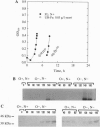
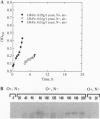
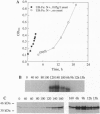
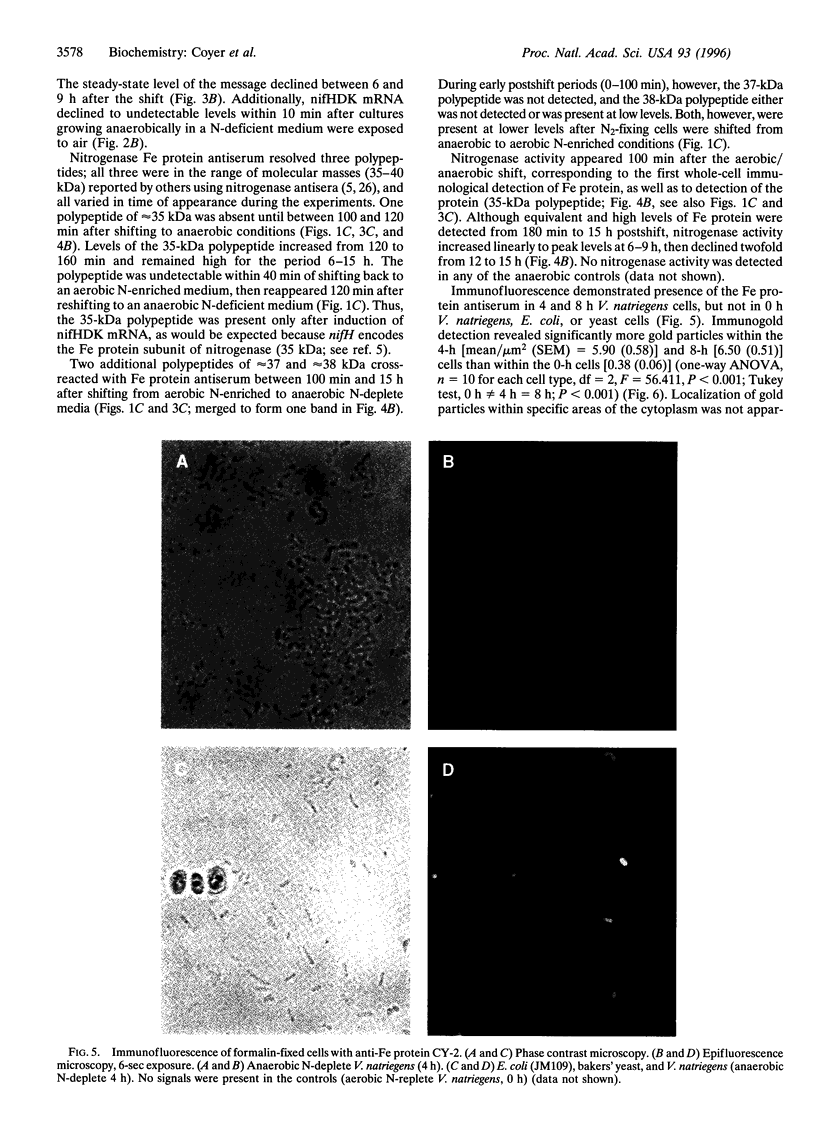
Abstract
Molecular and immunological techniques were used to examine N2 fixation in a ubiquitous heterotrophic marine bacterium, the facultative anaerobic Vibrio natriegens. When batch cultures were shifted from aerobic N-replete to anaerobic N-deplete conditions, transcriptional and post-translational regulation of N2 fixation was observed. Levels of nifHDK mRNA encoding the nitrogenase enzyme were highest at 140 min postshift and undetectable between 6 and 9 h later. Immunologically determined levels of nitrogenase enzyme (Fe protein) were highest between 6 and 15 h postshift, and nitrogenase activity peaked between 6 and 9 h postshift, declining by a factor of 2 after 12-15 h. Unlike their regulation in cyanobacteria, Fe protein and nitrogenase activity were present when nifHDK mRNA was absent in V. natriegens, indicating that nitrogenase is stored and stable under anaerobic conditions. Both nifHDK mRNA and Fe protein disappeared within 40 min after cultures were shifted from N2-fixing conditions (anaerobic, N-deplete) to non- N2-fixing conditions (aerobic, N-enriched) but reappeared when shifted to conditions favoring N2 fixation. Thus, unlike other N2-fixing heterotrophic bacteria, nitrogenase must be resynthesized after aerobic exposure in V. natriegens. Immunological detection based on immunoblot (Western) analysis and immunogold labeling correlated positively with nitrogenase activity; no localization of nitrogenase was observed. Because V. natriegens continues to fix N2 for many hours after anaerobic induction, this species may play an important role in providing "new" nitrogen in marine ecosystems.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannon F. C., Riedel G. E., Ausubel F. M. Overlapping sequences of Klebsiella pneumoniae nifDNA cloned and characterized. Mol Gen Genet. 1979 Jul 2;174(1):59–66. doi: 10.1007/BF00433306. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeLong E. F., Wickham G. S., Pace N. R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989 Mar 10;243(4896):1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- Drozd J., Postgate J. R. Effects of oxygen on acetylene reduction, cytochrome content and respiratory activity of Azotobacter chroococcum. J Gen Microbiol. 1970 Sep;63(1):63–73. doi: 10.1099/00221287-63-1-63. [DOI] [PubMed] [Google Scholar]
- EAGON R. G. Pseudomonas natriegens, a marine bacterium with a generation time of less than 10 minutes. J Bacteriol. 1962 Apr;83:736–737. doi: 10.1128/jb.83.4.736-737.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussin G. N., Ronson C. W., Ausubel F. M. Regulation of nitrogen fixation genes. Annu Rev Genet. 1986;20:567–591. doi: 10.1146/annurev.ge.20.120186.003031. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heery D. M., Gannon F., Powell R. A simple method for subcloning DNA fragments from gel slices. Trends Genet. 1990 Jun;6(6):173–173. doi: 10.1016/0168-9525(90)90158-3. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Toukdarian A. Genetics of azotobacters: applications to nitrogen fixation and related aspects of metabolism. Annu Rev Microbiol. 1987;41:227–258. doi: 10.1146/annurev.mi.41.100187.001303. [DOI] [PubMed] [Google Scholar]
- PAYNE W. J., EAGON R. G., WILLIAMS A. K. Some observations on the physiology of Pseudomonas natriegens nov. spec. Antonie Van Leeuwenhoek. 1961;27:121–128. doi: 10.1007/BF02538432. [DOI] [PubMed] [Google Scholar]
- PAYNE W. J. Studies on bacterial utilization of uronic acids. III. Induction of oxidative enzymes in a marine isolate. J Bacteriol. 1958 Sep;76(3):301–307. doi: 10.1128/jb.76.3.301-307.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Greenberg E. P., Hastings J. W. Planktonic marine luminous bacteria: species distribution in the water column. Appl Environ Microbiol. 1980 Feb;39(2):302–306. doi: 10.1128/aem.39.2.302-306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Haselkorn R. Activation of extra copies of genes coding for nitrogenase in Rhodopseudomonas capsulata. Nature. 1984 Jan 19;307(5948):289–292. doi: 10.1038/307289a0. [DOI] [PubMed] [Google Scholar]
- Tsang S. S., Yin X., Guzzo-Arkuran C., Jones V. S., Davison A. J. Loss of resolution in gel electrophoresis of RNA: a problem associated with the presence of formaldehyde gradients. Biotechniques. 1993 Mar;14(3):380–381. [PubMed] [Google Scholar]
- Zehr J. P., McReynolds L. A. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1989 Oct;55(10):2522–2526. doi: 10.1128/aem.55.10.2522-2526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr J. P., Wyman M., Miller V., Duguay L., Capone D. G. Modification of the Fe Protein of Nitrogenase in Natural Populations of Trichodesmium thiebautii. Appl Environ Microbiol. 1993 Mar;59(3):669–676. doi: 10.1128/aem.59.3.669-676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman R. K., Giebink G. S., Street H. B. Lack of money--barrier to immunization. Pediatrics. 1992 Dec;90(6):1007–1007. [PubMed] [Google Scholar]