur J Clin Invest. 2013 Mar;43(3):277–285. doi: 10.1111/eci.12042. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez PM, Patierno SR, Rickles FR. Tissue factor and fibrin in tumor angiogenesis. Semin Thromb Hemost. 2004;30:31–44. doi: 10.1055/s-2004-822969. [DOI] [PubMed] [Google Scholar]
- 21.Mohan Rao V. Tissue factor as a tumor procoagulant. Cancer and Metastasis Rev. 1992;11:249–266. doi: 10.1007/BF01307181. [DOI] [PubMed] [Google Scholar]
- 22.Mechiche H, Cornillet-Lefebvre P, Nguyen P. A subpopulation of human B lymphocytes can express a functional Tissue Factor in response to phorbol myristate acetate. Thromb Haemost. 2005 Jul;94(1):146–154. doi: 10.1160/TH04-12-0845. [DOI] [PubMed] [Google Scholar]
- 23.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin's lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol. 2011;29:1812–1826. doi: 10.1200/JCO.2010.32.8401. [DOI] [PubMed] [Google Scholar]
- 24.Haire WD, Pirruccello SJ, Carson SD. Monocyte tissue factor in treated Hodgkin's disease. Leuk Lymphoma. 1994;12:259–263. doi: 10.3109/10428199409059597. [DOI] [PubMed] [Google Scholar]
- 25.Gheldof D, Mullier F, Bailly N, Devalet B, Dogné JM, Chatelain B, Chatelain C. Microparticle bearing tissue factor: A link between promyelocytic cells and hypercoagulable state. Thromb Res. 2013 Nov 16; doi: 10.1016/j.thromres.2013.11.008. pii: S0049-3848(13)00541-0. [DOI] [PubMed] [Google Scholar]
- 26.López-Pedrera C, Jardí M, del Mar Malagón M, Inglés-Esteve J, Dorado G, Torres A, Félez J, Velasco F. Involvement of tissue factor (TF) and urokinase receptor (uPAR) in bleeding complications of leukemic patients. Thromb Haemost. 1997;77:62–70. [PubMed] [Google Scholar]
- 27.Kwaan HC, Magalhães-Rego E. Role of microparticles in the hemostatic dysfunction in acute promyelocytic leukemia. Semin Thromb Hemost. 2010;36:917–924. doi: 10.1055/s-0030-1267045. [DOI] [PubMed] [Google Scholar]
- 28.Cole M, Bromberg M. Tissue factor as a novel target for treatment of breast cancer. Oncologist. 2013;18:14–18. doi: 10.1634/theoncologist.2012-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Zhang D, Wang S, Tao L, Wang A, Chen W, Zhu Z, Zheng S, Gao X, Lu Y. Holothurian glycosaminoglycan inhibits metastasis and thrombosis via targeting of nuclear factorκB/tissue factor/Factor Xa pathway in melanoma B16F10 c ells. PLoS One. 2013;8:e56557. doi: 10.1371/journal.pone.0056557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribatti D. Tissue factor in hematological malignancies. Leukemia. 2006;20:1356–1357. doi: 10.1038/sj.leu.2404269. [DOI] [PubMed] [Google Scholar]
- 31.Tang YL, Zhou Y, Wang CB, Qin Y, Feng J, Qin J. Predictive value of tissue factor-associated platelet microparticles in thrombosis of patients with lymphoma. Zhongguo Shi Yan Xue Ye XueZaZhi. 2012;20:325–328. [PubMed] [Google Scholar]
- 32.López-Pedrera C, Barbarroja N, Dorado G, Siendones E, Velasco F. Tissue factor as an effector of angiogenesis and tumor progression in hematological malignancies. Leukemia. 2006;20:1331–1340. doi: 10.1038/sj.leu.2404264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.