Early, late, and cross tolerance
The occurrence of tolerance or host unresponsiveness in animals and humans administered multiple doses of microbe or microbial products has long been recognized by scientists and physicians with published reports appearing in professional journals dating back to the 19th century.1 Many of the very early observations focused largely upon the establishment of pyrogen tolerance in which animals treated with a microbe or a microbial product exhibited a refractory state for the development of fever, or a markedly diminished fever response, upon subsequent treatments with the same or a related microbe or product. Following the identification and purification of lipopolysaccharide (LPS) in the 20th century, it was determined that the microbial product, LPS itself, can be highly tolerogenic.2 In this respect, many of the pleiotropic effects of LPS, including fever, induction of cytokine production, and even mortality are absent or markedly diminished upon repeated administration of LPS. From these observations, it has been postulated that tolerance serves to protect the host from the detrimental consequences of the robust and extensive inflammatory responses that follow exposure to LPS. Tolerance cannot, however, be characterized as a global downregulation of responsiveness as some LPS-responsive characteristics remain unchanged or in some cases can actually be upregulated in experimental models of tolerance.
In studies carried out by numerous investigators over the years to clarify the concept of tolerance, a variety of terms have been used to designate the observed alteration in immune responses that occur upon repeated administration of LPS. Many alternative terminologies in lieu of the word “tolerance” have been suggested, these include reprogramming, deactivation, adaptation, refractoriness, hyporesponsiveness, and desensitization. All are arguably accurate to some extent, although many of these should not be used interchangeably, as some of these terms refer to tolerance that can be established only under specific conditions.
Numerous studies have been dedicated to the elucidation of LPS structures from various microbes in order to account at the molecular level for LPS tolerance. From these studies, it has been established that the lipid A moiety of LPS is responsible for almost all of the described activities of LPS.3-8 Accordingly, the induction of tolerance is also routinely observed with repeated administration of highly purified lipid A; responses to which are virtually indistinguishable from those observed with repeated LPS administration.9 In addition to tolerance to repeated lipid A/LPS treatment, cross-tolerance can be obtained in which other microbial products, such as lipoteichoic acid, induce tolerance to the effects of lipid A/LPS and vice versa.10-12 The concept of tolerance is also distinguished temporally on the basis of the time required to establish this phenomenon. In this regard, both early and late tolerance have been described. Early tolerance is usually observed within hours of LPS administration and may last up to a week or more. During early tolerance, suppression of cytokine production, fever, and endotoxin lethality occur independently of antibody production.13-15 Cross-tolerance is generally associated with early, but not late tolerance. In contrast to early tolerance, late tolerance is induced many days after LPS treatment when early tolerance has generally ceased or markedly waned. Late tolerance is characterized by the production of antibodies directed against LPS, which confers the specificity and lack of cross-tolerance observed in this phase. The following discussion will be driven primarily toward an examination of the consequences of early lipid A/LPS tolerance.
The consequences of the development of early lipid A/LPS tolerance on the therapeutic use of lipid A is two-fold. While subtoxic doses of lipid A may be used to either mitigate the side effects of lipid A/LPS treatment and/or protect against endotoxin shock and thus may be considered beneficial, the diminished therapeutic efficacy of lipid A in repeated administration over time may also be considered a deleterious effect. Consequently, a number of lipid A analogs have been developed to both decrease tolerance in order to increase efficacy as well as to increase tolerance to either diminish the associated toxicity of lipid A/LPS treatment or protect against endotoxin shock in patients susceptible to sepsis.9,16-20
The isolation of various lipid A structures and synthesis of analogs
An extensive array of lipid A molecules and structurally-related analogs have been isolated and/or synthesized in an effort to identify molecules that could act as competitive inhibitors and/or be used to reduce the toxicity of LPS. Indeed, comparisons of the biological potencies of lipid A structures derived from different bacterial strains and methods of preparation have been reported over the past several decades.21-27 Monophosphoryl lipid A (MPL) was among the first structures to be purified and structurally characterized by our laboratory.24,27 The results of these seminal studies led to the initial determination of the complete structure of lipid A. The subsequent development of a number of highly purified MPL analogs (with respect to number of fatty acyl groups) led to the analysis of numerous analogs in regard to toxicity and efficacy in tumor regression. MPL was found to be generally less toxic than diphosphoryl lipid A and intact LPS, which generated interest in its potential therapeutic use.26,27 The relative efficacy and toxicity of MPL is, however, somewhat dependent upon the purity of the preparation and the strain of bacteria from which it is derived. In general, hexaacylated lipid A structures were shown to be more toxic than the pentacylated or tetraacylated structures and the chain length of the fatty acyl groups was also considered an important factor in toxicity.18 Although generally less toxic than most diphosphoryl lipid A structures and LPS, MPL exhibits some agonist activity, albeit at a markedly lower level than that of intact LPS.28 In contrast, the pentaacyl diphosphoryl lipid A derived from LPS of Rhodobacter sphaeroides (RsDPLA) was purified and characterized in our laboratory for its ability to act as a nontoxic effective antagonist of LPS and agonist lipid A moieties in both human and murine cells.29-33 In addition to native lipid A structures, a relatively large number of synthetic lipid A analogs were later developed and screened for toxicity as well as LPS-mimetic or antagonistic activity.20,34,35
Relevance of tolerance to the use of LPS/lipid A in cancer
The phenomenon of tolerance in immunotherapy for the treatment of cancer has been observed as long as LPS/lipid A has been used as a cancer therapeutic. Indeed, to avoid the induction of tolerance, the late nineteenth century physician, Dr. William Coley, found it necessary to use incremental doses of a toxin formulation prepared from culture filtrates of bacteria in treating his sarcoma and carcinoma patients. “Coley’s toxin”, as the aforementioned preparation came to be called, was found to have mixed success, but was used for many years by physicians for a variety of different malignancies. The tolerance induced by Coley’s toxin may have been the result of LPS/lipid A tolerance, cross tolerance, or a combination of both as Coley’s toxin was comprised of killed bacteria of both gram-positive and gram-negative strains.1 While Coley’s toxin was comprised of a variety of microbial products that included LPS, LPS alone was also found to cause tumor regression.2 It was later determined that LPS-induced hemorrhagic necrosis of tumors is primarily due to the induction of a serum factor, termed tumor necrosis factor (TNF).36 Further investigation revealed that the lipid A portion of LPS was primarily responsible for the induction of TNFα.37 In the late twentieth century, the results of several clinical trials using LPS as a therapy were reported in cancer patients (Table 1). While purified LPS was confirmed to have positive antitumor activity in humans, both the toxicity of LPS as well as the relatively rapid induction of tolerance by LPS detracted from its overall utility as a cancer chemotherapeutic. The decreased antitumor activity of LPS due to tolerance was similar to the reduced antitumor activity observed with multiple administrations of TNFα, suggesting that the tolerance observed in vivo may well be due to both reduced TNFα activity as well as the diminished induction of TNFα by repeated LPS administration.38
Table 1.
The role of tolerance in the use of various lipid A moieties in cancer.
| Lipid A Moiety | Activity | Role of tolerance |
|---|---|---|
| LPS (lipid A) | Tumor regression/antimetastasis | Detrimental: diminishes efficacy |
| Tolerin | Protection against sepsis | Beneficial: prevents endotoxin shock |
| MPL/Detox | Adjuvant for cancer vaccine | Unknown |
| ONO-4007 | Tumor regression | Detrimental, however induces less tolerance than LPS |
| DT-5461 | Tumor regression/antimetastasis, protection against sepsis | Detrimental for anti-tumor activity, but beneficial for prevention of LPS shock |
| SDZ MRL 953 | Protection against sepsis | Beneficial: prevents endotoxin shock |
LPS in clinical and animal studies
As discussed previously, one of the primary problems with the use of LPS/lipid A as a therapeutic intervention in the treatment of cancer is the rapid induction of tolerance that diminishes the efficacy of the treatment. To address the problem of decreased antitumor activity of LPS after multiple administrations, Mackensen et al. evaluated the effect of endotoxin tolerance upon cytokine production in cancer patients following repeated daily LPS injections. Patients treated a second time with LPS, 24 hours after an initial LPS treatment, had significantly decreased levels of circulating TNFα, interleukin (IL)-6, IL-8, granulocyte colony stimulating factor (G-CSF), and macrophage colony stimulating factor (M-CSF). The levels of most of these mediators continued to decline to baseline levels upon subsequent daily injections of LPS for 5 days. Interestingly, IL-6 levels plateaued upon the third day of LPS injections with no further decline in days 4 and 5. The authors also reported that side effects, such as fever and chills, increased on the second day of LPS treatment, which did not correlate with the reported decreases in cytokine levels. In addition to tolerance induced by daily LPS injections, the authors also noted that tolerance can be induced with repeated LPS injections of 1 week and 2 week intervals as well.39
In initial clinical trials carried out by the same group to evaluate LPS therapeutic benefit, induction of tolerance was reduced by using escalating doses of LPS.40 In a subsequent phase I trial, tolerance was further reduced through the use of both escalating doses of LPS as well as an increased treatment interval of two weeks, which results in less tolerance induction than shorter treatment intervals. While moderate or strong antitumor activity with this treatment regimen was observed in a few of the reported cases, most cases did not demonstrate significant antitumor effects .41 The authors hypothesized that the relatively limited antitumor effect of LPS was, at least in part, due to the induction of LPS tolerance.42,43
The same group also conducted a phase I clinical study in which interferon γ (IFNγ) was administered in addition to LPS, in an effort to prevent LPS tolerance and thereby increase the therapeutic efficacy of the treatment. Pretreatment with IFNγ not only prevented LPS tolerance induction but, in fact, induced higher levels of TNFα, IL-6, and G-CSF than in the initial administration with LPS alone. Conversely, the downregulation of IL-8 upon repeated LPS administration was unaffected by IFNγ pretreatment. Whether the diminished LPS tolerance observed with IFNγ pretreatment correlated with improved antitumor activity was not reported, however.44
In addition to clinical trials in humans, the induction of LPS/lipid A-mediated tolerance has also been widely examined in experimental animal models. Numerous tactics have been found to delay or prevent tolerance to LPS/lipid A in animals, including treatment with recombinant IFNβ, nitric oxide synthase inhibitors, p38/stress-activated protein kinase-2 inhibitors, administration of flt3 ligand (a growth factor important for dendritic cell differentiation), and many other methods as well.45-48 Interestingly, one group recently attempted to counteract the effects of LPS/lipid A-mediated tolerance by using LPS in combination with cytotoxic drugs, such as 4’-(9-acridinylamino)-methansulfone-m-aniside, cyclophosphamide, 1-octadecyl-2-methoxy-rac-glycero-3-phosphocholine, and hexadecylphosphocholine. The authors reported, however, that they were unable to produce sufficient anticancer therapy with acceptable toxicity using this approach.49
While the anticancer activity of LPS in its native form has been extensively investigated, the potential therapeutic efficacy of irradiated LPS in cancer patients has also been reported. Presumably, the irradiation of LPS produces a variety of different lipid A structures. The overall goal of clinical studies with irradiated LPS, which is currently registered under the market name of Tolerin®, was somewhat different than the aforementioned studies with native LPS. Tolerin was designed to be administered with the purpose of inducing tolerance to LPS and at the same time, boosting natural immunity with the intent of protecting highly susceptible immunosuppressed cancer patients from sepsis and subsequent lethal septic shock. The results from these recently published studies have documented that Tolerin was well-tolerated by the patients and increased natural resistance which, importantly, correlated with a decreased incidence of infection in these patients.50
Although the majority of studies that have investigated LPS/lipid A in the treatment of cancer have focused upon their effects on inhibition of tumor growth and/or protection against sepsis, other studies have been directed toward the reverse, namely the effects of tumors upon endotoxin lethality. Interestingly, Berendt et al. reported an increased sensitivity to endotoxin in mice bearing LPS-sensitive tumors, which the authors maintain is analogous to increased endotoxin lethality in mice infected with pathogens that cause systemic macrophage activation. Furthermore, the authors report systemic activation of macrophages in mice bearing LPS-sensitive tumors which they correlate to the increased endotoxin lethality observed in these animals.51 In contrast, numerous studies have reported that many tumors are capable of inducing a tolerant state in immune cells.52-55 While the term “tolerance” is routinely used to describe both the hyporesponsive state of leukocytes induced by tumor cells as well as the diminished cytokine production, fever, and lethality following multiple administrations of LPS/lipid A, these are two separate phenomena. Although there may be similarities between these two different types of tolerance, the two phenomena can be distinguished from one another by the causative agents, which are presumably one or more factors produced by tumor cells and microbial products, respectively. In addition to these direct effects of the tumors themselves upon the ability of immune cells to respond to LPS, surgical removal of tumors can also impact responsiveness of immune cells to LPS. A general state of immunosuppression is routinely observed in humans and animals following surgery.56 Moreover, it has been reported recently that cryosurgery of tumor tissue induces tolerance to endotoxin-mediated lethality, suggesting that this surgical procedure may also be protective against septic shock in cancer patients.57 Collectively, these studies suggest that the effects of tumors upon immune cell activation overall, and LPS responsiveness specifically, appear to be complex and seem to differ based upon the nature of the tumor. In addition, surgical removal of tumors may well contribute to the induction of tolerance to LPS/lipid A, which may have the dual effect of diminishing the efficacy of lipid A treatments as well as protecting the patient from septic shock.
Monophosphoryl lipid A and lipid A adjuvants
Following the isolation, derivatization, and synthesis of various lipid A structures, it was quickly established that some of the beneficial effects of the identified nontoxic lipid A moieties had therapeutic potential. For instance, SDZ MRL 953 and MPL were found to enhance host defenses against subsequent bacterial infection through induction of G-CSF, M-CSF, and other cytokines.26,58-60 At the same time, however, these compounds induced refractoriness (tolerance) to LPS toxicity through reduced secretion of inflammatory cytokines, while host cell phagocytic activity was maintained. Some of the nontoxic lipid A moieties, such as MPL, also demonstrated tumor regression activity.61 Similar to LPS, however, MPL has also been shown to induce tolerance in both experimental animals as well as in patients enrolled in clinical trials.26,28,62-64 Due to these confounding factors, MPL is not currently in use clinically for its tumor regression activity per se, but remains important in cancer therapy as an immunoadjuvant in cancer vaccines (see chap. 10).65 Because macrophage activation is critical to the adjuvant activity of lipid A (and in the development of tolerance), lipid A adjuvancy may also be susceptible to tolerance, depending upon experimental conditions and treatment administration.
Synthetic lipid A analog, ONO-4007
In addition to the various native forms of lipid A derived from different bacterial strains, numerous lipid A analogs have been synthesized and screened for antitumor activity. Several synthetic lipid A analogs have demonstrated potential as cancer chemotherapeutics, but the induction of tolerance upon repeated administration has only been reported for a fraction of these. ONO-4007, a synthetic triacylated monosaccharidic lipid A analog, has been the focus of numerous studies in both animals and humans, which have shown that it exhibits lower toxicity than LPS and causes tumor regression.66-68 While ONO-4007 has been shown to induce tolerance in animals using several different models, it induces less tolerance than that induced by LPS and synthetic E. coli-type lipid A (LA-15-PP).17,69,70 Interestingly, differential tolerance to ONO-4007 was observed in different tissue types. While significant tolerance to ONO-4007-mediated cytokine induction was observed in serum and liver tissue, no tolerance was observed in tumor tissue extracts as assessed by TNFα production in tumor-bearing mice treated with ONO-4007 at 8, 12, and 15 days following tumor implantation.17
The results of further investigations have established that the tumor tissue did become hyporesponsive to stimulation with ONO-4007 as measured by TNFα induction, but tumoral responses recovered more quickly than responses in liver and serum and were completely responsive by 72 hours. Similarly, tumor infiltrating macrophages recovered from ONO-4007-mediated hyporesponsiveness within 72 hours after initial exposure. The authors hypothesized that the selective recovery of tumor tissues may, at least in part, be due to constant recruitment of macrophages to tumor tissue. In addition, the authors demonstrate that repeated injections of LA-15-PP into mice enhanced its clearance from blood circulation, whereas the clearance of ONO-4007 was stable even following multiple administrations, suggesting that pharmacokinetics also play a role in the differences in tolerance between these two lipid A structures. Moreover, TNFα tissue levels peaked 1-2 hours following ONO-4007 treatment and then decreased in the spleen and liver, but remained elevated for at least 6 hours in tumor tissue.71,72
Synthetic compound, DT-5461
Like LPS and ONO-4007, tolerance to the synthetic tetraacylated lipid A analog, DT-5461, is also observed. Tolerance to DT-5451 occurs one day after treatment with a return to responsiveness observed 3-5 days later.73 In experimental animal models of cancer using prostaglandin E2 (PGE2)-producing tumors, a combined treatment of indomethacin and DT-5461 was shown to have significant antitumor activity and an additive effect upon survival. The authors have hypothesized that the antitumor effect of the combined therapy is due to a combination of TNFα activity, as well as the inhibition of PGE2 production.74 Interestingly, PGE2 has also been hypothesized to be a mediator of LPS/lipid A-induced tolerance.75 Furthermore, and of some interest, cyclooxygenase inhibitors have been reported to prevent the induction of tolerance to LPS.76
While the majority of studies of DT-5461 have focused upon its antitumor activity, DT-5461 has also been shown to be protective against endotoxemia. DT-5461 pretreatment induced significant tolerance to lethal LPS exposure in mice.77 Similar to studies described earlier with Tolerin, the induction of tolerance by DT-5461 was found to be beneficial therapeutically for the protection of immunosuppressed patients from sepsis, including cancer patients undergoing chemotherapy or radiation treatment. In addition to the induction of tolerance by DT-5461 itself, LPS also induces tolerance to DT-5461 as well, strongly suggesting that these two compounds act through similar cellular mechanisms. In mice injected with LPS at daily intervals for a week, the antimetastatic activity of DT-5461 was significantly reduced, further supporting the conclusion that the mechanisms of tolerance by LPS and DT-5461 are identical or cross-tolerance occurs between the two mediators.78
Synthetic compound, SDZ MRL 953
Similar to Tolerin and DT-5461, the LPS tolerance induced by the synthetic triacylated lipid A analog, SDZ MRL 953, has been evaluated for its protective effects against sepsis in cancer patients.79 SDZ MRL 953 pretreatment inhibited LPS-induced TNFα, IL-1β, IL-8, IL-6, and G-CSF serum levels, suggesting that it induces tolerance to LPS and might be effective as a prophylactic treatment for patients who may otherwise be susceptible to sepsis.79 Further clinical studies will be needed, however, to determine the overall efficacy of SDZ MRL 953 in the prevention of gram-negative infections and septic shock in immunosuppressed cancer patients.
Mechanisms of early LPS/lipid A-mediated tolerance
Because many investigators have demonstrated that lipid A is the active component of LPS, and is also capable of inducing LPS tolerance, presumably most of the LPS-mediated activity that occurs during early tolerance can be attributed to the lipid A portion of the molecule.28,80,81 These conclusions are also strongly supported by the results of studies using a variety of lipid A analogs as described in previous sections. Despite hundreds of papers that have been published concerning LPS/lipid A-mediated early tolerance, the underlying mechanism still remains to be determined. Indeed, numerous hypotheses and supportive studies suggest that multiple mechanisms may play a role in tolerance. If multiple pathways do exist, they may be activated concurrently or at temporally distinct points in the activation/deactivation pathway sequences. Alternatively, different mechanisms may operate in a manner that is distinct from one another. The activation of one particular mechanism rather than another may depend upon the specific cell type involved, the local environment within the host, or the experimental conditions in vitro. While the majority of studies of early tolerance to LPS/lipid A have focused upon macrophages, a number of other cell types have also been shown to be susceptible to tolerance.82,83 Because detailed reviews of the numerous studies focusing upon the mechanisms of tolerance have recently been published, only a broad overview will be included in this chapter. The reader is referred to a number of excellent reviews for a more comprehensive discussion concerning the role of different LPS/lipid A signaling pathways in the induction of tolerance.13,75,84,85
Toll-like receptors, associated signaling molecules, and negative regulators
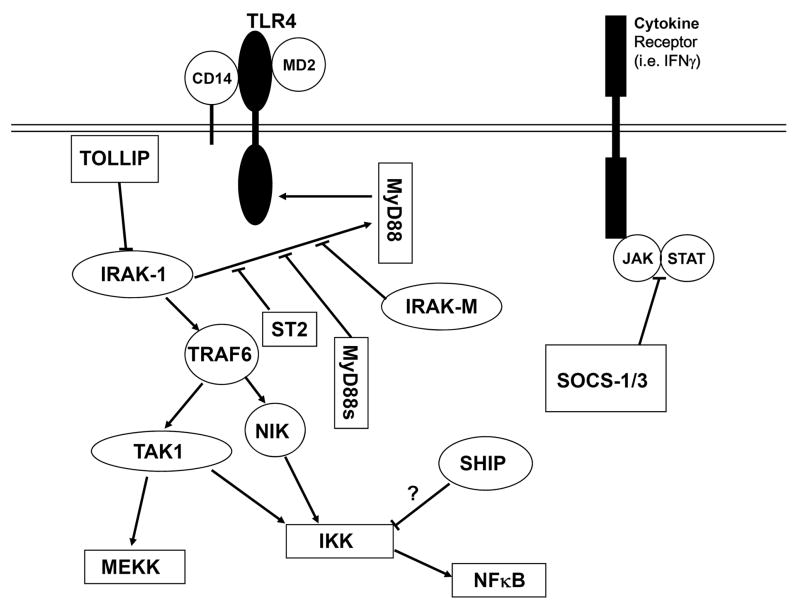
The members of the toll-like receptor (TLR) family are unique in their ability to recognize pathogen associated molecular patterns (PAMPs) found in variety of microbial products, including LPS/lipid A. LPS has been shown to activate cells through the TLR4 pathway and to a lesser extent, through TLR2. CD14 and MD-2 are both proteins that have been shown to associate with, and also be required for, TLR4-dependent activity and are therefore important for activation of cells by LPS/lipid A (Fig.1). Upon activation of TLR4 by LPS, the adapter protein, myeloid differentiation factor 88 (MyD88) is recruited to the cytoplasmic domain of TLR4, where it associates with and then activates the IL-1 receptor associated kinase-1 (IRAK-1). Activated IRAK-1 then dissociates from the TLR4 complex and subsequently binds and activates TNF receptor associated factor-6 (TRAF-6), which in turn activates TGFβ-activated kinase-1 (TAK1) and nuclear factor of κB-inducing kinase (NIK). The activation of TAK1 and NIK results in the activation of the mitogen activated protein kinase (MAPK) and nuclear factor of κB (NFκB) pathways. 75,85 The reader is referred to previous chapters of this volume for more information concerning the signaling pathways of lipid A/LPS.
Fig. 1.
Schematic diagram of hypothetical mechanisms of tolerance within the TLR4 pathway. While ST2, IRAK-M, and the splice variant of MyD88 (MyD88s) are thought to suppress association of IRAK-1 with MyD88 through competitive inhibition. TOLLIP has been shown to bind to IRAK-1 and inhibits its activity. SOCS-1 and SOCS-3 are inhibitors of the JAK/STAT pathway. While the mechanism by which SHIP induces tolerance is unknown, it is believed to inhibit the NFκB signaling pathway.
Numerous studies have reported the role of TLRs and associated proteins in the induction of tolerance. Although it would be intuitive that downregulation of TLR4 or another component of the TLR4 receptor complex may be responsible for LPS/lipid A-induced tolerance, this remains a somewhat controversial issue. While some studies have reported a downregulation of TLR4 receptor protein expression following LPS treatment, other studies have demonstrated that TLR4 expression is either unaffected or sometimes even increased upon LPS administration.83,86-93 In contrast to TLR4, studies of the effects of LPS on TLR2 expression have been more conclusive, with no downregulation of TLR2 observed in most models of tolerance.87-91 In addition, the expression levels of CD14 and MD-2 have also been evaluated in numerous studies. The results of most studies examining CD14 expression have shown no decrease in protein or transcript levels following LPS treatment.88,91,94-96 Similar to TLR4, however, the results with MD-2 have been inconsistent. While many studies demonstrate little change in MD-2 expression with LPS/lipid A treatment, some studies show decreased MD-2 transcription and surface expression of the TLR4/MD-2 complex.89,91,94,97 Interestingly, tolerance has been demonstrated in HEK293T cells in which TLR4, CD14 and MD-2 have all been overexpressed, suggesting that decreased CD14/TLR4/MD-2 expression is not required for the induction of tolerance in this model.98 Overall, these studies suggest that, while there may be decreased expression of TLR4/MD-2 upon LPS/lipid A administration under certain experimental conditions, it is by no means a universal requirement for tolerance.
The role of a number of different signaling molecules downstream of the CD14/TLR4/MD-2 complex has also been investigated. Although expression levels of MyD88 have not been found to be decreased in tolerant monocytes, a marked inhibition of MyD88/TLR4 association has been reported.90 In addition, a splice variant of MyD88 that is expressed upon LPS treatment, and functions to inhibit LPS signaling, has garnered considerable attention as a potential mechanism of tolerance induction.99,100 Similarly, IRAK-1 activity and MyD88/IRAK-1 association have been reported to be diminished in LPS-tolerant cells.90,101,102 Moreover, upregulation of the inactive kinase, IRAK-M, may also play a role in the induction of tolerance in monocytes. IRAK-M is markedly upregulated upon LPS treatment, causing inhibition of LPS signaling, possibly through competitive inhibition of IRAK-1 binding to MyD88. Of particular relevance, the induction of tolerance is significantly diminished in macrophages lacking IRAK-M as compared to macrophages derived from wild-type mice.103,104
In addition to IRAK-M, other negative regulators of LPS signaling have also been investigated. Suppressor of cytokine signaling-1 (SOCS-1) and SOCS-3 have been shown to be rapidly induced upon LPS exposure, and these proteins can serve as negative feedback regulators of the janus activated kinase (JAK)/signal transducers and activators of transcription (STAT) signaling cascade, which is the downstream mechanism of signal transduction of many cytokine receptors.105,106 The results of studies examining the role of SOCS-1 in tolerance have once again been mixed, with one report demonstrating an absence of tolerance to endotoxin lethality and LPS-mediated TNFα secretion in SOCS-1 -/- mice, while a different group demonstrated no difference in the induction of LPS tolerance to IL-12 secretion in SOCS-1 -/- macrophages. As a consequence, the role of SOCS-1 in tolerance remains inconclusive.107,108
ST2, a member of the Toll-IL1 superfamily, is another inhibitor of LPS signaling. There is evidence to suggest that ST2 suppresses signaling of both the TLR4 and the IL-1 receptor through the sequestration of MyD88 and Mal adaptor proteins. Although the role of ST2 in tolerance has not yet been extensively investigated, recent reports demonstrating a failure of ST2-deficient mice to develop tolerance to endotoxin lethality, as well as LPS-mediated IL-6 and IL-12 production, suggest that ST2 may also play a role in the induction of tolerance.109,110 These promising preliminary findings are likely to provide the basis for additional study of mechanisms of LPS/lipid A-mediated tolerance induction.
SH2-containing inositol phosphatase (SHIP) and toll interacting protein (TOLLIP) are also negative regulators of LPS signaling. While TOLLIP mRNA and protein levels have been reported to be upregulated in LPS-tolerant cells, the role of this protein in tolerance has not been extensively investigated.111,112 Interestingly, the results of recent studies with TOLLIP null mice indicate that TOLLIP can also act as an activator of LPS signaling, at least under certain circumstances.113 In comparison to TOLLIP, there is stronger evidence for involvement of SHIP in endotoxin tolerance. SHIP protein levels are significantly elevated following LPS treatment, a response which is mediated by LPS-induced TGFβ secretion. Accordingly, treatment with antibodies against TGFβ blocks LPS-induced tolerance. Notably, LPS-mediated tolerance cannot be induced in SHIP null mice.114
The suppressive effects of NO on cytokine production, as well as on immune cell proliferation and growth, have been extensively described.115 As a result, the effect of NO upon tolerance has also been investigated. Supportive evidence for NO involvement in the induction of tolerance includes increased NO production by tolerant peritoneal macrophages, inhibition of tolerance to endotoxin lethality by NO synthase inhibitors, and increased survival to a lethal dose of LPS in rats treated with an NO donor.116-120 While many studies report increased NO production and/or iNOS transcription in tolerant macrophages, other studies have shown decreased NO production and NO synthase activity by macrophages in experimental models of tolerance.121 Similarly, and perhaps not totally unexpectedly, contradictory evidence for the role of NO in tolerance is also found in investigations with knockout mice. While Dias et al. have recently reported that iNOS null mice do not become tolerant to LPS-mediated pyrogenicity, Zingarelli et al. earlier demonstrated tolerance to LPS-mediated lethality and TNFα production in iNOS null mice.122,123 The differences between the two studies may be dependent upon the different doses of LPS used, the endpoints measured, or the treatment regimens which also differed substantially between the two sets of experiments.
Corticosteroids, anti-inflammatory cytokines, and prostaglandins
In addition to participation of a number of components of the TLR4 pathway and the negative regulators associated with this pathway in the development of tolerance to LPS/lipid A, other mechanisms have also been proposed to mediate this phenomenon. LPS-induced glucocorticoid secretion has been well-documented and results in the suppression of a variety of different immune cell types. As such, the role of glucocorticoids as potential mediators of tolerance has remained an area of interest for decades. Despite numerous studies to address this hypothesis, however, strong evidence for the involvement of glucocorticoids in the induction of tolerance has been relatively sparce.13 While currently inconclusive, the role of glucocorticoids in tolerance continues to be investigated.
Anti-inflammatory cytokines, such as IL-10 and TGFβ, have also been suggested to contribute to the induction of tolerance to LPS/lipid A treatment. Support for this concept comes from the findings of studies showing significant downregulation of TNFα and other proinflammatory mediators by IL-10.124,125 Furthermore, treatment with an antibody specific for IL-10 results in an inhibition of the induction of LPS/lipid A-mediated tolerance.126 Conversely, tolerance to LPS/lipid A treatment can be readily induced in IL-10 knockout mice, suggesting that IL-10 cannot be the sole mediator of tolerance.127 Indeed, induction of IL-10 by LPS itself is diminished upon subsequent administration of LPS under certain experimental conditions.126 Similar to IL-10, pretreatment of isolated human peripheral blood mononuclear cells with recombinant TGFβ also suppresses LPS-mediated TNFα secretion, and a blocking antibody against TGFβ also diminishes the development of tolerance to LPS.126,128 In addition to immunosuppressive cytokines, the synthesis and release of immunosuppressive prostaglandins, such as PGE2, have also been hypothesized to contribute to the induction of tolerance in some experimental models. The basis for this hypothesis is that PGE2 levels have been shown to be highly elevated in tolerant cells reexposed to endotoxin in a number of different animal and human models. Moreover, PGE2 inhibits cytokine production in activated macrophages and lymphocytes.129-132 Further studies are needed to more completely elucidate the role of PGE2 in the induction of tolerance, however.
Transcriptional mediators
Peroxisome proliferator activated receptor γ (PPARγ) activation has been correlated with inhibition of macrophage activation by LPS, as assessed by cytokine and NO production.133-135 Consequently, the role of PPARγ in LPS-mediated tolerance has been examined. Increased PPARγ binding to the PPAR response element (PPRE) and PPARγ transcriptional activity have been observed in LPS-tolerant cells, suggesting possible involvement of PPARγ in endotoxin tolerance.136 Furthermore, the use of blocking oligonucleotides for the PPRE inhibited tolerance induced by LPS. Collectively, these preliminary studies suggest a potential role for PPARγ in tolerance, but more extensive investigations are required before definitive conclusions can be reached.
In part because so many LPS-responsive genes have been shown to be regulated by NFκB, there has been considerable interest in the numerous reports of decreased NFκB activation in the development of LPS tolerance, which has been widely observed in a variety of experimental models of tolerance. The results of some of these studies suggest that the decrease in NFκB activity may be due to an increase in the formation of p50/p50 homodimers, which are transactivationally inactive and therefore would be expected to antagonize the active p50/p65 heterodimer by competitively binding to κB sites in the promoters of LPS-responsive genes.95 This hypothesis is supported by the reportedly increased levels of p50/p50 homodimers in tolerant cells and the failure to induce tolerance in p50-deficient mice.137 Contradictory evidence has, however, also been presented, demonstrating that LPS tolerance could still be induced in p50 -/- mice, as assessed by IL-12 and TNFα production by splenocytes.138 Collectively, these data suggest that, while decreased NFκB transcriptional activity is likely to be a causative factor in the induction of tolerance in a number of different experimental models, current data suggest that p50/p50 homodimers are not likely to be the only mechanism responsible for the diminished NFκB activity in various models of LPS tolerance.
The role of the proteasome
Our recent data have provided convincing evidence for a prominent role of the proteasome, a cytoplasmic organelle with multiple protease activities, in LPS signaling and subsequent development of inflammatory and immune responses. Structurally, proteasomes exist as multi-subunit complexes, consisting of a number of distinct, well-characterized, proteins.139 The so-called 26S proteasome complex (2.5 MDa) is comprised of a 20S proteasome, which exhibits proteolytic activity, and a 19S proteasome, which provides regulatory functions.140 The 20S proteasome has been defined structurally as a hollow, cylindrical, multi-protein structure composed of 28 protein subunits that are derived from 14 distinct gene products.141,142 The protein subunits of the proteasome are arranged in four heptameric rings shaped approximately as a barrel. The three proteases of the proteasome are X (LMP7) (chymotrypsin-like protease activity), Y (LMP2) (post-glutamase protease activity), and Z (MECL-1) (trypsin-like protease activity) and these have been described in detail.142,143 The protease activities of the proteasome have been shown to be regulated by IFNγ. Subunits LMP7, LMP2, and MECL-1 of the 20S proteasome are recognized as IFNγ-inducible proteasome-associated β-subunits. There is an overproduction of these subunits due to IFNγ produced early on during an inflammatory response, resulting in the introduction of the subunits into newly assembled proteasomes, which have been termed immunoproteasomes. Immunoproteasomes appear to have enhanced capability for generating class I MHC-binding peptides, as compared with “standard” proteasomes, cleaving more efficiently after basic or hydrophobic residues and less efficiently after acidic residues.144
The role of the proteasome in LPS-induced inflammation had not been extensively pursued until our demonstration that LPS binds specifically to A1 (C2) and B4 (N3) proteins of the 20S proteasome complex.145 After demonstrating that LPS binds proteasome subunits, we then assessed the potential physiological relevance of these interactions. To this end, we first carried out studies to determine the extent to which LPS modulates the proteasome’s proteolytic activity. We demonstrated that the addition of LPS to partially purified proteasomes in vitro activated the chymotrypsin-like and post-glutamase activities of macrophage proteasomes.145,146 We next sought to determine the extent to which well-defined proteasome inhibitors might block LPS-induced inflammation.
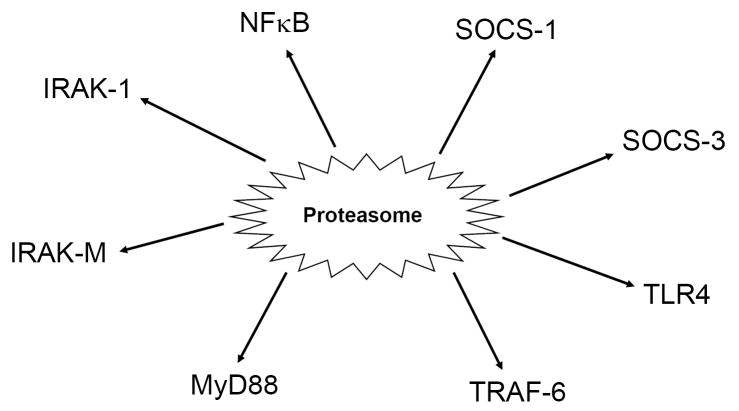
To address this question, we pretreated RAW 264.7 macrophages with the well-characterized proteasome inhibitor, lactacystin, and observed a dose-dependent inhibition of LPS-induced cytokine secretion.145,146 Furthermore, we found that pretreatment of primary murine macrophages with lactacystin inhibited the expression of a spectrum of LPS-inducible genes, including IL-1β, IL-6, IL-12 p40 and p35, COX-2, and iNOS. In addition, lactacystin also blocked the LPS-induced upregulation of TLR2 mRNA, and reduced constitutive levels of TLR4 mRNA expression.145 The net effect of proteasome activation would appear to be enhancement of TLR-mediated inflammatory responses, while proteasome inhibition would be predicted to suppress the inflammatory response. Our data demonstrate that more than 90% of LPS-responsive genes in peritoneal macrophages are regulated by the proteasome.147 Furthermore, studies from our laboratory and others suggest that the proteasome regulates a number of proteins involved in tolerance, including SOCS-1, SOCS-3, IRAK-M, IRAK-1, MyD88, TLR4, and others (Fig. 2).147,148 In addition, the proteasome also regulates NFκB, a critical transcription factor for many LPS-responsive genes that has been shown to be dysregulated in LPS-tolerant cells. The role of the proteasome in tolerance remains largely untested thus far, however.
Fig. 2.
Schematic diagram of tolerance-related mediators that are regulated by the proteasome at either the transcriptional or post-transcriptional levels.
Mechanisms of tolerance of other lipid A structures and LPS antagonists
In addition to lipid A moieties with agonist activity, there also exist a variety of lipid A analogs that that can function as LPS antagonists. The mechanism of the LPS antagonists is likely through the competitive inhibition of LPS binding to either LPS binding molecules, such as LPS binding protein (LBP), or the TLR complex. Indeed, evidence for this has been presented for RsDPLA, the biologically inactive lipid A molecule from Rhodobacter sphaeroides, using colloidal gold particles to label both LPS and RsDPLA and electron microscopy to monitor cellular binding and internalization. Our studies conducted thus far suggest that RsDPLA competes with LPS in binding to LBP, CD14, and TLR4.149-151 Other lipid A analogs, including ONO-4007 and others described earlier, have been postulated to induce tolerance to LPS and lipid A through upregulation of endogenous corticosteroids.69 In addition, the inhibition of suppressor T cell activity by MPL and RsDPLA has also been proposed as a mechanism of tolerance for these two lipid A molecules.152-154
Future directions
As summarized in this review, evidence has been presented to suggest that multiple LPS/lipid A-induced signal transduction intermediates and other mediators are involved in the induction of tolerance. It is likely that no single mechanism will emerge as playing the dominant role in this process. The continued investigation of the numerous factors implicated thus far in the development of tolerance to LPS/lipid A will help to determine to what extent and under which circumstances these various factors play a role in this phenomenon. In addition to the aforementioned factors that are currently being investigated, other factors, such as the proteasome that have not yet been widely studied in tolerance, may also be involved. Studies from this laboratory strongly suggest a key role for the proteasome in LPS/lipid A signaling. This evidence includes the modulation of proteasomal protease activity by LPS/lipid A, the degradation of IκB by the proteasome, and the subsequent activation of NFκB that ultimately upregulates inflammatory mediators. In addition to that, proteasomal proteases have also been shown to degrade various mediators of LPS signaling, including TLR4, IRAK-1, etc. Furthermore, inhibition of the proteasome modulates the gene expression of many mediators of LPS signaling, including many associated with tolerance (Table 2). The proteasome may also play a key role in tolerance such that when LPS-induced inflammatory mediators increase to a certain level, compensatory mechanisms are induced to trigger the development of tolerance, possibly by modulating the activities of individual proteasomal proteases.
Table 2.
LPS-modulated, proteasome-dependent cancer related genes. LPS-modulated, proteasome-dependent genes. Thioglycollate-elicited murine macrophages were treated with the compounds, LPS and/or lactacystin.
| LPS | LPS/LACT | LACT | DESCRIPTION | |
|---|---|---|---|---|
| SOCS3 | 256.51 | -4.02 | -3.49 | Suppressor of cytokine signaling 3 |
| SOCS1 | 52.35 | 12.87 | 2.34 | Suppressor of cytokine signaling 1 |
| TLR2 | 10.32 | 6.82 | -1.74 | Toll-like receptor 2 |
| NFKB2 | 10.20 | 3.60 | 1.23 | Nuclear factor of kappa light gene enhancer in B cells |
| STAT2 | 9.17 | 6.68 | -1.57 | Signal transducer and activator of transcription 2 |
| STAT1 | 6.77 | 4.84 | -1.48 | Signal transducer and activator of transcription 1 |
| TLR3 | 5.74 | 2.77 | -6.63 | Toll-like receptor 3 |
| MAP3K8 | 4.36 | 1.86 | -3.09 | Mitogen-activated protein kinase kinase kinase 8 |
| IRAKM | 3.46 | 1.66 | -1.38 | Interleukin-1 receptor associated kinase 3 |
| TAK1 | 3.32 | -3.09 | 2.18 | Mitogen-activated protein kinase kinase kinase 7 |
| TLR1 | 2.83 | -1.69 | -1.45 | Toll-like receptor 1 |
| MyD88 | 2.50 | 1.51 | -1.08 | Myeloid differentiation pathway primary response gene |
| CD14 | 2.24 | -1.22 | -2.31 | CD14 antigen |
| TLR4 | -2.83 | -6.47 | -3.89 | Toll-like receptor 4 |
| TRAF6 | -5.56 | -2.33 | 2.28 | TNF-receptor associated factor 6 |
The gene expression values are reported as average normalization ratios (modified from ref. 165). A data set containing gene identifiers and their corresponding expression values was uploaded as an Excel spreadsheet using the template provided in the application. Each gene identifier was mapped to its corresponding gene object in the Ingenuity Pathways Knowledge Base.
Interestingly, there is a great deal of overlap between many of the proposed mechanisms of tolerance and putative targets of cancer therapy. The roles of NFκB, IRAK-1, IRAK-M, SOCS-1, SOCS-3, and MyD88 have been evaluated both in tolerance as well as in several models of cancer, for example. Because proteasome inhibitors modulate gene expression of these signal transduction intermediates, they have also been investigated for their potential as cancer therapeutics. Indeed, the proteasome inhibitor, Bortezomib has recently been approved by the FDA for the treatment of refractory multiple myeloma and has also shown promise in the treatment of lung cancer, as well as various types of lymphoma.
As our knowledge of lipid A and its derivatives continues to expand, the therapeutic potential of these compounds has become evident. MPL has been used as an effective adjuvant for cancer vaccines and will likely continue to be used in future vaccine formulations. The development of nontoxic lipid A analogs, such as SDZ MRL 953, that induce tolerance to LPS and thereby protect susceptible patients against endotoxin shock also shows therapeutic promise. Moreover, the development of lipid A analogs that induce less tolerance than LPS and exhibit greater efficacy in tumor regression show potential as cancer therapeutics as well. Because lipid A-mediated tolerance is a particularly complicated phenomenon that plays dual and opposite roles in the efficacy of cancer therapeutics, elucidation of the mechanisms of tolerance is essential for the continued development of lipid A compounds into nontoxic, efficacious treatments for cancer patients.
Acknowledgments
This work was supported by NIH grants GM50870 (N.Q.), AI54962 (N.Q.) and AI44936 (DCM).
Reference List
- 1.Hoption Cann SA, van Netten JP, van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J. 2003;79(938):672–680. [PMC free article] [PubMed] [Google Scholar]
- 2.Shear MJ, Turner FC. Chemical treatment of tumors: isolation of hemorrhagic-producing fraction from Serratia marcescens (Bacillus prodigiosus) culture filtrate. J Natl Cancer Inst. 1943;4:81–97. [Google Scholar]
- 3.Raetz CR, Garrett TA, Reynolds CM, et al. Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. J Lipid Res. 2006;47(5):1097–1111. doi: 10.1194/jlr.M600027-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Galanos C. Physical state and biological activity of lipopolysaccharides. Toxicity and immunogenicity of the lipid A component. Z Immunitatsforsch Exp Klin Immunol. 1975;149(2-4):214–229. [PubMed] [Google Scholar]
- 5.Kanegasaki S, Kojima Y, Matsuura M, et al. Biological activities of analogues of lipid A based chemically on the revised structural model. Comparison of mediator-inducing, immunomodulating and endotoxic activities. Eur J Biochem. 1984;143(2):237–242. doi: 10.1111/j.1432-1033.1984.tb08364.x. [DOI] [PubMed] [Google Scholar]
- 6.Homma JY, Matsuura M, Kanegasaki S, et al. Structural requirements of lipid A responsible for the functions: a study with chemically synthesized lipid A and its analogues. J Biochem (Tokyo) 1985;98(2):395–406. doi: 10.1093/oxfordjournals.jbchem.a135294. [DOI] [PubMed] [Google Scholar]
- 7.Kotani S, Takada H, Tsujimoto M, et al. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli remutant. Infect Immun. 1985;49(1):225–237. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galanos C, Luderitz O, Rietschel ET, et al. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- 9.Galanos C, Lehmann V, Luderitz O, et al. Endotoxic properties of chemically synthesized lipid A part structures. Comparison of synthetic lipid A precursor and synthetic analogues with biosynthetic lipid A precursor and free lipid A. Eur J Biochem. 1984;140(2):221–227. doi: 10.1111/j.1432-1033.1984.tb08090.x. [DOI] [PubMed] [Google Scholar]
- 10.Dobrovolskaia MA, Medvedev AE, Thomas KE, et al. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR “homotolerance” versus “ heterotolerance” on NF-kappa B signaling pathway components. J Immunol. 2003;170(1):508–519. doi: 10.4049/jimmunol.170.1.508. [DOI] [PubMed] [Google Scholar]
- 11.Lehner MD, Morath S, Michelsen KS, et al. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J Immunol. 2001;166(8):5161–5167. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- 12.Sato S, Nomura F, Kawai T, et al. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165(12):7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 13.Schade FU, Flach R, Flohe S, et al. Endotoxin tolerance. In: Brade H, Opal SM, Vogel SN, Morrison D, editors. Endotoxin in Health and Disease. New York: Marcel Dekker, Inc.; 1999. pp. 751–767. [Google Scholar]
- 14.West MA, Heagy W. Endotoxin tolerance: a review. Crit Care Med. 2002;30(1 Suppl):S64–S73. [PubMed] [Google Scholar]
- 15.Greisman SE, Hornick RB. The nature of endotoxin tolerance. Trans Am Clin Climatol Assoc. 1975;86:43–50. [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuura M, Nakano M. Mechanisms to induce tolerance by synthetic monosaccharide lipid A analogues against LPS lethality in mice. Prog Clin Biol Res. 1995;392:539–548. [PubMed] [Google Scholar]
- 17.Satoh M, Tsurumaki K, Kagehara H, et al. Induction of intratumoral tumor necrosis factor by a synthetic lipid A analog, ONO-4007, with less tolerance in repeated administration and its implication in potent antitumor effects with low toxicity. Cancer Immunol Immunother. 2002;50(12):653–662. doi: 10.1007/s00262-001-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qureshi N, Takayama K. Structure and function of lipid A. In: Iglewski BH, Clark VL, editors. The bacteria. New York: Academic Press; 1990. pp. 319–338. [Google Scholar]
- 19.Golenbock DT, Hampton RY, Qureshi N, et al. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991;266(29):19490–19498. [PubMed] [Google Scholar]
- 20.Stutz P, Liehl E. Lipid A analogs aimed at preventing the detrimental effects of endotoxin. Infect Dis Clin North Am. 1991;5(4):847–873. [PubMed] [Google Scholar]
- 21.Ribi E, Haskins WT, Landy M, et al. Symposium on bacterial endotoxins. I. Relationship of chemical composition to biological activity. Bacteriol Rev. 1961;25:427–436. doi: 10.1128/br.25.4.427-436.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribi E, Haskins WT, Milner KC, et al. Physicochemical changes in endotoxin associated with loss of biological potency. J Bacteriol. 1962;84:803–814. doi: 10.1128/jb.84.4.803-814.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takayama K, Qureshi N, Raetz CR, et al. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infect Immun. 1984;45(2):350–355. doi: 10.1128/iai.45.2.350-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi N, Takayama K, Heller D, et al. Position of ester groups in the lipid A backbone of lipopolysaccharides obtained from Salmonella typhimurium. J Biol Chem. 1983;258(21):12947–12951. [PubMed] [Google Scholar]
- 25.Qureshi N, Mascagni P, Ribi E, et al. Monophosphoryl lipid A obtained from lipopolysaccharides of Salmonella minnesota R595. Purification of the dimethyl derivative by high performance liquid chromatography and complete structural determination. J Biol Chem. 1985;260(9):5271–5278. [PubMed] [Google Scholar]
- 26.Madonna GS, Peterson JE, Ribi EE, et al. Early-phase endotoxin tolerance: induction by a detoxified lipid A derivative, monophosphoryl lipid A. Infect Immun. 1986;52(1):6–11. doi: 10.1128/iai.52.1.6-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qureshi N, Takayama K, Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982;257(19):11808–11815. [PubMed] [Google Scholar]
- 28.Henricson BE, Benjamin WR, Vogel SN. Differential cytokine induction by doses of lipopolysaccharide and monophosphoryl lipid A that result in equivalent early endotoxin tolerance. Infect Immun. 1990;58(8):2429–2437. doi: 10.1128/iai.58.8.2429-2437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takayama K, Qureshi N, Beutler B, et al. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989;57(4):1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkland TN, Qureshi N, Takayama K. Diphosphoryl lipid A derived from lipopolysaccharide (LPS) of Rhodopseudomonas sphaeroides inhibits activation of 70Z/3 cells by LPS. Infect Immun. 1991;59(1):131–136. doi: 10.1128/iai.59.1.131-136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qureshi N, Takayama K, Kurtz R. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect Immun. 1991;59(1):441–444. doi: 10.1128/iai.59.1.441-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henricson BE, Perera PY, Qureshi N, et al. Rhodopseudomonas sphaeroides lipid A derivatives block in vitro induction of tumor necrosis factor and endotoxin tolerance by smooth lipopolysaccharide and monophosphoryl lipid A. Infect Immun. 1992;60(10):4285–4290. doi: 10.1128/iai.60.10.4285-4290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qureshi N, Jarvis BW, Takayama K. Nontoxic RsDPLA as a potent antagonist of toxic lipopolysaccharide. In: Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in health and disease. New York: Marcel Dekker, Inc.; 1999. pp. 687–698. [Google Scholar]
- 34.Rossignol DP, Hawkins LD, Christ WJ, et al. Synthetic endotoxin antagonists. In: Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in health and disease. New York: Marcel Dekker, Inc.; 1999. pp. 699–717. [Google Scholar]
- 35.Persing DH, Coler RN, Lacy MJ, et al. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002;10(10 Suppl):S32–S37. doi: 10.1016/s0966-842x(02)02426-5. [DOI] [PubMed] [Google Scholar]
- 36.Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haranaka K, Satomi N, Sakurai A, et al. Role of lipid A in the production of tumor necrosis factor and differences in antitumor activity between tumor necrosis factor and lipopolysaccharide. Tohoku J Exp Med. 1984;144(4):385–396. doi: 10.1620/tjem.144.385. [DOI] [PubMed] [Google Scholar]
- 38.Fraker DL, Sheppard BC, Norton JA. Impact of tolerance on antitumor efficacy of tumor necrosis factor in mice. Cancer Res. 1990;50(8):2261–2267. [PubMed] [Google Scholar]
- 39.Mackensen A, Galanos C, Wehr U, et al. Endotoxin tolerance: regulation of cytokine production and cellular changes in response to endotoxin application in cancer patients. Eur Cytokine Netw. 1992;3(6):571–579. [PubMed] [Google Scholar]
- 40.Engelhardt R, Mackensen A, Galanos C, et al. Biological response to intravenously administered endotoxin in patients with advanced cancer. J Biol Response Mod. 1990;9(5):480–491. [PubMed] [Google Scholar]
- 41.Engelhardt R, Mackensen A, Galanos C. Phase I trial of intravenously administered endotoxin (Salmonella abortus equi) in cancer patients. Cancer Res. 1991;51(10):2524–2530. [PubMed] [Google Scholar]
- 42.Otto F, Schmid P, Mackensen A, et al. Phase II trial of intravenous endotoxin in patients with colorectal and non-small cell lung cancer. Eur J Cancer. 1996;32A(10):1712–1718. doi: 10.1016/0959-8049(96)00186-4. [DOI] [PubMed] [Google Scholar]
- 43.Engelhardt R, Otto F, Mackensen A, et al. Endotoxin (Salmonella abortus equi) in cancer patients. Clinical and immunological findings. Prog Clin Biol Res. 1995;392:253–261. [PubMed] [Google Scholar]
- 44.Mackensen A, Galanos C, Engelhardt R. Modulating activity of interferon-gamma on endotoxin-induced cytokine production in cancer patients. Blood. 1991;78(12):3254–3258. [PubMed] [Google Scholar]
- 45.Kawasaki H, Moriyama M, Ohtani Y, et al. Restoration of normal febrile response to endotoxin in pyrogen-tolerant rabbits by injection with human beta interferon. Infect Immun. 1987;55(11):2574–2578. doi: 10.1128/iai.55.11.2574-2578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soszynski D. Inhibition of nitric oxide synthase delays the development of tolerance to LPS in rats. Physiol Behav. 2002;76(1):159–169. doi: 10.1016/s0031-9384(02)00693-5. [DOI] [PubMed] [Google Scholar]
- 47.Ropert C, Closel M, Chaves AC, et al. Inhibition of a p38/stress-activated protein kinase-2-dependent phosphatase restores function of IL-1 receptor-associate kinase-1 and reverses Toll-like receptor 2- and 4-dependent tolerance of macrophages. J Immunol. 2003;171(3):1456–1465. doi: 10.4049/jimmunol.171.3.1456. [DOI] [PubMed] [Google Scholar]
- 48.Wysocka M, Montaner LJ, Karp CL. Flt3 ligand treatment reverses endotoxin tolerance-related immunoparalysis. J Immunol. 2005;174(11):7398–7402. doi: 10.4049/jimmunol.174.11.7398. [DOI] [PubMed] [Google Scholar]
- 49.Berger MR, Petru E, Schmahl D. Therapeutic ratio of mono or combination bacterial lipopolysaccharide therapy in methylnitrosourea-induced rat mammary carcinoma. J Cancer Res Clin Oncol. 1987;113(5):437–445. doi: 10.1007/BF00390037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertok L. Radio-detoxified endotoxin activates natural immunity: a review. Pathophysiology. 2005;12(2):85–95. doi: 10.1016/j.pathophys.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Berendt MJ, Newborg MF, North RJ. Increased toxicity of endotoxin for tumor-bearing mice and mice responding to bacterial pathogens: macrophage activation as a common denominator. Infect Immun. 1980;28(2):645–647. doi: 10.1128/iai.28.2.645-647.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16(1):53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55(3):237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32(1-3):155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 55.Frey AB, Monu N. Effector-phase tolerance: another mechanism of how cancer escapes antitumor immune response. J Leukoc Biol. 2006;79(4):652–662. doi: 10.1189/jlb.1105628. [DOI] [PubMed] [Google Scholar]
- 56.Page GG. Surgery-induced immunosuppression and postoperative pain management. AACN Clin Issues. 2005;16(3):302–309. doi: 10.1097/00044067-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Joosten JJ, van Muijen GN, Wobbes T, et al. Cryosurgery of tumor tissue causes endotoxin tolerance through an inflammatory response. Anticancer Res. 2003;23(1A):427–432. [PubMed] [Google Scholar]
- 58.Chase JJ, Kubey W, Dulek MH, et al. Effect of monophosphoryl lipid A on host resistance to bacterial infection. Infect Immun. 1986;53(3):711–712. doi: 10.1128/iai.53.3.711-712.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schutze E, Hildebrandt J, Liehl E, et al. Protection of mice from mortality caused by living and heat-killed bacteria by SDZ MRL 953. Circ Shock. 1994;42(3):121–127. [PubMed] [Google Scholar]
- 60.Knopf HP, Otto F, Engelhardt R, et al. Discordant adaptation of human peritoneal macrophages to stimulation by lipopolysaccharide and the synthetic lipid A analogue SDZ MRL 953. Down-regulation of TNF-alpha and IL-6 is paralleled by an up-regulation of IL-1 beta and granulocyte colony-stimulating factor expression. J Immunol. 1994;153(1):287–299. [PubMed] [Google Scholar]
- 61.Takayama K, Ribi E, Cantrell JL. Isolation of a nontoxic lipid A fraction containing tumor regression activity. Cancer Res. 1981;41(7):2654–2657. [PubMed] [Google Scholar]
- 62.Astiz ME, Rackow EC, Still JG, et al. Pretreatment of normal humans with monophosphoryl lipid A induces tolerance to endotoxin: a prospective, double-blind, randomized, controlled trial. Crit Care Med. 1995;23(1):9–17. doi: 10.1097/00003246-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Henricson BE, Manthey CL, Perera PY, et al. Dissociation of lipopolysaccharide (LPS)-inducible gene expression in murine macrophages pretreated with smooth LPS versus monophosphoryl lipid A. Infect Immun. 1993;61(6):2325–2333. doi: 10.1128/iai.61.6.2325-2333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Astiz ME, Saha DC, Brooks K, et al. Comparison of the induction of endotoxin tolerance in endotoxemia and peritonitis by monophosphoryl lipid A and lipopolysaccharide. Circ Shock. 1993;39:194–198. [PubMed] [Google Scholar]
- 65.Ribi E, Cantrell JL, Takayama K, et al. Lipid A and immunotherapy. Rev Infect Dis. 1984;6(4):567–572. doi: 10.1093/clinids/6.4.567. [DOI] [PubMed] [Google Scholar]
- 66.Matsushita K, Kuramitsu Y, Ohiro Y, et al. ONO-4007, a synthetic lipid A analog, induces Th1-type immune response in tumor eradication and restores nitric oxide production by peritoneal macrophages. Int J Oncol. 2003;23(2):489–493. [PubMed] [Google Scholar]
- 67.Matsushita K, Kobayashi M, Totsuka Y, et al. ONO-4007 induces specific anti-tumor immunity mediated by tumor necrosis factor-alpha. Anticancer Drugs. 1998;9(3):273–282. doi: 10.1097/00001813-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Kuramitsu Y, Matsushita K, Ohiro Y, et al. Therapeutic effects of a new synthetic lipid A analog, ONO-4007, on rat hepatoma KDH-8 depend on tumor necrosis factor-sensitivity of the tumor cells. Anticancer Drugs. 1997;8(9):898–901. doi: 10.1097/00001813-199710000-00012. [DOI] [PubMed] [Google Scholar]
- 69.Ishida H, Irie K, Suganuma T, et al. A lipid A analog ONO-4007 induces tolerance to plasma leakage in mice. Inflamm Res. 2002;51(1):38–43. doi: 10.1007/pl00000280. [DOI] [PubMed] [Google Scholar]
- 70.Matsumoto N, Oida H, Aze Y, et al. Intratumoral tumor necrosis factor induction and tumor growth suppression by ONO-4007, a low-toxicity lipid A analog. Anticancer Res. 1998;18(6A):4283–4289. [PubMed] [Google Scholar]
- 71.Ueda H, Yamazaki M. Induction of tumor necrosis factor in a murine tumor by systemic administration of a novel synthetic lipid A analogue, ONO-4007. J Immunother. 1997;20(1):65–69. doi: 10.1097/00002371-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Morita S, Yamamoto M, Kamigaki T, et al. Synthetic lipid A produces antitumor effect in a hamster pancreatic carcinoma model through production of tumor necrosis factor from activated macrophages. Kobe J Med Sci. 1996;42(4):219–231. [PubMed] [Google Scholar]
- 73.Akimoto T, Kumazawa E, Jimbo T, et al. Antitumor effect of DT-5461a, a synthetic low-toxicity lipid A analog, involves endogenous tumor necrosis factor induction subsequent to macrophage activation. Int J Immunopharmacol. 1994;16(11):887–893. doi: 10.1016/0192-0561(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 74.Jimbo T, Akimoto T, Tohgo A. Effect of combined administration of a synthetic low-toxicity lipid A derivative, DT-5461a, and indomethacin in various experimental tumor models of colon 26 carcinoma in mice. Cancer Immunol Immunother. 1995;40(1):10–16. doi: 10.1007/BF01517230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10(2):71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 76.Chu E, Casey LC, Harris JE, et al. Suppression of the development of tumoricidal function in gamma interferon-treated human peripheral blood monocytes by lipopolysaccharide: the role of cyclooxygenase metabolites. J Clin Immunol. 1993;13(1):49–57. doi: 10.1007/BF00920635. [DOI] [PubMed] [Google Scholar]
- 77.Sato K, Yoo YC, Fukushima A, et al. A novel synthetic lipid A analog with low endotoxicity, DT-5461, prevents lethal endotoxemia. Infect Immun. 1995;63(8):2859–2866. doi: 10.1128/iai.63.8.2859-2866.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sato K, Yoo YC, Matsuzawa K, et al. Tolerance to the anti-metastatic effect of lipopolysaccharide against liver metastasis in mice. Int J Cancer. 1996;66(1):98–103. doi: 10.1002/(SICI)1097-0215(19960328)66:1<98::AID-IJC17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 79.Kiani A, Tschiersch A, Gaboriau E, et al. Downregulation of the proinflammatory cytokine response to endotoxin by pretreatment with the nontoxic lipid A analog SDZ MRL 953 in cancer patients. Blood. 1997;90(4):1673–1683. [PubMed] [Google Scholar]
- 80.Saha DC, Barua RS, Astiz ME, et al. Monophosphoryl lipid A stimulated up-regulation of reactive oxygen intermediates in human monocytes in vitro. J Leukoc Biol. 2001;70(3):381–385. [PubMed] [Google Scholar]
- 81.Kasravi FB, Lee DH, Weisgraber K, et al. Lipoprotein-bound endotoxin exerts an immunomodulatory effect on hepatocytes through the lipid A domain of LPS. J Endotoxin Res. 2005;11(1):19–24. doi: 10.1179/096805105225006731. [DOI] [PubMed] [Google Scholar]
- 82.Ogawa H, Rafiee P, Heidemann J, et al. Mechanisms of endotoxin tolerance in human intestinal microvascular endothelial cells. J Immunol. 2003;170(12):5956–5964. doi: 10.4049/jimmunol.170.12.5956. [DOI] [PubMed] [Google Scholar]
- 83.Parker LC, Jones EC, Prince LR, et al. Endotoxin tolerance induces selective alterations in neutrophil function. J Leukoc Biol. 2005;78(6):1301–1305. doi: 10.1189/jlb.0405236. [DOI] [PubMed] [Google Scholar]
- 84.Fujihara M, Muroi M, Tanamoto K, et al. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther. 2003;100(2):171–194. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Medvedev AE, Sabroe I, Hasday JD, et al. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12(3):133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- 86.Nomura F, Akashi S, Sakao Y, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164(7):3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 87.Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164(11):5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 88.Martin M, Katz J, Vogel SN, et al. Differential induction of endotoxin tolerance by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. J Immunol. 2001;167(9):5278–5285. doi: 10.4049/jimmunol.167.9.5278. [DOI] [PubMed] [Google Scholar]
- 89.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168(2):554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 90.Medvedev AE, Lentschat A, Wahl LM, et al. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169(9):5209–5216. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- 91.Oshikawa K, Sugiyama Y. Gene expression of Toll-like receptors and associated molecules induced by inflammatory stimuli in the primary alveolar macrophage. Biochem Biophys Res Commun. 2003;305(3):649–655. doi: 10.1016/s0006-291x(03)00837-4. [DOI] [PubMed] [Google Scholar]
- 92.Moreno C, Merino J, Vazquez B, et al. Anti-inflammatory cytokines induce lipopolysaccharide tolerance in human monocytes without modifying toll-like receptor 4 membrane expression. Scand J Immunol. 2004;59(6):553–558. doi: 10.1111/j.0300-9475.2004.01445.x. [DOI] [PubMed] [Google Scholar]
- 93.Wittebole X, Coyle SM, Kumar A, et al. Expression of tumour necrosis factor receptor and Toll-like receptor 2 and 4 on peripheral blood leucocytes of human volunteers after endotoxin challenge: a comparison of flow cytometric light scatter and immunofluorescence gating. Clin Exp Immunol. 2005;14(1):99–106. doi: 10.1111/j.1365-2249.2005.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tamai R, Sugawara S, Takeuchi O, et al. Synergistic effects of lipopolysaccharide and interferon-gamma in inducing interleukin-8 production in human monocytic THP-1 cells is accompanied by up-regulation of CD14, Toll-like receptor 4, MD-2 and MyD88 expression. J Endotoxin Res. 2003;9(3):145–153. doi: 10.1179/096805103125001540. [DOI] [PubMed] [Google Scholar]
- 95.Ziegler-Heitbrock HW, Wedel A, Schraut W, et al. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem. 1994;269(25):17001–17004. [PubMed] [Google Scholar]
- 96.Mathison J, Wolfson E, Steinemann S, et al. Lipopolysaccharide (LPS) recognition in macrophages. Participation of LPS-binding protein and CD14 in LPS-induced adaptation in rabbit peritoneal exudate macrophages. J Clin Invest. 1993;92(4):2053–2059. doi: 10.1172/JCI116801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Akashi S, Shimazu R, Ogata H, et al. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164(7):3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 98.Medvedev AE, Vogel SN. Overexpression of CD14, TLR4, and MD-2 in HEK 293T cells does not prevent induction of in vitro endotoxin tolerance. J Endotoxin Res. 2003;9(1):60–64. doi: 10.1179/096805103125001360. [DOI] [PubMed] [Google Scholar]
- 99.Burns K, Janssens S, Brissoni B, et al. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197(2):263–268. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Janssens S, Burns K, Vercammen E, et al. MyD88S, a splice variant of MyD88, differentially modulates NF-kappaB- and AP-1-dependent gene expression. FEBS Lett. 2003;548(1-3):103–107. doi: 10.1016/s0014-5793(03)00747-6. [DOI] [PubMed] [Google Scholar]
- 101.Li L, Cousart S, Hu J, et al. Characterization of interleukin-1 receptor-associated kinase in normal and endotoxin-tolerant cells. J Biol Chem. 2000;275(30):23340–23345. doi: 10.1074/jbc.M001950200. [DOI] [PubMed] [Google Scholar]
- 102.Noubir S, Hmama Z, Reiner NE. Dual receptors and distinct pathways mediate interleukin-1 receptor-associated kinase degradation in response to lipopolysaccharide. Involvement of CD14/TLR4, CR3, and phosphatidylinositol 3-kinase. J Biol Chem. 2004;279(24):25189–25195. doi: 10.1074/jbc.M312431200. [DOI] [PubMed] [Google Scholar]
- 103.Kobayashi K, Hernandez LD, Galan JE, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110(2):191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 104.Lopez-Collazo E, Fuentes-Prior P, Arnalich F, et al. Pathophysiology of interleukin-1 receptor-associated kinase-M: implications in refractory state. Curr Opin Infect Dis. 2006;19(3):237–244. doi: 10.1097/01.qco.0000224817.35105.7d. [DOI] [PubMed] [Google Scholar]
- 105.Stoiber D, Kovarik P, Cohney S, et al. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-gamma. J Immunol. 1999;163(5):2640–2647. [PubMed] [Google Scholar]
- 106.Berlato C, Cassatella MA, Kinjyo I, et al. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168(12):6404–6411. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- 107.Nakagawa R, Naka T, Tsutsui H, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17(5):677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 108.Gingras S, Parganas E, de Pauw A, et al. Re-examination of the role of suppressor of cytokine signaling 1 (SOCS1) in the regulation of toll-like receptor signaling. J Biol Chem. 2004;279(52):54702–54707. doi: 10.1074/jbc.M411043200. [DOI] [PubMed] [Google Scholar]
- 109.Brint EK, Xu D, Liu H, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5(4):373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 110.Liew FY, Liu H, Xu D. A novel negative regulator for IL-1 receptor and Toll-like receptor 4. Immunol Lett. 2005;96(1):27–31. doi: 10.1016/j.imlet.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 111.Zhang G, Ghosh S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem. 2002;277(9):7059–7065. doi: 10.1074/jbc.M109537200. [DOI] [PubMed] [Google Scholar]
- 112.Li T, Hu J, Li L. Characterization of Tollip protein upon Lipopolysaccharide challenge. Mol Immunol. 2004;41(1):85–92. doi: 10.1016/j.molimm.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 113.Didierlaurent A, Brissoni B, Velin D, et al. Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol Cell Biol. 2006;26(3):735–742. doi: 10.1128/MCB.26.3.735-742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sly LM, Rauh MJ, Kalesnikoff J, et al. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity. 2004;21(2):227–239. doi: 10.1016/j.immuni.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 115.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54(4):469–487. [PubMed] [Google Scholar]
- 116.Zhang X, Morrison DC. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993;177(2):511–516. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tominaga K, Saito S, Matsuura M, et al. Role of IFN-gamma on dissociation between nitric oxide and TNF/IL-6 production by murine peritoneal cells after restimulation with bacterial lipopolysaccharide. J Leukoc Biol. 1999;66(6):974–980. doi: 10.1002/jlb.66.6.974. [DOI] [PubMed] [Google Scholar]
- 118.Spitzer JA, Zheng M, Kolls JK, et al. Ethanol and LPS modulate NF-kappaB activation, inducible NO synthase and COX-2 gene expression in rat liver cells in vivo. Front Biosci. 2002;7:a99–108. doi: 10.2741/A744. [DOI] [PubMed] [Google Scholar]
- 119.Fahmi H, Ancuta P, Perrier S, et al. Preexposure of mouse peritoneal macrophages to lipopolysaccharide and other stimuli enhances the nitric oxide response to secondary stimuli. Inflamm Res. 1996;45(7):347–353. doi: 10.1007/BF02252947. [DOI] [PubMed] [Google Scholar]
- 120.Zingarelli B, Halushka PV, Caputi AP, et al. Increased nitric oxide synthesis during the development of endotoxin tolerance. Shock. 1995;3(2):102–108. [PubMed] [Google Scholar]
- 121.Severn A, Xu D, Doyle J, et al. Pre-exposure of murine macrophages to lipopolysaccharide inhibits the induction of nitric oxide synthase and reduces leishmanicidal activity. Eur J Immunol. 1993;23(7):1711–1714. doi: 10.1002/eji.1830230747. [DOI] [PubMed] [Google Scholar]
- 122.Dias MB, Almeida MC, Carnio EC, et al. Role of nitric oxide in tolerance to lipopolysaccharide in mice. J Appl Physiol. 2005;98(4):1322–1327. doi: 10.1152/japplphysiol.01243.2004. [DOI] [PubMed] [Google Scholar]
- 123.Zingarelli B, Hake PW, Cook JA. Inducible nitric oxide synthase is not required in the development of endotoxin tolerance in mice. Shock. 2002;17(6):478–484. doi: 10.1097/00024382-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 124.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Waal MR, Abrams J, Bennett B, et al. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Randow F, Syrbe U, Meisel C, et al. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;181(5):1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berg DJ, Kuhn R, Rajewsky K, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96(5):2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chantry D, Turner M, Abney E, et al. Modulation of cytokine production by transforming growth factor-beta. J Immunol. 1989;142(12):4295–4300. [PubMed] [Google Scholar]
- 129.Hafenrichter DG, Roland CR, Mangino MJ, et al. The Kupffer cell in endotoxin tolerance: mechanisms of protection against lethal endotoxemia. Shock. 1994;2(4):251–256. [PubMed] [Google Scholar]
- 130.Li MH, Seatter SC, Manthei R, et al. Macrophage endotoxin tolerance: effect of TNF or endotoxin pretreatment. J Surg Res. 1994;57(1):85–92. doi: 10.1006/jsre.1994.1115. [DOI] [PubMed] [Google Scholar]
- 131.Seatter SC, Li MH, Bubrick MP, et al. Endotoxin pretreatment of human monocytes alters subsequent endotoxin-triggered release of inflammatory mediators. Shock. 1995;3(4):252–258. doi: 10.1097/00024382-199504000-00002. [DOI] [PubMed] [Google Scholar]
- 132.Bulger EM, Maier RV. Lipid mediators in the pathophysiology of critical illness. Crit Care Med. 2000;28(4 Suppl):N27–N36. doi: 10.1097/00003246-200004001-00004. [DOI] [PubMed] [Google Scholar]
- 133.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 134.Ricote M, Li AC, Willson TM, et al. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 135.Uchimura K, Nakamuta M, Enjoji M, et al. Activation of retinoic X receptor and peroxisome proliferator-activated receptor-gamma inhibits nitric oxide and tumor necrosis factor-alpha production in rat Kupffer cells. Hepatology. 2001;33(1):91–99. doi: 10.1053/jhep.2001.21145. [DOI] [PubMed] [Google Scholar]
- 136.Von Knethen AA, Brune B. Delayed activation of PPARgamma by LPS and IFN-gamma attenuates the oxidative burst in macrophages. FASEB J. 2001;15(2):535–544. doi: 10.1096/fj.00-0187com. [DOI] [PubMed] [Google Scholar]
- 137.Bohuslav J, Kravchenko VV, Parry GC, et al. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. J Clin Invest. 1998;102(9):1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wysocka M, Robertson S, Riemann H, et al. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J Immunol. 2001;166(12):7504–7513. doi: 10.4049/jimmunol.166.12.7504. [DOI] [PubMed] [Google Scholar]
- 139.Hirsch C, Ploegh HL. Intracellular targeting of the proteasome. Trends Cell Biol. 2000;10(7):268–272. doi: 10.1016/s0962-8924(00)01768-2. [DOI] [PubMed] [Google Scholar]
- 140.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 141.Elenich LA, Nandi D, Kent AE, et al. The complete primary structure of mouse 20S proteasomes. Immunogenetics. 1999;49(10):835–842. doi: 10.1007/s002510050562. [DOI] [PubMed] [Google Scholar]
- 142.Dahlman B, Hendil KB, Kristensen P, et al. Subunit arrangement in the human proteasome. In: Hilt W, Wolf DH, editors. Proteasomes; The world of regulatory proteolysis. Georgetown, Texas: Landes Bioscience; 2000. pp. 37–47. [Google Scholar]
- 143.Groettrup M, Khan S, Schwarz K, et al. Interferon-gamma inducible exchanges of 20S proteasome active site subunits: why? Biochimie. 2001;83(3-4):367–372. doi: 10.1016/s0300-9084(01)01251-2. [DOI] [PubMed] [Google Scholar]
- 144.Gaczynska M, Goldberg AL, Tanaka K, et al. Proteasome subunits X and Y alter peptidase activities in opposite ways to the interferon-gamma-induced subunits LMP2 and LMP7. J Biol Chem. 1996;271(29):17275–17280. doi: 10.1074/jbc.271.29.17275. [DOI] [PubMed] [Google Scholar]
- 145.Qureshi N, Perera PY, Shen J, et al. The proteasome as a lipopolysaccharide-binding protein in macrophages: differential effects of proteasome inhibition on lipopolysaccharide-induced signaling events. J Immunol. 2003;171(3):1515–1525. doi: 10.4049/jimmunol.171.3.1515. [DOI] [PubMed] [Google Scholar]
- 146.Qureshi N, Vogel SN, Van WC, III, et al. The proteasome: a central regulator of inflammation and macrophage function. Immunol Res. 2005;31(3):243–260. doi: 10.1385/IR:31:3:243. [DOI] [PubMed] [Google Scholar]
- 147.Shen J, Reis J, Morrison DC, et al. Key inflammatory signaling pathways are regulated by the proteasome. Shock. 2006;25(5):472–484. doi: 10.1097/01.shk.0000209554.46704.64. [DOI] [PubMed] [Google Scholar]
- 148.Husebye H, Halaas O, Stenmark H, et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25(4):683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kutuzova GD, Albrecht RM, Erickson CM, et al. Diphosphoryl lipid A from Rhodobacter sphaeroides blocks the binding and internalization of lipopolysaccharide in RAW 264.7 cells. J Immunol. 2001;167(1):482–489. doi: 10.4049/jimmunol.167.1.482. [DOI] [PubMed] [Google Scholar]
- 150.Lien E, Means TK, Heine H, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105(4):497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jarvis BW, Lichenstein H, Qureshi N. Diphosphoryl lipid A from Rhodobacter sphaeroides inhibits complexes that form in vitro between lipopolysaccharide (LPS)-binding protein, soluble CD14, and spectrally pure LPS. Infect Immun. 1997;65(8):3011–3016. doi: 10.1128/iai.65.8.3011-3016.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baker PJ, Taylor CE, Stashak PW, et al. Inactivation of suppressor T cell activity by the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides. Infect Immun. 1990;58(9):2862–2868. doi: 10.1128/iai.58.9.2862-2868.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baker PJ, Hiernaux JR, Fauntleroy MB, et al. Ability of monophosphoryl lipid A to augment the antibody response of young mice. Infect Immun. 1988;56(12):3064–3066. doi: 10.1128/iai.56.12.3064-3066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baker PJ, Hiernaux JR, Fauntleroy MB, et al. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]