Abstract
Purpose
Previous research incorporating yoga (YG) into radiotherapy (XRT) for women with breast cancer finds improved quality of life (QOL). However, shortcomings in this research limit the findings.
Patients and Methods
Patients with stages 0 to III breast cancer were recruited before starting XRT and were randomly assigned to YG (n = 53) or stretching (ST; n = 56) three times a week for 6 weeks during XRT or waitlist (WL; n = 54) control. Self-report measures of QOL (Medical Outcomes Study 36-item short-form survey; primary outcomes), fatigue, depression, and sleep quality, and five saliva samples per day for 3 consecutive days were collected at baseline, end of treatment, and 1, 3, and 6 months later.
Results
The YG group had significantly greater increases in physical component scale scores compared with the WL group at 1 and 3 months after XRT (P = .01 and P = .01). At 1, 3, and 6 months, the YG group had greater increases in physical functioning compared with both ST and WL groups (P < .05), with ST and WL differences at only 3 months (P < .02). The group differences were similar for general health reports. By the end of XRT, the YG and ST groups also had a reduction in fatigue (P < .05). There were no group differences for mental health and sleep quality. Cortisol slope was steepest for the YG group compared with the ST and WL groups at the end (P = .023 and P = .008) and 1 month after XRT (P = .05 and P = .04).
Conclusion
YG improved QOL and physiological changes associated with XRT beyond the benefits of simple ST exercises, and these benefits appear to have long-term durability.
INTRODUCTION
Radiotherapy (XRT) is often the final step in the multimodal treatment regimen for women with breast cancer. Patients often experience treatment-related adverse effects (fatigue, pain, lymphedema, neuropathy, cardiotoxicity, sleep disturbances, and cognitive problems) that negatively affect physical, psychological, social, and spiritual aspects of quality of life (QOL)1,2 and may create negative health consequences.3
Research on yoga (YG) in patients with cancer has increased considerably in the last decade, and a variety of YG programs studied in cancer have reported improvements in stress and QOL,4 fatigue and emotional health,5,6 pain, vitality, and QOL,7 positive affect,5 joint pain, fatigue, and sleep disturbance,8,9 and fatigue in women with metastatic breast cancer10 and obese breast cancer survivors.11 Studies of a Patanjali-based integrated YG program for patients with breast cancer developed by the Vivekananda Yoga Anusandhana Samsthana (VYASA) have consistently reported improvement in anxiety, symptom severity, and distress,12–14 nausea and vomiting,15 and affect and global QOL14 as well as beneficial effects on natural-killer cell counts16 and radiation-induced DNA damage.13
Previous YG research has a number of limitations, including small sample size (ranging from 18 to 168); absence of objective outcome measures; lack of active control groups; and lack of long-term follow-up after the end of the YG program. One objective measure of interest is cortisol rhythm. Studies have revealed that both elevated levels of cortisol and a blunted, less steep, diurnal cortisol slope are associated with worse survival in women with breast cancer.17,18 Vadiraja et al14 also reported significantly reduced morning and overall mean cortisol levels in patients with breast cancer participating in a YG program.
We previously reported that participation in the VYASA YG program two times a week for patients with breast cancer undergoing XRT resulted in significantly better general health perception (GH) and physical functioning (PF) scores at the end of XRT and greater benefit finding 3 months after XRT than in women in a waitlist (WL) control group.19 This study tested the hypotheses that participation in YG three times a week during XRT would have long-term effects on physical and mental health (MH) aspects of QOL (primary outcomes), fatigue, depression, and sleep disturbances and result in steeper cortisol slope (secondary outcomes) relative to an active stretching (ST) or WL control groups.
PATIENTS AND METHODS
Patients
Women with stages 0 to III breast cancer were recruited before XRT. Inclusion criteria were ≥ 18 years old; ability to read, write, and speak English; and scheduled to undergo daily adjuvant XRT for 6 weeks at MD Anderson Cancer Center. Patients with lymphedema; metastatic bone disease; deep vein thrombosis; documented diagnosis of a formal thought disorder (eg, schizophrenia); extreme mobility problems; or who had practiced YG in the year before diagnosis were excluded. The protocol was approved by the institutional review board.
Randomization and Schedule
Eligible patients were identified through an institutional database or by referring physicians and were approached at their simulation appointment. After giving written informed consent, participants completed a baseline assessment including self-report measures and provided saliva samples to assess diurnal cortisol rhythm. Blood samples for future assays, an actigraphy watch (worn 24 hours a day for 7 days to assess sleep quality), and questionnaires assessing plausible mediators were also collected and will be reported in a subsequent article. Participants were then randomly assigned to one of three groups: 1) YG; 2) ST; or WL control by using a form of adaptive randomization,20 according to age, stage of disease, time since diagnosis, type of surgery, and chemotherapy (neoadjuvant or adjuvant). Follow-up assessments were conducted during the last week of treatment and 1, 3, and 6 months later. Participants were given a gift certificate ($20 value) after each assessment completion. Participants in the WL group received usual care, completed all assessments on the same timeline as the active groups, and were offered YG or ST classes at the end of their study participation. All participants were asked to refrain from participating in any other YG classes while on study.
Intervention Programs
Participants in the YG and ST groups attended up to three 60-minute classes per week during their 6 weeks of XRT. Classes were held near the radiation treatment center in large conference-style rooms dedicated to behavioral research. Classes were offered to accommodate participants' schedules, most often being given in a one-on-one just before or after XRT. Each participant received an audio CD and a written manual of the program to encourage at-home practice. Compliance was determined weekly during XRT and at each follow-up with an evaluation and practice log that asked about length and frequency of practice at home.
The integrated YG program, described previously,19 included the following: (1) preparatory warm-up synchronized with breathing; (2) selected postures, or asana (forward-, backward-, and side-bending asanas in sitting and standing position, cobra posture, crocodile, and half-shoulder-stand with support); (3) deep relaxation (supine posture); (4) alternate-nostril breathing, or pranayama; and (5) meditation. The program was taught by VYASA-trained teachers.
The ST program included exercises recommended specifically for women undergoing or recovering from breast cancer treatment.21,22 The exercises included standing, lying down, and sitting positions and approximated the gross movements of the YG exercises (eg, horizontal arm stretch, breast stroke, neck stretch, quarterback throwing a football). Participants were introduced the stretches in a stepped approach and learned all of the material over the course of the first four classes. Classes were taught by physiotherapists from Rehabilitative and Physical Therapy at MD Anderson.
Measures
General QOL was assessed by the Medical Outcomes Study 36-item short-form survey (SF-36). The SF-36 assesses PF, physical impediments to role functioning, bodily pain, GH, vitality, social functioning, emotional impediments to role functioning, MH, and includes an overall physical component scale (PCS) and mental component scale (MCS).23,24 The PCS and MCS were the primary outcomes. If the component scale was significant, then the subscales were analyzed as secondary outcomes. Higher scores reflect better QOL, with increases from baseline indicating improved QOL. Normed-based scoring is presented with a population mean = 50 and standard deviation = 10. A change of five points or more is considered clinically significant.25,26
Fatigue was assessed by using the Brief Fatigue Inventory,27 a questionnaire used in clinical settings to assess fatigue severity and its impact on QOL. Lower scores reflect less fatigue.
Sleep disturbances were assessed by using the Pittsburgh Sleep Quality Index (PSQI),28 a questionnaire that assesses sleep disturbances over a 1-month period. We report on the total score. Lower scores reflect fewer sleep disturbances.
Depression was assessed by using the Centers for Epidemiological Studies-Depression (CES-D) measures,29 a well-validated measure focusing on affective components of depression. Lower scores reflect fewer depressive symptoms.
Cortisol
Five saliva samples (waking, 45 minutes later, approximately 8 and 12 hours after waking, and at bedtime) were obtained for 3 consecutive days at each assessment. Participants chewed on a cotton swab (Salivette; Sarstedt, Newton, NC), placed it in a plastic tube (Sarstedt), and then it was frozen at −80°C for later time-resolved immunoassay with fluorescence detection performed at the University of Dresden. Values < 0.0001 and > 70 nmol/L were classified as missing. If patients missed a collection point, they were told to leave the tube empty. Of the data received, 2.8% of the saliva samples were classified as missing (either empty or not within range). Approximately 30% of the patients (21% to 34%, depending on the time point) did not provide saliva samples. There were no differences between patients providing samples and those who did not on the basis of group assignment, medical, demographic, or outcome measures. Slopes were calculated without the waking sample, using the other four samples throughout the day. A steeper, more negative cortisol slope indicates better cortisol regulation. Medical information was obtained from medical records.
Data Analyses
For analysis of the self-report measures, we examined change from baseline to follow-up. To test group differences, PROC MIXED procedures in SAS version 9.2 were used. Changes from baseline were regressed on group, time (treated as categorical), and group × time interaction; the intercept was treated as random effect; the covariance structure was unstructured. There were no significant group × time interactions, and group comparisons at each assessment are presented from the mixed models. Because of non-normality, cortisol levels were log-transformed, and slopes were calculated and regressed on saliva collection time (hours after waking up in the morning); the slopes were then used as the dependent variable in the general linear model analyses as described above, examining slopes covarying for baseline levels. All analyses were controlled for randomization factors. We also controlled for baseline SF-36 GH scores in the SF-36 GH analyses due to imbalances across groups. The primary and secondary outcomes remained the same, and we present the results without covariates.
The primary outcomes were the PCS and MCS subscales of the SF-36 at 1 month post-XRT. Although our pilot work only found group differences in PCS measures at the end of XRT,19 we hypothesized that increasing the amount of YG from two to three times a week would result in more lasting effects (ie, at least 1 month post-XRT). The end of XRT time point and the longer term follow-up at 3 and 6 months were designated as secondary time points. We adjusted the α level for significance to P = .029 by conducting a Bonferroni correction taking into account the correlation between the two variables.30 Assuming a two-tailed significance level of P = .029, with 50 patients per group and 80% power, we would detect differences between any pair of group means of 0.63 standard deviation units, a similar effect size to that found in our previous study (range, 0.44 to 0.47).19 The secondary outcomes of the subscales from the component scores of the SF-36, Brief Fatigue Inventory, PSQI, CES-D, and cortisol slope at each time point were regarded as exploratory analyses.
RESULTS
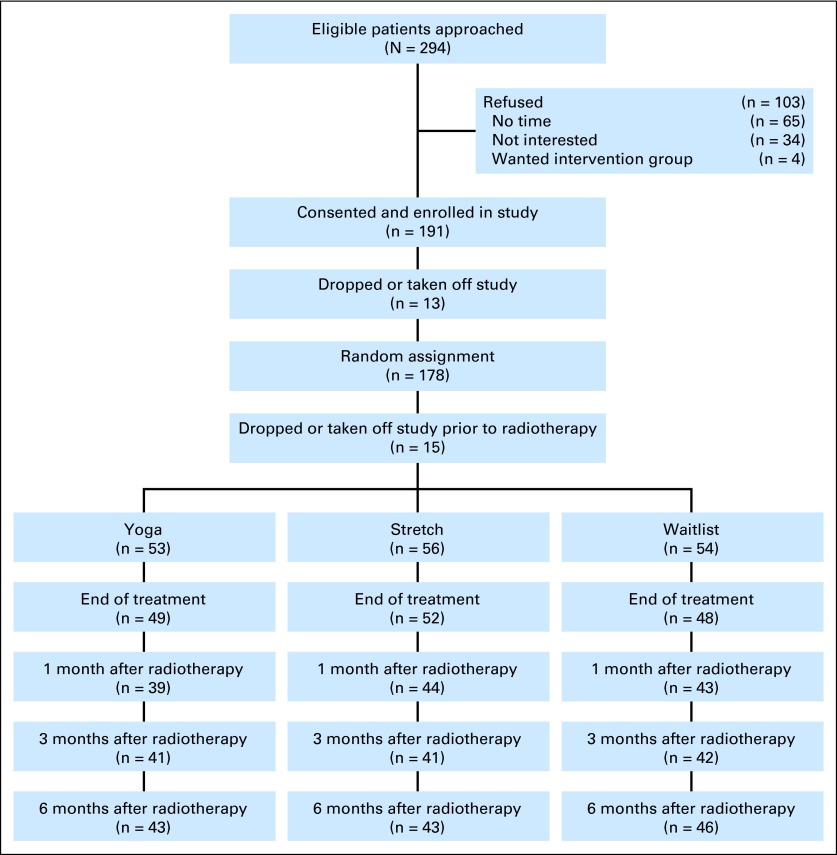
Two hundred ninety-four eligible women were approached and 191 consented to participate. Thirteen dropped out before, and 15 after, they were randomly assigned, for a final sample size of 163 (YG = 53, ST = 56, WL = 54; Fig 1). Retention was high, with no group differences in loss to follow-up or number of classes attended on the basis of whether patients provided follow-up data or not. In addition, there were no differences between patients with and without missing data on the basis of medical, demographic, or baseline outcome measures.
Fig 1.
Flow of study participants over study period.
All groups were similar in baseline demographic, medical, self-report measures (except for SF-36 GH), and cortisol slopes (Tables 1 and 2). Eighty-seven percent of YG and 85% of ST participants attended ≥ 12 classes (mean, YG = 13.8; ST = 14.7). Only three patients in each group attended fewer than half the classes. Practice outside of class was high (> twice per week) for the YG group 1 month post-treatment and then declined at 3 and 6 months (71%, 55%, and 45%, respectively). Practice outside of class (> twice per week) for the ST group was lower at 1 month and then increased somewhat at 3 and 6 months (53%, 69%, and 60%, respectively). Baseline and follow-up means of self-report measures are presented in Table 2.
Table 1.
Baseline Characteristics of Study Participants by Group
| Patient Demographics and Clinical Characteristics | Yoga (n = 53; 33%) |
Stretch (n = 56; 34%) |
Waitlist (n = 54; 33%) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age | .79 | ||||||
| Mean ± SE | 52.38 ± 1.35 | 51.14 ± 1.32 | 52.11 ± 1.34 | ||||
| Range, years | 26-77 | 25-79 | 30-69 | ||||
| Disease stage | .99 | ||||||
| 0 | 5 | 10 | 6 | 11 | 7 | 13 | |
| I | 16 | 30 | 18 | 32 | 17 | 31 | |
| II | 15 | 28 | 14 | 25 | 15 | 28 | |
| III | 17 | 32 | 18 | 32 | 15 | 28 | |
| Surgery | .71 | ||||||
| Mastectomy (without reconstruction) | 12 | 23 | 17 | 31 | 12 | 22 | |
| Mastectomy (with reconstruction) | 6 | 11 | 3 | 5 | 5 | 9 | |
| Breast conserving | 35 | 66 | 36 | 64 | 37 | 69 | |
| Chemotherapy | .73 | ||||||
| Yes | 36 | 68 | 34 | 61 | 34 | 63 | |
| No | 17 | 32 | 22 | 39 | 20 | 37 | |
| Marital status (n = 151) | |||||||
| Married and living together | 31 | 67 | 37 | 71 | 34 | 64 | .75 |
| Not cohabitating | 15 | 33 | 15 | 29 | 19 | 36 | |
| Ethnicity (n = 150)* | .56 | ||||||
| Black/African American | 9 | 19 | 9 | 17 | 7 | 13 | |
| White | 32 | 68 | 28 | 55 | 37 | 71 | |
| Latino/Hispanic/Mexican | 4 | 9 | 8 | 16 | 5 | 10 | |
| Asian/Pacific Islander | 2 | 4 | 4 | 8 | 1 | 2 | |
| Other | 0 | 0 | 2 | 4 | 2 | 4 | |
| Employment status (n = 140) | .1 | ||||||
| Employed full-time | 14 | 31 | 17 | 35 | 10 | 22 | |
| Employed part-time | 1 | 2 | 4 | 8 | 8 | 17 | |
| Employed, taken time off | 11 | 25 | 10 | 20 | 5 | 11 | |
| Not employed | 19 | 42 | 18 | 37 | 23 | 50 | |
| Education (n = 152) | .59 | ||||||
| High school or technical school | 10 | 21 | 12 | 23 | 17 | 32 | |
| Some college | 17 | 36 | 14 | 27 | 15 | 28 | |
| Higher education | 20 | 43 | 26 | 50 | 21 | 40 | |
| Income (n = 149) | .17 | ||||||
| > $75,000 | 31 | 67 | 26 | 51 | 26 | 50 | |
| < $75,000 | 15 | 33 | 25 | 49 | 26 | 50 | |
Minority representation reflects that of women diagnosed with breast cancer in Harris County.
Table 2.
Raw Means and Standard Deviations of Self-Report Measures at Baseline and Follow-Up Time Points
| Baseline |
Last Week of Treatment |
1 Month Post-Treatment |
3 Months Post-Treatment |
6 Months Post-Treatment |
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YG |
ST |
WL |
P | YG |
ST |
WL |
P | YG |
ST |
WL |
P | YG |
ST |
WL |
P | YG |
ST |
WL |
P | ||||||||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||||
| PCS | 41.8 | 1.3 | 43.0 | 1.1 | 44.9 | 1.4 | .20 | 42.3 | 1.3 | 44.5 | 1.1 | 44.1 | 1.2 | .47 | 47.0* | 1.3 | 46.8 | 1.4 | 45.5 | 1.2 | .04 | 48.2* | 1.2 | 47.4 | 1.4 | 46.1 | 1.2 | .05 | 46.9 | 1.4 | 47.5 | 1.4 | 46.6 | 1.1 | .18 |
| PF | 41.9 | 1.3 | 44.7 | 1.2 | 45.9 | 1.2 | .06 | 43.7 | 1.4 | 46.0 | 1.1 | 45.7 | 1.3 | .18 | 47.3*† | 1.3 | 46.6 | 1.3 | 47.0 | 1.1 | .005 | 48.7*† | 1.1 | 47.2‡ | 1.2 | 46.3 | 1.4 | .0001 | 47.8* | 1.4 | 47.4 | 1.4 | 46.5 | 1.2 | .005 |
| GH | 44.8 | 1.5 | 50.4 | 1.2 | 47.7 | 1.2 | .01 | 47.1 | 1.4 | 50.6 | 1.1 | 48.0 | 1.3 | .28 | 47.9*† | 1.5 | 49.2 | 1.4 | 46.9 | 1.5 | .003 | 49.2*† | 1.4 | 50.5 | 1.3 | 46.3 | 1.5 | .005 | 47.7 | 1.6 | 51.7 | 1.3 | 49.8 | 1.1 | .35 |
| RF | 36.8 | 1.5 | 37.1 | 1.3 | 38.3 | 1.6 | .70 | 39.1 | 1.4 | 41.1 | 1.4 | 40.8 | 1.5 | .54 | 44.2 | 1.5 | 43.8 | 1.7 | 43.5 | 1.5 | .50 | 45.0 | 1.6 | 45.5 | 1.7 | 44.3 | 1.4 | .66 | 44.3 | 1.6 | 46.4 | 1.5 | 44.1 | 1.3 | .28 |
| BP | 44.2 | 1.4 | 44.8 | 1.2 | 44.6 | 1.5 | 1.00 | 44.3 | 1.3 | 46.4 | 1.3 | 45.1 | 1.4 | .67 | 47.5 | 1.5 | 48.3 | 1.6 | 48.4 | 1.4 | .98 | 49.0 | 1.4 | 48.9 | 1.5 | 47.5 | 1.5 | .84 | 46.8 | 1.5 | 48.3 | 1.4 | 48.3 | 1.3 | .81 |
| MCS | 42.2 | 1.7 | 45.8 | 1.4 | 42.0 | 1.8 | .20 | 47.2 | 1.9 | 49.5 | 1.2 | 47.1 | 1.7 | .88 | 46.2 | 2.1 | 47.0 | 1.7 | 49.1 | 1.5 | .07 | 46.5 | 2.0 | 50.1 | 1.6 | 46.6 | 1.9 | .80 | 46.8 | 1.9 | 50.8 | 1.5 | 48.8 | 1.4 | .75 |
| CES-D | 15.4 | 1.5 | 11.7 | 0.8 | 15.1 | 1.4 | .07 | 17.3 | 1.4 | 17.8 | 1.1 | 15.8 | 1.4 | .53 | 13.1 | 1.7 | 11.6 | 1.4 | 12.3 | 1.3 | .19 | 13.9 | 1.7 | 9.6 | 1.4 | 12.9 | 1.6 | .68 | 13.9 | 1.8 | 10.4 | 1.4 | 11.5 | 1.3 | .23 |
| PSQI | 8.3 | 0.6 | 8.5 | 0.5 | 8.2 | 0.5 | .90 | 6.7 | 0.5 | 8.3 | 0.6 | 7.3 | 0.5 | .60 | 7.0 | 0.6 | 7.7 | 0.7 | 5.8 | 0.6 | .40 | 6.5 | 0.5 | 7.3 | 0.5 | 6.4 | 0.6 | .5 | 7.1 | 0.5 | 7.2 | 0.6 | 6.4 | 0.6 | 1.00 |
| BFI | 3.2 | 0.3 | 3.0 | 0.3 | 2.6 | 0.3 | .30 | 2.9* | 0.3 | 2.5‡ | 0.3 | 3.2 | 0.4 | .03 | 2.7 | 0.4 | 2.7 | 0.3 | 2.7 | 0.3 | .22 | 2.6 | 0.3 | 2.3 | 0.4 | 3.0 | 0.4 | .15 | 2.8 | 0.4 | 2.5 | 0.3 | 2.6 | 0.3 | .56 |
NOTE. The baseline P values are from analysis of variance of raw scores. The follow-up P values represent the group main effects from the MIXED models analyses of change scores and the noted group differences are from the same MIXED models. Significant group differences are labeled below.
Abbreviations: BFI, Brief Fatigue Inventory; BP, Medical Outcomes Study 36-Item Short-Form Survey (SF-36) Bodily Pain; CES-D, Center for Epidemiologic Studies-Depression; GH, SF-36 General Health; MCS, SF-36 Mental Component Score; PCS, SF-36 Physical Component Score; PF, SF-36 Physical Functioning; PSQI, Pittsburg Sleep Quality Index; RP, SF-36 Role-physical; ST, stretching; WL, waitlist; YG, yoga.
YG versus WL, P ≤ .05.
YG versus ST, P ≤ .05.
ST versus WL, P ≤ .05.
SF-36
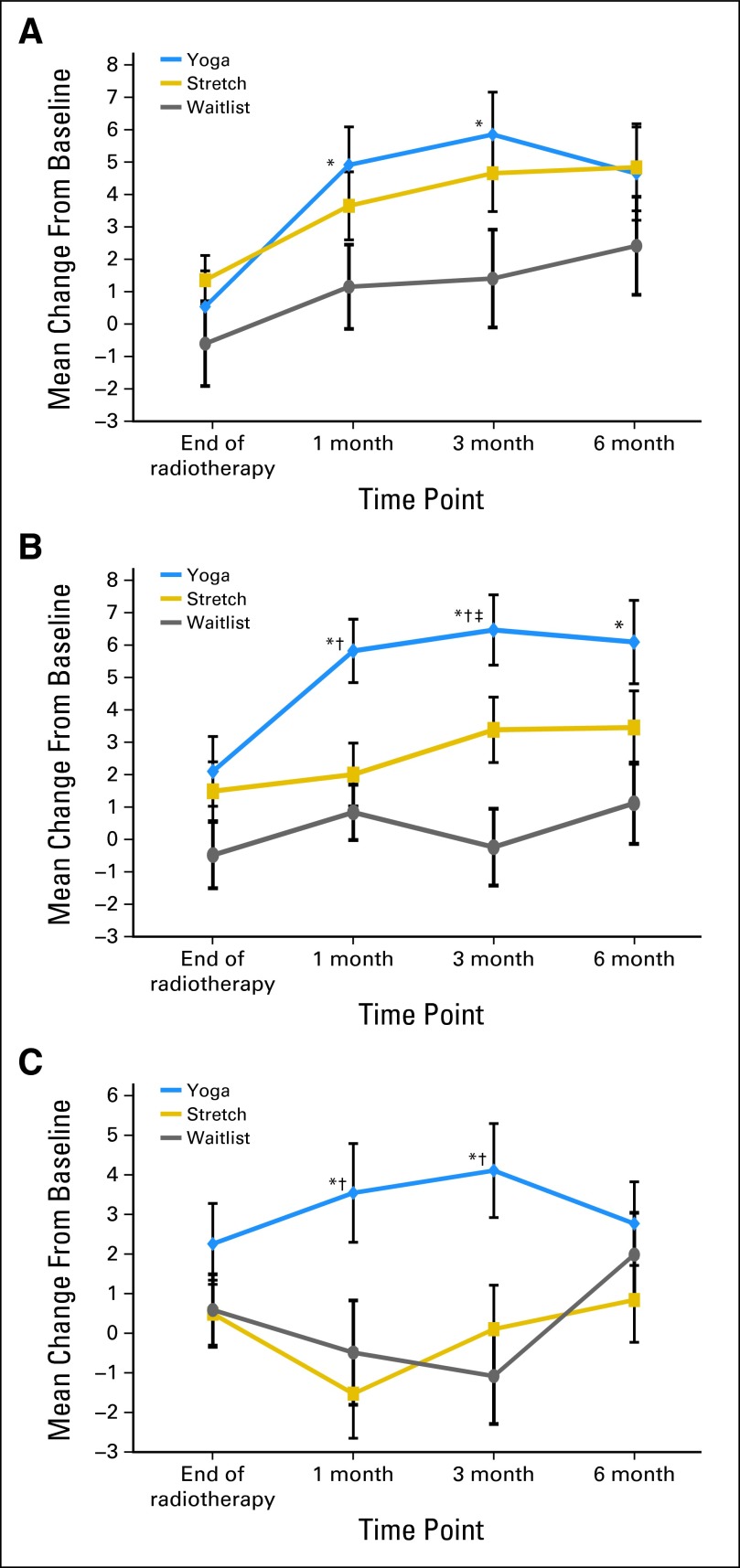
Significantly greater increases from baseline were observed in PCS scores for the YG group compared with the WL group at 1 and 3 months (P = .01 and P = .01, respectively; Fig 2). No other comparisons reached significance. There were no significant effects for the MCS.
Fig 2.
Change from baseline in Medical Outcomes Study 36-item short-form survey (SF-36) subscale scores. (A) SF-36 PCS change from baseline; (B) SF-36 physical function change from baseline; (C) SF-36 general health change from baseline. Significance values are from the MIXED models of change scores at each follow-up time point. (*) Yoga versus waitlist, P < .05. (†) Yoga versus stretch, P < .05. (‡) Stretch versus waitlist, P < .05.
Analyses of the PCS subscales revealed significant effects for the PF and GH. Group differences in PF scores revealed significantly greater increases for the YG group compared with the WL group at 1, 3, and 6 months (P < .002; P < .0001; P = .001, respectively), with marginal group differences at the end of treatment (P = .08); greater increases for the YG group compared with the ST group at 1 and 3 months (P = .01; P = .05, respectively), with marginal group differences at 6 months (P = .08); and greater increases in the ST group compared with the WL group at 3 months (P = .02; Fig 2). GH outcomes followed a similar pattern, with significantly greater increases in GH scores for the YG group compared with the WL and ST groups at 1 and 3 months (all P < .01; Fig 2). No significant group effects were found for other SF-36 subscales.
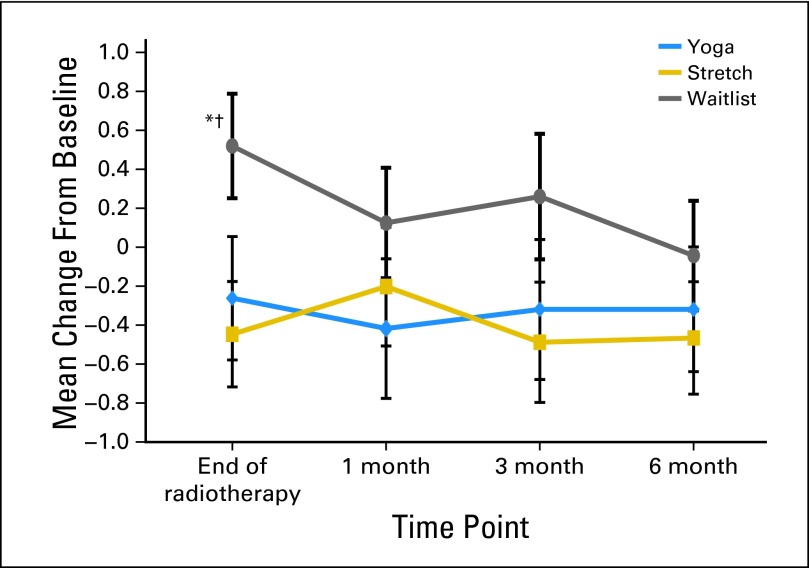
Fatigue
Significantly greater decreases in fatigue were observed for the YG and ST groups compared with the WL group by the end of treatment (P = .04; P = .02, respectively), with marginally significant differences observed for the YG group compared with the WL group at 1 month (P = .09) and for the ST group compared with the WL group at 3 months (P = .07; Fig 3). There were no significant group differences at any time point for CES-D or PSQI scores.
Fig 3.
Change from baseline for fatigue. Significance values are from the MIXED models of change scores at each follow-up time point. (*) Yoga versus waitlist, P < .05. (†) Stretch versus waitlist, P < .05.
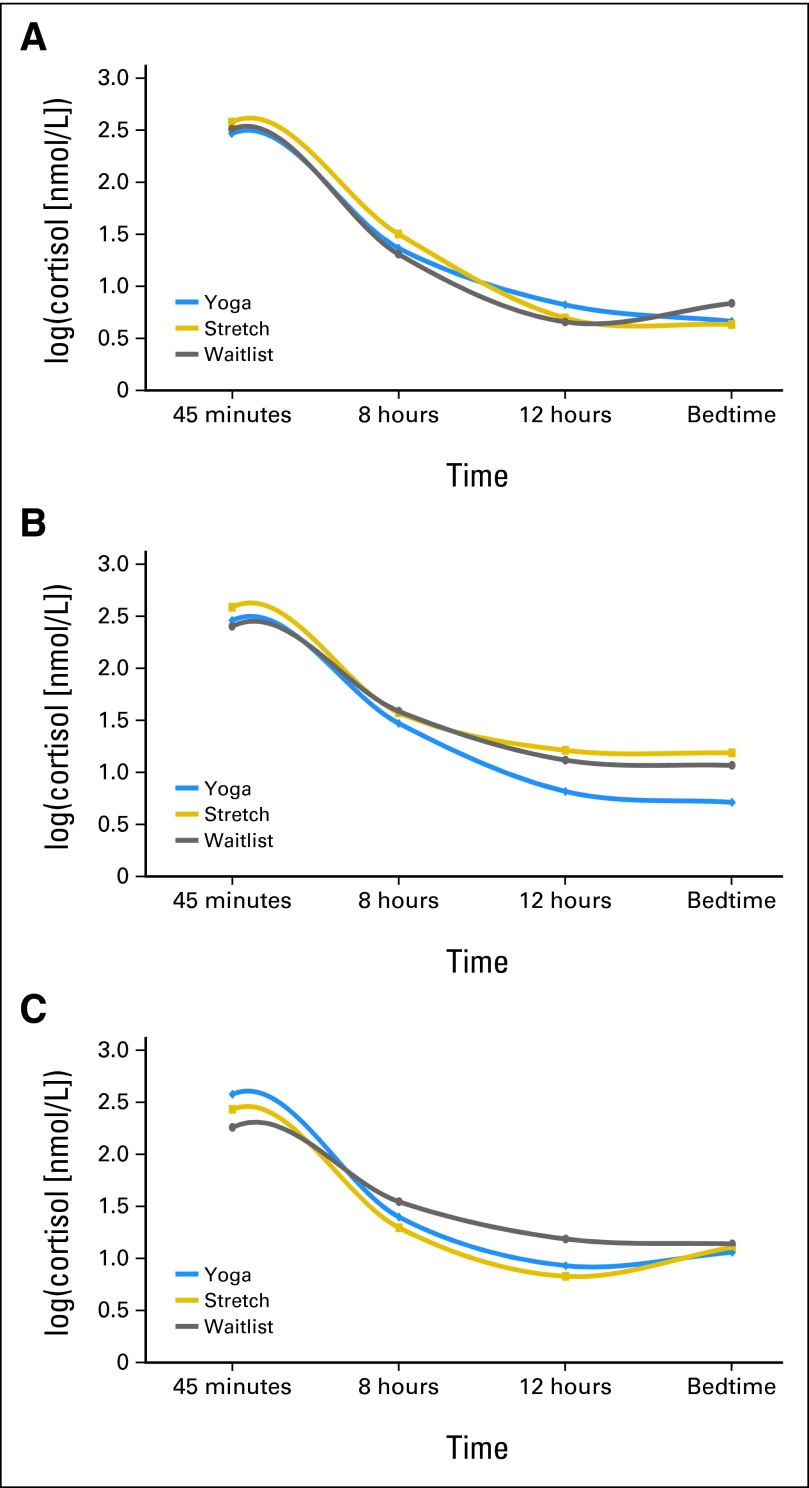
Salivary Cortisol
GLM analysis of cortisol slopes, covarying for baseline, revealed a group main effect at the end of treatment (adjusted means: YG −0.104, SE 0.011; ST −0.072, SE 0.009; WL −0.064, SE 0.010; P = .02), with the YG group having a significantly steeper slope than the ST and WL groups (P = .023 and P = .008, respectively). There was also a marginally significant group main effect at the 1-month follow-up (adjusted means: YG −0.104, SE 0.011; ST −0.073, SE 0.010; WL −0.073, SE 0.010; P = .07), with the YG group having a significantly steeper slope than the ST and WL groups (P = .05 and P = .04, respectively; Fig 4). There were no differences in slope at the other time points (data not shown) or waking cortisol levels at any time point (data not shown).
Fig 4.
Log-transformed cortisol level daily curves. (A) Daily log-cortisol mean at baseline. No group differences. (B) Daily log-cortisol mean at end of radiotherapy. Yoga has steeper slope than stretch and waitlist groups (P = .027 and P = .008, respectively). (C) Daily log-cortisol mean at 1 month. Yoga has steeper slope than waitlist (P = .05 and P = .04, respectively). Significance values are from the GLM analysis of cortisol slopes at each time point, covarying for baseline.
Although there were no group differences between patients with and without missing data on demographic, medical, or the outcome variables at baseline, we imputed the missing data by using multiple imputations (SAS version 9.2 MI procedure) with Markov Chain Monte Carlo method and then used the MIANALYZE procedure to generate statistical inferences. All the analyses remained the same or resulted in smaller P values except for cortisol slopes, but the pattern remained the same (end of treatment: YG v ST, P = .083; YG v WL, P = .015; 1-month follow-up: YG v ST, P = .21; YG v WL, P = .14).
DISCUSSION
To our knowledge, this is the first study to compare the effects of YG against active ST and WL control groups in a cancer population. Compared with the WL group, the YG group had higher PCS scores 1 and 3 months after XRT (primary outcome), better PF at 1, 3, and 6 months, better GH at 1 and 3 months, less fatigue by the end of XRT, and steeper cortisol slopes at the end of XRT and 1 month later. Compared with the ST group, the YG group reported better PF 1 and 3 months after XRT, GH at 1 and 3 months, and had steeper cortisol slopes by the end of treatment. The improvement in PF in the YG group is also considered clinically significant because there was ≥ five-point increase, which was not the case for the other two groups.25,26 Although the ST group reported less fatigue by the end of XRT and improved PF 3 months after treatment relative to the WL group, no other differences emerged between the ST and WL groups. There were no significant group differences for MH outcomes or sleep disturbances.
The present findings of improvements in PF and GH are consistent with the results of the pilot study using the same YG intervention.19 There was, however, a more lasting effect of delivering YG three times a week in the current study versus two times a week in the pilot study, with group differences in PF lasting through the 6-month follow-up versus just 1 week after the end of XRT. Although the minimum frequency of YG practice to achieve positive benefits has yet to be determined, it is generally believed that daily practice is ideal. Class attendance was extremely high, as was observed in the pilot study,19 and higher than that reported by other studies.31,32
The lack of benefit for measures of MH, fatigue, and sleep disturbances is also consistent with the pilot trial.19 Even though the YG program included components to address aspects of MH through relaxation and meditation, this was a minor component relative to the physical movements the women practiced. In addition, MCS scores of the women by the end of XRT were not clinically significantly different than the general population, and they improved across the course of 6 months, suggesting a possible ceiling effect for MH. The same pattern was seen for fatigue and sleep disturbances. Although some studies show that YG improves these outcomes, most research has been conducted in cancer survivors after treatment has ended, used YG programs that were perhaps less physical with more of a focus on relaxation,5,6 or targeted specific symptoms.9,33,34 In addition, improvements in PF may become more apparent over time with other outcomes being more stable.35 Yet, it may be beneficial to examine if programs placing a greater emphasis on relaxation and meditation may have resulted in improved MH and sleep quality outcomes.
The current study also examined an objective measure of stress arousal by assessing the diurnal changes in circulating cortisol levels during waking hours. Although there was a blunting of the cortisol slope by the end of XRT, participants in the YG group had a significantly steeper cortisol slope than the other groups. Although the clinical significance of this finding is unclear, it does suggest the positive effects of YG on the stress hormone cortisol. There is evidence that a blunted cortisol slope is associated with tumor progression18 and decreased survival17 in patients with breast cancer, so maintaining a sustained steep cortisol slope may therefore have prognostic implications.
Although this study controlled for the ST and attention components associated with the YG program, the ST group did not learn any aspects of relaxation. However, the YG group resulted in greater improvement in PF, likely resulting from the physical aspects of YG. Study groups were not blinded, and treatment expectations were not assessed. In addition, because of the number of secondary outcomes and multiple comparisons, significant group differences for secondary outcomes (fatigue and cortisol slope) should be interpreted cautiously.
The current study found that, for some outcomes, YG yielded better subjective and objective results than either ST or usual care. There were fewer differences between ST and WL groups. Although physical therapy is a reimbursable expense in the United States and will likely help patients recover faster, expanding to include services such as YG should be considered. Future studies should examine methods to increase practice frequency outside of class, examine the benefits of different YG components by using appropriate controls, conduct such trials in a blinded manner, assess expectations, and conduct multilevel cost-benefit analyses.
Acknowledgment
We thank Mira Rao and Daksha Shah for their wonderful work teaching the yoga program to the participants, and we thank the Department of Scientific Publications, The University of Texas MD Anderson Cancer Center for their helpful editorial comments on the article.
Footnotes
See accompanying article on page 1040
Supported in part by Grants No. R21CA102385 and R01CA138800 from the National Cancer Institute; the National Cancer Institute Cancer Center Support Grant No. CA016672; National Cancer Institute Grant No. R25CA10618; and philanthropic support for the Integrative Medicine Program, The University of Texas MD Anderson Cancer Center.
Presented in part at the American Psychosomatic Society Annual Meeting, San Antonio, TX, March 2011; the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2011; and the International Congress of North American Consortium of Complementary Medicine and Health, Portland, OR, May 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kavita D. Chandwani, George Perkins, Hongasandra Ramarao Nagendra, Nelamangala V. Raghuram, Raghuram Nagarathna, Banu Arun, G. Stephen Morris, Janet Scheetz, Alejandro Chaoul, Lorenzo Cohen
Financial support: Lorenzo Cohen
Provision of study materials or patients: George Perkins, Banu Arun, Clemens Kirschbaum, G. Stephen Morris, Janet Scheetz, Lorenzo Cohen
Collection and assembly of data: Kavita Chandwani, Amy Spelman, Kayla Johnson, Adoneca Fortier, Qi Wei, Clemens Kirschbaum
Data analysis and interpretation: Kavita Chandwani, Qi Wei, Robin Haddad, Lorenzo Cohen
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Irvine D, Brown B, Crooks D, et al. Psychosocial adjustment in women with breast cancer. Cancer. 1991;67:1097–1117. doi: 10.1002/1097-0142(19910215)67:4<1097::aid-cncr2820670438>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Ganz PA, Lee JJ, Sim MS, et al. Exploring the influence of multiple variables on the relationship of age to quality of life in women with breast cancer. J Clin Epidemiol. 1992;45:473–485. doi: 10.1016/0895-4356(92)90096-6. [DOI] [PubMed] [Google Scholar]
- 3.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulger O, Yagli NV. Effects of yoga on the quality of life in cancer patients. Complement Ther Clin Pract. 2010;16:60–63. doi: 10.1016/j.ctcp.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: Findings from a randomized pilot study. Psychooncology. 2009;18:360–368. doi: 10.1002/pon.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banasik J, Williams H, Haberman M, et al. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011;23:135–142. doi: 10.1111/j.1745-7599.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- 7.Speed-Andrews AE, Stevinson C, Belanger LJ, et al. Pilot evaluation of an Iyengar yoga program for breast cancer survivors. Cancer Nurs. 2010;33:369–381. doi: 10.1097/NCC.0b013e3181cfb55a. [DOI] [PubMed] [Google Scholar]
- 8.Carson JW, Carson KM, Porter LS, et al. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: Results from a randomized trial. Support Care Cancer. 2009;17:1301–1309. doi: 10.1007/s00520-009-0587-5. [DOI] [PubMed] [Google Scholar]
- 9.Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233–3241. doi: 10.1200/JCO.2012.43.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson JW, Carson KM, Porter LS, et al. Yoga for women with metastatic breast cancer: Results from a pilot study. J Pain Symptom Manage. 2007;33:331–341. doi: 10.1016/j.jpainsymman.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Littman AJ, Bertram LC, Ceballos R, et al. Randomized controlled pilot trial of yoga in overweight and obese breast cancer survivors: Effects on quality of life and anthropometric measures. Support Care Cancer. 2012;20:267–277. doi: 10.1007/s00520-010-1066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao MR, Raghuram N, Nagendra HR, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: A randomized controlled trial. Complement Ther Med. 2009;17:1–8. doi: 10.1016/j.ctim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6:242–250. doi: 10.1177/1534735407306214. [DOI] [PubMed] [Google Scholar]
- 14.Vadiraja HS, Raghavendra RM, Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: A randomized controlled trial. Integr Cancer Ther. 2009;8:37–46. doi: 10.1177/1534735409331456. [DOI] [PubMed] [Google Scholar]
- 15.Raghavendra RM, Nagarathna R, Nagendra HR, et al. Effects of an integrated yoga programme on chemotherapy-induced nausea and emesis in breast cancer patients. Eur J Cancer Care (Engl) 2007;16:462–474. doi: 10.1111/j.1365-2354.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 16.Rao RM, Telles S, Nagendra HR, et al. Effects of yoga on natural killer cell counts in early breast cancer patients undergoing conventional treatment. Comment to: Recreational music-making modulates natural killer cell activity, cytokines, and mood states in corporate employees Masatada Wachi, Masahiro Koyama, Masanori Utsuyama, Barry B. Bittman, Masanobu Kitagawa, Katsuiku Hirokawa. Med Sci Monit, 2007; 13: CR57-70. Med Sci Monit. 2008;14:LE3–LE4. [PubMed] [Google Scholar]
- 17.Sephton SE, Sapolsky RM, Kraemer HC, et al. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 18.Filipski E, King VM, Li X, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94:690–697. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- 19.Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8:43–55. [PubMed] [Google Scholar]
- 20.Pocock SJ. Clinical Trials: A practical Approach. New York, NY: John Wiley & Sons; 1983. [Google Scholar]
- 21.Davis SL. Thriving After Breast Cancer: Essential Healing Exercises for Body and Mind. New York, NY: Broadway Books; 2002. [Google Scholar]
- 22.Halverstadt ALA. Essential Exercises for Breast Cancer Survivors. Boston, MA: The Harvard Common Press; 2000. [Google Scholar]
- 23.Ware JE, Jr, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Med Care. 1995;33:AS264–AS279. [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Snow KK, Kosinski M, et al. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center Hospitals, Inc; 1993. [Google Scholar]
- 25.Sloan JA, Frost MH, Berzon R, et al. The clinical significance of quality of life assessments in oncology: A summary for clinicians. Support Care Cancer. 2006;14:988–998. doi: 10.1007/s00520-006-0085-y. [DOI] [PubMed] [Google Scholar]
- 26.Sloan JA, Vargas-Chanes D, Kamath CC, et al. Detecting worms, ducks and elephants: A simple approach for defining clinically relevant effects in quality-of-life measures. J Cancer Integ Med. 2003;1:41–47. [Google Scholar]
- 27.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Radloff LS. The CES-D scale: A new self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 30.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen L, Warneke C, Fouladi RT, et al. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 32.Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: Effects on quality of life. J Clin Oncol. 2007;25:4387–4395. doi: 10.1200/JCO.2006.06.6027. [DOI] [PubMed] [Google Scholar]
- 33.Bower JE, Garet D, Sternlieb B. Yoga for persistent fatigue in breast cancer survivors: Results of a pilot study. Evid Based Complement Alternat Med. 2011;2011:623168. doi: 10.1155/2011/623168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2012;118:3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cramer H, Lauche R, Langhorst J, et al. Quality of life and mental health in patients with chronic diseases who regularly practice yoga and those who do not: A case-control study. Evid Based Complement Alternat Med. 2013;2013:702914. doi: 10.1155/2013/702914. [DOI] [PMC free article] [PubMed] [Google Scholar]