Abstract
Biochemical markers, specifically enzymes of the first phase of xenobiotic transformation - cytochrome P450 and ethoxyresorufin-O-deethylase (EROD) - were used to determine the quantities of persistent organic pollutants (POPs) in fish muscle (PCB, HCB, HCH, OCS, DDT). Eight rivers were monitored (Orlice, Chrudimka, Cidlina, Jizera, Vltava, Ohře and Bílina; and the River Blanice was used as a control). The indicator species selected was the chub (Leuciscus cephalus L.). There were no significant differences in cytochrome P450 content between the locations monitored. The highest concentration of cytochrome P450 in fish liver was in the Vltava (0.241 nmol mg-1 protein), and the lowest was in the Orlice (0.120 nmol mg-1 protein). Analysis of EROD activity showed a significant difference between the Blanice and the Vltava (P< 0.05), and also between the Orlice and the Vltava (P< 0.01), the Orlice and the Bílina (P< 0.01), and the Orlice and the Ohře (P< 0.05). The highest EROD activity in fish liver was in the Vltava (576.4 pmol min-1 mg-1 protein), and the lowest was in the Orlice (63.05 pmol min-1 mg-1 protein). In individual locations, results of chemical monitoring and values of biochemical markers were compared. A significant correlation (P< 0.05) was found between biochemical markers and OCS, and PCB. Among the tributaries studied those that contaminated the Elbe most were the Vltava and the Bílina. These tributaries should not be considered the main sources of industrial contamination of the River Elbe, because the most important contamination sources were along the river Elbe itself.
Keywords: cytochrome P450, EROD, Leuciscus cephalus L., persistent organic pollutants, River Elbe
1. Introduction
The dramatic increase in anthropogenic activity since the early 20th century has had a negative impact on all parts of the environment. The aquatic environment has become an easily accessible disposal site for xenobiotics and pollutants such as organochlorine compounds - PCBs and chlorinated pesticides. Contamination of water with industrial and agricultural pollutants influences the biochemical processes of aquatic organisms. An effective monitoring system using biochemical markers has been established to demonstrate these xenobiotics in the environment. The cytochrome P450 system has proved to be a very suitable tool for biochemical and environmental monitoring [1]. Suitable markers have been used to assess contamination in the River Elbe, which is ranked among the very important European aquatic ecosystems. The river is 1 103.5 km long and its catchment covers 148 268 km2. The river flows through two countries, the Czech Republic (51 336 km2) and Germany (96 932 km2). Contamination remains a focus of attention [2-7].
A project of intensive research into contamination of the River Elbe, called Projekt Labe I (1991-1994), was launched in 1991. At present, Projekt Labe IV is in progress, and is centred on potential sources of the river's contamination, that is on the important tributaries. These were the Orlice, Chrudimka, Cidlina, Jizera, Vltava, Ohře and Bílina. The River Blanice was used as a control.
The indicator species selected was the chub (Leuciscus cephalus L.). Fish were caught at sites along the lower reaches of the tributaries and upstream of the first migration barrier before their discharge to the Elbe. The effect of contamination on fish populations was assessed using biochemical markers (specifically enzymes of the first stage of xenobiotic conversion, namely cytochrome P450 and ethoxyresorufin-O-deethylase) and chemical analyses of fish muscle tissue.
Cytochrome P450 (CYP 450) is an important biochemical marker and indicator of contamination with some pollutants [8]. It is particularly sensitive to a broad spectrum of industrial contaminants (e.g. dioxins, polychlorinated biphenyls - PCB, polycyclic aromatic hydrocarbons - PAH) [9,10]. These pollutants accumulate in large quantities in river sediments [11], and from there they get in aquatic organisms via the food chain. The most important indicator of aquatic environment contamination seems to be subfamily 1A of cytochrome P450 [12].
Another enzyme, ethoxyresorufin-O-deethylase (EROD) is functionally linked to cytochrome P450. This enzyme transforms substrates into products emitting a measurable fluorescent signal and is more sensitive than the determination of CYP 450. EROD enzyme activity was also determined as part of aquatic environment contamination monitoring [4,5,13,14].
The aim of the study was to assess the degree of contamination in selected tributaries of the Elbe, using biochemical markers in male chub; to compare results of biochemical and chemical monitoring; and to assess the relationship between CYP 450 and EROD.
2. Methods
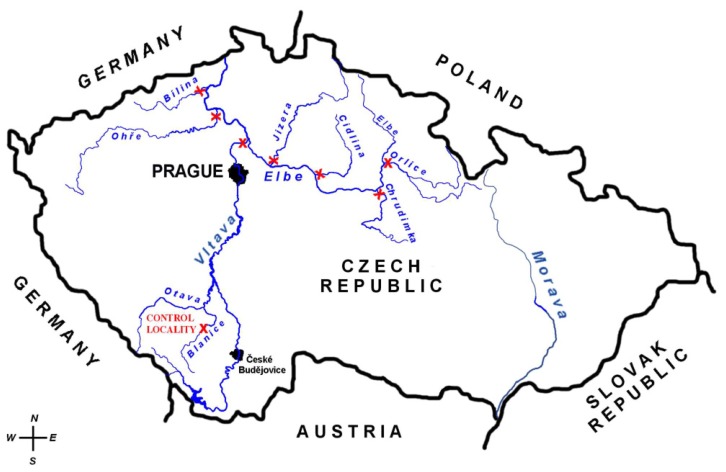
In summer (from May to June) 2006, male chub were caught in the Orlice (992 km river's length), the Chrudimka (967 km river's length), the Cidlina (907 km river's length), the Jizera (868 km river's length), the Vltava (837 km river's length), the Ohře (792 km river's length), and the Bílina (765 km river's length), and at a location on the control River Blanice. These individual locations are shown in Figure 1.
Figure 1.
Map of the Czech Republic and location of sampling sites and the control site.
At each location, 3-10 chub were caught. The biometric characteristics of these fish are given in Table 1. The fish were killed, measured, weighed, aged from scales, and their health status determined by macroscopic examination. Individual liver samples were taken for analysis of biochemical markers (cytochrome P450, EROD) and individual muscle samples for POPs (PCB, DDT, HCB, HCH, OCS) concentrations. Immediately after collection, liver samples were placed in cryotubes into liquid nitrogen and stored at -86° C, where they were kept until use. Muscle samples were stored at -20° C.
Table 1.
Biometric characteristics of male chub (Leuciscus cephalus L.), n = number of fish examined.
| Location | (n) | Weight (g) mean±SD | Age (years) (mean of the total) (min-max) |
|---|---|---|---|
| ORLICE | 6 | 323±39 | 3.8 (3–4) |
| CHRUDIMKA | 10 | 180±11 | 3.2 (3–4) |
| CIDLINA | 9 | 238±53 | 3.7 (2–5) |
| JIZERA | 3 | 377±189 | 4.0 (3–5) |
| VLTAVA | 7 | 290±30 | 3.3 (3–4) |
| OHŘE | 10 | 541±64 | 4.8 (3–7) |
| BÍLINA | 10 | 121±18 | 2.3 (2–3) |
| BLANICE (control location) | 10 | 339±39 | 4.8 (3–6) |
2.1. Liver sample processing
Liver samples were homogenized in homogenizing buffer (0.25 M saccharose, 0.01 M TRIS and 0.1 mM EDTA). Processed samples were then poured into centrifugation test tubes and centrifuged at 10 000 g for 20 min at 4° C. The supernatant was carefully pipetted to ultracentrifugation tubes and centrifuged again at 100 000 g for 1 h at 4° C. The supernatant was drained, pellets were washed with buffer and, then, resuspended in buffer. This suspension was put into individual eppendorf tubes and stored at – 80° C until use. Before the enzymes were assayed, microsomal protein concentrations were determined by the Lowry method [15].
2.2. Quantitative determination of cytochrome P450
Total cytochrome P450 was determined by visible light spectrophotometry at 400–490 nm wavelength range on the basis of the difference between absorbance readings at 450 and 490 nm, and the values obtained were then transformed to final concentrations. Measurements were made after cytochrome reduction by sodium dithionite and after the complex with carbon oxide was formed. The method is described in detail in [5].
2.3. EROD activity determination
Activity of the enzyme ethoxyresorufin-O-deethylase (EROD) was measured by spectrofluorometry. The method is described in detail in [5]. In the presence of the enzyme, its activity transforms the substrate ethoxyresorufin to resurufin in the presence of NADPH. Measurements were made using the Perkin-Elmer Fluorescence Spectrophotometer 203.
2.4. Determination of POPs in muscle samples
Polychlorinated biphenyl (PCB) indicator congeners – IUPAC numbers 28, 52, 101, 118, 138, 153 and 180, hexachlorbenzene (HCB), α-, β-, γ-isomers of hexachlorocyklohexane (HCH), octachlorostyrene (OCS) and DDT and its degradation products DDD and DDE were determined in individual muscle samples by means of two-dimensional capillary gas chromatography (2D/HRGC) employing two parallel columns of equal dimension differing in selectivity (DB-5 and DB-17) and two electron-capture detectors (ECD). Isolation of target analytes from fish muscle was carried out by Soxhlet extraction into a hexane:dichloromethane (1:1, v/v) solvent mixture. The clean-up of extracts was performed, as for alkylphenols, by GPC on a Bio-Beads S-X3 column and mobile phase ethylacetate:cyclohexane (1:1, v/v). ). The method is described in detail in [16].
2.5. Statistical methods
Statistical analysis of the data was performed using the program STATISTICA 6.1 for Windows (StatSoft ČR). The data were assessed by non-parametric methods because data normality was not proven. The Kruskal-Wallis test was used to compare contamination levels of the exogenous substances monitored in individual profiles. The same test was used to compare biochemical markers of contamination between individual profiles. Whenever the Kruskal-Wallis test showed statistically significant differences between profiles (P<0.05), multiple comparisons of all profiles were subsequently performed. Relations between individual parameters were assessed using Spearman's correlation coefficient (R).
3. Results
3.1. Macroscopic assessment of health status of fish
The health status of the fish was examined before tissue samples were collected. Eye damage (exophthalmos and corneal opacity) was found in two fish from the Vltava. Pathological changes of the gonads (neoplasms and an atrophy of part of the gonads) were found in fish from the Cidlina (1 fish), the Orlice (2 fish) and the Ohře (1 fish). Macroscopic examination of chub from the Blanice and from the remaining locations revealed no pathological changes in the fish.
3.2. Biochemical markers
The highest cytochrome P450 concentrations in fish liver were in the Vltava (0.241 nmol mg-1 protein), while the lowest concentrations were in the Blanice (0.152 nmol mg-1 protein) and the Orlice (0.120 nmol mg-1 protein). Statistical analysis showed no significant differences in cytochrome P450 concentrations in the liver of indicator fish between locations.
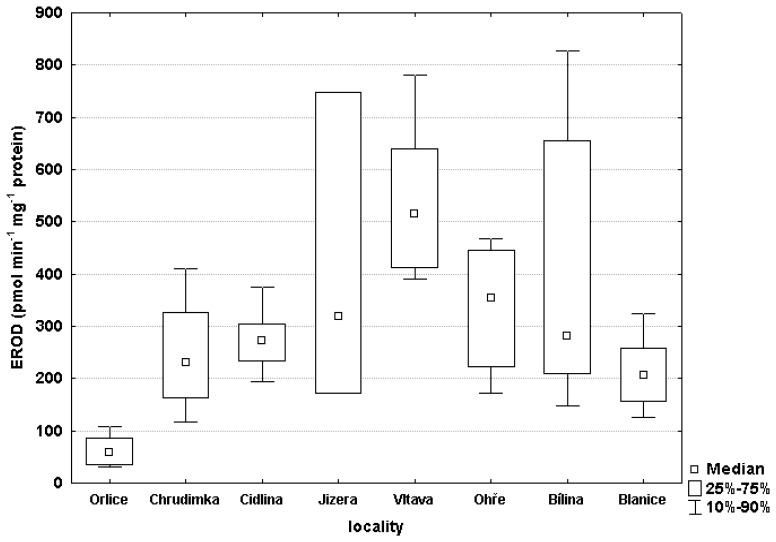
The highest EROD activity in fish liver was in the Vltava (576.4 pmol min-1.mg-1 protein), and the lowest level was in the Orlice (63.05 pmol min-1.mg-1 protein) (Figure 2). The Blanice, which was the control, was 213.7 pmol min-1.mg-1 protein. Statistical analysis of EROD activity showed a significant difference (P< 0.05) between the Blanice control and the Vltava, and also a significant difference between the Orlice and the Vltava (P< 0.01), the Bílina (P< 0.01) and the Ohře (P< 0.05).
Figure 2.
EROD activity in liver samples of male chub
Significant correlations (R = 0.687) between EROD activity and cytochrome P450 were found in fish from all locations at P< 0.01.
3.2. Chemical monitoring
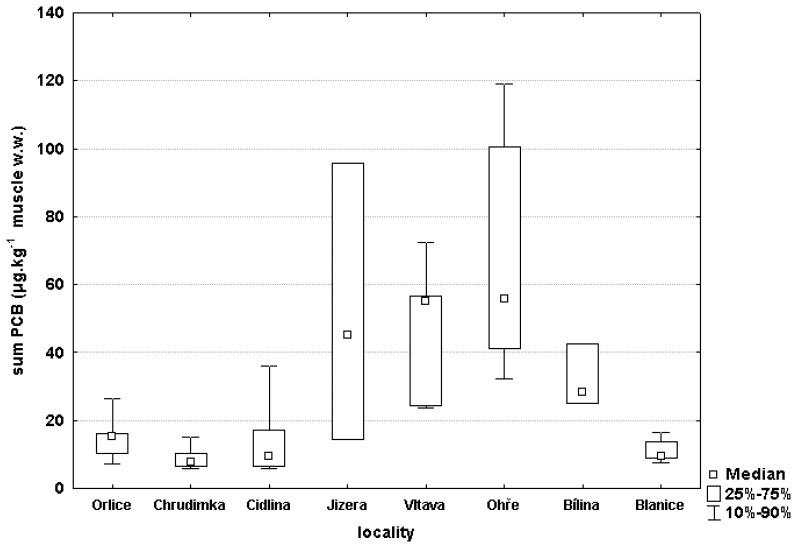
Results of chemical monitoring are summarized in Table 2 and Figure 3. The highest concentrations of pollutants monitored were in fish from the Ohře and the Vltava. The lowest concentrations were in the Blanice and in the Chrudimka.
Table 2.
Content of persistent organic pollutants in male chub (Leuciscus cephalus L.) (μg kg-1 of muscle, w.w.).
| LOCATION | PCB* | DDT** | HCB | HCH*** | OCS |
|---|---|---|---|---|---|
| ORLICE | 15.1±2.66 | 10.6±1.54 | 1.51±0.23 | 0.31±0.1 | 0.028±0.004 |
| CHRUDIMKA | 9.1±1.18 | 13.0±1.27 | 1.57±0.10 | 0.22±0.04 | 0.041±0.003 |
| CIDLINA | 13.7±3.24 | 8.1±1.67 | 0.52±0.05 | 0.45±0.10 | 0.072±0.02 |
| JIZERA | 51.9±23.73 | 23.2±10.21 | 1.22±0.36 | 0.20±0.02 | 0.24±0.13 |
| VLTAVA | 46.1±7.11 | 25.1±3.23 | 1.55±0.19 | 0.21±0.03 | 0.12±0.02 |
| OHŘE | 68.6±10.95 | 44.0±5.51 | 2.82±0.38 | 0.56±0.21 | 0.46±0.23 |
| BÍLINA | 31.9±2.92 | 14.4±1.73 | 2.93±0.38 | 0.32±0.01 | 0.10±0.02 |
| BLANICE (control location) | 10.8±1.06 | 35.0±4.09 | 1.75±0.20 | 0.18±0.03 | 0.03±0.008 |
sum of 7 indicator congeners (28, 52, 101, 118, 138, 153, 180);
sum of DDT (o,p'- DDE; p,p'- DDE; o,p'- DDD; p,p'- DDD; o,p' - DDT; p,p'- DDT);
sum of HCH isomers (α, β, γ)
Figure 3.
Content of PCB (sum of 7 indicator congeners) in male chub muscle samples (wet weight)
PCB concentrations in muscle of indicator fish from the Ohře (P< 0.01) and the Vltava (P< 0.05) were significantly higher than those found in the fish from the Blanice. Significant differences were also found between the Chrudimka and the Vltava (P< 0.01) and the Ohře (P< 0.05), and between the Ohře and the Cidlina (P< 0.05). Among all the indicator PCB congeners, the large majority (70-80 %) were the higher chlorinated PCB congeners 138, 153 and 180.
The lowest muscle DDT concentrations were in indicator fish from the Cidlina. The river with the highest DDT pollution was the Ohře (44.01 μg kg-1 muscle). DDT muscle concentrations in the Orlice and the Cidlina were significantly lower (P< 0.05) than those found in the Blanice. Significant (P< 0.01) differences were found between the Ohře and the Chrudimka, the Ohře and the Orlice, and the Ohře and the Cidlina.
The highest HCB muscle concentrations were in chub from the Ohře and the Bílina, the lowest in chub from the Cidlina. Concentration of HCB in fish muscle from the Cidlina were significantly lower than those in the Blanice (P< 0.01), and in fish from the Chrudimka (P< 0.05), the Ohře (P< 0.01) and the Bílina (P< 0.01).
Statistical analysis showed no significant differences in HCH muscle concentrations between locations. The highest HCH concentrations in muscle of indicator fish were found in the Cidlina, and the lowest HCH concentrations were in the Blanice tributary. The most abundant were the β-isomer (35–50%) and γ-isomer (i.e. lindane) (35–50 %), while the least abundant was the α-HCH isomer (approx. 15 %).
The lowest OCS concentrations in muscle were found in the Blanice, the highest in the Ohře and the Jizera. OCS muscle concentrations in chub from the Ohře were significantly higher (P< 0.05) than those from fish from the Blanice, the Orlice and the Chrudimka.
3.3. Correlations between biochemical markers and pollutant concentrations
Correlating results of chemical monitoring and the results of biochemical marker measurements revealed significant relationships (P<0.05) between cytochrome P450 concentrations and the concentrations of PCB (R=0.317) and OCS (R=0.329) in muscle of indicator fish. A significant correlation (P<0.05) was also found between EROD activity and the concentrations of PCB (R=0.396) and OCS (R=0.436) in muscle of indicator fish.
4. Discussion
Biochemical monitoring showed that the most contaminated tributaries of the Elbe were the rivers Vltava, Ohře, Bílina and Jizera. EROD activity in the liver of chub ranged between 63.1 pmol min-1 mg-1 protein (the Orlice) and 576.4 pmol min-1 mg-1 protein (the Vltava), cytochrome P450 concentrations were between 0.120 nmol mg-1 protein (the Orlice) and 0.241 nmol mg-1 protein (the Vltava). The broad range of EROD activity observed in fish from the sampling locations may be due to the typical induction of cytochrome P450, where small differences in levels of cytochrome inductor exposure will be translated into relatively large differences in enzymatic activity. Organisms exposed to the natural environment are affected by a number of biotic and abiotic factors, which sometimes result in considerable variations in cytochrome P450 values and EROD activity [17]. Substances responsible for the activation of biochemical markers were present in all the locations monitored. Biochemical markers reflect the exposure of the organism to pollutants in their environment and, because they are easy to measure, they are often used as aquatic environment contamination assessment indicators [14] – the river Mures in Rumania, [18] – contamination of rivers in southern Belgium, [19] – Karnaphuly River in Bangladesh, [20] – small streams in an urban area. Biochemical markers for the assessment of the contamination of the River Elbe in the Czech Republic with organic pollutants have also been used by [4,5]. The highest values of EROD activity along the Elbe were reported by these authors from Valy (263.2 pmol min-1 mg-1 protein), a location negatively affected by the chemical industry. The least contaminated was the control site, the river Blanice. The highest cytochrome P450 concentration was recorded in Lysá nad Labem in the middle reaches of the Elbe (0.48 nmol mg-1 protein). The assessment of organic pollutants contamination of the River Elbe on German territory using biochemical markers has been conducted by, e.g. [3,21-23] found higher values of biochemical markers (EROD and cytochrome system) at locations along the Elbe near the Czech Republic compared with locations situated further downstream. Cytochrome P450 values found at locations monitored in Germany are comparable with values ascertained in this study in the Czech Republic. Broeg et al. used EROD activity as one of several parameters they monitored when they assessed contamination in four locations [21]. They found the highest EROD activity levels in the Elbe estuary, indicating that the River Elbe is contaminated with xenobiotics along its entire length.
Biochemical markers are important indicators of organic pollutant contamination of the environment. Monitoring these markers showed differences in contamination levels in different locations. These differences were confirmed by the results of chemical monitoring. Results of biochemical monitoring indicated that the most contaminated tributaries were the rivers Jizera, Vltava, Ohře and Bílina. At the same time, correlation was demonstrated (P<0.05) between concentrations of biochemical markers and the amounts of chemical pollutants (specifically between cytochrome P450 and ΣPCB, cytochrome P450 and OCS, EROD activity and ΣPCB and EROD activity and OCS). PCB is one of important cytochrome P450 inductors. The most abundantly represented PCB congeners included higher chlorinated PCB congeners (Nos 138, 153 and 180). Higher chlorinated PCBs in the Elbe have also been reported in Germany where Heinisch et al. monitored PCB in fish and sediment along the entire river [24]. The frequent occurrence of higher chlorinated PCB congeners in the environment is the result of past use of technical mixes (e.g. Delor 106), which contain relatively high amounts of higher chlorinated congeners. In Czechoslovakia, 21 482 tonnes of PCB were produced between 1959 and 1984, of which Delor 106 contributed about 20 % (4 381 tonnes) [25].
The highest muscle concentrations of DDT were in fish from the Ohře, and the lowest were from the Cidlina. In all the samples examined, the dominant proportion in the sum of substances derived from DDT was made up of the isomer p,p'-DDE (75–90 %). Large amounts of the p,p'-DDE metabolite are suggestive of old contamination because the DDT to DDE conversion is relatively slow and, moreover, the parent substance DDT/DDE metabolite ratio is very low. p,p'-DDE isomer is the final product of metabolic transformation of the p,p'-DDT insecticide. A role in this transformation, that takes place in the liver, is played by the P450 cytochrome system [26]. The levels of other substances decreased in the order: DDE >> DDD >> DDT. All tests showed only minimum quantities of o,p'- isomers. o,p'-isomers are less stable than p,p' –isomers and are therefore detected in low concentrations in the environment. The o,p'-DDT isomer has a mild oestrogenic activity [27,28]. Some DDT isomers can be classified as environmental endocrine disruptors because they may cause disruption of the endocrine system [29,30]. Bioaccumulation of DDT and its metabolites in the brain causes an induction of liver enzymes [31] and a disruption of hormonal mechanisms [32].
HCB, another major environmental contaminant, used to be used as a fungicide for cereal seed. Nowadays it is brought into the environment as an intermediate product of a number of chlorinated compounds, particularly of lower chlorinated benzenes and of some pesticides [33]. Hexachlorobenzene is an omnipresent substance produced during combustion. The European parliament regulation 2000/60/EC identifies HCB as a priority hazardous substance. The main source of HCB for the Elbe's aquatic ecosystem is the waste site at Chabařovice, which is an abandoned brown coal open pit where municipal wastes and about 80 000 barrels of HCB-containing chemical waste from chemical plants were dumped between 1978 and 1990. Chlorobenzenes in various locations along the River Elbe (in water and in the sediment) in the Czech Republic and in Germany were monitored by Heinisch et al. [34]. HCB in the Elbe was demonstrated in bream (Abramis brama) and eel (Anguilla anguilla) as early as the 1980s [35,36]. OCS and HCB are considered the main organochlorine contaminants of the Elbe. Marth et al. measured levels of chlorinated hydrocarbon contaminants (HCB, OCS, DDT and PCB) in muscle and liver of bream at various locations along the Elbe in Germany [37]. Higher levels of organochlorine pollutant contamination were found mainly in fish from locations in the upper reaches of the river.
The lowest OCS concentrations in muscle were in the Blanice tributary, the highest in the Ohře and the Jizera. These concentrations were, however, lower by two orders of magnitude than those reported by Bester et al. in the liver of bream in the Elbe in Germany [38]. Though octachlorostyrene is a pollutant that is known to be prevalent in the environment, there may be sources that are still emitting significant amounts of chlorostyrenes along the River Elbe. Generally, such industrial sources exhibit their own chemical profile in their emissions.
Of the three HCH isomers, the most abundantly represented were the β isomer and γ-isomer. (i.e. lindane). Lindane, has been used frequently in agriculture for its insecticidal action, and is still found in the environment because of its bioaccumulation in soil. Investigating bream from locations along the Elbe in Germany, Marth et al. found that the most abundant HCH isomers were a-HCH and b-HCH isomers [37].
Although the manufacture and use of PCB and organochlorinated pesticides is banned or considerably restricted, these persistent compounds continue to be found even today among the pollutants contaminating aquatic environments all over the world [39-42]. It was noted that substances responsible for the activation of biochemical markers were present in all the locations monitored. The results of chemical monitoring also showed that locations with the highest values of biochemical markers were also those with the highest xenobiotic contaminations.
5. Conclusion
Analyses of biochemical markers of contamination and of chemical analyses of indicator fish showed that of the tributaries studied the ones that most contaminate the Elbe are the Jizera, Vltava, Ohře and Bílina. In most cases, elevated biomarker values were found together with elevated levels of the pollutants monitored. On the basis of concentrations of xenogenous substances found, these tributaries should not, however, be generally considered the main sources of industrial contamination of the River Elbe because the most important contamination sources are along the river Elbe itself.
Acknowledgments
This research was supported by the Ministry of the Environment of the Czech Republic SP/2e7/229/07 and by the Ministry of Education, Youth and Sports of the Czech Republic (MSM 621 571 2402 and MSM 600 766 5809).
References
- 1.Payne J.F., Fancey L.L., Rahimtula A.D., Porter E.L. Review and perspective on the use of mixed-function oxygenase enzymes in biological monitoring. Comp. Biochem. Physiol. Pt C. 1987;86:233–245. doi: 10.1016/0742-8413(87)90074-0. [DOI] [PubMed] [Google Scholar]
- 2.Adams M.S., Ballin U., Gaumert T., Hale B.W., Kausch H., Kruse R. Monitoring selected indicators of ecological change in the Elbe River since the fall of the Iron Curtain. Environ. Conserv. 2001;28:333–344. [Google Scholar]
- 3.Hecker M., Sanderson J.T., Karbe L. Suppression of aromatase activity in populations of bream (Abramis brama) from the River Elbe, Germany. Chemosphere. 2007;66:542–552. doi: 10.1016/j.chemosphere.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 4.Randak T., Zlabek V., Kolarova J., Svobodova Z., Hajslova J., Siroka Z., Janska M., Pulkrabova J., Cajka T., Jarkovsky J. Biomarkers detected in chub (Leuciscus cephalus L.) to evaluate contamination of the Elbe and Vltava Rivers, Czech Republic. B. Environ. Contam. Tox. 2006;76:233–241. doi: 10.1007/s00128-006-0912-3. [DOI] [PubMed] [Google Scholar]
- 5.Siroka Z., Krijt J., Randak T., Svobodova Z., Peskova G., Fuksa J., Hajslova J., Jarkovsky J., Janska M. Organic pollutant contamination of the River Elbe as assessed by biochemical markers. Acta Vet. BRNO. 2005;74:293–303. [Google Scholar]
- 6.Stachel B., Ehrhorn U., Heemken O.P., Lepom P., Reincke H., Sawal G., Theobald N. Xenoestrogens in the River Elbe and its tributaries. Environ. Pollut. 2003;124:497–507. doi: 10.1016/s0269-7491(02)00483-9. [DOI] [PubMed] [Google Scholar]
- 7.Zlabek V., Svobodova Z., Randak T., Valentova O. Mercury content in the muscle of fish from the Elbe River and its tributaries. Czech J. Anim. Sci. 2005;50:528–534. [Google Scholar]
- 8.Jung D.K.J., Klaus T., Fent K. Cytochrome P450 induction by nitrated polycyclic aromatic hydrocarbons, azaarenes, and binary mixtures in fish hepatoma cell line PLHC-1. Environ. Toxicol. Chem. 2001;20:149–159. [PubMed] [Google Scholar]
- 9.van der Oost R., Beyer J., Vermeulen N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Pharm. 2003;13:57–149. doi: 10.1016/s1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- 10.White R.D., Shea D., Stegeman J.J. Metabolism of the aryl hydrocarbon receptor agonist 3,3',4,4'tetrachlorobiphenyl by the marine fish scup (Stenotomus chrysops) in vivo and in vitro. Drug Metab. Dispos. 1997;25:564–572. [PubMed] [Google Scholar]
- 11.Malins D.C., McCain B.B., Brown D.W., Chan S.L., Myers M.S., Landahl J.T., Prohaska P.G., Friedman A.J., Rhodes L.D., Burrows D.G., Gronlund W.D., Hodgins H.O. Chemical-pollutants in sediments and diseases of bottom-dwelling fish in Puget Sound, Washington. Environ. Sci. Technol. 1984;18:705–713. [Google Scholar]
- 12.Anzenbacherova E., Anzenbacher P. Cytochromy P450 a metabolismus xenobiotik. Bull. Ceske Spol. Biochem. Mol. Biol. 1999;1:4–33. [Google Scholar]
- 13.Flammarion P., Devaux A., Nehls S., Migeon B., Noury P., Garric J. Multibiomarker responses in fish from the Moselle River (France) Ecotox. Environ. Safe. 2002;51:145–153. doi: 10.1006/eesa.2001.2134. [DOI] [PubMed] [Google Scholar]
- 14.Koehler H.R., Sandu C., Scheil V., Nagy-Petrica E.M., Segner H., Telcean I., Stan G., Triebskorn R. Monitoring pollution in River Mures, Romania, Part III: biochemical effect markers in fish and integrative reflection. Environ. Monit. Assess. 2007;127:47–54. doi: 10.1007/s10661-006-9257-y. [DOI] [PubMed] [Google Scholar]
- 15.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Hajslova J., Schoula R., Holadova K., Poustka J. Analysis of PCBs in biotic matrices by 2-dimensional GC-ECD. Int. J. Environ. An. Ch. 1995;60:163–173. [Google Scholar]
- 17.Goksoyr A., Forlin L. The cytochrome P-450 system in fish, aquatic toxicology and environmental monitoring. Aquat. Toxicol. 1992;22:287–311. [Google Scholar]
- 18.Mayon N., Bertrand A., Leroy D., Malbrouck C., Mandiki S.N.M., Silvestre F., Goffart A., Thome J.P., Kestemont P. Multiscale approach of fish responses to different types of environmental contaminations: A case study. Sci. Total Environ. 2006;367:715–731. doi: 10.1016/j.scitotenv.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Al-Arabi S.A.M., Adolfsson-Erici M., Waagbo R., Ali M.S., Goksoyr A. Contaminant accumulation and biomarker responses in caged fish exposed to effluents from anthropogenic sources in the Karnaphuly River, Bangladesh. Environ. Toxicol. Chem. 2005;24:1968–1978. doi: 10.1897/04-383r.1. [DOI] [PubMed] [Google Scholar]
- 20.Behrens A., Segner H. Cytochrome P4501A induction in brown trout exposed to small streams of an urbanised area: results of a five-year-study. Environ. Pollut. 2005;136:231–242. doi: 10.1016/j.envpol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Broeg K., Zander S., Diamant A., Korting W., Kruner G., Paperna I., von Westernhagen H. The use of fish metabolic, pathological and parasitological indices in pollution monitoring - 1. North Sea. Helgoland Mar. Res. 1999;53:171–194. [Google Scholar]
- 22.Jedamskigrymlas J., Kammann U., Tempelmann A., Karbe L., Siebers D. Biochemical responses and environmental contaminants in breams (Abramis-brama L) caught in the River Elbe. Ecotox. Environ. Safe. 1995;31:49–56. doi: 10.1006/eesa.1995.1042. [DOI] [PubMed] [Google Scholar]
- 23.Jedamskigrymlas J., Lange U., Siebers D., Karbe L. Induction of the hepatic biotransformation system of golden ide [Leuciscus-idus (L)] after exposure in the River Elbe. Ecotox. Environ. Safe. 1994;28:35–42. doi: 10.1006/eesa.1994.1032. [DOI] [PubMed] [Google Scholar]
- 24.Heinisch E., Kettrup A., Bergheim W., Holoubek I., Wenzel S. PCB in aquatic ecosystems of the river Elbe and Berlin waters - Source oriented monitoring. Fresen. Environ. Bull. 2003;12:103–110. [Google Scholar]
- 25.Breivik K., Sweetman A., Pacyna J.M., Jones K.C. Towards a global historical emission inventory for selected PCB congeners - A mass balance approach. 3. An update. Sci. Total Environ. 2007;377:296–307. doi: 10.1016/j.scitotenv.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura S., Yoshida M., Sugihara K., Ohta S. Reductive dechlorination of p,p '-DDT mediated by hemoproteins in the hepatopancreas and blood of goldfish, Carassius auratus. J. Health Sci. 1999;45:217–221. [Google Scholar]
- 27.Ackermann G.E., Brombacher E., Fent K. Development of a fish reporter gene system for the assessment of estrogenic compounds and sewage treatment plant effluents. Environ. Toxicol. Chem. 2002;21:1864–1875. [PubMed] [Google Scholar]
- 28.Toppari J., Larsen J.C., Christiansen P., Giwercman A., Grandjean P., Guillette L.J., Jegou B., Jensen T.K., Jouannet P., Keiding N., Leffers H., McLachlan J.A., Meyer O., Muller J., RajpertDeMeyts E., Scheike T., Sharpe R., Sumpter J., Skakkebaek N.E. Male reproductive health and environmental xenoestrogens. Environ. Health Perspect. 1996;104:741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leanos-Castaneda O., Van Der Kraak G., Rodriguez-Canul R., Gold G. Endocrine disruption mechanism of o,p'-DDT in mature male tilapia (Oreochromis niloticus) Toxicol. Appl. Pharm. 2007;221:158–167. doi: 10.1016/j.taap.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Metcalfe T.L., Metcalfe C.D., Kiparissis Y., Niimi A.J., Foran C.M., Benson W.H. Gonadal development and endocrine responses in Japanese medaka (Oryzias latipes) exposed to o,p '-DDT in water or through maternal transfer. Environ. Toxicol. Chem. 2000;19:1893–1900. [Google Scholar]
- 31.Nims R.W., Lubet R.A., Fox S.D., Jones C.R., Thomas P.E., Reddy A.B., Kocarek T.A. Comparative pharmacodynamics of CYP2B induction by DDT, DDE, and DDD in male rat liver and cultured rat hepatocytes. J. Toxicol. Env. Health Pt A. 1998;53:455–477. doi: 10.1080/009841098159187. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez A., Piferrer F. Aromatase activity in the European sea bass (Dicentrarchus labrax L.) brain. Distribution and changes in relation to age, sex, and the annual reproductive cycle. Gen. Comp. Endocr. 2003;132:223–230. doi: 10.1016/s0016-6480(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 33.Bailey R.E. Global hexachlorobenzene emissions. Chemosphere. 2001;43:167–182. doi: 10.1016/s0045-6535(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 34.Heinisch E., Kettrup A., Bergheim W., Martens D., Wenzel S. Persistent chlorinated hydrocarbons (PCHC), source-oriented monitoring in aquatic media. 4. The chlorobenzenes. Fresen. Environ. Bull. 2006;15:148–169. [Google Scholar]
- 35.Luckas B., Oehme M. Characteristic contamination levels for polychlorinated hydrocarbons, dibenzofurans and dibenzo-para-dioxins in bream (Abramis brama) from the River Elbe. Chemosphere. 1990;21:79–89. [Google Scholar]
- 36.Oxynos K., Schramm K.W., Marth P., Schmitzer J. Chlorinated hydrocarbons (CHC) and PCDD/F-levels in sediments and breams (Abramis-brama) from the River Elbe (A contribution to the German Environmental Specimen Banking) Fresenius J. Anal. Chem. 1995;353:98–100. doi: 10.1016/s0045-6535(97)00077-5. [DOI] [PubMed] [Google Scholar]
- 37.Marth P., Oxynos K., Schmitzer J., Schramm K.W., Kettrup A. Levels of chlorinated hydrocarbons (CHC) in breams (Abramis brama) from the River Elbe (A contribution to the Federal Environmental Specimen Bank) Chemosphere. 1997;34:2183–2192. doi: 10.1016/s0045-6535(97)00077-5. [DOI] [PubMed] [Google Scholar]
- 38.Bester K., Biselli S., Ellerichmann T., Huhnerfuss H., Moller K., Rimkus G., Wolf M. Chlorostyrenes in fish and sediment samples from the River Elbe. Chemosphere. 1998;37:2459–2471. doi: 10.1016/s0045-6535(98)00302-6. [DOI] [PubMed] [Google Scholar]
- 39.Deboer J., Vandervalk F., Kerkhoff M.A.T., Hagel P., Brinkman U.A.T. 8-year study on the elimination of PCBs and other organochlorine compounds from eel (Anguilla-anguilla) under natural conditions. Environ. Sci. Technol. 1994;28:2242–2248. doi: 10.1021/es00062a007. [DOI] [PubMed] [Google Scholar]
- 40.Ferrante M.C., Cirillo T., Naso B., Clausi M.T., Lucisano A., Cocchieri R.A. Polychlorinated biphenyls and organochlorine pesticides in seafood from the Gulf of Naples (Italy) J. Food Protect. 2007;70:706–715. doi: 10.4315/0362-028x-70.3.706. [DOI] [PubMed] [Google Scholar]
- 41.Vorkamp K., Riget F., Glasius M., Pecseli M., Lebeuf M., Muir D. Chlorobenzenes, chlorinated pesticides, coplanar chlorobiphenyls and other organochlorine compounds in Greenland biota. Sci. Total Environ. 2004;331:157–175. doi: 10.1016/j.scitotenv.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 42.Yang N.Q., Matsuda M., Kawano M., Wakimoto T. PCBs and organochlorine pesticides (OCPs) in edible fish and shellfish from China. Chemosphere. 2006;63:1342–1352. doi: 10.1016/j.chemosphere.2005.09.029. [DOI] [PubMed] [Google Scholar]