Abstract
Purpose
Cumulative neurotoxicity is a prominent toxicity of oxaliplatin-based therapy. Intravenous calcium and magnesium have been extensively used to reduce oxaliplatin-induced neurotoxicity. This trial was designed to definitively test whether calcium/magnesium decreases oxaliplatin-related neurotoxicity.
Patients and Methods
In all, 353 patients with colon cancer undergoing adjuvant therapy with FOLFOX (fluorouracil, leucovorin, and oxaliplatin) were randomly assigned to intravenous calcium/magnesium before and after oxaliplatin, a placebo before and after, or calcium/magnesium before and placebo after. The primary end point was cumulative neurotoxicity measured by the sensory scale of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Chemotherapy-Induced Peripheral Neuropathy 20 tool.
Results
There were no statistically significant neuropathy differences among the study arms as measured by the primary end point or additional measures of neuropathy, including clinician-determined measurement of the time to grade 2 neuropathy by using the National Cancer Institute Common Terminology Criteria for Adverse Events scale or an oxaliplatin-specific neuropathy scale. In addition, calcium/magnesium did not substantially decrease oxaliplatin-induced acute neuropathy.
Conclusion
This study does not support using calcium/magnesium to protect against oxaliplatin-induced neurotoxicity.
INTRODUCTION
Neuropathy is the most prominent dose-limiting toxicity of oxaliplatin, an agent used frequently in adjuvant and palliative settings; it can cause substantial symptoms that can last for years.
Oxaliplatin-associated neuropathy is different from cisplatin-associated neuropathy. Although both cause a peripheral stocking-glove neuropathy that worsens with increasing dose exposure, oxaliplatin is also associated with an acute neuropathic problem that is generally associated with each oxaliplatin dose and largely abates after a few days. This acute neuropathy commonly consists of cold intolerance, muscle cramps, and throat discomfort.1 Although this acute neuropathy can be quite bothersome, it is the more chronic neuropathy that is generally more problematic and dose-limiting.
High doses of intravenous calcium and magnesium (CaMg), given before and after FOLFOX (fluorouracil, leucovorin, and oxaliplatin) therapy, represent the most thoroughly studied and the most commonly used clinical regimen for the prevention of FOLFOX neuropathy. French investigators initially proposed that CaMg would be helpful for preventing oxaliplatin-induced neuropathy; it was hypothesized that the reason for the difference between the neurotoxicity of oxaliplatin and cisplatin was that oxalate was metabolized from oxaliplatin, and oxalate was known to chelate calcium and magnesium, elements involved in the function of ion channels in nerve membranes. Thus, calcium and/or magnesium might prevent or ameliorate oxaliplatin-induced neurotoxicity.2
In 2004, Gamelin et al2 reported the results of a retrospective review of 161 patients receiving oxaliplatin therapy, with or without concurrent intravenous CaMg therapy. Neurotoxicity-related discontinuation of oxaliplatin occurred in only 4% of patients receiving CaMg versus 31% of patients who did not receive this treatment (P < .001). Overall, neurotoxicity was reported less frequently with the use of CaMg (20% v 45%; P = .003). Benefit was reported for both the acute neuropathy and for chronic cumulative neurotoxicity, with no evident interferences with the anticancer activity of FOLFOX. Subsequently, many oncologists included intravenous CaMg as part of clinical practice for patients receiving FOLFOX.
In addition, prospective clinical trials were developed to address this question. One trial, labeled CONcePT, had a 2 × 2 study design whereby patients were randomly assigned to receive CaMg versus placebos while they were also randomly assigned to receive either continuous FOLFOX therapy or a “stop-and-go” treatment (eight cycles of FOLFOX plus bevacizumab followed by a maintenance period of fluorouracil and leucovorin plus bevacizumab and subsequent planned reintroduction of oxaliplatin). In view of poor accrual, the trial was amended, with the CaMg question being dropped in favor of treating all patients with CaMg, since investigators considered this to most likely be beneficial.
A short time after the decision to stop the CaMg part of that study, the study data monitoring committee noted that patients who received CaMg had significantly lower response rates than did patients who received placebo.3 This raised marked concern that led to trial closure. This concern also led to early closure of another clinical trial, N04C7, being conducted in the adjuvant setting. Further review of the CONcePT data, however, indicated that patients randomly assigned to CaMg and those randomly assigned to placebo had virtually identical response rates.4
Data analyses from both of these prematurely discontinued trials have been reported. The CONcePT results supported that CaMg did not decrease either acute or chronic oxaliplatin-associated neuropathy.5 Although the results of N04C7 also did not suggest any CaMg-related decrease in oxaliplatin-caused acute neuropathy, they did support that CaMg decreased cumulative sensory neurotoxicity seen in the first 100 days of therapy.1
Given the early discontinuation of these two clinical trials, the divergent results, and the prominent use of CaMg in clinical practice, this clinical trial was developed to determine the value of CaMg in preventing oxaliplatin-induced neuropathy.
PATIENTS AND METHODS
Patients considered for this clinical trial had to be adults with adenocarcinoma of the colon who, after curative-intent resection, were scheduled to receive 6 months (12 cycles) of adjuvant FOLFOX chemotherapy (ie, fluorouracil, leucovorin, and oxaliplatin [FOLFOX4] or modified fluorouracil, leucovorin, and oxaliplatin [mFOLFOX6]), involving oxaliplatin 85 mg/m2 every 2 weeks.6 Participants needed to have adequate hematologic parameters to allow chemotherapy, along with serum total bilirubin and creatinine ≤ 1.5× the upper limit of normal and calcium and magnesium 1.2× the upper limit of normal. Women of childbearing potential needed to have a negative pregnancy test. A central venous access device was required before starting chemotherapy and protocol treatment.
Patients were not allowed on trial if they had a pre-existing peripheral neuropathy of any grade; had received prior treatment with neurotoxic chemotherapy such as oxaliplatin, cisplatin, a taxane, or a vinca alkaloid; had a history of second- or third-degree atrioventricular heart block; were receiving digoxin, carbamazepine, phenytoin, valproic acid, gabapentin, pregabalin, venlafaxine, desvenlafaxine, milnacipran, duloxetine, a tricyclic antidepressant, or any other agent specifically being given to prevent or treat neuropathy; had a family history of a genetic/familial neuropathy; had other medical conditions which, in the opinion of the treating physician, would make the protocol unreasonably hazardous for the patient; or were not considered to be able to comply with the protocol. The protocol was approved per US federal guidelines, and patients needed to provide appropriate informed written consent. This trial was reviewed at least twice yearly by a data and safety monitoring committee.
At study entry, laboratory tests (serum Ca, Mg, Na, K, creatinine, AST, ALP, and total bilirubin) were obtained. At study initiation and before each 2-week cycle of chemotherapy, a history, physical examination, and CBC were performed for each patient. At the same time, neurotoxicity assessments were obtained by several separate means. The primary neuropathy assessment was measured by the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Chemotherapy-Induced Peripheral Neuropathy 20 (EORTC QLQ-CIPN20)7 sensory neuropathy score. EORTC QLQ-CIPN20 motor and autonomic neurotoxicity scores were also collected. In addition, an investigator-determined neuropathy score was assigned by using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0, with standardized questions regarding neurotoxic symptoms and examples of answers (Data Supplement), to allow a more accurate classification of patient symptoms as grade 1, 2, 3, or 4. Another neuropathy assessment method used an oxaliplatin-specific scale that generated an investigator-assigned score focusing on the reversibility of neurotoxicity symptoms between treatment cycles.8
The QLQ-CIPN20 was developed by the EORTC to assess chemotherapy-induced peripheral neuropathy.7 Because this tool has been well validated7,9 and it is recognized that patient-reported outcomes are better tools for measuring symptoms than are clinician-determined means, it was decided to use the QLQ-CIPN20 instrument as the primary end point, rather than the NCI-CTCAE grading scale.
Patients completed daily questionnaires before each dose of FOLFOX and for an additional 5 days after the initiation of each cycle of FOLFOX to provide data regarding the acute, more transient neuropathy observed with oxaliplatin therapy.1 By using this questionnaire, patients provided answers relating to the previous 24 hours on a numerical analog scale ranging from 0 to 10 that addressed sensitivity touching cold items, discomfort swallowing cold items, throat discomfort, and muscle cramps.
At study entry and before each 2-week cycle of chemotherapy, patients were monitored for adverse events. This included patient-reported outcome variables evaluated by questionnaires for diarrhea, abdominal cramping, constipation, bowel problems, and dysphagia, all using numerical analog scales ranging from 0 to 10.
Patients were randomly allocated to receive intravenous calcium gluconate plus magnesium sulfate (1 g of each) in 100 mL of D5W over 30 minutes immediately before and after each dose of oxaliplatin, an identical-appearing placebo immediately before and after each dose of oxaliplatin, or intravenous calcium gluconate plus magnesium sulfate immediately before and placebo immediately after each dose of oxaliplatin. Patients and all clinical study personnel who interacted with them were blinded to the treatment arm. Patients were stratified by age (< 65 years v 65 years or older), sex, colon cancer stage (II v III v IV), and whether they were to receive FOLFOX4 or modified FOLFOX6.
If the patient developed any clinically significant adverse effect attributed to CaMg (placebo), the CaMg (placebo) was to be stopped and the event recorded. The patient then continued to be observed according to the protocol.
Although oxaliplatin dose modifications were not dictated within this study, recommendations were included as guidelines for patient treatment. It was suggested that for patients who experienced persistent grade 2 sensory neuropathy that did not resolve within 2 weeks, a dose reduction of oxaliplatin to 65 mg/m2 should be considered; for patients with persistent grade 3 sensory neuropathy, discontinuing oxaliplatin should be considered. A dose reduction of oxaliplatin to 65 mg/m2 and of fluorouracil by 20% should be considered for patients after recovery from grade 3 to 4 GI toxicity or grade 4 neutropenia or grade 3 to 4 thrombocytopenia, and the next dose should be delayed until neutrophils are ≥ 1.5 × 109/L and platelets are ≥ 75 × 109/L.
Statistical Methodology
A three-arm, randomized, placebo-controlled, double-blind, parallel group design was used. Patients were randomly assigned by a dynamic allocation procedure to balance the marginal distributions of the stratification factors. The primary end point was the area under the curve (AUC) of the EORTC QLQ-CIPN20 sensory scale during the chemotherapy. This summary measure of AUC was used to compare the AUC of the CIPN20 sensory scale between each of the two schedules of CaMg infusions versus the placebo arms. Intermittent missing data were implicitly imputed following the trapezoidal rule, and terminal missing data were prorated by the actual number of chemotherapy cycles patients received. Two Wilcoxon rank sum tests were used at the 2.5% significance level. Thus, the overall type I error rate of falsely concluding that at least one of the two CaMg infusion treatments was significantly different from placebo was, at most, a 5% chance by using the Bonferroni approach. Statistical analyses were conducted by the Alliance Statistics and Data Center, which ensured data quality. Data for this analysis were frozen on November 6, 2012.
Early use of the EORTC QLQ-CIPN20 instrument in another North Central Cancer Treatment Group (NCCTG) trial10 did not provide enough preliminary data on the AUC of CIPN20 sensory scale because it was used only in patients with established neuropathy; however, data support that there is a positive correlation between the EORTC QLQ-CIPN20 and NCI-CTCAE CIPN grade.10a Instead of guessing the clinically meaningful difference in terms of AUC and its variation, the percentages of grade 2+ chronic sensory neuropathy during the treatment, measured by CTCAE criteria, were adopted as a key secondary analysis for the sample size calculation. Results from the N04C7 trial1 indicated that an expected level of grade 2+ chronic neurotoxicity after oxaliplatin-based chemotherapy would be roughly 40% for the placebo arm. On the basis of a two-sided Fisher's exact test at a significance level of 2.5%, we needed a sample size of 107 patients per arm to provide 80% power to detect a difference in incidence of grade 2+ neuropathic toxicity from 40% in the placebo arm to 20% in either schedule of the CaMg infusion arms. The sample size was inflated by 10% to account for patient ineligibility, cancellation, or major protocol violations.
Numerous secondary end points were compared between either of the CaMg infusion arms and placebo in an exploratory manner. The CIPN20 motor and autonomic scales were analyzed by using the same approach as in the primary analysis. The percentage of patients experiencing grade 2+ chronic CIPN, according to NCI-CTCAE version 4.0, were compared by using the χ2 test, and times to onset of grade 2+ chronic cumulative neurotoxicity were examined by using Kaplan-Meier survival curves and log-rank testing. The percentage of acute neuropathy associated with oxaliplatin was summarized by using descriptive statistics and statistical plots.
RESULTS
Baseline Characteristics
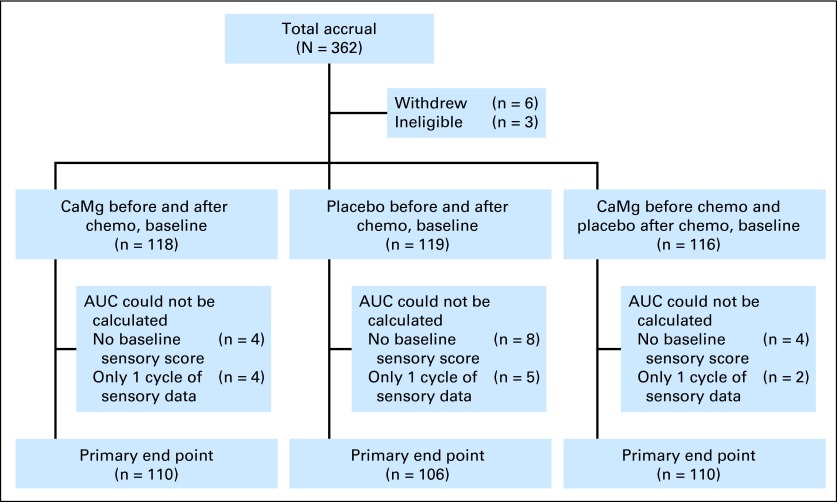
This study accrued 353 patients between June 22, 2010, and June 8, 2012, from more than 50 individual sites. Baseline patient characteristics (detailed in Table 1) were equivalent in the three treatment groups. Patient study flow is illustrated in a CONSORT diagram (Fig 1).
Table 1.
Baseline Patient Characteristics
| Characteristic | CaMg Before and After Chemotherapy (n = 118) |
CaMg Before and Placebo After Chemotherapy (n = 116) |
Placebo (n = 119) |
Total (n = 353) |
P | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean | SD | Median | No. | % | Mean | SD | Median | No. | % | Mean | SD | Median | No. | % | Mean | SD | Median | ||
| Age, years | 57.0 | 57.0 | 56.0 | 56.0 | .9058* | ||||||||||||||||
| Age group < 65 years | 88 | 75 | 87 | 75 | 90 | 76 | 265 | 75 | .9823† | ||||||||||||
| Male sex | 56 | 48 | 56 | 48 | 57 | 48 | 169 | 48 | .9922† | ||||||||||||
| Race/ethnicity | .4543‡ | ||||||||||||||||||||
| White | 96 | 81 | 99 | 85 | 105 | 88 | 300 | 85 | |||||||||||||
| Black or African American | 16 | 14 | 15 | 13 | 10 | 8 | 41 | 12 | |||||||||||||
| Asian | 4 | 3 | 1 | 1 | 2 | 2 | 7 | 2 | |||||||||||||
| American Indian or Alaska Native | 2 | 2 | 0 | 0 | 1 | 1 | 3 | 1 | |||||||||||||
| Not reported (patient refused or not available) | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | |||||||||||||
| Stage | .9627† | ||||||||||||||||||||
| II | 22 | 19 | 22 | 19 | 22 | 19 | 66 | 19 | |||||||||||||
| III | 89 | 75 | 88 | 76 | 88 | 74 | 265 | 75 | |||||||||||||
| IV | 7 | 6 | 6 | 5 | 9 | 8 | 22 | 6 | |||||||||||||
| Regimen | .9394† | ||||||||||||||||||||
| FOLFOX4 | 7 | 6 | 8 | 7 | 9 | 8 | 24 | 7 | |||||||||||||
| mFOLFOX6 | 111 | 94 | 108 | 93 | 110 | 92 | 329 | 93 | |||||||||||||
| Oral CaMg supplement | .9709† | ||||||||||||||||||||
| Missing | 2 | 0 | 3 | 5 | |||||||||||||||||
| Yes | 12 | 10 | 13 | 11 | 13 | 11 | 38 | 11 | |||||||||||||
| No | 104 | 90 | 103 | 89 | 103 | 89 | 310 | 89 | |||||||||||||
| Total EORTC QLQ-CIPN20 score | |||||||||||||||||||||
| Sensory neuropathy | 98.4 | 4.0 | 100.0 | 97.6 | 5.9 | 100.0 | 97.8 | 6.4 | 100.0 | 97.9 | 5.5 | 100.0 | .2654* | ||||||||
| Autonomic neuropathy | 95.0 | 10.1 | 100.0 | 92.3 | 12.6 | 100.0 | 91.8 | 13.0 | 100.0 | 93.0 | 12.0 | 100.0 | .1155* | ||||||||
| Motor neuropathy | 97.9 | 3.9 | 100.0 | 97.0 | 7.7 | 100.0 | 98.0 | 3.9 | 100.0 | 97.6 | 5.5 | 100.0 | .9789* | ||||||||
| Sensitivity to touching cold items | 95.9 | 14.2 | 100.0 | 94.4 | 17.0 | 100.0 | 96.0 | 14.5 | 100.0 | 95.4 | 15.3 | 100.0 | .3876* | ||||||||
| Discomfort swallowing cold liquids | 96.2 | 13.4 | 100.0 | 92.6 | 18.9 | 100.0 | 94.2 | 17.7 | 100.0 | 94.4 | 16.8 | 100.0 | .1173* | ||||||||
| Throat discomfort | 97.1 | 11.1 | 100.0 | 93.7 | 17.5 | 100.0 | 96.0 | 13.4 | 100.0 | 95.6 | 14.3 | 100.0 | .2685* | ||||||||
| Muscle cramps | 97.9 | 8.6 | 100.0 | 97.5 | 8.9 | 100.0 | 96.8 | 11.4 | 100.0 | 97.4 | 9.7 | 100.0 | .5322* | ||||||||
| Difficulties buttoning/tying | 99.7 | 2.1 | 100.0 | 98.8 | 5.9 | 100.0 | 99.8 | 1.3 | 100.0 | 99.5 | 3.7 | 100.0 | .0930* | ||||||||
Abbreviations: CaMg, calcium and magnesium; EORTC QLQ-CIPN20, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Chemotherapy-Induced Peripheral Neuropathy 20; FOLFOX4, fluorouracil, leucovorin, and oxaliplatin; mFOLFOX6, modified fluorouracil, leucovorin, and oxaliplatin; SD, standard deviation.
Kruskal-Wallis test.
χ2 test.
Fisher's exact test.
Fig 1.
CONSORT diagram. AUC, area under the curve; CaMg, calcium and magnesium; chemo, chemotherapy.
Neuropathy Data
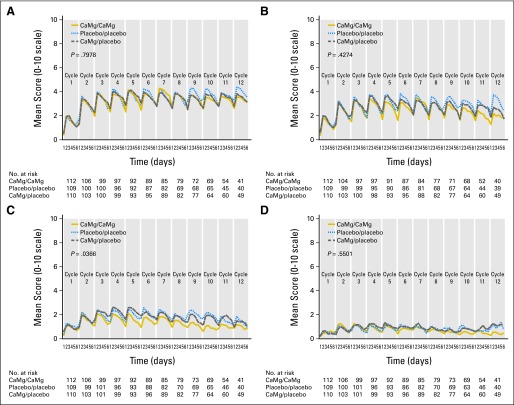
Patient-reported acute neuropathy data are illustrated in Figure 2 for 5 days after each oxaliplatin dose regarding sensitivities to touching cold items, discomfort swallowing cold liquids, muscle cramps, and throat discomfort. This figure illustrates that there were no significant differences among the three study arms in any the first three items, but there was some indication of a decrease of throat discomfort with CaMg (Fig 2C), with a raw P value of .036 (without adjustment for multiple comparisons).
Fig 2.
Acute symptoms regarding (A) sensitivities to touching cold items, (B) discomfort swallowing cold liquids, (C) throat discomfort, and (D) muscle cramps. P values are derived from a repeated measures analysis of a variance model. CaMg, calcium and magnesium.
Chronic Peripheral Neurotoxicity
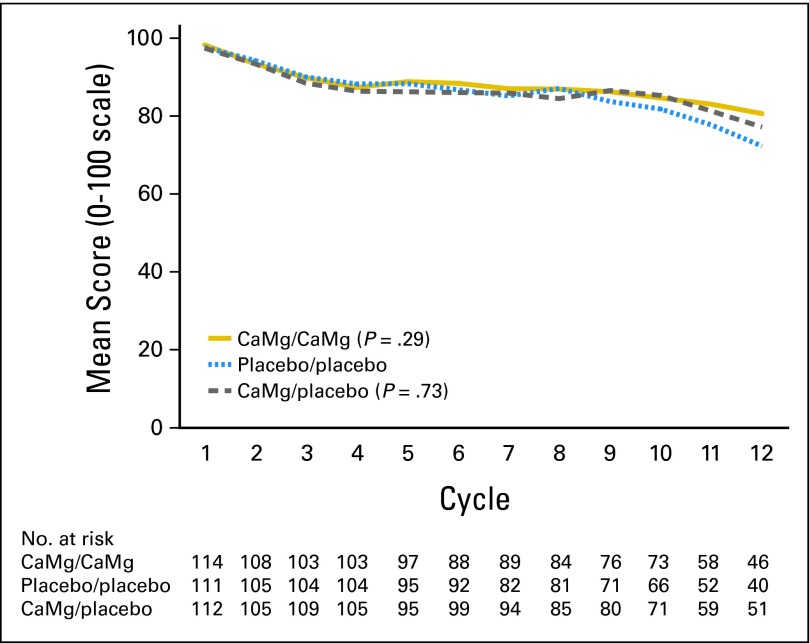
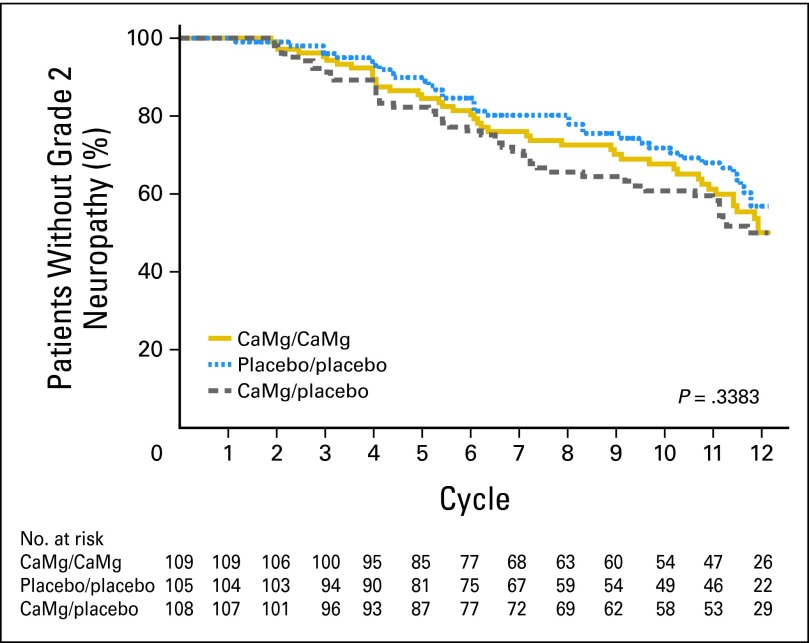
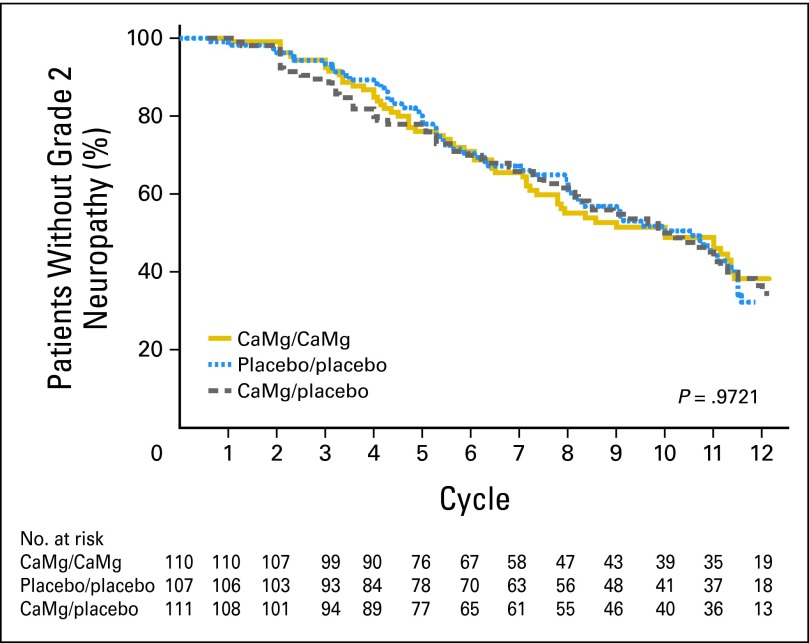
Peripheral neuropathy data for the three study arms using the EORTC QLQ-CIPN20 sensory neuropathy scale (primary end point; Fig 3) illustrate that there were no statistically significant differences in the AUC among the three study arms (P = .727 and P = .292 for comparing each CaMg arm with the placebo arm). Similarly, there were no evident differences for the EORTC QLQ-CIPN20 motor neuropathy scale (P = .294 and P = .251 for comparing each CaMg arm with the placebo arm) or the EORTC QLQ-CIPN20 autonomic neuropathy scale (P = .054 and P = .270 for comparing each CaMg arm with the placebo arm). In addition, no significant differences were shown (Fig 4) when neurotoxicity was assessed by physicians using CTCAE for the time to grade 2 or worse neurotoxicity (P = .338 for comparing all three arms with each other). The incidence rates of CTCAE grade 2 or worse neurotoxicity were 43%, 46%, and 45% for CaMg/CaMg, CaMg/placebo, and placebo/placebo arms, respectively. No substantial differences were seen when CIPN was measured by the oxaliplatin-specific neuropathy scale (Fig 5) for the time to grade 2 or worse neuropathy (P = .972 for comparing all three arms with each other).
Fig 3.
Peripheral sensory neuropathy changes for the three study arms, as measured by the sensory scale of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Chemotherapy-Induced Peripheral Neuropathy 20 instrument. Wilcoxon rank sum test P = .73 and P = .29 for comparing each calcium and magnesium (CaMg) arm with the placebo arm.
Fig 4.
Times until grade 2 or worse chemotherapy-induced peripheral neuropathy, using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4 instrument. CaMg, calcium and magnesium.
Fig 5.
Times until grade 2 or worse chemotherapy-induced peripheral neuropathy, using the oxaliplatin-specific neuropathy instrument. CaMg, calcium and magnesium.
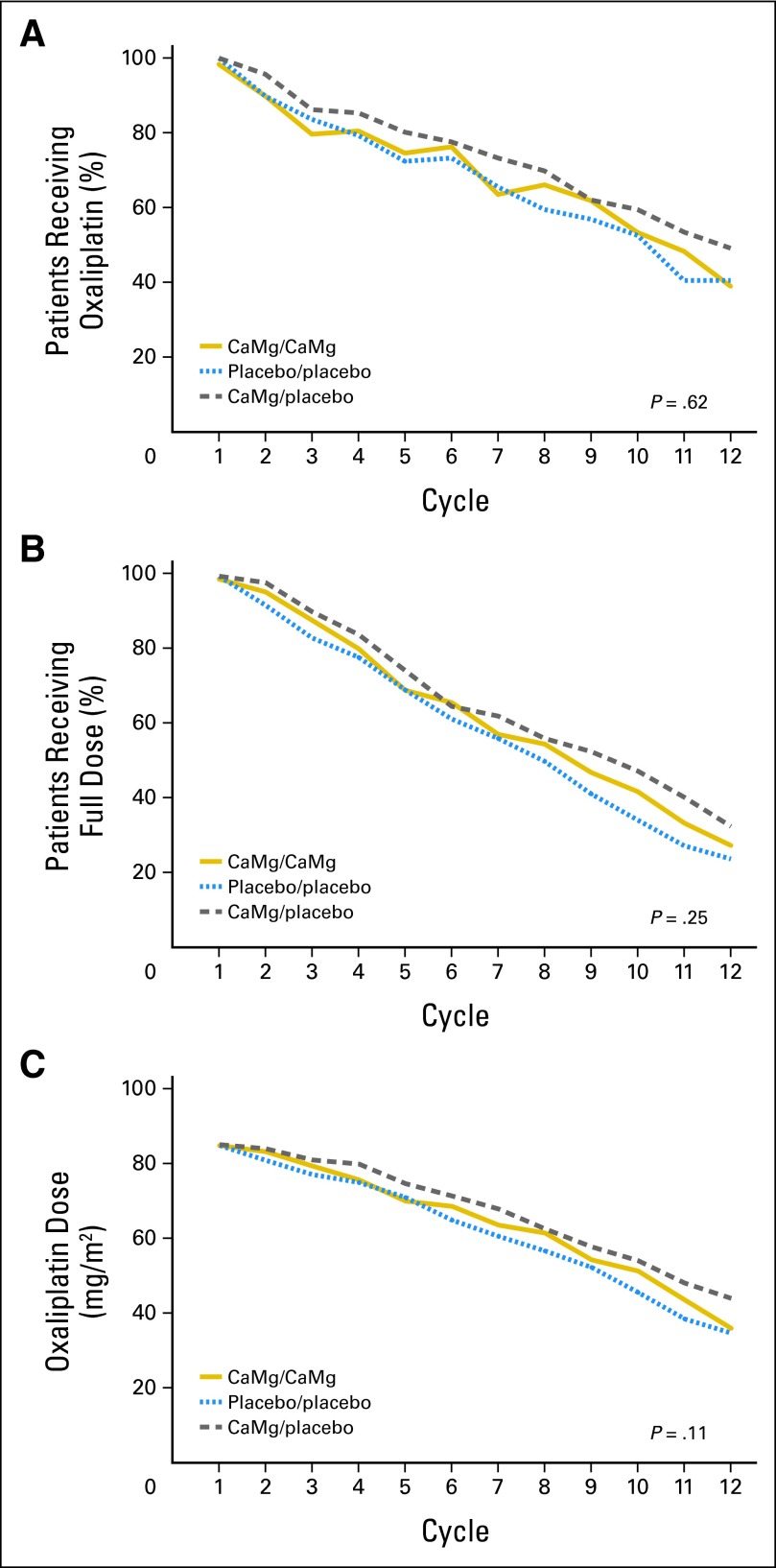
There were no significant differences with regard to the use of FOLFOX, or specifically oxaliplatin, over time; oxaliplatin use per treatment arm is illustrated in Figure 6. The median times on full-dose oxaliplatin and median numbers of cycles to discontinuation for the three arms are provided in Table 2.
Fig 6.
Percentages of patients in each study arm continuing to use (A) oxaliplatin over time (χ2 test P = .62) or (B) full doses of oxaliplatin over time (χ2 test P = .25). (C) Mean of oxaliplatin doses in each study arm over time (Wilcoxon rank sum test P = .11). In each situation, the denominators are the numbers of evaluable patients who started each study arm. CaMg, calcium and magnesium.
Table 2.
FOLFOX Dose Reduction and Discontinuation by Treatment Arm
| Variable | CaMg Before and After Chemotherapy (n = 118) |
Placebo (n = 119) |
CaMg Before and Placebo After Chemotherapy (n = 116) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean | SD | Median | Q1, Q3 | Range | P | No. | % | Mean | SD | Median | Q1, Q3 | Range | No. | % | Mean | SD | Median | Q1, Q3 | Range | P | |
| No. of cycles | 117 | 9.5 | 3.2 | 11.0 | 8.0, 12.0 | 1.0-12.0 | .8077* | 116 | 9.2 | 3.4 | 11.0 | 6.5, 12.0 | 1.0-12.0 | 115 | 9.9 | 2.9 | 12.0 | 8.0, 12.0 | 1.0-12.0 | .1941* | |||
| Dose reduction | .9462† | .9491† | |||||||||||||||||||||
| Missing | 1 | 3 | 1 | ||||||||||||||||||||
| Yes | 60 | 51.3 | 60 | 51.7 | 59 | 51.3 | |||||||||||||||||
| No | 57 | 48.7 | 56 | 48.3 | 56 | 48.7 | |||||||||||||||||
| Dose reduction cycle | 60 | 5.7 | 2.7 | 5.0 | 3.5, 8.0 | 2.0-12.0 | .3749* | 60 | 6.2 | 2.9 | 6.0 | 3.5, 9.0 | 2.0-12.0 | 59 | 6.6 | 3.2 | 6.0 | 4.0, 9.0 | 2.0-12.0 | .5637* | |||
| Discontinued | .5223† | .6637† | |||||||||||||||||||||
| Missing | 17 | 14 | 15 | ||||||||||||||||||||
| Yes | 35 | 34.7 | 32 | 30.5 | 28 | 27.7 | |||||||||||||||||
| No | 66 | 65.3 | 73 | 69.5 | 73 | 72.3 | |||||||||||||||||
| Cycle discontinued | 34 | 8.1 | 3.0 | 9.0 | 6.0, 11.0 | 1.0-12.0 | .8917* | 32 | 8.4 | 2.5 | 9.0 | 6.5, 10.0 | 2.0-12.0 | 28 | 8.0 | 2.6 | 8.0 | 6.0, 10.0 | 3.0-12.0 | .4960* | |||
Abbreviations: CaMg, calcium and magnesium; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; Q1, quartile 1; SD, standard deviation.
Kruskal-Wallis test.
χ2 test.
Evaluation of CaMg Toxicity
There were neither statistically significant nor clinically apparent toxicity differences among the three study arms with regard to diarrhea, constipation, stomach cramping, bowel problems, or laboratory parameters.
Subgroup Analyses
Subgroup analyses evaluated age, sex, disease stage, and specific FOLFOX regimens without revealing any good evidence of benefit in any subgroup.
DISCUSSION
Given the convincingly negative results of this clinical trial, has the question of the utility of CaMg as a potential neuro-protectant for oxaliplatin been definitively answered? A review of other related trials is necessary to answer this question.
There have been three published observational studies of the utility of CaMg as a potential neuro-protectant for oxaliplatin. In addition to the study by Gamelin et al,2 which yielded positive results, Knijn et al11 studied 732 patients randomly treated on a clinical trial with capecitabine, oxaliplatin, and bevacizumab versus capecitabine, oxaliplatin, and cetuximab, with the use of CaMg being left to the discretion of the treating oncologist. In a retrospective analysis, for 551 patients who received CaMg during at least their first treatment cycle, there was a slightly lower incidence of any physician-judged neurotoxicity (85% v 92%, respectively; P = .02) and physician-judged grade 2 neurotoxicity (40% v 45%; P = .22), versus those who did not receive CaMg (181 patients).
In a third retrospective analysis, 90 patients receiving FOLFOX also received goshajinkigan, a traditional Japanese herbal medicine, CaMg alone, CaMg plus goshajinkigan, or neither agent. No benefit was shown for CaMg.12
What about prospective trial results? Two other prospective double-blind published trials, both prematurely discontinued, have already been discussed in the Introduction. The CONcePT5 trial did not demonstrate any significant benefit, but the N04C7 trials suggested benefit for CaMg.1
Three other small trials have also attempted to address this issue, two of which were quite similar to each other.13,14 Both were randomized, double-blind, placebo-controlled trials that planned to have 30 to 31 patients per study arm. Both were stopped early with only approximately 50% of planned accrual because of the CONcePT trial results; thus, both were underpowered. In both trials, the patients in the control arms did numerically better than the patients receiving CaMg regarding subjective and objective measures of neuropathy. The third double-blind, placebo-controlled clinical trial had three study arms, with about 30 patients per study arm. Patients received CaMg, glutathione, or placebo. There were no statistically significant differences in neuropathy observed among the study arms.15
Another piece of tantalizing data suggesting that CaMg was beneficial was published by Gamelin et al4 in a letter to the editor in 2008. This report discussed preliminary data from the NEUROXA trial involving 52 patients with metastatic colorectal cancer; 50% of patients received CaMg and 50% did not. This report noted a difference in the rate of grade 3 neurotoxicity between the two study arms (5% v 24%; P < .001), but did not unblind the study arms. More than 4 years later, there is still no unblinded study report publically available from this trial.
Thus, the benefit of CaMg is primarily supported by the results of the initial retrospective report by Gamelin et al,2 the mildly positive retrospective review from Knjin et al,11 and the preliminary results of the N04C7 trial, which was prematurely stopped.1
Given the more definitive results of this trial and the lack of observed benefit in the CONCepT trial and the other three small, randomized, placebo-controlled trials, the bulk of available data do not support the continued use of intravenous CaMg to prevent oxaliplatin-induced neuropathy.
Of note, informal surveys during the last few years suggest that, with regional differences worldwide, approximately 50% of oncologists have been using CaMg in their practices. This is supported by UpToDate,15a which currently notes that CaMg can be considered for prevention of oxaliplatin neuropathy. The results of this trial may change that recommendation, saving time and expense for future patients.
Given that CaMg does not appear to be the solution to the problem of oxaliplatin-induced neuropathy, a prominent chronic problem for some patients, what options are there to further address this problem? One is to define a patient's risk for developing neuropathy on the basis of genetic factors (work ongoing from data on this study, since DNA was collected). A second is to look at other agents that might prevent this toxicity; efforts are ongoing to study the benefits of a serotonin/norepinephrine reuptake inhibitor as a means of preventing oxaliplatin neuropathy based on preliminary evidence suggesting that this approach may be beneficial.16,17
Supplementary Material
Appendix
The following are additional participating institutions: Geisinger Clinic & Medical Center Community Clinical Oncology Program (CCOP), Danville, PA (Maged Khalil, MD); Siouxland Hematology-Oncology Associates, Sioux City, IA (Donald Wender, MD); Toledo Community Hospital Oncology Program (Rex B. Mowat, MD); Medical College of Georgia, Augusta, GA (Anand P. Jillella, MD); Iowa Oncology Research Association CCOP, Des Moines, IA (Robert J. Behrens, MD); Colorado Cancer Research Program, Denver, CO (Keren Sturtz, MD); Medcenter One Health Systems, Bismarck, ND (John T. Reynolds, MD); Carle Cancer Center CCOP, Urbana, IL (Kendrith M. Rowland Jr, MD); Essentia Duluth CCOP, Duluth, MN (Daniel A. Nikcevich, MD); Marshfield Clinical Research Foundation, Minocqua, WI (Matthias Weiss, MD); Missouri Valley Cancer Consortium, Omaha, NE (Gamini S. Soori, MD); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN (Robin T. Zon, MD); Illinois Oncology Research Association CCOP, Peoria, IL (Nguyet Anh Le-Lindqwister, MD); Altru Health Systems, Grand Forks, ND (Grant Seeger, MD); St Vincent Regional Cancer Center CCOP, Green Bay, WI (Anthony J. Jaslowski, MD); Hawaii Minority-Based CCOP, Honolulu, HI (Jeffrey L. Berenberg, MD); Heartland Cancer Research CCOP, St Louis, MO (Alan P. Lyss, MD); Edward Comprehensive Cancer Center, Huntington, WV (Maria Rosalia B. Tri Tirona, MD); Lehigh Valley Hospital, Allentown, PA (Suresh Nair, MD); Montana Cancer Consortium, Billings, MT (Benjamin T. Marchello, MD); and Cancer Care Associates, Tulsa, OK (Alan M. Keller, MD).
Footnotes
Supported in part by Grants No. CA-25224, CA-37404, CA-35431, CA-63848, CA-63849, CA-35113, CA-35415, CA-35448, CA-35269, CA-35103, CA-63844, CA-35101, CA31946, CA33601, and CA-35195 from the Public Health Service.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT01099449.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Axel Grothey, sanofi-aventis Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Charles L. Loprinzi, Rui Qin, Axel Grothey
Administrative support: Charles L. Loprinzi
Provision of study materials or patients: Charles L. Loprinzi, Rui Qin, Shaker R. Dakhil, Louis Fehrenbacher, Kathleen A. Flynn, Pamela Atherton, Rubina Qamar, Grant C. Lewis, Axel Grothey
Collection and assembly of data: Rui Qin, Shaker R. Dakhil, Louis Fehrenbacher, Kathleen A. Flynn, Drew Seisler, Rubina Qamar, Grant C. Lewis, Axel Grothey
Data analysis and interpretation: Charles L. Loprinzi, Rui Qin, Pamela Atherton, Drew Seisler, Axel Grothey
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Grothey A, Nikcevich DA, Sloan JA, et al. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol. 2011;29:421–427. doi: 10.1200/JCO.2010.31.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gamelin L, Boisdron-Celle M, Delva R, et al. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: A retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res. 2004;10:4055–4061. doi: 10.1158/1078-0432.CCR-03-0666. [DOI] [PubMed] [Google Scholar]
- 3.Hochster HS, Grothey A, Childs BH. Use of calcium and magnesium salts to reduce oxaliplatin-related neurotoxicity. J Clin Oncol. 2007;25:4028–4029. doi: 10.1200/JCO.2007.13.5251. [DOI] [PubMed] [Google Scholar]
- 4.Gamelin L, Boisdron-Celle M, Morel A, et al. Oxaliplatin-related neurotoxicity: Interest of calcium-magnesium infusion and no impact on its efficacy. J Clin Oncol. 2008;26:1188–1189. doi: 10.1200/JCO.2007.15.3767. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A, Hart LL, Rowland KM, et al. Intermittent oxaliplatin (oxali) administration and time-to-treatment-failure (TTF) in metastatic colorectal cancer (mCRC): Final results of the phase III CONcePT trial. J Clin Oncol. 2008;26(suppl):180s. abstr 4010. [Google Scholar]
- 6.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 7.Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer. 2005;41:1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Lévi FA, Zidani R, Vannetzel JM, et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: A randomized multi-institutional trial. J Natl Cancer Inst. 1994;86:1608–1617. doi: 10.1093/jnci/86.21.1608. [DOI] [PubMed] [Google Scholar]
- 9.Lavoie Smith EM, Barton DL, Qin R, et al. Assessing patient-reported peripheral neuropathy: The reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res. doi: 10.1007/s11136-013-0379-8. [epub ahead of print on March 30, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton DL, Wos EJ, Qin R, et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer. 2011;19:833–841. doi: 10.1007/s00520-010-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Puttabasavaiah S, Liu H, Qin R, et al. A comparison of simple single-item measures and the NCI Common Toxicity Criteria version 3.0 measure of peripheral neuropathy. J Clin Oncol. 2012;30(suppl):99s. abstr 1557. [Google Scholar]
- 11.Knijn N, Tol J, Koopman M, et al. The effect of prophylactic calcium and magnesium infusions on the incidence of neurotoxicity and clinical outcome of oxaliplatin-based systemic treatment in advanced colorectal cancer patients. Eur J Cancer. 2011;47:369–374. doi: 10.1016/j.ejca.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Kono T, Mamiya N, Chisato N, et al. Efficacy of goshajinkigan for peripheral neurotoxicity of oxaliplatin in patients with advanced or recurrent colorectal cancer. Evid Based Complement Alternat Med. 2011;2011:418481. doi: 10.1093/ecam/nep200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chay WY, Tan SH, Lo YL, et al. Use of calcium and magnesium infusions in prevention of oxaliplatin induced sensory neuropathy. Asia Pac J Clin Oncol. 2010;6:270–277. doi: 10.1111/j.1743-7563.2010.01344.x. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi K, Okada N, Miyazaki T, et al. Effect of calcium and magnesium on neurotoxicity and blood platinum concentrations in patients receiving mFOLFOX6 therapy: A prospective randomized study. Int J Clin Oncol. 2010;15:82–87. doi: 10.1007/s10147-009-0015-3. [DOI] [PubMed] [Google Scholar]
- 15.Dong M, Xing PY, Liu P, et al. Assessment of the protective effect of calcium-magnesium infusion and glutathione on oxaliplatin-induced neurotoxicity [in Chinese] Zhonghua Zhong Liu Za Zhi. 2010;32:208–211. [PubMed] [Google Scholar]
- 15a.Wolters Kluwer Health. UpToDate. http://www.uptodate.com.
- 16.Durand JP, Deplanque G, Montheil V, et al. Efficacy of venlafaxine for the prevention and relief of oxaliplatin-induced acute neurotoxicity: Results of EFFOX, a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2012;23:200–205. doi: 10.1093/annonc/mdr045. [DOI] [PubMed] [Google Scholar]
- 17.Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA. 2013;309:1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.