Abstract
Background
Structural remodeling is associated with the fibroinflammatory process in the atrial extracellular matrix. In the present study we aimed to investigate whether serum levels of new circulating remodeling markers differ in patients with atrial fibrillation (AF) compared to patients with sinus rhythm.
Material/Methods
The study population included 52 patients diagnosed with non-valvular AF and 33 age-matched patients with sinus rhythm. Serum levels of Galectin-3, matrix metalloproteinase-9 (MMP-9), lipocalin-2 (Lcn2/NGAL), N-terminal propeptide of type III procollagen (PIIINP), Hs-Crp, and neutrophil-to-lymphocyte ratio (NLR) were measured. The left atrial volume (LAV) was calculated by echocardiographic method and LAV index was calculated.
Results
Galectin-3, MMP-9, and PIIINP levels were significantly higher in AF patients except NGAL levels (1166 pg/ml (1126–1204) and 1204 pg/ml (1166–1362) p=0.001, 104 (81–179) pg/ml and 404 (162–564) pg/ml p<0.0001, and 1101 (500–1960) pg/ml and 6710 (2370–9950) pg/ml p<0.0001, respectively). The NLR and Hs-CRP levels were also higher in AF (2.1±1.0 and 2.7±1.1 p=0.02 and 4.2±1.9 mg/L and 6.0±4.7 mg/L p=0.04, respectively). In correlation analyses, NLR showed a strongly significant correlation with LAVi, but Hs-CRP did not (p=0.007 r=0.247, Pearson test and p=0.808 r=0.025, Pearson test, respectively). Moreover, Galectin-3, MMP-9, and PIIINP had a strong positive correlation with LAVi (p=0.021 r=640, Spearman test and p=0.004 r=0.319 Pearson test, and p=0.004 r=0.325 Pearson test, respectively).
Conclusions
Novel fibrosis and inflammation markers in AF are correlated with atrial remodeling. Several unexplained mechanisms of atrial remodeling remain, but the present study has taken the first step in elucidating the mechanisms involving fibrosis and inflammation markers.
Keywords: Atrial Fibrillation, Atrial Remodeling, Collagen Type III, Galectin 3, Lipocalins, Matrix Metalloproteinase 9
Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia and increases the risk of stroke and death [1]. Inflammation is an important factor related to the initiation, maintenance, and recurrence of AF. This abnormal inflammation may cause a prothrombotic state, finally resulting in thromboembolism [2]. In persistent AF, atrial enlargement and structural and electrical remodeling form the basis of atrial changes. Structural remodeling is associated with an activated and gradually progressive fibrosis and inflammation process in the atrial extracellular matrix (ECM) [3–5]. Matrix metalloproteinases (MMPs) are major mediators of normal ECM remodeling [3–5, 7]. Moreover, studies have increased our understanding of the role of inflammation in AF by showing that C-reactive protein (CRP) [6–8] and the neutrophil-to-lymphocyte ratio (NLR) are associated with AF [9–11].
However, the exact mechanism of the pathogenesis and progression of AF remains to be elucidated. Various mediators may play a role in the pathogenesis. A growing body of evidence is showing that galectin-3 [12–14], lipocalin-2/neutrophil gelatinase-B-associated lipocalin (Lcn2/NGAL) [15–17] and N-terminal propeptide of type III procollagen (PIIINP) [18–20] seem to play important roles in the cardiovascular inflammation and fibrosis that result in cardiac remodeling.
In the present study, we aimed to investigate whether serum levels of galectin-3, Lcn2/NGAL, PIIINP, and NLR differ in patients with AF compared with patients with a sinus rhythm, with guidance of known markers such as serum MMP-9 and Hs-CRP levels. We also evaluated the associations of these markers with atrial structural remodeling, which was interpreted by measuring the left atrial volume index.
Material and Methods
Patient selection
The study population included 85 patients who were seen in our outpatient clinic between March 2012 and January 2013. Fifty-two patients diagnosed with non-valvular (mitral and aortic valve) persistent AF (AF duration longer than 1 month) were recruited into the AF group. End-stage hepatic or renal disease, malignancy, any prior blood transfusions, carotid artery disease, prior transient ischemic attack and ischemic or hemorrhagic stroke, oral anticoagulant usage, and NYHA functional class III or IV patient were exclusion criteria in our study. Thirty-three age-matched patients with sinus rhythm were recruited into the control group. A clinical history of risk factors such as diabetes mellitus, hypertension, hypercholesterolemia, and cardiovascular disease were recorded. For each patient, we calculated height, weight, body mass index (BMI), and body surface area (BSA). Based on the serum creatinine level on admission, estimated glomerular filtration rate (e-GFR) was calculated by using the Modification of Diet in Renal Disease (MDRD) formula. The ethical implications regarding the study were approved by the local ethics committee and informed consent was obtained from each patient.
Echocardiographic assessment
The LV ejection fraction (EF) was assessed by the modified biplane Simpson method. Cardiac dimensions were measured according to the recommendations of the American Society of Echocardiography by M-mode and 2-dimensional echo [21]. LV mass (LVM) was calculated from 2D echocardiographic measurements by using Devereux formula: LVM=1.04* [(LVsw + LVpw + LVEDD)3 – (LVEDD)3]–13.6 and was indexed to body surface area [22]. Two cardiologists, blinded to patient clinical history, performed the physical examinations, measured outcome variables, interpreted all echocardiograms, and verified LV
volumetric analyses. Left ventricular ejection fraction (LVEF) was calculated using the biplane method of discs (modified Simpson’s rule) in the apical 4- and 2-chamber views at end systole and end diastole. Measurements were obtained as the mean value from the apical 4- and 2-chamber views. The left atrial volume was calculated by using the biplane method of discs (modified Simpson’s rule) in the apical 4- and 2-chamber views at end diastole of the atria. Measurements were obtained as the mean value from the apical 4- and 2-chamber views. The LAVI was then calculated as LAV divided by body surface area [4,23].
Blood samples and biochemical measurements
Venous blood samples were drawn after an outpatient clinical examination. The samples were kept at room temperature for 15 min to permit coagulation and were then centrifuged at 1000× g for 15 min. The serum was separated with the aid of a pipette, transferred to Eppendorf tubes, and kept at −40°C until analysis.
The serum levels of galectin-3, MMP-9, Lcn2/NGAL, and PIIINP were measured using commercial enzyme-linked immunoassay kits, and each assay was carried out in duplicate. The galectin-3 level was determined using sandwich ELISA (Human Galectin-3 ELISA kit; eBioscience), NGAL levels (Human Lipocalin-2/NGAL ELISA kit; BioVendor Research and Diagnostic Products), MMP-9 levels (Human Matrix Metalloproteinase 9; Bio-Medical Assay), and a PIIINP kit (Human Procollagen III N-Terminal Propeptide; Bio-Medical Assay). The minimal measurable concentrations for these detection systems are 120 pg/ml for galectin-3, 20 pg/ml for NGAL, 60 pg/ml for PIIINP, and 50 pg/ml for MMP-9.
The hemoglobin level and white blood cell count were determined as part of the automated complete blood count using a Sysmex XT-1800i (USA) hematology analyzer. The baseline NLR level was measured by dividing the neutrophil count by the lymphocyte count. A white blood cell count of >12,000 cells/μl or <4,000 cells/μl and high body temperature of >38°C were excluded from the study to ensure a subclinical inflammatory status.
Statistical analyses
Continuous variables are expressed as mean ±SD or median (interquartile range) when appropriate. Categorical variables are expressed as percentages. To compare parametric continuous variables, Student’s t-test was used; to compare nonparametric continuous variables, the Mann-Whitney U-test was used. To compare categorical variables, the chi-square-test was used. The Pearson and Spearman correlation coefficient were used to determine parametric and nonparametric measure of statistical dependence between 2 variables. Multivariate regression analysis was used to identify the independent predictors of higher LAVi value >48 mm3\m2 (mean LAVi value is 48 mm3\m2). All variables showing significance values of less than 0.1 on univariate analysis were included in the model. A 2-tailed P-value of less than 0.05 was considered to indicate statistical significance. The statistical analyses were performed using software (SPSS 15.0, SPSS Inc, Chicago, IL).
Results
Baseline characteristics
The baseline characteristics of the groups (mean age, 71±8 years; minimum age, 42 years; maximum age, 85 years; 62% female) are presented in Table 1. There were no differences between the groups in terms of baseline characteristics, excluding congestive heart failure, and no differences in the conventional laboratory findings. Aspirin and digitalis use was significantly higher in the NVAF group, but there were no differences in the remaining medications.
Table 1.
Baseline characteristics of groups.
| Variables | Control group (n: 33) | AF group (n: 52) | P-value |
|---|---|---|---|
| Patients characteristics | |||
| Age | 70±10 | 70±10 | 0.942 |
| Female/Male n | 20/13 | 34/18 | 0.656 |
| BMI kg/m2 | 29.5±4.4 | 30±5.2 | 0.620 |
| BSA m2 | 1.87±0.21 | 1.86±0.22 | 0.934 |
| Diabetes n | 9/33 | 11/52 | 0.603 |
| Hypertension n | 17/33 | 39/52 | 0.035 |
| Hyperlipidemia n | 7/33 | 5/52 | 0.135 |
| CHF n | 3/33 | 12/52 | 0.005 |
| CAD n | 7/33 | 12/52 | 0.842 |
| Laboratory | |||
| Creatinine mg/dl | 1.0±0.3 | 1.0±0.3 | 0.808 |
| eGFR mL/min | 72±20 | 69±20 | 0.491 |
| LDL mg/dl | 120±30 | 116±38 | 0.703 |
| Hb gr/dl | 13.1±1.8 | 13.3±1.6 | 0.625 |
| Platelet ×103 | 247±67 | 231±60 | 0.265 |
| Echocardiography | |||
| EF% | 63±5 | 52±13 | <0.001 |
| LVMass gr | 221±49 | 256±65 | 0.02 |
| LAV mean mm3 | 57±21 | 100±40 | <0.0001 |
| LAVi mm3/m2 | 31±10 | 54±22 | <0.0001 |
| Admission medication | |||
| Aspirin | 12/33 | 31/52 | 0.037 |
| Beta blocker | 7/33 | 26/52 | 0.008 |
| Digitals | 0/33 | 9/52 | 0.011 |
| Ace/ARB | 14/33 | 27/52 | 0.393 |
| Statin | 7/33 | 5/52 | 0.135 |
| Diuretics | 3/33 | 10/52 | 0.206 |
| Clopidogrel | 1/33 | 1/52 | 0.743 |
| CaCB | 4/33 | 12/52 | 0.206 |
| OAD | 7/33 | 8/52 | 0.492 |
| Insulin | 1/33 | 1/52 | 0.743 |
| NSAI | 5/33 | 14/52 | 0.062 |
ACE – Angiotensin converting enzyme; ARB – angiotensin receptor blocker; BMI – body mass index; BSA – body surface area; CHF – congestive heart failure; CAD – coronary artery disease; NLR – neutrophil to lymphocyte ratio; NSE – neuron specific enolase; Hs-CRP – high sensitive C reactive protein; MPV – mean platelet volume; eGFR – estimated glomerular filtration rate; EF – ejection fraction; LV – left ventricle; LAV – left atrial volume; LAVi – left atrial volume index; CaCB – Ca channel blocker; OAD – oral anti-diabetics; NSAI – non-steroid anti-inflammatory. Results are expressed as mean ±SD or frequency (with in group percentage) and median (Interquartile range). Student-t and Chi-square-test are used.
Echocardiographic measurements
The EF was significantly lower and the LVMass, LAV, and LAVi were significantly higher in patients with NVAF (Table 1).
Inflammatory and remodeling markers
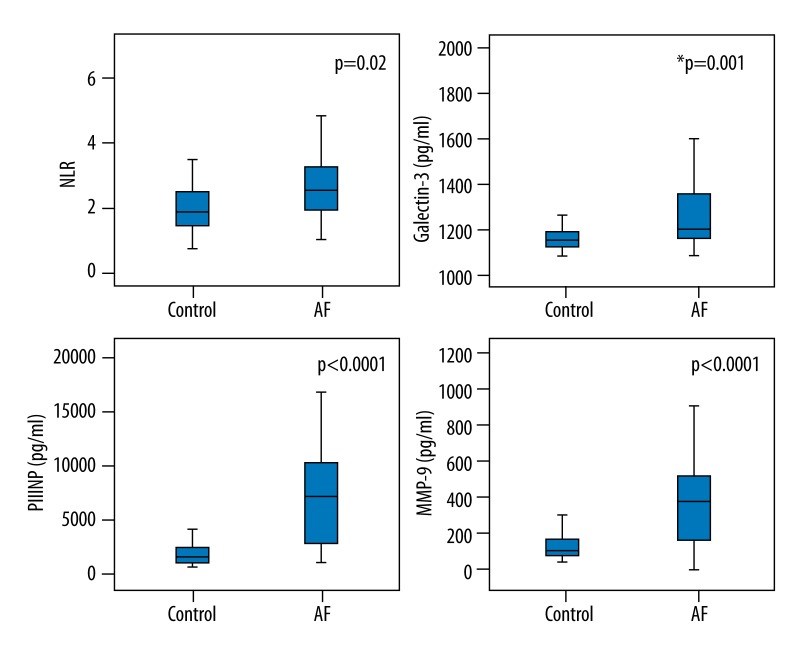
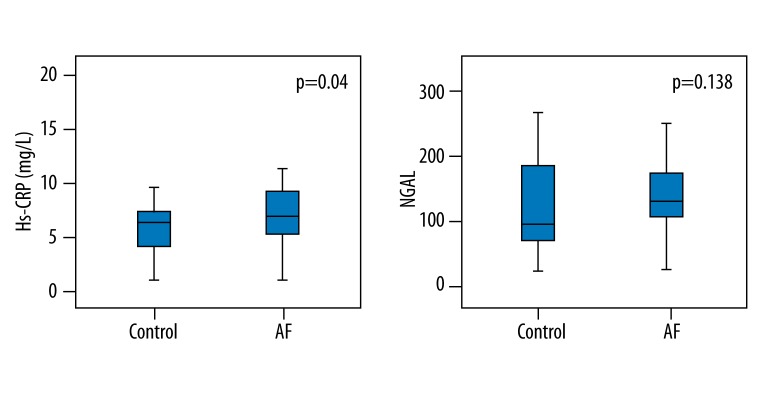
There were significant differences between the groups in terms of inflammatory and remodeling markers, with the exception of the NGAL levels (Table 2). The galectin-3, MMP-9, and PIIINP levels were significantly higher in patients with NVAF (1166 pg/ml (1126–1204) and 1204 pg/ml (1166–1362) p=0.001, 104 (81–179) pg/ml and 404 (162–564) pg/ml p<0.0001, and 1101 (500–1960) pg/ml and 6710 (2370–9950) pg/ml p<0.0001 respectively, Mann-Whitney U test fol all. The NLR and Hs-CRP levels were also higher in patients with NVAF (2.1±1.0 vs. 2.7±1.1 mg/l, p=0.02, Student’s t-test and 4.2±1.9 vs. 6.0±4.7 mg/l, p=0.04, Student’s t-test, respectively) (Figures 1 and 2).
Table 2.
Values of fibro-inflammatory markers of groups.
| Variables | Control group (n: 33) | AF group (n: 52) | P-value* |
|---|---|---|---|
| Hs-CRP mg/L | 4.2±1.9 | 6.0 ±4.7 | 0.04 |
| NLR | 2.1±1.0 | 2.7±1.1 | 0.02 |
| Galectin-3 pg/ml | 1166 (1126–1204) | 1204 (1166–1362) | 0.001# |
| Lipocalin-2/NGAL pg/ml | 132±63 | 152±55 | 0.138 |
| MMP-9 pg/ml | 104 (81–179) | 404 (162–564) | <0.0001# |
| PIIINP pg/ml | 1101 (500–1960) | 6710 (2370–9950) | <0.0001# |
NLR – neutrophil to lymphocyte ratio; NGAL – neutrophil gelatinase-associated lipocalin; Hs-CRP – high sensitive C reactive protein; MMP-9 – matrix metalloproteinase-9; PIIINP – procollagen III N-terminal propeptide. Results are expressed as mean ±SD and median (interquartile range).
Student-t test;
Mann-Whitney U test.
Figure 1.
Mean values of fibrosis and inflammation markers in groups: MMP-9 – matrix metalloproteinase-9, PIIINP – N-terminal propeptide of type III procollagen, NLR – neutrophil-to-lymphocyte ratio (Student-t test was used), and Galectin-3 (Mann-Whitney U test was used).
Figure 2.
Mean values of fibrosis and inflammation markers in groups: Hs-CRP – high sensitive CRP, Lcn2/NGAL – lipocalin-2/neutrophil gelatinase-B associated lipocalin (Student-t test was used for both).
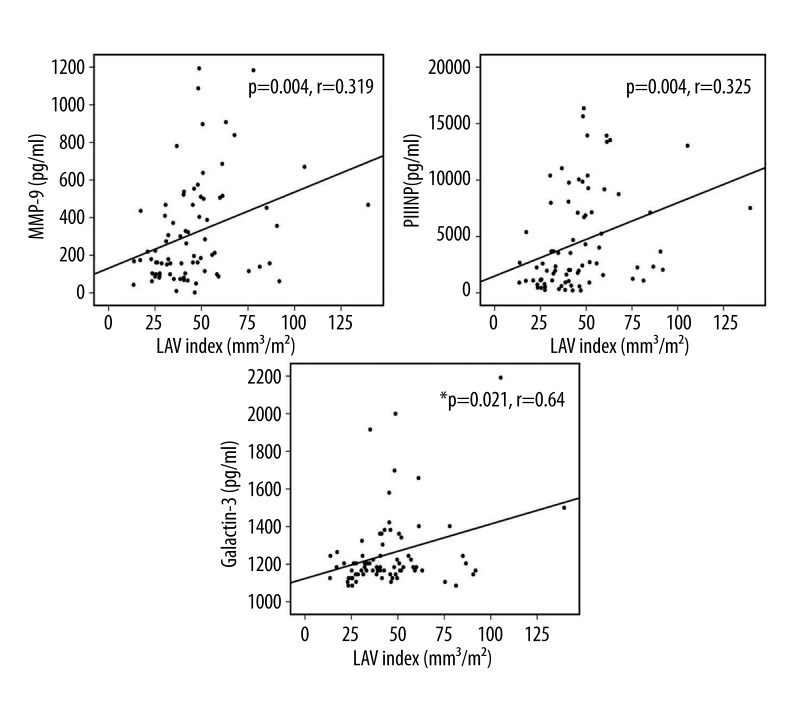
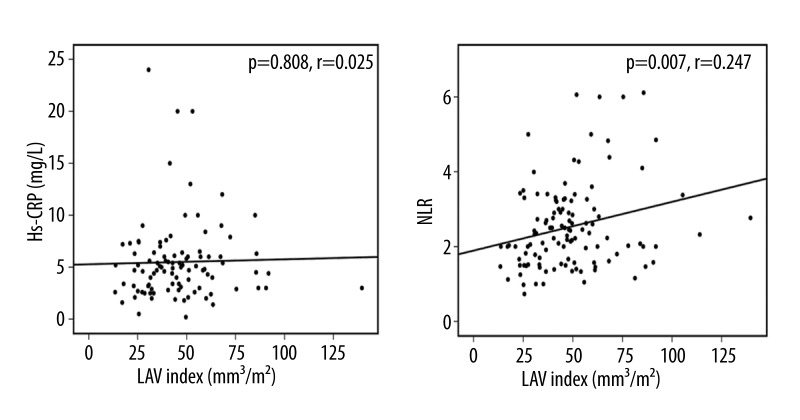
Figures 3 and 4 demonstrate a correlation between LAVi and the inflammatory and remodeling markers as shown by the correlation analyses. NLR showed a significant correlation with LAVi, whereas Hs-CRP did not (p=0.007, r=0.247, Pearson’s test and p=0.808, r=0.025, Pearson’s test, respectively). Moreover, galectin-3, MMP-9, and PIIINP showed a strong positive correlation with LAVi (p=0.021, r=640, Spearman’s test; p=0.004, r=0.319, Pearson’s test; and p=0.004, r=0.325, Pearson’s test, respectively).
Figure 3.
Correlation analyses between LAVi (Left Atrial Volume Index) and fibrosis and inflammation markers: MMP-9 – Matrix metalloproteinase-9, PIIINP – N-terminal propeptide of type III procollagen, Pearson correlation analysis was used for both, Galectin-3 (Spearman correlation analysis was used). Figure reflects the entire (patient and control) group.
Figure 4.
Correlation analyses between LAVi (Left Atrial Volume Index) and fibrosis and inflammation: Hs-CRP – high sensitive CRP, NLR – neutrophil to lymphocyte ratio, Pearson correlation analysis was used for both. Figure reflects the entire (patient and control) group.
In univariate analysis, a cut point was determined as mean LAVi value, MMP-9,PIIINP, NLR, and EF were correlated with high LAVi (LAVi >48 mm3/m2) (Table-3). In multivariate analysis, PIIINP [odds ratio (OR)=1.22, 95% confidence interval (CI) (1.11–1.41), P=0.001] was the only independent factor associated with high LAVi.
Table 3.
Independent predictors of LAVi (>48 mm3\m2) in multivariate regression analysis.
| Variables | Univariate OR (95% CI) | P-value | Multivariate OR (95% CI) | P-value |
|---|---|---|---|---|
| Age | – | 0.309 | ||
| Female | 1.19 (0.47–2.99) | 0.453 | ||
| Diabetes mellitus | 0.78 (0.34–1.8) | 0.577 | ||
| Hypertension | 0.74 (0.33–1.6) | 0.466 | ||
| Coronary Artery Disease | 0.97 (0.33–2.80) | 0.831 | ||
| E-GFR | – | 0.146 | ||
| EF | – | 0.001 | ||
| Hs-CRP | – | 0.724 | ||
| NLR | – | 0.034 | ||
| Lipocalin-2/NGAL | – | 0.589 | ||
| Galectin-3 | – | 0.132 | ||
| MMP-9 | – | 0.001 | ||
| PIIINP | – | 0.001 | 1.22 (1.11–1.41) | 0.001 |
EF – ejection fraction; LAVi – left atrial volume index; eGFR – estimated glomerular filtration rate; NLR – neutrophil to lymphocyte ratio; NGAL – neutrophil gelatinase-associated lipocalin; Hs-CRP – high sensitive C reactive protein; MMP-9 – matrix metalloproteinase-9; PIIINP – procollagen III N-terminal propeptide; CI – confidence interval; OR – odds ratio.
Discussion
The most important finding of our study is the new fibrosis and inflammation markers in patients with AF: galectin-3, PNIIIP, Lcn2/NGAL, Hs-CRP, and NLR. We have shown higher levels of these fibrosis and inflammation markers with the guidance of the serum levels of MMP-9, Hs-CRP, and echocardiographic measurements such as LAVi in patients with AF compared with patients with a sinus rhythm.
ECM components and turnover are regulated by MMPs [4]. During collagen synthesis and degradation, MMPs are upregulated, indicating that the unbalanced expression of the MMP/TIMP system may have a role in the process of atrial structural remodeling. Structural remodeling can be shown using the echocardiographic measurement LAV/LAVi [4,23]. We found higher levels of MMP-9 in patients with AF. Our findings confirm those of previous studies in which enhanced MMP-9 activity was proposed as a major molecular mechanism contributing to the dilation of the atria [4,24,25].
The role of galectin-3 in the pathogenesis of cardiac fibrosis involves the recruitment of additional macrophages, myofibroblasts, and fibroblasts, resulting in cellular proliferation and secretion of procollagen after mechanical and neurohormonal stimuli, which results in secretion of galectin-3 from macrophages [26]. Galectin-3 has been shown to mediate cell-to-cell and cell-to-ECM interactions and acts as a novel chemo-attractant for monocytes and macrophages [12,26]. To the best of our knowledge, galectin-3 levels have not been evaluated in patients with AF, especially with LAVi. We found higher levels of galectin-3 in patients with AF than in the control group, indicating that galectin-3 may play a crucial role in the migration of inflammatory cells. Based on these data, we suggest that galectin-3 can be used as a novel target in patients with AF to decrease the degree of fibrosis and inflammation in the atria.
Lipocalin-2/NGAL is a proinflammatory adipokine [27]. Cardiomyocytes, vascular wall cells, and fibroblasts in myocarditis strongly express lipocalin-2/NGAL, which is associated with insulin resistance [16]. Lipocalin-2/NGAL has been studied in various cardiovascular diseases (e.g., myocarditis and coronary heart disease) but not yet in AF [17]. We found no significant difference in the levels of lipocalin-2/NGAL between patients with AF and the control group. There are several possible explanations for this. Lipocalin-2/NGAL levels are generally higher in obese patients [15], and in our patient population there was no significant difference in the mean body mass index. In addition, statin use reduces the plasma lipocalin-2/NGAL level [28]. Therefore, in this study, statin use in the control group was slightly higher.
One of the serum markers of collagen synthesis is PIIINP, which reflects collagen turnover. Its role as a marker of collagen synthesis has been shown in various cardiovascular diseases, but to date few study in AF has been performed [18–20,29,30]. Swartz et al. have demonstrated that PIIINP levels correlate with presence of LA fibrosis and may be a predictor for post-operative AF [30]. PIIINP is a cardiac remodeling marker and is used to monitor the anti-remodeling effect of various drugs such as aldosterone antagonists [31,32]. In this study, we showed significantly higher levels of PIIINP in patients with AF compared with the control group, indicating that during collagen turnover, PIIINP may play a role in the remodeling of the atria in patients with AF.
Previous studies have contributed to our understanding of the role of inflammation in AF by showing that Hs-CRP [6–8,33] and NLR are associated with AF [9–11]. According to the review by Bhat et al., the association of NLR with AF has not been evaluated in detail. Recently, in association between NLR and AF, few studies have focussed on cardioversion success, ablation therapy results, and thromboembolic stroke risk [34]. In our study, NLR showed a significant correlation with LAVi, whereas Hs-CRP did not. According to our study results, NLR is an inflammatory marker that plays a role in remodeling of the atria. On a molecular and cellular level, galectin-3 and various other markers activate and increase the numbers of monocytes and macrophages involved in cell-to-cell and cell-to-ECM interactions during atrial remodeling.
The renin-angiotensin-aldosterone system plays an important role in structural atrial remodeling [35]. The current literature indicates that angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) prevent the development of AF (primary prevention) [36,37]. Contrary to these data, the significance of the role of ACEIs and ARBs in upstream therapy (secondary prevention) is not clear. Some results have been encouraging, whereas others have been very unfavorable; however, some data show a positive anti-remodeling effect in patients with AF [38,39]. In light of recent reports, and according to our data, PIIINP, galectin-3, and NLR levels could be important in determining the presence of underlying heart disease (remodeling status) in patients who are candidates for medical therapy. A reduction in new-onset AF has been reported in patients with remarkable underlying heart disease treated with ACEIs or ARBs, but the benefit was minimal in patients with moderate structural heart disease and recurrent AF. A GISSI-AF echocardiographic sub-study revealed that during 1 year ARB for did not reverse LA remodeling or prevent AF recurrence [23]. Therefore, early anti-remodeling therapy should be recommend to patients with persistent AF before irreversible ECM remodeling occurs, as pointed-out by Gramley et al. [3]. It is important to identify the time at which atrial remodeling starts in patients likely to develop AF in the short term. Camm et al. reported a purpose for new clinical concepts of AF in the facilitation of intensive clinical monitoring and management [40]. Based on our results, we suggest that LAVi and new fibrosis and inflammation markers such as galactin-3, PIIINP, and NLR may predict the beginning of the atrial remodeling process.
Study limitations
Two limitations must be considered. The main limitation is the lack of histologic correlation, because we did not perform a histopathological examination of atrial tissue. Also, our study included a small population. However, research on fibrosis and inflammation markers at the tissue level would contribute additional information.
Conclusions
Certain fibrosis and inflammation markers in AF are correlated with atrial remodeling. Several unexplained mechanisms of atrial remodeling remain, but the present study has taken the first step in elucidating the mechanisms involving fibrosis and inflammation markers. Although targeting fibrosis and inflammation as early anti-remodeling therapy is not supported by the current data, present study findings also suggest that galectin-3, PIIINP, and NLR may contribute to the clinical and therapeutic management of AF as novel targets for therapies that aim to decrease fibrosis and inflammation in the atria.
Footnotes
Source of support: The abstract of this manuscript has been accepted and presented as an oral presentation in The 29th National Cardiology Congress, October 26-29/2013, Antalya, Turkey
Declaration of conflicting interest
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 2.Lip GY, Patel JV, Hughes E, Hart RG. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke. 2007;38:1229–37. doi: 10.1161/01.STR.0000260090.90508.3e. [DOI] [PubMed] [Google Scholar]
- 3.Gramley F, Lorenzen J, Plisiene J, et al. Decreased plasminogen activator inhibitor and tissue metalloproteinase inhibitor expression may promote increased metalloproteinase activity with increasing duration of human atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1076–82. doi: 10.1111/j.1540-8167.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- 4.Kalogeropoulos AS, Tsiodras S, Rigopoulos AG, et al. Novel association patterns of cardiac remodeling markers in patients with essential hypertension and atrial fibrillation. BMC Cardiovasc Disord. 2011;11:77. doi: 10.1186/1471-2261-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gai X, Lan X, Luo Z, et al. Association of MMP-9 gene polymorphisms with atrial fibrillation in hypertensive heart disease patients. Clin Chim Acta. 2009;408:105–59. doi: 10.1016/j.cca.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Licata G, Tuttolomondo A, Di Raimondo D, et al. Immuno-inflammatory activation in acute cardio-embolic strokes in comparison with other subtypes of ischaemic stroke. Thromb Haemost. 2009;101:929–37. [PubMed] [Google Scholar]
- 7.Ehrlich JR, Kaluzny M, Baumann S, et al. Biomarkers of structural remodelling and endothelial dysfunction for prediction of cardiovascular events or death in patients with atrial fibrillation. Clin Res Cardiol. 2011;100:1029–36. doi: 10.1007/s00392-011-0337-9. [DOI] [PubMed] [Google Scholar]
- 8.Pena JM, MacFadyen J, Glynn RJ, Ridker PM. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur Heart J. 2012;33:531–37. doi: 10.1093/eurheartj/ehr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ertas G, Sonmez O, Turfan M, et al. Neutrophil/lymphocyte ratio is associated with thromboembolic stroke in patients with non-valvular atrial fibrillation. J Neurol Sci. 2013;324:49–52. doi: 10.1016/j.jns.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Aribas A, Akilli H, Gul EE, et al. Can neutrophil/lymphocyte ratio predict recurrence of non-valvular atrial fibrillation after cardioversion? Anadolu Kardiyol Derg. 2013;13:123–30. doi: 10.5152/akd.2013.036. [DOI] [PubMed] [Google Scholar]
- 11.Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 12.McCullough PA, Olobatoke A, Vanhecke TE. Galectin-3: a novel blood test for the evaluation and management of patients with heart failure. Rev Cardiovasc Med. 2011;12:200–10. doi: 10.3909/ricm0624. [DOI] [PubMed] [Google Scholar]
- 13.Gruson D, Ko G. Galectins testing: new promises for the diagnosis and risk stratification of chronic diseases? Clin Biochem. 2012;45:719–26. doi: 10.1016/j.clinbiochem.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Lok DJ, Van Der Meer P, de la Porte PW, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010;99:323–28. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanhoutte PM. Endothelial dysfunction in obesity. Ann Pharm Fr. 2013;71:42–50. doi: 10.1016/j.pharma.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Ding L, Hanawa H, Ota Y, et al. Lipocalin-2/neutrophil gelatinase-B associated lipocalin is strongly induced in hearts of rats with autoimmune myocarditis and in human myocarditis. Circ J. 2010;74:523–30. doi: 10.1253/circj.cj-09-0485. [DOI] [PubMed] [Google Scholar]
- 17.Choi KM, Lee JS, Kim EJ, et al. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur J Endocrinol. 2008;158:203–7. doi: 10.1530/EJE-07-0633. [DOI] [PubMed] [Google Scholar]
- 18.Diez J, Laviades C, Mayor G, et al. Increased serum concentrations of procollagen peptides in essential hypertension. Relation to cardiac alterations. Circulation. 1995;91:1450–56. doi: 10.1161/01.cir.91.5.1450. [DOI] [PubMed] [Google Scholar]
- 19.Dellegrottaglie S, Sands RL, Gillespie BW, et al. Association between markers of collagen turnover, arterial stiffness and left ventricular hypertrophy in chronic kidney disease (CKD): the Renal Research Institute (RRI)-CKD study. Nephrol Dial Transplant. 2011;26:2891–98. doi: 10.1093/ndt/gfr186. [DOI] [PubMed] [Google Scholar]
- 20.Gluba A, Bielecka A, Mikhailidis DP, et al. An update on biomarkers of heart failure in hypertensive patients. J Hypertens. 2012;30:1681–89. doi: 10.1097/HJH.0b013e3283569a9c. [DOI] [PubMed] [Google Scholar]
- 21.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 22.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–18. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 23.Staszewsky L, Wong M, Masson S, et al. Left atrial remodeling and response to valsartan in the prevention of recurrent atrial fibrillation: the GISSI-AF echocardiographic substudy. Circ Cardiovasc Imaging. 2011;4:721–28. doi: 10.1161/CIRCIMAGING.111.965954. [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, Zhang W, Guo CH, et al. Effects of matrix metalloproteinase-9 and tissue inhibitor-1 of metalloproteinase expression on atrial structural remodeling during chronic atrial fibrillation. Zhonghua Yi Xue Za Zhi. 2005;85:45–48. [PubMed] [Google Scholar]
- 25.Zhao Y, Zhou X, Liao X, Yang Z. Expression and significance of matrix metalloproteinase-1,9, tissue inhibitor of metalloproteinase-4 and extracellular matrix metalloproteinase inducer in the myocardium of congestive heart failure in patients with rheumatic heart diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34:790–95. [PubMed] [Google Scholar]
- 26.Lin YH, Lin LY, Wu YW, et al. The relationship between serum galectin-3 and serum markers of cardiac extracellular matrix turnover in heart failure patients. Clin Chim Acta. 2009;409:96–99. doi: 10.1016/j.cca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev. 2013;18(5):631–44. doi: 10.1007/s10741-012-9337-8. [DOI] [PubMed] [Google Scholar]
- 28.Fassett RG, Robertson IK, Ball MJ, et al. Effects of atorvastatin on NGAL and cystatin C in chronic kidney disease: a post hoc analysis of the LORD trial. Nephrol Dial Transplant. 2012;27:182–89. doi: 10.1093/ndt/gfr193. [DOI] [PubMed] [Google Scholar]
- 29.Romero JR, Vasan RS, Beiser AS, et al. Association of carotid artery atherosclerosis with circulating biomarkers of extracellular matrix remodeling: the Framingham Offspring Study. J Stroke Cerebrovasc Dis. 2008;17:412–17. doi: 10.1016/j.jstrokecerebrovasdis.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swartz MF, Fink GW, Sarwar MF, et al. Elevated pre-operative serum peptides for collagen I and III synthesis result in post-surgical atrial fibrillation. J Am Coll Cardiol. 2012;60(18):1799–806. doi: 10.1016/j.jacc.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards NC, Ferro CJ, Kirkwood H, et al. Effect of spironolactone on left ventricular systolic and diastolic function in patients with early stage chronic kidney disease. Am J Cardiol. 2010;106:1505–11. doi: 10.1016/j.amjcard.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Udelson JE, Feldman AM, Greenberg B, et al. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circ Heart Fail. 2010;3:347–53. doi: 10.1161/CIRCHEARTFAILURE.109.906909. [DOI] [PubMed] [Google Scholar]
- 33.Sahin T, Acar E, Celikyurt U, et al. Relation of hs-CRP and BNP levels with the atrial spontaneous echo contrast and thrombi in permanent atrial fibrillation patients with different etiologies. Med Sci Monit. 2012;18(2):CR78–87. doi: 10.12659/MSM.882461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canpolat U, Aytemir K, Yorgun H, et al. Role of Preablation Neutrophil/Lymphocyte Ratio on Outcomes of Cryoballoon-Based Atrial Fibrillation Ablation. Am J Cardiol. 2013 doi: 10.1016/j.amjcard.2013.04.015. pii: S0002-9149(13)00989-2. [DOI] [PubMed] [Google Scholar]
- 35.Goette A, Staack T, Rocken C, et al. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35:1669–77. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Zhang P, Mu Y, et al. The role of renin-angiotensin system blockade therapy in the prevention of atrial fibrillation: a meta-analysis of randomized controlled trials. Clin Pharmacol Ther. 2010;88:521–31. doi: 10.1038/clpt.2010.123. [DOI] [PubMed] [Google Scholar]
- 37.Durin O, Pedrinazzi C, Inama G. Focus on renin-angiotensin system modulation and atrial fibrillation control after GISSI AF results. J Cardiovasc Med (Hagerstown) 2010;11:912–18. doi: 10.2459/JCM.0b013e32833cdd6f. [DOI] [PubMed] [Google Scholar]
- 38.Sasamura H, Kitamura Y, Nakamura M, et al. Effects of the angiotensin receptor blocker candesartan on arterial stiffness and markers of extracellular matrix metabolism in patients with essential hypertension. Clin Exp Hypertens. 2006;28:511–20. doi: 10.1080/10641960600798721. [DOI] [PubMed] [Google Scholar]
- 39.Yoon N, Cho JG, Kim KH, et al. Beneficial effects of an angiotensin-II receptor blocker on structural atrial reverse-remodeling in a rat model of ischemic heart failure. Exp Ther Med. 2013;5:1009–16. doi: 10.3892/etm.2013.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camm AJ, Al-Khatib SM, Calkins H, et al. A proposal for new clinical concepts in the management of atrial fibrillation. Am Heart J. 2013;164:292–302. doi: 10.1016/j.ahj.2012.05.017. [DOI] [PubMed] [Google Scholar]