SUMMARY
A critical aspect of gut morphogenesis is initiation of a leftward tilt. Failure to do so leads to gut malrotation and volvulus. The direction of tilt is specified by asymmetric cell behaviors within the dorsal mesentery (DM), which suspends the gut tube, and is downstream of Pitx2, the key transcription factor responsible for the transfer of left-right (L-R) information from early gastrulation to morphogenesis. Although Pitx2 is a master regulator of L-R organ development, its cellular targets that drive asymmetric morphogenesis are not known. Using laser microdissection and targeted gene misexpression in the chicken DM, we show that Pitx2-specific effectors mediate Wnt signaling to activate the formin Daam2, a key Wnt effector and itself a Pitx2 target, linking actin dynamics to cadherin-based junctions, to ultimately generate asymmetric cell behaviors. Our work highlights how integration of two conserved cascades may be the ultimate force through which Pitx2 sculpts L-R organs.
INTRODUCTION
The generation of asymmetry is fundamental to vertebrate development. Most organs develop with a characteristic left-right (L-R) anatomy that is critical to function and coordination of overall organ situs within the body cavity. How global L-R information is translated into asymmetries of cell behavior and coordinated with organ polarity is largely unknown (Gray et al., 2011). Initial L-R symmetry-breaking decisions are made at the primitive node and lead to left-sided expression of the highly conserved TGFβ-related Nodal throughout the left splanchnic mesoderm (Levin et al., 1995). While this restricted expression is transient, Nodal induces the homeobox transcription factor Pitx2, whose expression persists to maintain left-side identity of all organ primordia to which the splanchnic mesoderm contributes. This Pitx2-driven asymmetry is evolutionarily conserved, and altered Pitx2 activity disrupts L-R patterning resulting in reversed or isomerised growth of organs. In spite of enormous progress made towards understanding upstream patterning events, mechanisms by which Pitx2 expression leads to asymmetric changes in tissue organization remain largely unknown (Shiratori and Hamada, 2006). Focusing on the midgut, our goal has been to define the transcriptional targets and cellular mechanisms through which Pitx2 manifests asymmetric morphogenesis in higher vertebrates.
The primitive gut, a straight epithelial tube surrounded by mesenchymal cells (Fig. 1A, yellow), is divided into foregut, midgut, and hindgut along the rostral-caudal axis. Importantly, the midgut lengthens disproportionately to the embryo, resulting in the formation of a primary midgut loop, which herniates ventrally into the base of the umbilicus (in mammals) or yolk stalk (in birds). A highly conserved counterclockwise rotation accompanies midgut herniation (Fig. 1A, curved arrow). This carries the caudal half of the loop cranially on the left, then across the abdomen, before it again passes caudally on the right side, completing a total rotation through 270 degrees. This asymmetric rotation brings the future intestines into the familiar adult position upon retraction into the abdomen.
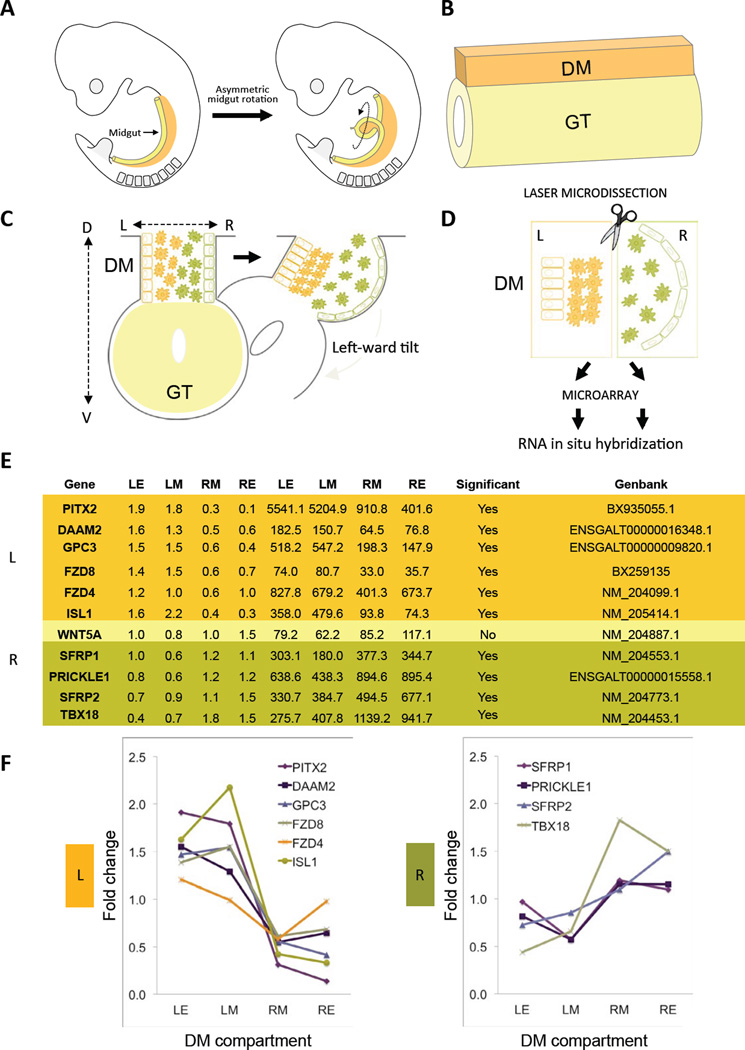
FIG. 1. DM and laser capture microdissection (LCM).
A Gut tube (GT, yellow; chicken HH20; mouse E10.0) undergoes counterclockwise rotation at HH21. B Gut tube is suspended by DM (orange) along the D-V axis. C Pitx2-driven deformation of DM at HH21 along the L-R axis initiates gut rotation. D LCM of the DM. E Selected microarray expression values from epithelium (E) and mesenchyme (M) of the Left (L) or Right (R) chicken DM (at HH21). Raw and normalized expression values are shown. Left genes (orange), right (green), and unbiased (yellow) are grouped. F Plot of normalized expression fold changes across the L-R axis.
Chiral midgut rotation in mammals and birds is driven by asymmetric cellular behavior within the dorsal mesentery (DM, Fig. 1AB), a bridge of mesoderm connecting the gut tube along its entire axial length to the dorsal body wall (Davis et al., 2008; Hecksher-Sorensen et al., 2004; Kurpios et al., 2008). The embryonic DM consists of four juxtaposed and molecularly distinct cellular compartments: left epithelium, left mesenchyme, right mesenchyme, and right epithelium (Fig. 1C). Subsequent cellular changes in each compartment are required to initiate gut rotation. In the chicken, DM forms on day 3 (Hamburger-Hamilton [HH] stage 19) (Hamburger and Hamilton, 1992), and initially these compartments are bilaterally symmetric. However, within 10–12 hours (HH21) DM cells rapidly reorganize via a combination of epithelial shape changes and mesenchymal condensation (left) or expansion (right). Consequently, relative to the dorsal-ventral (D-V) axis the left DM shortens while the right side lengthens, deforming the DM and shifting the attached gut tube to the left (Fig. 1C). This leftward tilt provides a directional L-R bias for counterclockwise gut rotation. Importantly, there are no asymmetries in cell number, proliferation or cell death within the DM showing that gut rotation is strictly a consequence of differential cell behavior across the L-R axis.
Previous studies in birds and mice have established that Pitx2 is essential to induce the left-specific gene expression and cell behavior within the DM (Davis et al., 2008; Kurpios et al., 2008). Pitx2-null mice are unable to generate the leftward tilt and exhibit randomized chirality of gut rotation (Davis et al., 2008; Shiratori and Hamada, 2006). These studies highlight the DM as a central player in the transfer of early L-R patterning, but leave unresolved the mechanisms by which this initial molecular asymmetry leads to asymmetric cell behavior.
Towards defining these processes, we made use of the binary cellular organization of the DM and its accessibility in ovo and performed laser capture microdissection (LCM) and microarray analysis of the left (Pitx2 positive) and right (Pitx2 negative) chicken mesenteric compartments (Fig. 1D). This indicated that genes involved in both positive and negative regulation of the Wnt pathway were differentially expressed across the L-R axis. RNA in situ hybridization (ISH) validated the spatial accuracy of these data and highlighted gradients of gene expression along the L-R and orthogonal D-V axis of the DM. Using targeted gene misexpression studies in the chicken DM and mouse genetics, we demonstrate that expression of the formin Daam2, a key cellular effector of Wnt signal transduction, is a downstream target of Pitx2, that is both necessary and sufficient for Pitx2-directed cell behavior in the mesenchyme of the left DM. We propose a model for Pitx2 regulation of asymmetric organ morphogenesis: Pitx2 potentiates asymmetric Wnt signaling via Daam2 activation, to induce polarized condensation in the left DM necessary to initiate gut rotation.
RESULTS
Asymmetric organization of a Wnt signaling network across the L-R axis of the DM
Our objective was to identify cellular effectors in the chicken DM that exhibit a spatial expression profile similar to Pitx2 and which may therefore represent Pitx2 targets responsible for the cellular behavior in the left DM (Fig. 1D). The detailed methodology of LCM together with the microarray data will be described elsewhere. Implicating the role of Wnt signaling, many of the most asymmetric gene expression profiles were those of the Wnt pathway (Fig. 1EF). Using ISH, we confirmed that the Frizzled (Fzd) receptors Fzd4 and Fzd8 are exclusively left-sided (Fig. 2AB, purple). Moreover, Fzd4/8 are expressed in a nested pattern within the left DM, with Fzd4 expressed in a broader domain that encompasses the more ventrally restricted Fzd8, suggesting a gradient of signaling potential exists along the D-V axis of the left DM (Fig. 2AB). The specific combination of Fzd4/8 has been shown to cooperatively mediate noncanonical Wnt signaling during development (Ye et al., 2011). Whereas we also identified a number of Fzd receptors that exhibit bilateral expression in the DM (such as Fzd1, Fig. S1A), notably, we found no Fzd receptors expressed only on the right.
FIG. 2. Asymmetric organization of a Wnt signaling network across the L-R DM.
A ISH, purple reveals positive (Daam2, Gpc3, Wnt5a, Fzd8, Fzd4) and negative (Sfrp1, and Sfrp2) Wnt pathway components in the DM and gut. B Gene expression schematic from A. Also see Fig. S1. C Left-sided Daam2 and absence of Daam2 in Pitx2−/− DM. D Left-sided Daam2 and Gpc3 prior to tilt. E Daam2tm1a(KOMP)Wtsi mice shows left-specific β-galactosidase activity. F Schematic of in ovo electroporation: DNA microinjected into the coelomic cavity (HH14) and electroporated to target the right splanchnic mesoderm (see IL). Arrow represents current-driven plasmid movement. G WT left-sided Daam2 expression. H pCAG-Pitx2 induces right-sided Daam2, marked by GFP in I. J WT left-sided Gpc3 expression. K pCAG-Pitx2 induces right-sided Gpc3, marked by GFP in L. Scale bars: A (100 µm); CIJKL (100 µm) D (50 µm) E (20 µm) See also Figure S1.
Wnt signaling is spatially modulated by heparan sulfate proteoglycans (Yan and Lin, 2009). For example, Wnt signaling is positively regulated by Gpc3 (Capurro et al., 2005b; De Cat et al., 2003; Song et al., 2005; Stigliano et al., 2009), a member of the glypican family that is mutated in Simpson-Golabi-Behmel syndrome (Pilia et al., 1996). Gpc3 is one of the most differentially expressed genes on the left side of the DM (Fig. 1E) and ISH on adjacent sections for Pitx2 and Gpc3 revealed striking similarities in expression pattern at the time of tilting (HH21, Fig. 2AB) and just prior to it (HH19–20, Fig. 2C). The clinical features of Simpson-Golabi-Behmel syndrome include instances of gut malrotation and other intestinal defects both in human and in Gpc3-null mice (Cano-Gauci et al., 1999; Golabi and Rosen, 1984). Importantly, Gpc3-null mice exhibit elevated canonical but reduced noncanonical Wnt signaling in vivo (Capurro et al., 2005a; Song et al., 2005) suggesting Gpc3 in the left midgut DM may act as a positive modulator of noncanonical Wnt signaling to establish the asymmetric cell behaviors of the leftward tilt, a finding supported by the combined expression of Fzd4 and Fzd8 in the same domain (Fig. 2AB) (Ye et al., 2011).
The dishevelled associated activator of morphogenesis (Daam) family of formin homology proteins consists of Daam1 (Habas et al., 2001; Khadka et al., 2009; Li et al., 2011; Miller et al., 2011; Nishimura et al., 2012; Sato et al., 2006; Zhu et al., 2012) and the largely uncharacterized Daam2 (Lee and Deneen, 2012). Daam1/2 are key intracellular effectors of Wnt signal transduction. We found that Daam2 expression is restricted to the left mesenteric compartment undergoing mesenchymal condensation (Fig. 2AB). ISH on adjacent sections for Pitx2, Gpc3 and Daam2 both prior to (HH19–20, Fig. 2C) and during the leftward tilt (HH21, Fig. 2AB) highlights expression overlap. Upon signal dependent activation, formins polymerize unbranched filamentous actin (F-actin) and form stress fibers necessary for cell polarity, cytoskeletal rearrangements, and adhesion (Habas et al., 2001; Kobielak et al., 2004; Liu et al., 2008). We hypothesized that Daam2-dependent regulation of the actin cytoskeleton drives mesenchymal condensation and thus provides the key missing link in the transmission of early L-R patterning signals to the forces driving gut rotation.
The secreted Frizzled-related proteins (Sfrp), which share structural homology to the Wnt binding domain of Fzd but are not membrane bound, compete for ligand binding and function to spatially attenuate Wnt signaling (Moon et al., 1997; Wolf et al., 2008). We found that expression of Sfrp1 and Sfrp2 is exclusive to the right side of the DM (Fig. 2AB). Interestingly, the noncanonical Wnt/PCP core gene Prickle-1 (Gray et al., 2011) was expressed in a decreasing R-to-L gradient (Fig. S1B). Collectively, this complementary left vs. right expression pattern of positive and negative Wnt pathway components establish differentially permissive environments for Wnt signaling via asymmetric responsiveness to Wnt signaling cues. For example, the expression of Fzd4/8, Gpc3 and Daam2 on the left side may function to potentiate Wnt signaling, leading to Wnt activation of Daam2 and condensation of the left mesenchyme. Simultaneously, these effects may be antagonized within the right DM by the presence of Wnt inhibitors Sfrp1 and Sfrp2, whereas graded Prickle-1 expression may further modulate Wnt output (Carreira-Barbosa et al., 2003; Chan et al., 2006; Veeman et al., 2003b).
Wnt5a, considered a prototypical noncanonical Wnt ligand, is expressed in the adjacent midgut at the onset of its morphogenesis (Cervantes et al., 2009; Yamaguchi et al., 1999). Whereas Wnt5a is critical for elongation of the midgut, whether it plays a role during L-R gut morphogenesis isn’t known. We hypothesized that Wnt5a expression in the midgut is the source of directional Wnt ligand responsible for Daam2 activation in the left DM (Liu et al., 2008). Indeed, Wnt5a was not found in the DM but was robustly expressed immediately ventral to the DM in the midgut mesenchyme (Fig. 2AB). We also observed that the most dorsal domain of Wnt5a expression overlaps with the most ventral domain of left-sided Fzd8 and Gpc3 (Fig. 2AB). We reasoned that mesenchymal cells on the left undergo condensation and drive the tilt in response to Wnt5a secreted from the adjacent gut mesenchyme, a mechanism that may temporally coordinate intestinal elongation and coincident looping morphogenesis.
Oriented mesenchymal condensation underlies the leftward tilt of the midgut
Specific Wnt-Fzd complex activation of canonical vs. noncanonical Wnt signaling is both highly context dependent and influenced by multiple factors (van Amerongen and Nusse, 2009; Veeman et al., 2003a). Noncanonical Wnt signaling primarily alters cellular behavior whereas the canonical pathway induces changes in cell proliferation and differentiation (Veeman et al., 2003a). We previously showed that there are no asymmetries in cell number, proliferation or cell death within the four mesenteric compartments at the time of tilting (Davis et al., 2008), arguing against a role of canonical signaling during gut tilting. Furthermore, formation of the leftward tilt involves polarized cell shape changes of the left epithelial compartment (Davis et al., 2008). While this observation favors noncanonical Wnt signaling, whether the condensing mesenchymal compartment of the left DM exhibits signs of tissue polarity characteristic of a noncanonical Wnt response isn’t known. To resolve this, we used cell polarity XY vectors defined by the location of the golgi apparatus (GM130/Golga2 staining) relative to the nucleus (DAPI, Fig. 3AB) in the DM. This analysis confirmed previously identified apical-basal polarity in the left epithelium compared to the random polarity of the right (Fig. 3B; n=75 L-side, n=70; R-side, p value < 0.0001). Moreover, we now show that left mesenchymal cells are oriented to the left across the L-R axis of the DM (Fig. 3B; n=322; p<0.009). In contrast, right mesenchymal cells show no significant orientation bias (Fig. 3B; n=270; p<0.6).
FIG. 3. Cellular behavior in the DM is polarized.
A WT left and right DM stained with GM130 (golgi) and DAPI. B Cell orientation measured by the angle of the golgi with respect to the nucleus (X-axis: L-R, Y-axis: D-V, Radial-axis: number of cells per bin). Polarized left mesenchyme is oriented to the left (L: p<0.009; R: p<0.6). For L vs. R epithelium: p value < 0.0001). C Electroporation of pTOP-nRFP (red) into (C) the left and right DM; (D) neural tube; and (E) yolk sack (Also see Fig. S2). GFP identifies electroporated cells. Blue is DAPI. Scale bars: A (10 µm; insets are 5 µm); C (10µm).
Our results support active noncanonical Wnt signaling in the DM and agree well with previous reports showing a lack of TOPGAL activity during midgut morphogenesis (Cervantes et al., 2009; Matsuyama et al., 2009). However, to rule out canonical Wnt activity in the DM we employed three widely published β-catenin dependent Wnt reporters in vivo (Barolo, 2006; Biechele et al., 2009). All three reporters showed no activity in the left or right DM by comparison to report in tissues known to undergo canonical Wnt signaling (dorsal neural tube, the yolk sac, and neural ectoderm (Griffin et al., 2011; Lassiter et al., 2007; Lee and Deneen, 2012) (Fig. 3C–E and Fig. S3AB). These results argue against canonical Wnt activity during asymmetric gut rotation and favor a model where Pitx2 asymmetrically induces Fzd4/8, Gpc3 and Daam2, enabling the DM to respond to noncanonical Wnt.
Wnt components in the DM are conserved downstream of Pitx2
The asymmetric architecture and molecular readout of the DM are evolutionarily conserved among birds and mice. In Pitx2 mutant mice, the left DM fails to condense (Davis et al., 2008) and we therefore asked whether this defect is associated with altered expression of Daam2. At E10.75, we observed left-sided Daam2 expression in WT mouse DM, akin to that observed in the chick (Fig. 2D). Importantly, expression of Daam2 was lost in the DM of Pitx2−/− mutant embryos (Fig. 2D, dotted line).
We have generated Daam2tm1a(KOMP)Wtsi mice harboring a targeted disruption of Daam2; the complete characterization of these mice will be reported independently. However, this allele harbors an insertion of lacZ under control of the endogenous Daam2 promoter, and whole mount β-galactosidase assays confirm left-restricted expression of Daam2 in the DM of Daam2+/− embryos (Fig. 2E), further arguing for a conserved role of Daam2 in L-R asymmetric gut morphogenesis.
To learn whether Pitx2 is sufficient to drive Daam2 expression we induced ectopic Pitx2 using in ovo DNA electroporation (Davis et al., 2008; Kurpios et al., 2008) (Fig. 2F). We found that Pitx2 strongly induced Wnt signaling components on the right side, including both Daam2 and Gpc3 (Fig. 2G–L).
In an effort to discern between direct and indirect Pitx2-dependent transcription, we performed comparative genomics and computational sequence analysis to search for highly conserved Pitx2 binding sites in the promoters of candidate Pitx2 targets. We confirmed Pitx2 binding sites at known Pitx2 targets and predicted conserved Pitx2 binding sites at Gpc3, Fzd4 and Daam2 (Fig. S2).
Daam2 activation is required for mesenchymal condensation in the DM
Daam1 and Daam2 contain identical structural domains and share a high degree of protein similarity (Nakaya et al., 2004). In the absence of signaling, Daam proteins are autoinhibited by interactions between their N-terminal GTPase binding domain (GBD), located within the diaphanous inhibitory domain (DID), and a C-terminal diaphanous autoinhibitory domain (DAD; Fig. 4A) (Habas et al., 2001). Wnt signal-dependent binding of Dishevelled to the DAD domain is a key step in Daam1 activation (Liu et al., 2008). To address the function of Daam2 during L-R gut morphogenesis, we generated protein truncation mutants (Fig. 4A) based upon the previous functional dissection of Daam1 (Habas et al., 2001). Mutants consisting of the N-terminus alone (N-Daam1) exhibit dominant-negative activity (Habas et al., 2001; Liu et al., 2008), whereas mutants missing only the GBD domain (CA-Daam1) are constitutively active even in the absence of Wnt signaling. The ability of activated Daam1 (and CA-Daam1) to elicit specific cytoskeletal changes in cultured cells such as polarized stress fiber formation has been well documented. Briefly, CA-Daam1 enhances, whereas N-Daam1 disrupts, the formation of actin stress fibers. We employed similar in vitro analysis to validate the utility our CA-Daam2 and N-Daam2 mutant constructs for the functional dissection of Daam2 during gut morphogenesis (Fig. S4 and Methods).
FIG. 4. Daam2 activation is required for mesenchymal condensation in the DM.
A Wnt signal-dependent binding of Dishevelled to DAD activates Daam. B In ovo electroporations: N-Daam2 is targeted to the left, CA-Daam2 to the right (See also Fig. S4 for construct validation). C–H CA-Daam2 targeting of the right DM: C WT DM with left-specific condensation (yellow line) with asymmetric F-actin (red in F, Phalloidin); D CA-Daam2 induces condensation (green line, GFP coelectroporated in E) and (G) increased F-actin (GFP coelectroporated in H). I Right-sided Daam2 electroporation in the DM has no effect on condensation, marked by GFP in J. K–O’ N-Daam2 targeting of the left DM. K Loss of condensation (green boundary from L). White arrows in L show N-Daam2-electroporated cells separating from the left. M Control (pCAG-GFP) highlights tightly compacted cells compared to dispersed cells expressing N-Daam2 (N), which produce filopodia-like extensions (white arrows) and (O) exhibit decreased F-actin (red, Phalloidin). P Calculated mesenchymal cell densities of WT and electroporated tissue sections (mean ± SEM, all p-values <0.0155). Scale bars: C–H (50 µm); I–J (100 µm); K–L (50 µm); M–O’ (15µm). *, p<0.05. See also Figure S4–7.
To learn whether Daam2 activation is required for mesenchymal condensation, we initially introduced CA-Daam2 into the right DM (Fig. 4B). This induced robust aggregation of mesenchymal cells (Fig. 4DEP; n=5; p<0.006) similar to that normally seen on the left side (Fig. 4CP), while non-electroporated right-sided cells remained dispersed (Fig. 4DE). Furthermore, compared to non-electroporated cells and consistent with the role of formins in regulating the actin cytoskeleton (Habas et al., 2001; Kobielak et al., 2004; Liu et al., 2008) CA-Daam2-positive cells exhibited increased F-actin staining (Fig. 4F–H), an effect also observed in vitro (not shown). Electroporation of WT Daam2 into the right side had no effect on cell behavior in the DM (Fig. 4IJ; n=7), in agreement with the autoinhibition of Daam proteins in the absence of Wnt, further arguing that the right DM is a non-permissive environment for Wnt signaling. To confirm the requirement for Daam2 during condensation, we introduced N-Daam2 to the left DM (Fig. 4B). This disrupted condensation (Fig. 4KLP; n=3; p<0.05) and was accompanied by reduced levels of F-actin (Fig. 4OO’; n=6). In contrast to extensive cell-cell compaction and a well-defined L-R boundary in WT (Fig. 4C) or control GFP-electroporated left mesenchyme (Fig. 4M), cells expressing N-Daam2 remained dispersed (Fig. 4N vs. Fig. 4M) and were often found separating from the left compartment (Fig. 4L, arrows). Moreover, long, filopodia extensions were present on Daam2-inhibited cells, mimicking the normal behavior of right-sided mesenchyme (Fig. 4N, arrows), while co-electroporation of the right DM with both N-Daam2 and Pitx2 interferes with the cellular effects of misexpressed Pitx2 alone in the right DM suggesting that epistatically, Daam2 is functionally downstream of Pitx2 (Fig. S7). Our experiments suggest the ability of N-Daam2 electroporated mesenchymal cells to maintain cell-cell contact was severely compromised. We also noted that N-Daam2 electroporated epithelial cells lost their columnar organization (Fig. S5). This is consistent with the previously characterized role of the actin cytoskeleton in the DM epithelial compartment (Davis et al., 2008; Plageman et al., 2011).
To confirm that the effects of N-Daam2 electroporation are a direct consequence of perturbed Daam2 function, we performed Daam2 knockdown experiments with the use of a previously published RCAS-shRNA system (Deneen et al., 2006). The effect of knockdown of endogenous Daam2 was verified using ISH (Fig. S6A–C’). Electroporating a scrambled shRNA had no effect on expression of endogenous Daam2 (Fig. S6BB’ vs. S6A) and did not alter condensation in the DM (Fig. S6EE’I vs. S6DD’I; n=3). In contrast, electroporating Daam2-shRNA perturbed mesenchymal condensation, consistent with our N-Daam2 results (Fig. S6FF’I vs. S6EE’I, n=6; p< 0.005). Hence, N-Daam2 interferes with the cellular effects of Pitx2 supporting that Daam2 is a key mediator of Pitx2 during mesenchymal condensation.
Daam2 affects mesenchymal condensation by lengthening cadherin-based junctions
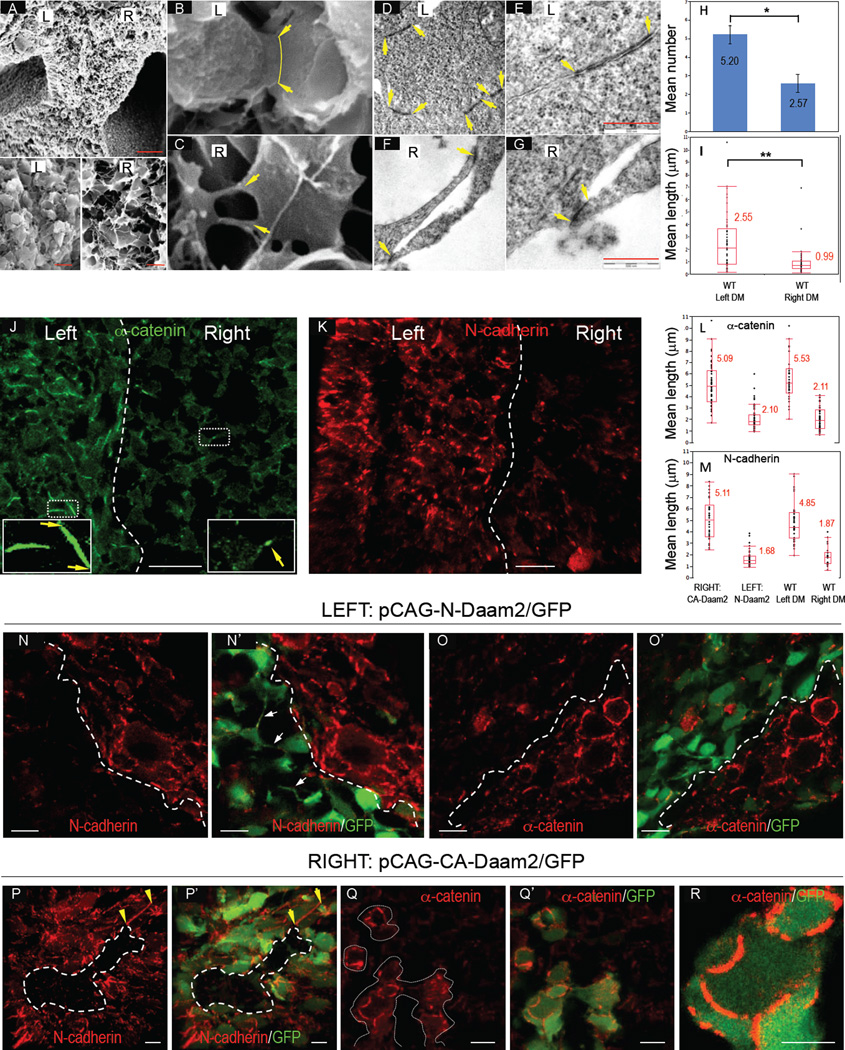
Asymmetric cell behaviors in the DM depend in part on the cell adhesion molecule N-cadherin (Kurpios et al., 2008; Plageman et al., 2011). We therefore asked whether differences exist in the organization of cell-cell junctions in the L-R mesenchymal compartments, and whether such differences are dependent on Daam2. Scanning and transmission electron microscopy (EM) highlighted that cell-cell junctions differed markedly on the two sides of the DM (Fig. 5A–C). We observed extensive membrane contacts between adjacent mesenchymal cells on the left (Fig. 5B, yellow arrowhead), while junctions between cells on the right consisted of thin filopodia extensions (Fig. 5C). We used transmission EM to count and measure the length of cell-cell contacts in the DM (Fig. 5D–G). We found an increased number of contacts per cell on the left vs. right side (Fig. 5DFH; 5.2 vs. 2.6 cell contacts; n=5 left and n=7 right; p<0.05). Moreover, there was a significant increase in the length of the left-sided contacts compared to the right (Fig. 5EGI; 2.55 vs. 0.99 μm, n=49 left and n=35 right; p<0.01).
FIG. 5. Daam2 lengthens cell-cell junctions.
A–G Scanning (A–C) and transmission (DG) EM show differences in left (ABDE) vs. right (ACFG) mesenchymal cell junction morphologies (yellow arrowheads). HI Cell-cell contacts on the left are increased in number (H, mean ± SEM) and length (I) vs. the right (p<0.05 & <0.01, respectively). (JK) Differences in cell junction organization highlighted by α-catenin (green, J) and N-cadherin staining (red, K, high magnifications inset); L-R boundary indicated (dotted line). LM Morphometric analysis of mean cell junction lengths in WT and electroporated tissues stained with α-catenin (top) and N-cadherin (bottom). Right-sided CA-Daam2 promotes lengthened junctions (p<0.0001) not statistically different from WT left cells (p>0.17), while left-sided N-Daam2 reduces junction length (p<0.0001) to resemble right-sided WT cells (p>0.59). N–O’ N-Daam2 (left) impairs adhesion and accumulation of both N-cadherin (red, NN’, note filopodia-like extensions, white arrows in N’) and α-catenin (red, OO’) to cell-cell contacts (GFP-marked electroporation boundary depicted). P–R CA-Daam2 (right) induces accumulation of both N-cadherin (red, PP’, yellow arrowheads) and α-catenin (red, Q–R). Scale bars: A (30µm top and 5µm bottom panels); EG (500nm); JK (10µm); NO’ (10µm); PP’ (5µm); QQ’ (10µm); R (5µm). Boxplots represent quartiles and median of the data, whiskers indicate extreme values. *, p<0.05, **, p<0.001.
α-Catenin is an adherens junction (AJ) protein that binds to β-catenin and is necessary for AJ formation and maintenance (Vasioukhin et al., 2000). Localization of α-catenin (Fig. 5J) and N-cadherin (Fig. 5K) in the DM highlights differences in the length of junctions across the L-R axis. We measured the length of junctions on the left vs. the right DM using fluorescently labeled cell junction complexes (Fig. 5L, α-catenin; Fig. 5M, N-cadherin). Consistent with EM data, the length of contacts on the left was ~2.6 (2.62, 2.59 vs. 2.57 [EM]) fold higher by comparison to the right (Fig. 5LM; n=11; p< 0.0001).
To determine whether junctions are dependent on Daam2, we introduced N-Daam2 to the left DM and followed with morphometric analyses as described above (Fig. 5LM). This significantly perturbed intercellular adhesion and resulted in a failure to accumulate both N-cadherin (Fig. 5NN’) and α-catenin (Fig. 5OO’L) to points of cell-cell contact. Moreover, the length of N-cadherin- or α-catenin-labeled cell junctions was significantly reduced in cells electroporated with N-Daam2 by comparison to non-electroporated cells (Fig. 5LM, n=6, p< 0.0001, N-cadherin; p< 0.0001, α-catenin). Long, filopodial cell-cell contacts were present between Daam2-inhibited left mesenchymal cells, mimicking the normal appearance of right-sided mesenchyme (Fig. 5N’, white arrows). In contrast, electroporation of CA-Daam2 into the right DM resulted in accumulation of N-cadherin (Fig. 5PP’) and α-catenin (Fig. 5QR) at cell-cell contacts and significantly increased the length of junctions (Fig. 5LM, n=5, p<0.0001, N-cadherin; p<0.0001, α-catenin). We conclude that Daam2 directs the formation and size of cadherin-based junctions during mesenchymal condensation.
Daam2 physically interacts with α-catenin and N-cadherin
Formin-1 (Fmn1), the founding member of the formin superfamily, is a critical binding partner for α-catenin in the skin, linking actin cytoskeleton to AJs (Kobielak et al., 2004). The modulation of junctions containing α-catenin downstream of Daam2 activation prompted us to determine whether Daam2 protein is physically associated with these junctions and to explore whether, like Fmn1, Daam2 and α-catenin physically interact. We turned first to the localization of Daam2 and α-catenin in mammalian cells, and show that Flag-Daam2 is found both in the cytoplasm and at cell borders (Fig. 6A), and co-localizes with staining for endogenous α-catenin (Fig. 6A, rectangles). To test whether Daam2 and α-catenin physically interact, we transfected 293T cells with either Daam2, Fmn1, or with CA-Daam2 (all flag-tagged). As expected, Fmn1 immunoprecipitated in a complex with antibodies against α-catenin (Fig. 6B). Importantly, both Daam2 and CA-Daam2 co-immunoprecipitated with endogenousα-catenin (Fig. 6B). Furthermore, endogenous Daam2 co-immunoprecipitated with endogenous α-catenin and N-cadherin in untransfected 293T cells (Fig. 6C). Together, these data reveal that Daam2 is a binding partner of α-catenin and N-cadherin, and suggest that the Daam2/α-catenin interaction is cadherin-complex dependent and likely to occur at the cell membrane.
FIG. 6. Daam2 physically interacts with junctional proteins.
A Flag-Daam2 (red) and endogenous α-catenin (green) co-localize at the cell surface in HeLa cells. Dotted rectangles (1 and 2) are magnified. B Overexpressed WT- and CA-Daam2, and positive control Fmn1 bind endogenous α-catenin. C Reciprocal IP experiments show Daam2 interacts with both α-catenin and N-cadherin (endognous, untransfected lysates). Scale bars: A (10µm).
Wnt5a−/− embryos fail to initiate the leftward tilt of the midgut
Wnt5a is required for ventral closure of the primitive gut and for subsequent midgut elongation, independent of canonical Wnt/β-catenin (Cervantes et al., 2009; Yamaguchi et al., 1999). To determine if Wnt5a is also required in the DM we examined this structure in Wnt5a−/− mutants (Fig. 7A–E’). While the DM formed in Wnt5a−/− embryos, its ventral outgrowth was arrested (Fig. 7FG, n=6/6, dotted rectangles). Importantly, the gut tube of Wnt5a−/− mutants also failed to initiate the asymmetric leftward tilt (Fig. 7F–I, n=6/6, single arrow). These defects were not accompanied by changes in cell proliferation or cell death in the DM, or Pitx2 expression (Fig. S8; Fig. S1C).
FIG. 7. Wnt5a−/− embryos fail to initiate the leftward tilt of the midgut.
A Wnt5a−/− and WT embryos (E10.5, arrow marks DM). BC Higher magnification of A highlights arrested DM development in Wnt5a−/− embryo (C, dotted lines). D–E’ Dissection of embryos from A highlights DM (orange) and midgut (yellow). FH Normal leftward tilt (arrow) and loss of tilt in Wnt5a−/− embryo (GI). Dotted rectangles highlight arrested ventral outgrowth of the DM at the level of the midgut (F, WT; G, Wnt5a−/−) and defective morphology of the DM (H, WT; I, Wnt5a−/−) posterior to the midgut. J–M Chicken DM with N-Daam2 on the left (JL, GFP, electroporated). KM Coelomic cavity defects (black arrows) in N-Daam2 electroporated chick embryos are reminiscent of Wnt5a−/− embryos (I, black arrows). N Model for the role of Wnt5a in midgut loop formation: Ventral closure of the gut occurs at the initiation of midgut elongation and results in formation of the vitelline duct (vd). Delayed closure and arrested DM outgrowth in Wnt5a−/− forces the elongating midgut to branch rather than loop. Scale bars: B–E’ (500 µm); F (100 µm); GH (50 µm); I (100 µm); J–M (50 µm). See also Figure S5 and S1.
In WT mice, elongation of the midgut to form the primary ventral loop is preceded by ventral closure and occurs concurrently with outgrowth of the DM (Fig. 7N, WT, curved arrows). Our data provide further insight into the etiology of intestinal defects in Wnt5a−/− mutants, suggesting that arrested DM outgrowth (Fig. 7N, Wnt5a−/−, straight arrows) imposes a restriction that, accompanied by delayed ventral closure of the midgut, forces the elongating gut tube to form a branch rather than a midgut loop as previously reported (Cervantes et al., 2009) (Fig. 7N). This agrees with the described physical restraint placed on the lengthening gut tube by the DM, a process required for the proper topology of intestinal loops during later stages (Savin et al., 2011).
Importantly, in some Wnt5a-null embryos there was defective remodeling of the lining of the coelomic cavity involving aberrant foldings and adhesions of serosal epithelial layers (Fig. 7I, arrowheads), a phenotype also noted in the left DM of some chick embryos electroporated with N-Daam2 (Fig. 7J–M, arrows). The results of these experiments demonstrate a requirement for Wnt5a to initiate asymmetric gut morphogenesis in mice and suggest that Wnt5a in the gut is the noncanonical signal required to activate Daam2 in the DM (Fig. 8).
FIG. 8. Model for L-R gut rotation.
Transcriptional regulation of Wnt pathway genes by Pitx2 leads to Daam2 activation. Daam2 mediates adhesion at cell junctions by binding α-catenin and N-cadherin. Subsequent actin remodeling and lengthening of junctions cause left condensation. Wnt5a produced in the adjacent gut orients condensation relative to the D-V axis. Antagonized Wnt signaling causes right mesenchymal cells to remain dispersed.
DISCUSSION
The goal of this research has been to define the transcriptional targets and cellular mechanisms through which Pitx2 manifests asymmetric gut morphogenesis in higher vertebrates. Although Pitx2 has been thoroughly studied in development, its downstream targets are unknown. Leveraging the binary L-R organization of the DM we performed laser microdisection of the left and right to identify Fzd4, Gpc3 and Daam2 as downstream Pitx2 targets. We show that positive regulation of these genes by Pitx2 acts to potentiate asymmetric Wnt signaling via Daam2 activation, inducing polarized condensation in the left DM necessary to initiate gut rotation. Conversely, these effects are antagonized within the right DM by the presence of the Wnt inhibitors Sfrp1 and Sfrp2. This work provides new insight into the molecular effectors of gut morphogenesis and uncovers an important link between the earliest L-R signals initiated during gastrulation, and Daam2 activation downstream of Wnt signaling, as the ultimate driving force through which Pitx2 initiates asymmetric development (Fig. 8).
The role of noncanonical Wnt signaling in L-R gut morphogenesis
The mammalian Wnt signaling family consists of 19 ligands and 10 receptors (van Amerongen and Nusse, 2009). The ability of specific Wnt-Frizzled complexes to activate canonical vs. noncanonical signaling is inherently complex and depends heavily on cellular context including the composition of co-receptors at the cell surface, the presence of extracellular antagonists, and the intracellular expression of various signaling intermediates (van Amerongen and Nusse, 2009). Components at almost every level of the Wnt pathway have been shown to affect both β-catenin-dependent and -independent responses. Ultimately, signal integration determines the appropriate cell behavior in response. In the midgut DM, we show left-sided expression of Fzd4/8, Gpc3, and Daam2 (Wnt-permissive) with contrasting expression of Wnt antagonists Sfrp1 and Sfrp2 on the right. Not surprisingly, all of these genes have been linked to canonical and noncanonical Wnt signaling (Capurro et al., 2005a; Chan et al., 2006; Song et al., 2005; Veeman et al., 2003b; Wawrzak et al., 2007; Ye et al., 2011). The noncanonical pathway primarily affects cellular behavior such as shape, adhesion and polarity, whereas the canonical Wnt pathway influences cell fate decisions and changes in cell proliferation and differentiation. We rule out canonical signaling in the DM by reporting an absence of any asymmetry in conventional metrics of β-catenin-dependent signaling: cell number, proliferation or cell death within the DM are equal, while several widely used canonical Wnt reporters show no activity in the DM. Instead, we show that the leftward tilt is initiated by Daam2-dependent regulation of the actin cytoskeleton and cadherin-based cell adhesion leading directly to changes in cell behavior that manifest as greater or lesser cell packing on the left or right sides, respectively. Hence, the left-specific expression of Fzd4/8, Gpc3 and Daam2 likely functions via noncanonical Wnt signaling to establish asymmetric cell behaviors underlying the leftward tilt.
Our evidence that noncanonical Wnt signaling is responsible for the L-R asymmetries includes the demonstration that Daam2-positive mesenchymal cells are oriented to the left across the L-R embryonic axis. In contrast, Daam2-negative cells of the right show no specific orientation. Tissue polarity is a key cellular feature of noncanonical Wnt/PCP signaling in both vertebrates and invertebrates (Gray et al., 2011). Whereas in Drosophila much has been learned of the PCP pathway (Vladar et al., 2009), in vertebrates the definition of what constitutes PCP signaling, particularly in mesenchymal cells, is not entirely clear (Wallingford, 2012). Interestingly, our work revealed that one of the components of the PCP pathway, Prickle-1, is asymmetrically expressed in a R-L gradient in the DM. In Drosophila wing disc, Prickle (Pk) is asymmetrically localized across the intercellular proximal-distal interface to antagonize Fzd receptor activity and to generate asymmetry of Fzd-Dishevelled activity across each proximal-distal cell boundary during PCP signaling (Tree et al., 2002). In zebrafish, the asymmetric localization of Pk and Dishevelled fusion proteins to the anterior and posterior cell edges (Yin et al., 2008) parallels localization of these proteins in Drosophila. At present, the subcellular localization of Prickle-1, Dishevelled or other core PCP proteins in the DM has not been explored and the mechanistic function of this restricted right-sided expression remains unclear. It is attractive to speculate that in the DM, Prickle-1 may function either upstream or in parallel to Daam2 to establish the distinct mesenchymal cell orientation during the leftward tilt of the midgut.
Coordinating cell behaviors in the DM with embryonic polarity
Expression of Wnt5a in the adjacent gut mesenchyme raises the possibility that Wnt5a diffusion may form a gradient, coordinating the D-V outgrowth of the DM with the establishment of L-R asymmetry. Indeed, the L-R changes in the DM are oriented relative to the orthogonal D-V axis such that the left side shortens while the right side lengthens. Among the genes whose expression we have examined in situ, Fzd8, Gpc3, and Islet1 (Davis et al., 2008), show a D-V bias, i.e. closer to the source of Wnt5a. A likely candidate responsible for Wnt5a extracellular distribution and gradient formation is Gpc3. Glypicans modulate Wnt signaling and this is highly dependent on complex post-translational modifications of the core Gpc protein (Yan and Lin, 2009). We have recently identified the left-specific, ventrally restricted expression of enzymes involved in biosynthesis and posttranslational modification of Gpc3 at the time when the DM deforms (unpublished), raising the possibility that functional editing of Gpc3 serves to concentrate diffusible Wnt5a. Indeed, in vitro studies have shown that Gpc3 binds Wnt5a with high affinity and enhances Wnt5a-dependent Jnk activation in mesothelioma cells and in mice (Song et al., 2005). In zebrafish, Gpc4 ortholog knypek is part of the Wnt/PCP pathway required to establish polarized cell behaviors underlying convergent extension (Topczewski et al., 2001). Interestingly, Drosophila homologs of Gpc3/4, Dally and Dally-like, respectively, play an important role in organizing the cellular distribution of extracellular Wnt/Wingless (Baeg et al., 2001). Future work will be necessary to establish the relationship between mammalian glypicans, their subcellular localization, and the noncanonical Wnt/PCP pathway to understand how glypicans can impact global tissue morphogenesis in higher vertebrates.
Wnt5a itself is required for midgut morphogenesis (Cervantes et al., 2009; Yamaguchi et al., 1999). We show that DM morphogenesis and initiation of asymmetric gut rotation is also disrupted in Wnt5a−/− mutants, arguing that Wnt5a links the morphogenetic program of these two adjacent tissues. These defects occur precisely in the region that forms a branch instead of a midgut loop in Wnt5a−/− embryos (Cervantes et al., 2009), suggesting that disrupted ventral outgrowth of the DM contributes to the formation of the aberrant branch (Fig. 6N). This combination of orthogonal D-V and L-R morphogenic defects in Wnt5a−/− embryos supports our model where Wnt5a in the gut mesenchyme provides a directional cue linking the D-V axis with L-R patterning in order to coordinate morphogenesis of the DM with developing midgut. Conserved looping morphogenesis depends on physical interaction between the growing gut tube and DM (Savin et al., 2011) underscoring the importance of coordinated growth of these adjacent tissues.
Examples of noncanonical Wnt/PCP-mediated molecular crosstalk between embryonic axes are few. Most recently, PCP signaling has been shown to position basal bodies of cilia at the posterior margin of node cells and Kupffer’s vesicle, providing the posterior tilt and thus leftward fluid flow it generates to bathe the left lateral plate with the Nodal ligand (Hashimoto and Hamada, 2010). This provided a partial answer to how anterior-posterior information is translated into L-R organ polarity. However, how established L-R patterning information is further translated into asymmetries of cell behavior and coordinated with organ polarity has remained elusive. Here we begin to answer this question, showing that integration of noncanonical Wnt signaling with embryonic polarity may be a general mechanism through which Pitx2 sculpts the growth of asymmetric organs, a mechanism warranting further investigation in other L-R asymmetric viscera.
Role of the formin Daam2 in the DM
Our data show that cadherin-based junctions involving α-catenin and N-cadherin are primary candidates responsible for the asymmetric condensation in the DM. Cadherin-based junctions are dynamic, and their stability arises from the coordinated assembly, remodeling, and recycling of junctional proteins that interact with the actin cytoskeleton. For example Dia1 (Diaphanous), the founding member of the subfamily of formins to which Daam2 belongs, is required for both the stability and length of AJs at points of cell-cell contact (Carramusa et al., 2007). Moreover, Formin-1 is recruited to nascent AJs via interaction with α-catenin, and this recruitment is required for intercellular adhesion in the skin (Kobielak et al., 2004). We show that Daam2 similarly interacts with both α-catenin and N-cadherin, while Daam2 activity is required for cell-cell adhesion, organizing N-cadherin and α-catenin at cell junctions. These data raise the possibility that individual formin proteins, as nucleators of filamentous actin, may function broadly during development as tissue-specific modulators of cell adhesion via their interaction with junctional complex proteins.
Daams have also been shown to regulate small GTPases such as RhoA to influence cytoskeletal architecture (Habas et al., 2001). However, debate continues as to whether Daams act upstream, downstream, or in parallel to small GTPases (Aspenstrom et al., 2006; Higashi et al., 2008). While mutation of mouse Daam1 results in neonatal lethality (Li et al., 2011), the disruption of cytoskeletal architecture due to Daam1 mutation is independent of RhoA, Rac1, or Cdc42. Instead, Daam1 organizes N-cadherin-dependent adhesion of cardiomyocytes during heart morphogenesis (Li et al., 2011). Interestingly, Daam2 was recently shown to mediate formation of Dvl3/Axin signaling complexes required for patterning the dorsal neural tube downstream of canonical Wnt signaling (Lee and Deneen, 2012), highlighting a diverse Daam signaling repertoire during vertebrate development that depends heavily on cellular context. Thus the specific contribution of these related formins to L-R laterality in other organ systems is of great interest.
Midgut rotation and clinical implications
Midgut malrotation is a birth defect of unknown genetic origin predisposing affected babies to catastrophic volvulus (Lampl et al., 2009). Understanding how L-R asymmetry is initiated and executed during gut rotation may shed light onto the origin of midgut malrotation and other classes of human gut disorders. Our work has shown that mutations in two genes associated with gut malrotation in humans are differentially expressed in the DM: transcription factor PITX2, which causes Rieger syndrome (Lu et al., 1999), and GPC3, which causes Simpson-Golabi-Behmel syndrome (Pilia et al., 1996). Both disorders are pleiotropic and difficult to study in humans. Thus the ability to isolate and perturb these genes and their downstream targets in vivo is a singular advantage of the DM, enabling us to link genetic cause with anatomic outcome. Moreover, lessons learned from these experiments will impact the study of other regions of the gut, and other tubular organs, some of which share strikingly similar features of morphogenesis and genetic patterning with the vertebrate midgut.
EXPERIMENTAL PROCEDURES
Animals
Mouse embryos, with the morning of the plug defined as E0.5, were collected from Pitx2hd allele (Lu et al., 1999), Wnt5a mutant B6:129S7-Wnt5atm1Amc/J (Yamaguchi et al., 1999), and Daam2−/− (Daam2tm1a(KOMP)Wtsi ). BAT-GAL reporter mice were generously provided by Dr. Courtney Griffin (Griffin et al., 2011). See Supplemental Material for more information. Fertile White Leghorn eggs from the Cornell Poultry Research Farm were incubated at 38°C and staged (Hamburger and Hamilton, 1992).
In Ovo Electroporation
Plasmid DNA (0.6–3.0µg/µl) was microinjected into the left or right coelomic cavity of HH14 chicken embryos (Kurpios et al., 2008), with coelectroporated pCAG-GFP or mCherry to visualize targeted cells. Platinum electrodes delivered 3 sequential 10 ms pulses of 50V delivered from a BTX electroporator. RNAi was performed using described reagents (Deneen et al., 2006; Lee and Deneen, 2012). The effect of knockdown using RCAS-Daam2-shRNA electroporation was verified using ISH compared to a mutant shRNA control with 5bp substitutions.
RNA in Situ Hybridization
250µm thick embryo slices for whole mount ISH was performed as described (Brent et al., 2003). Section ISH was performed as described (http://geisha.arizona.edu/geisha/protocols.jsp) using chromogenic detection.
Electron Microscopy
EM specimens were dissected, fixed ON with either 4% PFA and 2.5% glutaraldehyde in 0.1M Sodium Cacodylate (SC) buffer, pH 7.4 (TEM) or with 2.0% glutaraldehyde in 0.05M SC buffer, pH7.4 (SEM). Several washes with 0.1M SC were followed by 1.5% Osmium Tetroxide (OsO4) staining. SEM samples were EtOH-dehydrated, subjected to critical point drying with CO2, mounted on stubs and sputter coated with gold/palladium. Following OsO4 staining, TEM samples were stained with 2% Uranyl Acetate, embedded in LX 112 resin (Ladd Research), and ultrathin sections were collected. Electron micrographs were acquired with a Leica 440 scanning EM or a FEI T12 Spirit transmission EM (Cornell).
Cell Behavior Quantification
Transmission electron micrographs in which the cell body and nucleus were completely in frame were used to estimate the mean number and length of cell-cell contacts within each compartment. Measurements (Imaris) were normalized relative to the scale bar. Cell behavior was quantified from 15μm sections of WT and electroporated embryos stained with DAPI and antibodies to α-catenin or N-cadherin. Fluorescently labeled cell junctions were measured (ImageJ) for electroporated (GFP) and non-electroporated cell contacts (control). Condensation was quantified (ImageJ) as nuclei per 100μm2 within WT or electroporated tissue. Cell polarity was scored from z-stacks of 20µm paraffin sections stained with DAPI and anti-GM130 (golgi). Vectors were drawn (Imaris) from the center of mass of the nucleus (point A) to that of the golgi (point B). XY-plane vector coordinates were transformed into unit vectors, combined, and plotted on an angle histogram with point A at the center. Vectors were grouped in 30° “bins” based on angle of orientation across the L-R axis (L, 225–315°; R, 45–135°).
Statistical Analysis
Student’s t-test was used to compare means; error bars are standard error of the mean.
Cloning, Plasmids, and Oligonucleotides
cDNAs and probes for RNA ISH were TA-cloned using oligo-dT primed cDNA reverse transcription from pooled RNA of chicken (HH19 and 21) or mouse (E8.5–18.5). Cloned DNA was sequence-verified. Chicken EST clones (ChESTs) were generously provided by Amitabha Bandyopadhyay and Jonaki Sen; pCMV-Fmn1(IV)-Flag by Elaine Fuchs (Kobielak et al., 2004), pTOP-nRFP, RCAS-Daam2-shRNA, RCAS-RNA-mut-shRNA by Benjamin Deneen (Lee & Deneen, 2012), and pBS-BARvs by Randall Moon (Biechele and Moon, 2008). See Supplemental Material for more information.
Transfections, IP, and Blotting
Cells were cultured in DMEM containing 10% fetal bovine serum, under humidified conditions in 5% CO2 at 37°C. 293FT or HeLa cells were transfected with Lipofectamine 2000 (Invitrogen). Cells were washed twice with PBS and lysates prepared in the presence of protease inhibitors (Roche). For IP, 1.0mg of lysate was cleared with protein G sepharose (GE) for 1 hour at 4°C then incubated ON with protein G sepharose and either 8.0µg of α-catenin (Thermo MA1–2000), 7.0 µg of N-cadherin (BD Sciences, 610921), or 7.0µg of Daam2 antibodies (LSBio LS-C100232). Primary antibodies for immunoblotting: Flag (1:15,000), α-catenin (1:1000), N-cadherin (1:4000), or Daam2 antibodies (1:5000). Signal was detected with a 1:15,000 dilution of anti-mouse (GE Healthcare) or anti-rabbit IgG HRP (Invitrogen) and ECL Select reagent (GE).
Immunofluorescence
Frozen sections or cultured cells were permeabilized with 0.1% Triton X-100/PBS, blocked in 3% BSA/PBS, and incubated with antibodies (primary at 4°C ON, secondary for 1 hour at RT) using standard protocols. Primary antibodies used: α-catenin (Sigma, C2081), N-cadherin (DSHB, clone 6b3 or BD Biosciences, 610921), GFP (Abcam, ab290), Flag (Agilent, 200472), phospho-Histone-H3 (Abcam, ab5176), GM-130 (BD, 610822) and cleaved-Caspase3 (Millipore, AB3623). Samples were incubated with mouse or rabbit Alexa Fluor 488 or Alexa Fluor 594 secondary antibodies, counterstained with Phalloidin and DAPI and mounted with Prolong Gold antifade (all Invitrogen). Confocal images were acquired using identical exposures within the same staining experiment. Confocal z-stacks were acquired with a Zeiss LSM 710. Imaris (Bitplane) was used for images and z-stack analyses. Bright field micrographs were acquired with a Zeiss Observer.Z1 and AxioCam HRc camera.
Supplementary Material
HIGHLIGHTS.
Daam2 is a cellular target of Pitx2 required for L-R asymmetric gut morphogenesis
Pitx2 targets Frizzled, Daam2 and Gpc3 mediate noncanonical Wnt signaling
Daam2 binds α-catenin and N-Cadherin to drive adhesion in the gut dorsal mesentery
Pitx2 and noncanonical Wnt signaling provide a mechanism for L-R gut morphogenesis
ACKNOWLEDGEMENTS
We thank Drs. Drew Noden and Randall Moon for reading the manuscript and helpful suggestions. We thank Dr. Richard Maas for access to Laser Microdissection; Drs. Habas, Fuchs, Griffin, Bandyopadhyay, Sen and Deneen for reagents described above; and Megan Carpenter and Kate Lew for technical assistance. This work was supported by Cornell Comparative Cancer Biology Training Program, I.C.W.; March of Dimes, N.A.K. (1-FY11-520); NSF CU-ADVANCE, N.A.K. (0547373); NIH, N.A.K. (R01 DK092776), and NIH institutional support for I.C.W. (HD057854). Daam2tm1a(KOMP)Wtsi targeted ES cells were funded by NIH grants to Velocigene (U01HG004085) and the CSD Consortium (U01HG004080).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aspenstrom P, Richnau N, Johansson AS. The diaphanous-related formin DAAM1 collaborates with the Rho GTPases RhoA and Cdc42, CIP4 and Src in regulating cell morphogenesis and actin dynamics. Exp Cell Res. 2006;312:2180–2194. doi: 10.1016/j.yexcr.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Barolo S. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene. 2006;25:7505–7511. doi: 10.1038/sj.onc.1210057. [DOI] [PubMed] [Google Scholar]
- Biechele TL, Adams AM, Moon RT. Transcription-based reporters of Wnt/beta-catenin signaling. Cold Spring Harbor protocols. 2009;2009 doi: 10.1101/pdb.prot5223. pdb prot5223. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S, et al. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol. 1999;146:255–264. doi: 10.1083/jcb.146.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro MI, Shi W, Sandal S, Filmus J. Processing by convertases is not required for glypican-3-induced stimulation of hepatocellular carcinoma growth. J Biol Chem. 2005a;280:41201–41206. doi: 10.1074/jbc.M507004200. [DOI] [PubMed] [Google Scholar]
- Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005b;65:6245–6254. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD. Mammalian diaphanous-related formin Dia1 controls the organization of Ecadherin-mediated cell-cell junctions. J Cell Sci. 2007;120:3870–3882. doi: 10.1242/jcs.014365. [DOI] [PubMed] [Google Scholar]
- Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- Cervantes S, Yamaguchi TP, Hebrok M. Wnt5a is essential for intestinal elongation in mice. Dev Biol. 2009;326:285–294. doi: 10.1016/j.ydbio.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DW, Chan CY, Yam JW, Ching YP, Ng IO. Prickle-1 negatively regulates Wnt/beta-catenin pathway by promoting Dishevelled ubiquitination/degradation in liver cancer. Gastroenterology. 2006;131:1218–1227. doi: 10.1053/j.gastro.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Davis NM, Kurpios NA, Sun X, Gros J, Martin JF, Tabin CJ. The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Dev Cell. 2008;15:134–145. doi: 10.1016/j.devcel.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cat B, Muyldermans SY, Coomans C, Degeest G, Vanderschueren B, Creemers J, Biemar F, Peers B, David G. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol. 2003;163:625–635. doi: 10.1083/jcb.200302152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron. 2006;52:953–968. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Golabi M, Rosen L. A new X-linked mental retardation-overgrowth syndrome. Am J Med Genet. 1984;17:345–358. doi: 10.1002/ajmg.1320170128. [DOI] [PubMed] [Google Scholar]
- Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin CT, Curtis CD, Davis RB, Muthukumar V, Magnuson T. The chromatin-remodeling enzyme BRG1 modulates vascular Wnt signaling at two levels. Proc Natl Acad Sci U S A. 2011;108:2282–2287. doi: 10.1073/pnas.1013751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hamada H. Translation of anterior-posterior polarity into left-right polarity in the mouse embryo. Curr Opin Genet Dev. 2010;20:433–437. doi: 10.1016/j.gde.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Hecksher-Sorensen J, Watson RP, Lettice LA, Serup P, Eley L, De Angelis C, Ahlgren U, Hill RE. The splanchnic mesodermal plate directs spleen and pancreatic laterality, and is regulated by Bapx1/Nkx3.2. Development. 2004;131:4665–4675. doi: 10.1242/dev.01364. [DOI] [PubMed] [Google Scholar]
- Higashi T, Ikeda T, Shirakawa R, Kondo H, Kawato M, Horiguchi M, Okuda T, Okawa K, Fukai S, Nureki O, et al. Biochemical characterization of the Rho GTPase-regulated actin assembly by diaphanous-related formins, mDia1 and Daam1, in platelets. J Biol Chem. 2008;283:8746–8755. doi: 10.1074/jbc.M707839200. [DOI] [PubMed] [Google Scholar]
- Khadka DK, Liu W, Habas R. Non-redundant roles for Profilin2 and Profilin1 during vertebrate gastrulation. Dev Biol. 2009;332:396–406. doi: 10.1016/j.ydbio.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E. Mammalian forming-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpios NA, Ibanes M, Davis NM, Lui W, Katz T, Martin JF, Izpisua Belmonte JC, Tabin CJ. The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc Natl Acad Sci U S A. 2008;105:8499–8506. doi: 10.1073/pnas.0803578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl B, Levin TL, Berdon WE, Cowles RA. Malrotation and midgut volvulus: a historical review and current controversies in diagnosis and management. Pediatr Radiol. 2009;39:359–366. doi: 10.1007/s00247-009-1168-y. [DOI] [PubMed] [Google Scholar]
- Lassiter RN, Dude CM, Reynolds SB, Winters NI, Baker CV, Stark MR. Canonical Wnt signaling is required for ophthalmic trigeminal placode cell fate determination and maintenance. Dev Biol. 2007;308:392–406. doi: 10.1016/j.ydbio.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Deneen B. Daam2 is required for dorsal patterning via modulation of canonical Wnt signaling in the developing spinal cord. Dev Cell. 2012;22:183–196. doi: 10.1016/j.devcel.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Johnson RL, Stern CD, Kuehn M, Tabin C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell. 1995;82:803–814. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- Li D, Hallett MA, Zhu W, Rubart M, Liu Y, Yang Z, Chen H, Haneline LS, Chan RJ, Schwartz RJ, et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development. 2011;138:303–315. doi: 10.1242/dev.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, Habas R. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A. 2008;105:210–215. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Matsuyama M, Aizawa S, Shimono A. Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS Genet. 2009;5:e1000427. doi: 10.1371/journal.pgen.1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Canny SG, Jang CW, Cho K, Ji H, Wagner DS, Jones EA, Habas R, McCrea PD. Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling. J Am Soc Nephrol. 2011;22:1654–1664. doi: 10.1681/ASN.2010101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Brown JD, Yang-Snyder JA, Miller JR. Structurally related receptors and antagonists compete for secreted Wnt ligands. Cell. 1997;88:725–728. doi: 10.1016/s0092-8674(00)81915-7. [DOI] [PubMed] [Google Scholar]
- Nakaya MA, Habas R, Biris K, Dunty WC, Jr, Kato Y, He X, Yamaguchi TP. Identification and comparative expression analyses of Daam genes in mouse and Xenopus. Gene Expr Patterns. 2004;5:97–105. doi: 10.1016/j.modgep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–1097. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet. 1996;12:241–247. doi: 10.1038/ng0396-241. [DOI] [PubMed] [Google Scholar]
- Plageman TF, Jr, Zacharias AL, Gage PJ, Lang RA. Shroom3 and a Pitx2-N-cadherin pathway function cooperatively to generate asymmetric cell shape changes during gut morphogenesis. Dev Biol. 2011;357:227–234. doi: 10.1016/j.ydbio.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB, Habas R. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development. 2006;133:4219–4231. doi: 10.1242/dev.02590. [DOI] [PubMed] [Google Scholar]
- Savin T, Kurpios NA, Shyer AE, Florescu P, Liang H, Mahadevan L, Tabin CJ. On the growth and form of the gut. Nature. 2011;476:57–62. doi: 10.1038/nature10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori H, Hamada H. The left-right axis in the mouse: from origin to morphology. Development. 2006;133:2095–2104. doi: 10.1242/dev.02384. [DOI] [PubMed] [Google Scholar]
- Song HH, Shi W, Xiang YY, Filmus J. The loss of glypican-3 induces alterations in Wnt signaling. J Biol Chem. 2005;280:2116–2125. doi: 10.1074/jbc.M410090200. [DOI] [PubMed] [Google Scholar]
- Stigliano I, Puricelli L, Filmus J, Sogayar MC, Bal de Kier Joffe E, Peters MG. Glypican-3 regulates migration, adhesion and actin cytoskeleton organization in mammary tumor cells through Wnt signaling modulation. Breast Cancer Res Treat. 2009;114:251–262. doi: 10.1007/s10549-008-0009-2. [DOI] [PubMed] [Google Scholar]
- Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003a;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Current biology : CB. 2003b;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell's compass. Cold Spring Harbor perspectives in biology. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annual review of cell and developmental biology. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- Wawrzak D, Metioui M, Willems E, Hendrickx M, de Genst E, Leyns L. Wnt3a binds to several sFRPs in the nanomolar range. Biochem Biophys Res Commun. 2007;357:1119–1123. doi: 10.1016/j.bbrc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Wolf V, Endo Y, Rubin JS. Purification and Wnt-inhibitory activities of secreted frizzled-related proteins. Methods Mol Biol. 2008;468:31–44. doi: 10.1007/978-1-59745-249-6_3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harbor perspectives in biology. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Rattner A, Nathans J. Genetic mosaic analysis reveals a major role for frizzled 4 and frizzled 8 in controlling ureteric growth in the developing kidney. Development. 2011;138:1161–1172. doi: 10.1242/dev.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol. 2008;180:221–232. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tian Y, Du J, Hu Z, Yang L, Liu J, Gu L. Dvl2-dependent activation of Daam1 and RhoA regulates Wnt5a-induced breast cancer cell migration. PLoS One. 2012;7:e37823. doi: 10.1371/journal.pone.0037823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.