Abstract
Purpose
To evaluate yoga's impact on inflammation, mood, and fatigue.
Patients and Methods
A randomized controlled 3-month trial was conducted with two post-treatment assessments of 200 breast cancer survivors assigned to either 12 weeks of 90-minute twice per week hatha yoga classes or a wait-list control. The main outcome measures were lipopolysaccharide-stimulated production of proinflammatory cytokines interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and interleukin-1β (IL-1β), and scores on the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF), the vitality scale from the Medical Outcomes Study 36-item Short Form (SF-36), and the Center for Epidemiological Studies-Depression (CES-D) scale.
Results
Immediately post-treatment, fatigue was not lower (P > .05) but vitality was higher (P = .01) in the yoga group compared with the control group. At 3 months post-treatment, fatigue was lower in the yoga group (P = .002), vitality was higher (P = .01), and IL-6 (P = .027), TNF-α (P = .027), and IL-1β (P = .037) were lower for yoga participants compared with the control group. Groups did not differ on depression at either time (P > .2). Planned secondary analyses showed that the frequency of yoga practice had stronger associations with fatigue at both post-treatment visits (P = .019; P < .001), as well as vitality (P = .016; P = .0045), but not depression (P > .05) than simple group assignment; more frequent practice produced larger changes. At 3 months post-treatment, increasing yoga practice also led to a decrease in IL-6 (P = .01) and IL-1β (P = .03) production but not in TNF-α production (P > .05).
Conclusion
Chronic inflammation may fuel declines in physical function leading to frailty and disability. If yoga dampens or limits both fatigue and inflammation, then regular practice could have substantial health benefits.
INTRODUCTION
Cancer survivors are more than twice as likely as individuals without a cancer history to have poor health and disability.1 Reduced physical activity during cancer treatment can decrease the capacity for physical performance, and activity may be further limited by the late effects of cancer and its treatment in survivors.2,3 With deconditioning, normal activities become more fatiguing, resulting in greater weariness and lessened functional capacity over time.2,3 Breast cancer survivors with lower levels of physical activity have a higher risk for premature death.4
Inflammation is one of the key candidate mechanisms for age-related decrements in physical function and disability.5–7 Chronic inflammation signals a heightened risk for disability and mortality, even in the absence of clinical disease.5,6,8,9
Inflammation is lower in active than in sedentary individuals.10 Indeed, when cardiorespiratory fitness is assessed objectively by maximal exercise testing, better physical fitness is associated with lower inflammation.11,12 In this context, it is noteworthy that cancer survivors' average cardiorespiratory fitness is consistently approximately 30% lower than that of their sedentary age mates without a cancer history.13,14
About a third of breast cancer survivors report that fatigue interferes with daily activities.15 Persistent fatigue in survivors may be related in part to overactivation of the inflammatory network.16 Regular exercise reduces fatigue17 as well as inflammation.7,18 However, fatigue and pain often limit survivors' physical activity.19,20 Yoga provides graded exercise that can be tailored for individuals who have been sedentary, and the postures can be modified to accommodate functional limitations.20 In addition, studies with cancer survivors suggest that yoga practice lowers fatigue and improves mood and sleep quality.20–25
Accordingly, this study assessed the impact of yoga on inflammation, mood, and fatigue, our primary outcomes. We hypothesized that yoga would decrease inflammation, depressive symptoms, and fatigue in contrast to those characteristics in the wait-list control group.
PATIENTS AND METHODS
Study Design
This randomized controlled trial (RCT) compared a 12-week hatha yoga intervention with a wait-list control condition. Questionnaires and fasting blood samples were collected in the Clinical Research Center at baseline, immediately post-treatment, and 3 months post-treatment. The institutional review board approved this study, and each participant provided informed consent.
Participants
The 200 stage 0 to IIIa breast cancer survivors ranged in age from 27 to 76 years (Table 1). They had completed cancer treatment within the past 3 years (except for tamoxifen/aromatase inhibitors) and were at least 2 months post-surgery or adjuvant therapy or radiation, whichever occurred last. Women were recruited through oncologists' referrals, community print and web-based announcements, and breast cancer groups and events.
Table 1.
Demographic and Medical Characteristics of Breast Cancer Survivors Randomly Assigned to Yoga or Wait-List Conditions
| Characteristic | Overall (N = 200) |
Yoga (n = 100) |
Wait-List (n = 100) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean | SD | No. | % | Mean | SD | No. | % | Mean | SD | |
| Age, years | 51.6 | 9.2 | 51.8 | 9.8 | 51.3 | 8.7 | ||||||
| BMI, kg/m2 | 27.8 | 5.7 | 27.9 | 5.3 | 27.6 | 6.0 | ||||||
| SAD, cm | 20.8 | 3.4 | 20.7 | 3.3 | 21.0 | 3.6 | ||||||
| Race/ethnicity | ||||||||||||
| White | 176 | 88 | 88 | 89 | 88 | 88 | ||||||
| Black | 18 | 9 | 8 | 8 | 10 | 10 | ||||||
| Asian | 5 | 3 | 3 | 3 | 2 | 2 | ||||||
| Current smoker | 12 | 6 | 4 | 4 | 8 | 8 | ||||||
| Usage of cardiac medication | 33 | 17 | 17 | 17 | 16 | 16 | ||||||
| CHAMPS activity, total hours/week | 6.3 | 5.7 | 6.8 | 6.3 | 5.8 | 5.1 | ||||||
| MFSI-SF fatigue | 15.8 | 20.1 | 14.3 | 19.6 | 17.3 | 20.5 | ||||||
| SF-36 vitality | 46.5 | 20.6 | 48.6 | 20.2 | 44.4 | 20.9 | ||||||
| CES-D depressive symptoms | 10.7 | 8.2 | 10.2 | 8.2 | 11.2 | 8.2 | ||||||
| Marital status | ||||||||||||
| Single | 26 | 13 | 18 | 18 | 8 | 8 | ||||||
| Married | 140 | 70 | 68 | 68 | 72 | 72 | ||||||
| Separated/divorced | 29 | 14 | 14 | 14 | 15 | 15 | ||||||
| Widowed | 5 | 3 | 0 | 0 | 5 | 5 | ||||||
| Education level | ||||||||||||
| High school or less | 12 | 6 | 5 | 5 | 7 | 7 | ||||||
| Some college | 49 | 25 | 27 | 27 | 22 | 22 | ||||||
| College graduate | 62 | 31 | 29 | 29 | 34 | 34 | ||||||
| Postgraduate | 77 | 38 | 39 | 39 | 38 | 38 | ||||||
| Employment status | ||||||||||||
| Employed full or part time | 137 | 68 | 71 | 71 | 66 | 66 | ||||||
| Unemployed | 35 | 18 | 15 | 15 | 20 | 20 | ||||||
| Retired | 28 | 14 | 14 | 14 | 14 | 14 | ||||||
| Income level, $ | ||||||||||||
| < 25,000 | 10 | 5 | 3 | 3 | 7 | 7 | ||||||
| 25,000-< 50,000 | 33 | 17 | 18 | 18 | 15 | 15 | ||||||
| 50,000-< 75,000 | 35 | 18 | 17 | 17 | 18 | 18 | ||||||
| 75,000-< 100,000 | 46 | 23 | 23 | 23 | 23 | 23 | ||||||
| ≥ 100,000 | 59 | 29 | 30 | 30 | 29 | 29 | ||||||
| No report | 17 | 8 | 9 | 9 | 8 | 8 | ||||||
| Type of treatment | ||||||||||||
| Surgery only | 26 | 13 | 13 | 13 | 13 | 13 | ||||||
| Surgery plus radiation | 52 | 26 | 28 | 28 | 24 | 24 | ||||||
| Surgery plus chemotherapy | 46 | 23 | 23 | 23 | 23 | 23 | ||||||
| Surgery plus radiation plus chemotherapy | 76 | 38 | 36 | 36 | 40 | 40 | ||||||
| Cancer stage | ||||||||||||
| 0 | 18 | 9 | 9 | 9 | 9 | 9 | ||||||
| I | 89 | 45 | 46 | 46 | 43 | 43 | ||||||
| IIA | 52 | 26 | 27 | 27 | 25 | 25 | ||||||
| IIB | 23 | 11 | 10 | 10 | 13 | 13 | ||||||
| IIIA | 18 | 9 | 8 | 8 | 10 | 10 | ||||||
| HER2 receptor status | ||||||||||||
| Positive | 35 | 18 | 18 | 18 | 17 | 17 | ||||||
| Negative | 146 | 73 | 74 | 74 | 72 | 72 | ||||||
| Unknown | 19 | 9 | 8 | 8 | 11 | 11 | ||||||
| Progesterone receptor status | ||||||||||||
| Positive | 142 | 71 | 73 | 73 | 69 | 69 | ||||||
| Negative | 49 | 25 | 23 | 23 | 26 | 26 | ||||||
| Unknown | 9 | 4 | 4 | 4 | 5 | 5 | ||||||
| Estrogen receptor status | ||||||||||||
| Positive | 159 | 80 | 81 | 81 | 78 | 78 | ||||||
| Negative | 32 | 16 | 15 | 15 | 17 | 17 | ||||||
| Unknown | 9 | 4 | 4 | 4 | 5 | 5 | ||||||
| Tamoxifen/aromatase inhibitors | 143 | 72 | 72 | 72 | 71 | 71 | ||||||
| Postmenopausal | 153 | 81 | 76 | 76 | 77 | 77 | ||||||
| Time since diagnosis, months | 17.3 | 8.1 | 16.3 | 7.5 | 18.4 | 8.5 | ||||||
| Time since treatment, months | 10.9 | 7.9 | 9.9 | 7.1 | 11.8 | 8.5 | ||||||
Abbreviations: BMI, body mass index; CES-D, Center for Epidemiological Studies–Depression; CHAMPS, Community Healthy Activities Model Program for Seniors questionnaire; HER2, human epidermal growth factor receptor 2; MFSI-SF, Multidimensional Fatigue Symptom Inventory–Short Form; SAD, sagittal abdominal diameter; SD, standard deviation; SF-36, Short Form-36.
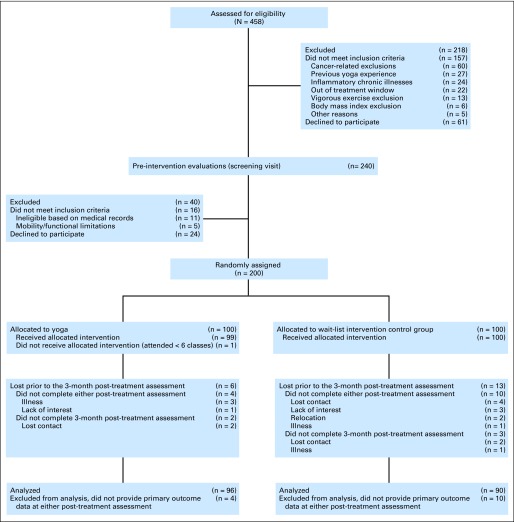
Exclusions included a prior history of breast or any other cancer except basal or squamous cell skin cancer, inflammatory breast cancer, anemia, diabetes, chronic obstructive pulmonary disease, uncontrolled hypertension, evidence of liver or kidney failure, symptomatic ischemic heart disease, conditions involving the immune system such as autoimmune and/or inflammatory diseases, cognitive impairment, alcohol/drug abuse, current yoga practice (within the last 6 months), and/or previous yoga practice for more than 3 months. Women reporting 5 hours or more of vigorous physical activity per week were excluded. Figure 1 shows screening, randomization, and participant flow by group.
Fig 1.
CONSORT diagram showing screening, random assignment, and participant flow by group.
Randomization and Masking
After women had completed the baseline assessment, the data manager stratified participants by cancer stage (0 v I v II and IIIA) as well as radiation therapy received or not, and then used an online randomization program to obtain the block randomization sequence (six per block) for assignment to yoga or control within strata. The data manager had no participant contact. Participants were told not to mention their group assignment to study personnel during their post-treatment assessments; questionnaires were administered via computer. The technicians who analyzed blood samples were blind to all other data.
Yoga and Wait-List Control Conditions
Women who were randomly assigned to yoga participated in two 90-minute sessions per week. The protocol outlined poses for the 24 sessions (Appendix Table A1, online only). A senior yoga teacher conducted the initial group, which was videotaped and used to train the subsequent six Yoga Alliance–certified instructors. The 25 yoga groups included 4 to 20 women per group.
To evaluate and limit protocol drift, sessions were audiotaped, and 50% were randomly assessed for omissions from the predetermined poses for the week. Protocol deviations were discussed with teachers.
To maximize adherence, the yoga teacher called any woman who missed a class. Home practice was strongly encouraged, and women recorded their total home plus class practice time in weekly logs.
Participants assigned to the wait-list control were told to continue performing their usual activities, and to refrain from beginning any yoga practice. After their final assessment they were offered the yoga classes.
Measures
The total score on the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) reflected behavioral, cognitive, physical, and affective expressions of fatigue in the last week.26,27 The energy/fatigue (vitality) scale from the Medical Outcomes Study 36-item short-form health survey (SF-36) measured vitality over the last month.20,28,29 The Center for Epidemiological Studies Depression Scale (CES-D) assessed depressive symptomatology in the last week.30 The Pittsburgh Sleep Quality Index (PSQI) evaluated sleep quality and disturbances over a 1-month interval.31 The increased social interaction provided by the intervention could diminish feelings of fatigue32; thus, we measured perceived support by using the Interpersonal Support Evaluation List.33 The Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire assessed the weekly frequency and duration of various physical activities.34,35 The Women's Health Initiative Food Frequency Questionnaire (FFQ) provided data on foods and beverages consumed in the past 90 days.36 All these measures have well-established reliability and validity.
Immunologic Assays
Fasting blood samples were collected between 7:00 and 9:00 am to control for diurnal variation. Longer-term exercise reduces mononuclear cell production of proinflammatory cytokines10,11; thus, lipopolysaccharide-stimulated production of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and interleukin-1β (IL-1β) from isolated peripheral blood mononuclear cells was multiplexed and measured by using an electrochemiluminescence method with Meso Scale Discovery kits.37 Each patient's frozen samples were assayed for all cytokines in one run by using the same controls for all time points for each person.
Sample Size
Sample size was based on detection of meaningful differences in primary end points with 80% power and a two-sided 5% significance level. Effect sizes were based on previously published studies,15,16 and data we collected from an earlier study of older adults. We estimated expected effect sizes for each of our primary outcomes and based the sample size calculation on the CES-D, which had the smallest estimated effect size and therefore required the largest sample size. The estimated group difference in CES-D was 0.7 (standard deviation, 1.6), requiring 85 patients per group. We expected 15% attrition, so 100 patients per group was required.
Statistical Methods
Baseline demographic and cancer-related characteristics were compared across randomized groups by using t tests and χ2 tests as appropriate. Mixed effect models were used to test the intervention's effect on primary outcomes. Fixed effects included visit, intervention group, and their interaction and baseline outcome levels. Of primary interest were preplanned contrasts comparing group means at each of the two postrandomization time points. Random effects included a patient-specific random intercept that accounted for within-patient correlation (repeated study visits) and a random effect for yoga class assignment that accounted for the partially nested data arising from small clusters being present in the intervention arm but not in the control arm. This explicitly models the within-group correlation among members of the yoga intervention groups while allowing the control patients to remain independent since they were not placed into groups.38 The intraclass correlation coefficient (ICC) was estimated from the mixed models for each outcome to quantify this within-group correlation. The Kenward-Roger adjustment to the df was used to control type I error rates.39 Lipopolysaccharide-stimulated cytokine responses were natural log (ln) transformed to better approximate normality of residuals. Cohen's d as a measure of effect size was calculated for the MFSI-SF fatigue score, SF-36 vitality scale, and CES-D depressive symptoms. Secondary analyses used frequency of continuous yoga practice in place of intervention group to account for differences in frequency of yoga practice in class and at home. For these secondary analyses, all models controlled for age and sagittal abdominal diameter (SAD)40 as potential confounders. A two-sided significance level of α = .05 was used for all tests. Analyses were performed by using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Study Population, Baseline Data
Table 1 shows baseline characteristics of the study sample. Groups were balanced on demographic and disease-related characteristics at baseline (P > .1 for all tests). Importantly, there were no significant differences between groups on activity and fatigue (P > .25 for both) or SAD and body mass index (P > 0.5 for both).
Protocol Adherence
Of the 200 randomly assigned patients, 186 women received the allocated 12-week intervention and completed the immediate post-treatment assessment, and 3-month post-treatment data were obtained on 181 patients (91%; Fig 1). There were no demographic differences between women who had post-treatment data and women who had baseline data only (dropouts). At baseline, the dropouts had significantly higher fatigue than women with post-treatment data (27.4 v 14.9; P = .02). There were no differences for any other outcomes at baseline (vitality, depression, cytokines). However, dropout appeared to be nondifferential since there were no differences among the dropouts between the yoga and control groups.
In the yoga group, patients attended a mean of 18.1 (75.4%) of 24 classes with a median of 19 (79.1%) of 24 classes and reported an average of 24.69 minutes per day of total home plus class practice across 12 weeks. None of the women in the control group reported yoga practice during their wait-list period.
The yoga intervention was administered with high fidelity. In randomly audited sessions, an average of 97% (standard deviation, 5%) of poses were taught as scheduled.
Intervention Effects on Fatigue, Vitality, and Depressive Symptoms
Results for total fatigue, vitality, and depressive symptoms are summarized in Figure 2; unadjusted group means for all outcome variables at each of the three measurement times are available in the Appendix Table A2. After adjusting for baseline levels, mean fatigue was not significantly lower in the yoga group compared with the control group at the immediate post-treatment visit (6.1 v 10.3; P = .058; Cohen's d = −0.22) but was significantly lower at the 3-month post-treatment visit (5.4 v 12.4; P = .002; Cohen's d = −0.36). The average vitality score was higher in the yoga group at the immediate post-treatment visit (58.7 v 52.3; P = .01; Cohen's d = 0.31) and at the 3-month post-treatment visit (58.1 v 51.6; P = .01; Cohen's d = 0.32). Depressive symptoms were not significantly different between groups (immediately post treatment: 8.1 v 9.2; P = .28; Cohen's d = −0.13; 3-month post treatment: 8.5 v 9.7; P = .21; Cohen's d = −0.16).
Fig 2.
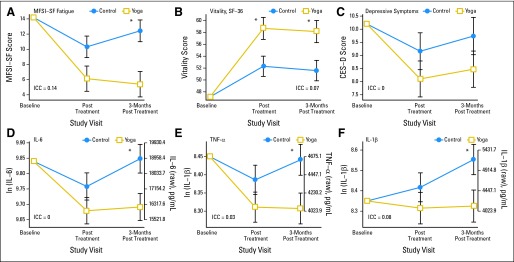
Changes in (A) Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF) fatigue scores, (B) vitality scores (36-item short form [SF-36]), (C) depressive symptoms (Center for Epidemiological Studies–Depression [CES-D]), and (D, E, F) lipopolysaccharide-stimulated cytokine production (interleukin-6 [IL-6], tumor necrosis factor alpha [TNF-α], and interleukin-1β [IL-1β]) immediately post treatment and 3 months post treatment in the yoga and control groups. Results shown are mean ± SE from mixed models adjusting for baseline levels. All models conditioned on baseline outcome levels as specified a priori in the trial protocol. ICC, intraclass correlation coefficient. (*) P < .05 for group comparison. ln, natural log.
Table 2 displays the results of preplanned secondary analyses by using yoga practice frequency (minutes per day) during the intervention in place of group assignment, controlling for age and SAD. Overall, frequency of yoga practice showed stronger associations with total fatigue, vitality, and depressive symptoms than simple group assignment, with more frequent yoga practice producing larger changes on all these dimensions (Fig 3). The association with both fatigue and vitality was stronger 3 months post-treatment. A 10-minute-per-day increase in yoga practice was associated with a 1.7-point decrease in MFSI-SF fatigue immediately post-treatment (P = .019) and a 2.8-point decrease at 3 months post-treatment (P < .001). Similarly, a 10 minute-per-day increase in yoga practice was associated with a 2.1-point increase in vitality immediately post-treatment (P = .016) and a 2.5-point increase at 3 months post-treatment (P = .0045).
Table 2.
Change in Primary Outcomes for Each 10-Minute Increase in Frequency of Yoga Practice, Adjusting for Baseline Outcome Levels, Age, and SAD
| Outcome | No. of Patients | Estimate | SE | 95% CI | P |
|---|---|---|---|---|---|
| MFSI-SF fatigue | 186 | ||||
| Immediately post treatment | −1.7 | 0.70 | −3.1 to −0.28 | .019 | |
| 3 months post treatment | −2.8 | 0.71 | −4.2 to −1.4 | < .001 | |
| SF-36 vitality | 186 | ||||
| Immediately post treatment | 2.1 | 0.85 | 0.40 to 3.75 | .016 | |
| 3 months post treatment | 2.5 | 0.85 | 0.77 to 4.14 | .0045 | |
| CES-D | 186 | ||||
| Immediately post treatment | −0.66 | 0.34 | −1.3 to 0.0039 | .051 | |
| 3 months post treatment | −0.56 | 0.34 | −1.2 to 0.10 | .098 | |
| Stimulated TNF-α* | 176 | ||||
| Immediately post treatment | −0.021 | 0.020 | −0.060 to 0.018 | .28 | |
| 3 months post treatment | −0.038 | 0.020 | −0.079 to 0.0021 | .063 | |
| Stimulated IL-6* | 176 | ||||
| Immediately post treatment | −0.022 | 0.021 | −0.063 to 0.019 | .30 | |
| 3 months post treatment | −0.056 | 0.022 | −0.098 to −0.013 | .01 | |
| Stimulated IL-1β* | 176 | ||||
| Immediately post treatment | −0.034 | 0.035 | −0.10 to 0.035 | .33 | |
| 3 months post treatment | −0.078 | 0.036 | −0.15 to −0.0074 | .030 |
NOTE. Models used frequency of continuous yoga practice in place of group assignment to predict primary outcomes.
Abbreviations: CES-D, Center for Epidemiological Studies–Depression; IL-1β, interleukin-1 beta; IL-6, interleukin-6; MFSI-SF, Multidimensional Fatigue Symptom Inventory–Short Form; SAD, sagittal abdominal diameter; SF-36, Short Form-36; TNF-α, tumor necrosis factor alpha.
Stimulated cytokines are natural log transformed.
Fig 3.
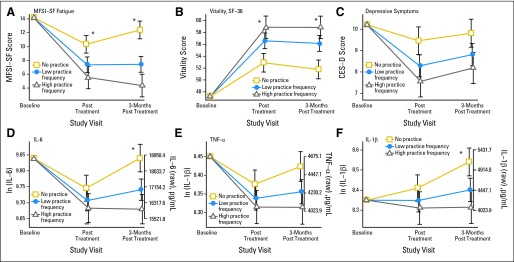
Changes in (A) Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF) fatigue scores, (B) vitality scores (36-item short form [SF-36]), (C) depressive symptoms (Center for Epidemiological Studies–Depression [CES-D]), and (D, E, F) lipopolysaccharide-stimulated cytokine production (interleukin-6 [IL-6], tumor necrosis factor alpha [TNF-α], and interleukin-1β [IL-1β]) immediately post treatment and 3 months post treatment as a function of yoga practice frequency. Results shown are mean ± SE from mixed models adjusting for baseline levels, age, and sagittal abdominal diameter at yoga practice frequency levels of 0 minutes per day (control, no practice), 18 minutes per day (25th percentile; low practice), and 29 minutes per day (75th percentile; high practice). (*) P < .05 for the slope estimate in yoga practice frequency. ln, natural log.
Intervention Effects on Inflammation
Figure 2 shows estimated mean Kenward-Roger adjustment stimulated cytokines post-treatment, after adjusting for baseline levels. For all three cytokines, there were no significant group differences immediately post-treatment, but by 3 months post-treatment, the yoga group had significantly reduced cytokine levels compared with the control group. At 3 months post-treatment, the estimated mean ln TNF-α was 0.13 units lower for the yoga group compared with controls (13% lower TNF-α geometric mean; P = .027); the estimated mean ln IL-6 was 0.16 units lower for the yoga group compared with controls (15% lower IL-6 geometric mean; P = .027); and the yoga group also had lower mean ln IL-1β, with a group difference of 0.23 units (20% lower IL-1β geometric mean; P = .037).
In secondary analyses, the pattern was similar, with significant effects of the frequency of yoga practice on cytokine levels 3 months post-treatment but not immediately post-treatment (controlling for age and SAD). At 3 months post-treatment, a 10-minute-per-day increase in yoga practice was associated with a 5% decrease in the IL-6 geometric mean (P = .01) and an 8% decrease in the IL-1β geometric mean (P = .03; Table 2 and Fig 3).
Health Behaviors and Support
Analyses of dietary data, body mass index, and weight did not show differential group changes (Table 3), nor did social support (P = .34). However, yoga group participants reported significantly improved sleep (ICC, 0; P = .03) compared with the control group.
Table 3.
Group Difference in Health Behaviors and Support, Adjusted for Baseline Outcome Levels
| Outcome | Control |
Yoga |
Group Difference |
95% CI | P | |||
|---|---|---|---|---|---|---|---|---|
| Least Squares Means | SE | Least Squares Means | SE | Least Squares Means | SE | |||
| BMI, kg/m2 | 27.8 | 0.10 | 27.8 | 0.11 | 0.017 | 0.15 | −0.28 to 0.32 | .91 |
| Weight, kg | 75.2 | 0.27 | 75.2 | 0.29 | 0.0068 | 0.40 | −0.79 to 0.81 | .99 |
| Social support, ISEL total score | 93.8 | 0.98 | 95.2 | 1.1 | 1.5 | 1.5 | −1.0 to 4.5 | .34 |
| Pittsburgh Sleep Quality Index | 7.0 | 0.23 | 6.3 | 0.22 | −0.71 | 0.32 | −1.3 to −0.082 | .03 |
| Total physical activity, hours per week | 9.1 | 0.64 | 11.2 | 0.78 | 2.1 | 1.0 | 0.10 to 4.1 | .04 |
| FFQ dietary data* | ||||||||
| Calories, kcal/kg | 25.5 | 0.71 | 24.6 | 0.70 | −0.87 | 1.0 | −2.8 to 1.1 | .39 |
| Fiber, g/1,000 kcal | 12.4 | 0.33 | 11.9 | 0.33 | −0.55 | 0.47 | −1.5 to 0.38 | .24 |
| Total fat, g/1,000 kcal | 36.2 | 0.79 | 35.9 | 0.78 | −0.31 | 1.1 | −2.5 to 1.9 | .78 |
| Protein, g/1,000 kcal | 40.4 | 0.80 | 41.1 | 0.78 | 0.69 | 1.1 | −1.5 to 2.9 | .54 |
| Saturated fat, g/1,000 kcal | 12.2 | 0.32 | 12.1 | 0.32 | −0.15 | 0.46 | −1.1 to 0.76 | .75 |
| Monounsaturated fat, g/1,000 kcal | 13.1 | 0.36 | 13.1 | 0.35 | 0.0002 | 0.50 | 1.0 to −1.0 | .99 |
| Polyunsaturated fats, g/1,000 kcal | 7.6 | 0.19 | 7.5 | 0.18 | −0.13 | 0.26 | 0.66 to 0.39 | .61 |
| Omega-3 fatty acids, g/1,000 kcal | 0.87 | 0.029 | 0.87 | 0.029 | −0.0003 | 0.04 | −0.082 to 0.081 | .99 |
| Linoleic acid, g/1,000 kcal | 0.062 | 0.0025 | 0.062 | 0.0024 | 0.0003 | 0.0035 | −0.0067 to 0.0073 | .93 |
NOTE. Group difference calculated as covariate-adjusted least squares means (SE). Least squares means were adjusted for baseline values, averaged across the two post-treatment visits for outcomes other than Food Frequency Questionnaire (FFQ) data.
Abbreviations: BMI, body mass index; ISEL, International Support Evaluation List.
FFQ data were measured only once post treatment.
The yoga group reported 1.65 more total physical activity hours per week immediately post-treatment than at baseline but returned to baseline levels 3 months post-treatment. Control participants reported 1.36 fewer hours per week immediately post-treatment, a decrease that was maintained 3 months post-treatment (ICC, 0.13; P = .04 for group effect). The yoga group's increased activity reflected more yoga practice, not additional activities; across the 12-week intervention, yoga participants reported a decrease in non–yoga activity (P < .001) from 23.78 to 12.65 minutes per day, consistent with other reports of a downward trend in physical activity among survivors.41
Adverse Events
Four recurrent breast cancers (two per group) were not considered intervention related. Two events appeared potentially attributable to the yoga intervention: two women reported the recurrence of chronic back and/or shoulder problems.
DISCUSSION
Yoga practice substantially reduced fatigue and inflammation. Immediately post-treatment, vitality was higher in the yoga group compared with the control group. At 3 months post-treatment, the yoga group's fatigue was lower, vitality was higher, and IL-6, TNF-α, and IL-1β were lower for yoga participants compared with controls. More frequent practice produced greater benefits in fatigue, vitality, and inflammation.
This is the first physical activity trial with breast cancer survivors to show significant inflammatory changes. Indeed, although observational studies reliably show that people who report more frequent and more intense physical activity have lower inflammation than their sedentary counterparts,7,10 RCT data demonstrating that exercise training reduces inflammation are sparse and inconsistent.42,43 The discrepancies are a function of underpowered trials, differences in how much the interventions altered body fat, disparities in intensity and duration, participants' variable levels of baseline inflammation, and absent or inappropriate control groups.43 In fact, two recent reviews concluded that exercise RCTs produce little or no change in inflammatory markers in healthy people who do not lose weight.42,43
Despite the fact that our participants' weight did not change and our trial did not include aerobic or resistance exercise, cytokine production decreased significantly in yoga participants compared with the wait-list group. Blood mononuclear cells provide a direct source for data on whole body inflammation, and their function may provide a proxy for inflammatory responses of macrophages in adipose tissue.10 Leukocyte cytokine production increases with age,7 and persistent subclinical inflammation appears to increase risk for chronic disease and disability among older adults.5–7,44–47 In related work, reductions in leukocyte nuclear factor kappa B activity followed participation in a yogic meditation intervention48; those data suggest one possible mechanism for the inflammatory changes we observed that persisted through 3 months post-treatment.
Among the many cancer-related fatigue treatments, exercise has been the most consistently beneficial. Our effect sizes for fatigue and depressive symptoms are comparable to those reported in meta-analyses for aerobic and/or resistance exercise,49,50 suggesting that yoga confers similar benefits on these dimensions. These meta-analyses show that better adherence is associated with greater improvement, paralleling our yoga practice frequency results.
Up to 60% of cancer survivors report sleep problems during survivorship, a rate that is two or three times as high as that in similar adults without a cancer history.51,52 Disturbed sleep elevates inflammation as well as fatigue,52,53 and thus the improved sleep reported by yoga group participants likely contributed to the positive changes on these dimensions, as well as their continuance through the 3-month post-treatment visit.
Strengths of this study include excellent adherence with minimal attrition; only 9% failed to complete the full trial. Randomization produced groups that were well-balanced on all key dimensions. Adverse effects were infrequent.
We did not compare the yoga group to an active control group, and it is possible that increased attention or group support produced nonspecific treatment benefits,20 a limitation. However, yoga participants did not report the changes in social support that would be expected if support were a key mechanism. In addition, women who practiced yoga more frequently showed greater improvements in inflammation, mood, and fatigue than those who practiced less frequently, and these dose-response effects support yoga's efficacy rather than nonspecific treatment benefits.
Fatigue and depressive symptoms were not used as part of the inclusion criteria, and thus women who were less fatigued and less depressed had less room to show positive change,20 another limitation. Accordingly, our data may underestimate yoga's potential benefit.
Persistent subclinical inflammation appears to enhance risk for chronic disease and disability among older adults.5–7,44–47 Cancer survivors have a greater risk for secondary cancers as well as several chronic diseases, including cardiovascular disease, diabetes, and osteoporosis compared with individuals without a cancer history.54,55 Chronic inflammation has been linked to a spectrum of health problems including cancer, cardiovascular disease, diabetes, and osteoporosis.46,56 In fact, more globally, chronic inflammation has been suggested as one key biologic mechanism that may fuel fatigue as well as declines in physical function leading to frailty and disability.5–9,16 If yoga dampens or limits fatigue and inflammation, then regular practice could have substantial health benefits.
Supplementary Material
Acknowledgment
We are grateful to Marcia Miller of Yoga on High who designed the breast cancer survivor yoga protocol.
Glossary Terms
- cytokines:
Cell communication molecules that are secreted in response to external stimuli.
- interleukin-6 (IL-6):
Produced predominantly by activated immune cells such as microglia. IL-6 is involved in the amplification of inflammatory reactions.
- TNF-α (tumor necrosis factor alpha):
A cytokine with pleiotropic activities. TNF-α (originally called cachexin) is secreted by several types of cells (eg, macrophages, monocytes, neutrophils, T cells) and in response to a variety of stimuli (eg, interferons, interleukin-2, platelet-activating factor). It acts as a cytolytic and cytostatic agent on several cell types. In addition, by promoting thrombotic processes, TNF-α is significantly involved in pathologic processes, including venous thomboses and arteriosclerosis. It is also a potent activator of angiogenesis in vivo.
Appendix
Table A1.
Yoga Poses and Timing Across the Intervention
| Pose | Benefits | Week |
Total Classes | |||||
|---|---|---|---|---|---|---|---|---|
| 1/2 | 3/4 | 5/6 | 7/8 | 9/10 | 11/12 | |||
| On the floor | ||||||||
| Pavana Muktasana (releasing of the winds pose) | X | X | X | X | X | X | 24 | |
| Pavana Muktasana with arms reaching overhead | U | X | X | X | X | X | 20 | |
| Supta Padangusthasana (reclined big toe pose) | X | X | X | X | X | X | 24 | |
| Active Setu Bandha Sarvangasana (bridge pose) | U, D, F, | X | X | 8 | ||||
| Jathara Parivartanasana (abdomen turning pose) | U, I | X | X | X | 12 | |||
| Balasana (child's pose) | F | X | X | X | X | 16 | ||
| Vajrasana (puppy pose) | U | X | X | X | 12 | |||
| Marjarasana (cat-cow pose) | I | X | X | 8 | ||||
| Bhujangasana (cobra pose) | D | X | X | X | 12 | |||
| Adho Mukha Svanasana (downward facing dog pose) | I | X | X | X | X | 16 | ||
| Standing | ||||||||
| Mukha Svanasana (dog pose) standing | U, D, I | X | X | X | 12 | |||
| Chaturanga Dandasana (four-limb staff pose) at wall | U | X | X | 8 | ||||
| Arm(s) up wall, side to wall, or facing wall | U | X | X | X | X | X | 20 | |
| Chest stretch | U | X | X | X | X | 16 | ||
| Prasarita Padottanasana (wide-leg forward extension) | U, D, I | X | X | 8 | ||||
| Tadasana (mountain pose) | D, U | X | X | X | X | X | X | 24 |
| Seated (in chair or on blankets on floor) | ||||||||
| Seated twist with legs in Sukhasana (simple pose) | I | X | X | X | 12 | |||
| Janushirasana (head-to-knee pose) | D, I | X | X | X | 12 | |||
| Paschimottanasana (full forward bend) | I | X | X | X | 12 | |||
| Restorative | ||||||||
| Savanasana (corpse pose) | D, F, I | X | X | X | X | X | X | 24 |
| Supta Baddha Konasana (reclined bound angle pose) | D, F, I | X | X | X | 12 | |||
| Viparita karani (restful inversion) | D, F, I | X | X | X | X | X | X | 24 |
| Supported Setu Bandha Sarvangasana (bridge pose) | U, D, F, I | X | X | X | 12 | |||
| Breathing practices | ||||||||
| Deerga Swasam (3-part breath) | D, I | X | X | X | X | X | 20 | |
| Ujjayi (extreme conquering) breathing | D, F, I | X | X | 8 | ||||
| Nodi Sodhana (alternate nostril) breathing | D, I | X | X | 8 | ||||
| Prana Sukha (breath of joy) | D, I | X | X | X | X | X | 20 | |
Abbreviations: D, depression; F, fatigue; I, immune function; U, upper body.
Table A2.
Unadjusted Group Means at Each Time Point for Each Primary Outcome
| Outcome | No. of Patients | Group Effects on Primary Outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Yoga |
Group Difference |
95% CI | P* | ICC | |||||
| Unadjusted Least Squares Mean | SE | Unadjusted Least Squares Mean | SE | Unadjusted Least Squares Mean | SE | |||||
| MFSI-SF fatigue | 200 | 0 | ||||||||
| Baseline | 17.3 | 2.0 | 14.3 | 2.0 | −3.0 | 2.8 | −8.6 to 2.5 | .28 | ||
| Immediate post treatment | 12.7 | 2.0 | 6.3 | 2.0 | −6.3 | 2.9 | −12.0 to −0.68 | .028 | ||
| 3 months post treatment | 14.7 | 2.0 | 5.8 | 2.0 | −9.0 | 2.9 | −14.7 to −3.3 | .002 | ||
| Energy scale (SF-36; vitality) | 200 | 0.1 | ||||||||
| Baseline | 44.4 | 2.0 | 48.1 | 2.4 | 3.7 | 3.2 | −2.6 to 10.1 | .24 | ||
| Immediate post treatment | 50.7 | 2.1 | 58.9 | 2.4 | 8.3 | 3.2 | 1.8 to 14.7 | .01 | ||
| 3 months post treatment | 49.9 | 2.1 | 58.3 | 2.5 | 8.3 | 3.2 | 1.8 to 14.8 | .01 | ||
| CES-D | 200 | 0.01 | ||||||||
| Baseline | 11.2 | 0.083 | 10.3 | 0.87 | −0.94 | 1.2 | −3.3 to 1.4 | .44 | ||
| Immediate post treatment | 9.8 | 0.86 | 8.1 | 0.88 | −1.7 | 1.2 | −4.1 to 0.75 | .17 | ||
| 3 months post treatment | 10.4 | 0.87 | 8.5 | 0.88 | −1.8 | 1.2 | −4.3 to 0.64 | .15 | ||
| TNF-α† | 199 | 0.06 | ||||||||
| Baseline | 8.44 | 0.043 | 8.45 | 0.048 | 0.012 | 0.065 | −0.12 to 0.14 | .85 | ||
| Immediate post treatment | 8.38 | 0.046 | 8.30 | 0.049 | −0.077 | 0.067 | −0.21 to 0.056 | .25 | ||
| 3 months post treatment | 8.43 | 0.048 | 8.30 | 0.050 | −0.13 | 0.069 | −0.26 to 0.0097 | .068 | ||
| IL-6† | 200 | 0 | ||||||||
| Baseline | 9.84 | 0.047 | 9.83 | 0.047 | −0.0042 | 0.067 | −0.14 to 0.13 | .95 | ||
| Immediate post treatment | 9.76 | 0.050 | 9.67 | 0.049 | −0.088 | 0.070 | −0.23 to 0.049 | .21 | ||
| 3 months post treatment | 9.83 | 0.052 | 9.69 | 0.050 | −0.14 | 0.072 | −0.28 to 0.0033 | .056 | ||
| IL-1β† | 200 | 0.09 | ||||||||
| Baseline | 8.25 | 0.071 | 8.39 | 0.083 | 0.14 | 0.11 | −0.072 to 0.36 | .19 | ||
| Immediate post treatment | 8.37 | 0.076 | 8.33 | 0.085 | −0.048 | 0.11 | −0.27 to 0.18 | .67 | ||
| 3 months post treatment | 8.49 | 0.079 | 8.34 | 0.086 | −0.16 | 0.12 | −0.39 to 0.077 | .19 | ||
Abbreviations: CES-D, Center for Epidemiological Studies–Depression; ICC, intraclass correlation coefficient; IL-1β, interleukin-1 beta; IL-6, interleukin-6; MFSI-SF, Multidimensional Fatigue Symptom Inventory–Short Form; SF-36, Short Form-36; TNF-α, tumor necrosis factor alpha.
P value for group by visit interaction: MFSI-SF fatigue, P = .014; SF-36 vitality scale, P = .10; CES-D depressive symptoms, P = .66; TNF-α, P = .087; IL-6, P = .18; IL-1β, P = .03.
Stimulated cytokines are natural log-transformed.
Footnotes
See accompanying article on page 1058
Supported in part by Grants No. R01 CA126857, R01 CA131029, K05 CA172296, UL1RR025755, and CA016058 from the National Institutes of Health.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00486525.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Janice K. Kiecolt-Glaser, Charles F. Emery
Financial support: Janice K. Kiecolt-Glaser
Administrative support: Janice K. Kiecolt-Glaser
Provision of study materials or patients: Charles L. Shapiro, William B. Malarkey, Charles F. Emery, Rachel Layman, Ewa E. Mrozek
Collection and assembly of data: Janice K. Kiecolt-Glaser, Jeanette M. Bennett, Charles L. Shapiro, William B. Malarkey, Rachel Layman, Ewa E. Mrozek, Ronald Glaser
Data analysis and interpretation: Janice K. Kiecolt-Glaser, Rebecca Andridge, Juan Peng
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: Age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 2.Mock V. Evidence-based treatment for cancer-related fatigue. J Natl Cancer Inst Monogr. 2004;32:112–118. doi: 10.1093/jncimonographs/lgh025. [DOI] [PubMed] [Google Scholar]
- 3.Jones LW, Eves ND, Haykowsky M, et al. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 4.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 5.Payette H, Roubenoff R, Jacques PF, et al. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: The Framingham Heart Study. J Am Geriatr Soc. 2003;51:1237–1243. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- 6.Taekema DG, Westendorp RG, Frölich M, et al. High innate production capacity of tumor necrosis factor-alpha and decline of handgrip strength in old age. Mech Ageing Dev. 2007;128:517–521. doi: 10.1016/j.mad.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Nicklas BJ, Brinkley TE. Exercise training as a treatment for chronic inflammation in the elderly. Exerc Sport Sci Rev. 2009;37:165–170. doi: 10.1097/JES.0b013e3181b7b3d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 10.Gleeson M, Bishop NC, Stensel DJ, et al. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 11.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 12.Kullo IJ, Khaleghi M, Hensrud DD. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol. 2007;102:1374–1379. doi: 10.1152/japplphysiol.01028.2006. [DOI] [PubMed] [Google Scholar]
- 13.Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: A meta-analysis. Oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 16.Collado-Hidalgo A, Bower JE, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 17.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134:700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 18.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 19.Brown JK, Byers T, Doyle C, et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin. 2003;53:268–291. doi: 10.3322/canjclin.53.5.268. [DOI] [PubMed] [Google Scholar]
- 20.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2012;118:3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen L, Warneke C, Fouladi RT, et al. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 22.Streeter CC, Gerbarg PL, Saper RB, et al. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571–579. doi: 10.1016/j.mehy.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Harder H, Parlour L, Jenkins V. Randomised controlled trials of yoga interventions for women with breast cancer: A systematic literature review. Support Care Cancer. 2012;20:3055–3064. doi: 10.1007/s00520-012-1611-8. [DOI] [PubMed] [Google Scholar]
- 24.Buffart LM, van Uffelen JG, Riphagen II, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012;12:559. doi: 10.1186/1471-2407-12-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cramer H, Lange S, Klose P, et al. Yoga for breast cancer patients and survivors: A systematic review and meta-analysis. BMC Cancer. 2012;12:412. doi: 10.1186/1471-2407-12-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein KD, Martin SC, Hann DM, et al. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 29.Puetz TW. Physical activity and feelings of energy and fatigue: A review of epidemiological evidence. Sports Med. 2006;36:767–780. doi: 10.2165/00007256-200636090-00004. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 31.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S, Hoberman HM. Positive events and social supports as buffers of life change stress. J Appl Soc Psychol. 1983;13:99–125. [Google Scholar]
- 34.Stewart AL, Mills KM, King AC, et al. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Harada ND, Chiu V, King AC, et al. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33:962–970. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women's Health Initiative Food Frequency Questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 37.Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain Behav Immun. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer DJ, Sterba SK, Hallfors DD. Evaluating group-based interventions when control participants are ungrouped. Multivariate Behav Res. 2008;43:210–236. doi: 10.1080/00273170802034810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 40.Clasey JL, Bouchard C, Teates CD, et al. The use of anthropometric and dual-energy x-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes Res. 1999;7:256–264. doi: 10.1002/j.1550-8528.1999.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 41.Emery CF, Yang HC, Frierson GM, et al. Determinants of physical activity among women treated for breast cancer in a 5-year longitudinal follow-up investigation. Psychooncology. 2009;18:377–386. doi: 10.1002/pon.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106:S5–S78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 43.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411:785–793. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan ZS, Beiser AS, Vasan RS, et al. Inflammatory markers and the risk of Alzheimer disease: The Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 45.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: The Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Trompet S, de Craen AJ, Mooijaart S, et al. High innate production capacity of proinflammatory cytokines increases risk for death from cancer: Results of the PROSPER Study. Clin Cancer Res. 2009;15:7744–7748. doi: 10.1158/1078-0432.CCR-09-2152. [DOI] [PubMed] [Google Scholar]
- 47.van den Biggelaar AH, Gussekloo J, de Craen AJ, et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Black DS, Cole SW, Irwin MR, et al. Yogic meditation reverses NF-κB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2013;38:348–355. doi: 10.1016/j.psyneuen.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment: A meta-analysis. Am J Prev Med. 2012;43:e1–e24. doi: 10.1016/j.amepre.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 50.Brown JC, Huedo-Medina TB, Pescatello LS, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: A meta-analysis. PLoS One. 2012;7:e30955. doi: 10.1371/journal.pone.0030955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savard J, Ivers H, Villa J, et al. Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. J Clin Oncol. 2011;29:3580–3586. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 52.Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: Sex differences. Brain Behav Immun. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ganz PA. Late effects of cancer and its treatment. Semin Oncol Nurs. 2001;17:241–248. doi: 10.1053/sonu.2001.27914. [DOI] [PubMed] [Google Scholar]
- 55.Demark-Wahnefried W, Morey MC, Clipp EC, et al. Leading the way in exercise and diet (Project LEAD): Intervening to improve function among older breast and prostate cancer survivors. Control Clin Trials. 2003;24:206–223. doi: 10.1016/s0197-2456(02)00266-0. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.