Abstract
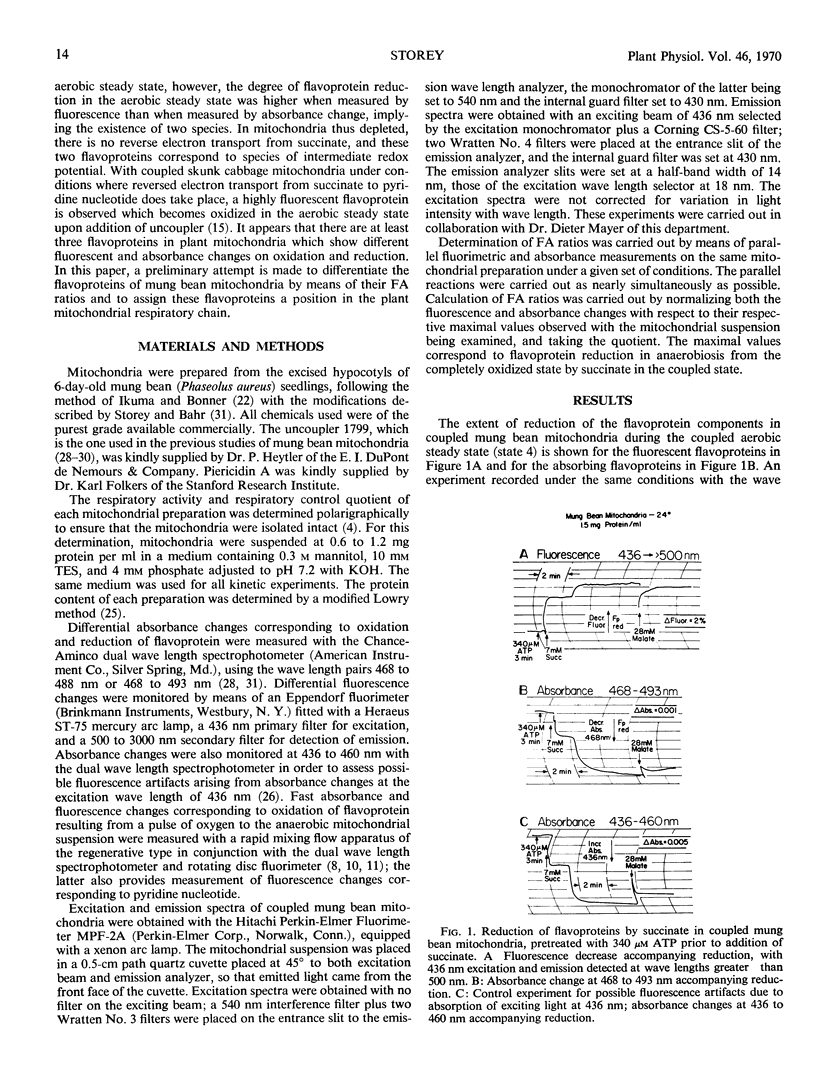
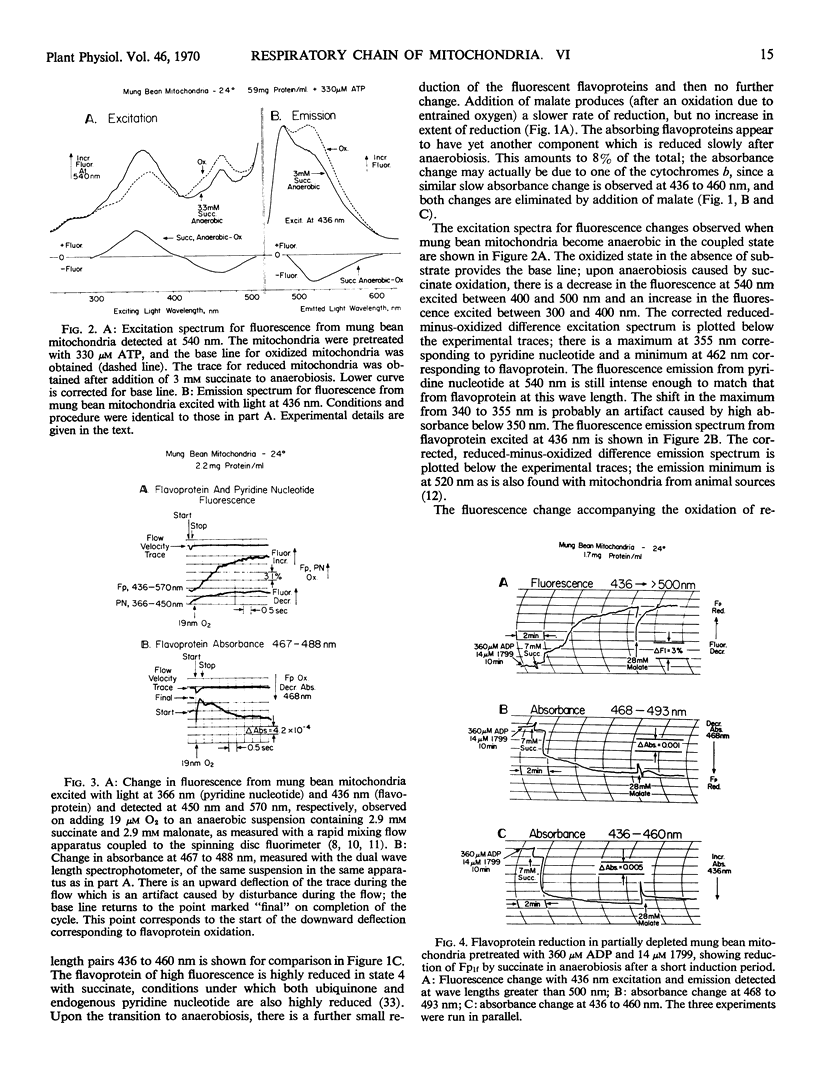
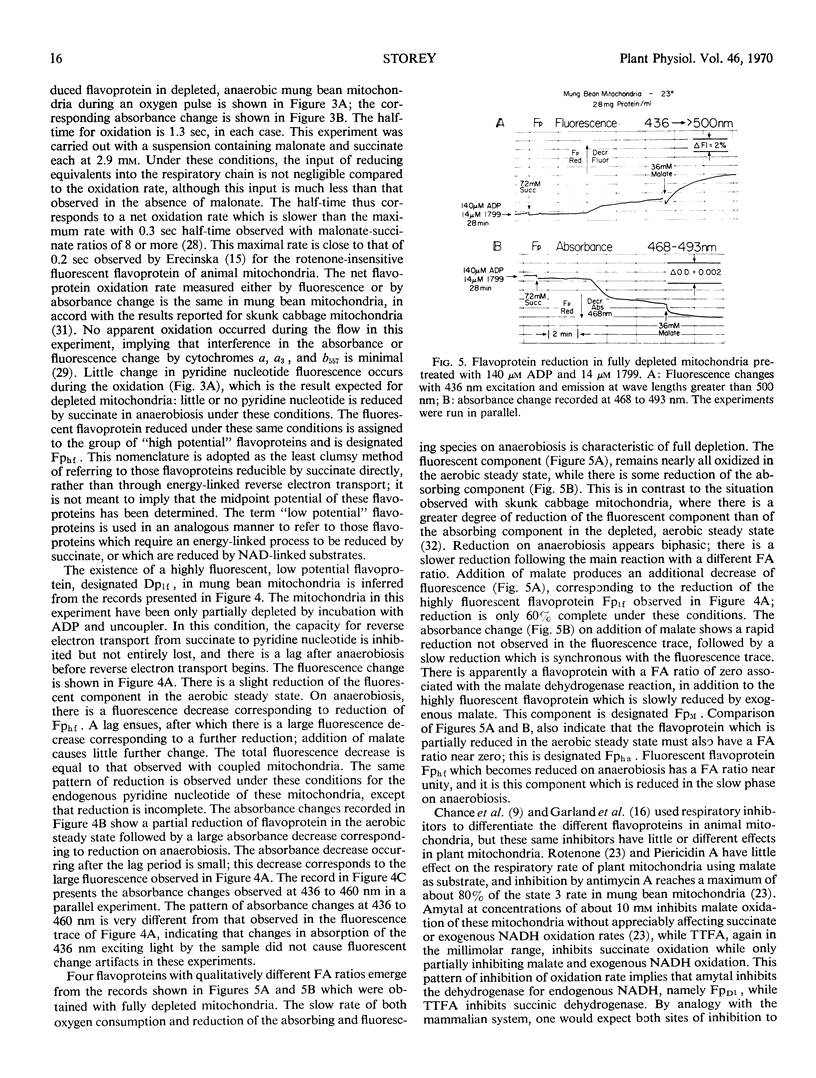
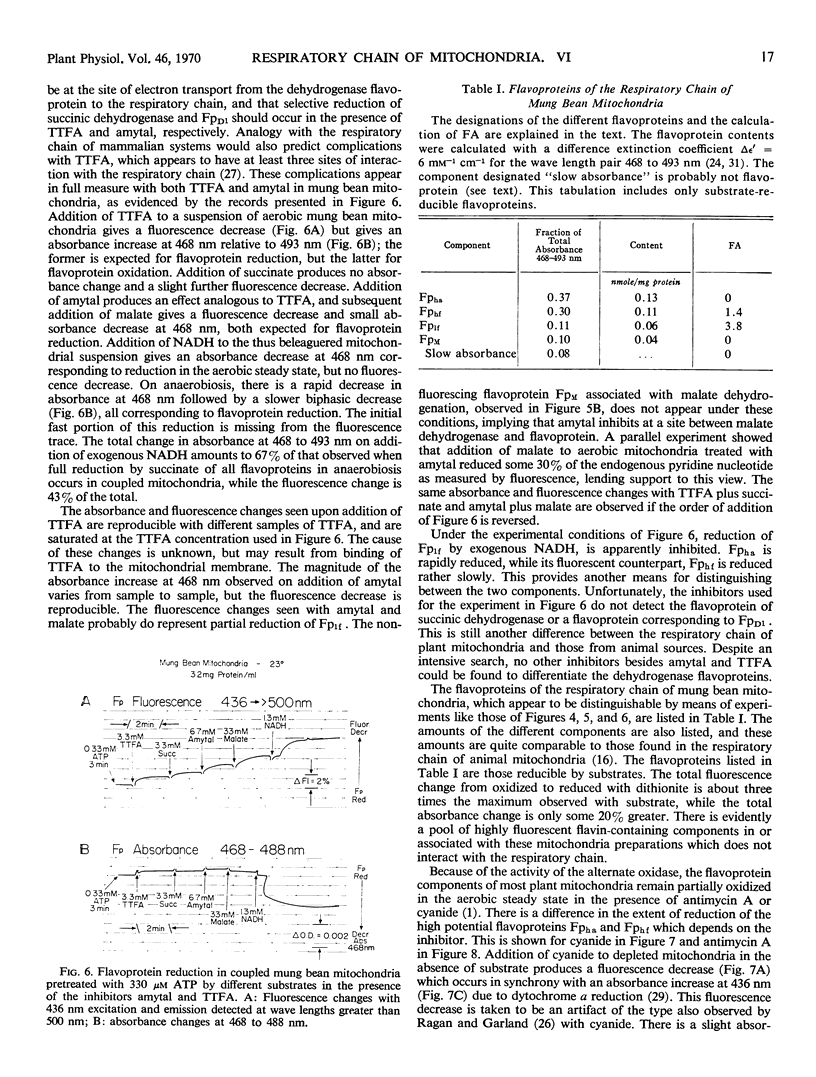
Redox changes of the flavoproteins of mung bean (Phaseolus aureus) mitochondria were measured by differential absorbance at 468 to 493 nanometers and by fluorescence emission above 500 nanometers excited at 436 nanometers. Four flavoproteins are distinguishable by the ratio of their fluorescence to absorbance changes, and by their requirement, or lack of it, for energy-linked reverse electron transport for reduction by succinate. Two flavoproteins are reduced by succinate in fully depleted mitochondria which lack the capacity for reverse electron transport. These are designated Fpha and Fphf and have fluorescence to absorbance ratios of 0 and 1.4, respectively. The two flavoproteins have the same half-time for oxidation, but Fphf is reduced more slowly than Fpha by substrate in the presence of cyanide. One flavoprotein with a fluorescence to absorbance ratio of 0 is not reduced by succinate in anaerobic, fully depleted mitochondria, but is rapidly reduced on subsequent addition of malate; it is designated Fpm. The fourth distinguishable flavoprotein component is reducible by succinate in an energy-linked reaction, even in partially depleted mitochondria. This component has a fluorescence to absorbance ratio of 3.8 and is designated Fp1f. In addition to these four flavoproteins reducible by substrates, there is a highly fluorescent flavin-containing component in or associated with these mitochondria, which is rapidly reduced by dithionite.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chance B., Bonner W. D. Kinetics of Cytochrome Oxidation in Skunk Cabbage Mitochondria. Plant Physiol. 1965 Nov;40(6):1198–1204. doi: 10.1104/pp.40.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Ernster L., Garland P. B., Lee C. P., Light P. A., Ohnishi T., Ragan C. I., Wong D. Flavoproteins of the mitochondrial respiratory chain. Proc Natl Acad Sci U S A. 1967 May;57(5):1498–1505. doi: 10.1073/pnas.57.5.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Garland P. B., Chance B., Ernster L., Lee C. P., Wong D. Flavoproteins of mitochondrial fatty acid oxidation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1696–1702. doi: 10.1073/pnas.58.4.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett D. P., Haas D. W. Oxidative Phosphorylation and Functional Cytochromes in Skunk Cabbage Mitochondria. Plant Physiol. 1958 Jan;33(1):27–32. doi: 10.1104/pp.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmey M. A., Ikuma H., Bonner W. D. Near ultra-violet spectrum of white potato mitochondria. Nature. 1966 Jan 8;209(5019):174–175. doi: 10.1038/209174a0. [DOI] [PubMed] [Google Scholar]
- Hassinen I., Chance B. Oxidation-reduction properties of the mitochondrial flavoprotein chain. Biochem Biophys Res Commun. 1968 Jun 28;31(6):895–900. doi: 10.1016/0006-291x(68)90536-6. [DOI] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. E. RECONSTITUTION OF RESPIRATORY CHAIN ENZYME SYSTEMS. XI. USE OF ARTIFICIAL ELECTRON ACCEPTORS IN THE ASSAY OF SUCCINATE-DEHYDROGENATING ENZYMES. J Biol Chem. 1963 Dec;238:4032–4036. [PubMed] [Google Scholar]
- Ragan C. I., Garland P. B. The intra-mitochondrial localization of flavoproteins previously assigned to the respiratory chain. Eur J Biochem. 1969 Oct;10(3):399–410. doi: 10.1111/j.1432-1033.1969.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria. III. Oxidation Rates of the Cytochromes c and b in Mung Bean Mitochondria Reduced With Succinate. Plant Physiol. 1969 Mar;44(3):413–421. doi: 10.1104/pp.44.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. IV. Oxidation rates of the respiratory carriers of mung bean mitochondria in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):447–454. doi: 10.1104/pp.45.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. V. Reaction of reduced cytochromes a and a3 in mung bean mitochondria with oxygen in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):455–460. doi: 10.1104/pp.45.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]



