Abstract
The generation of functional endodermal lineages, such as hepatocytes and pancreatic endocrine cells, from pluripotent stem cells remains a challenge. One strategy to enhance the purity, yield and maturity of endodermal derivatives is to expand endoderm committed stem or progenitor cell populations derived from pluripotent stem cells prior to final differentiation. Recent studies have shown that this is in fact a viable option both for expanding pure populations of endodermal cells as well as for generating more mature derivative tissues, as highlighted in the case of pancreatic beta cells.
Introduction
Human pluripotent stem cells (PSC)s, including embryonic stem cells (ESC)s and induced pluripotent stem cells (iPSC)s, hold tremendous promise for both the study of and treatment of a wide variety of diseases due to virtually unlimited proliferative capacity and their potential to differentiate into any cell type in the body. The in vitro differentiation of ESCs/iPSCs seems to mimic the process of development that occurs during embryogenesis. PSC populations proceed down developmental intermediaries with successively restricted potential until mature cell types are generated, recapitulating the events that occur in vivo (reviewed in [1,2]). This review will focus on the generation of expandable endodermal populations from PSCs.
During embryonic development the process of gastrulation leads to the formation of the primary germ layers, ectoderm, mesoderm, and endoderm (reviewed in [3,4]). Definitive endoderm (DE) is generated as epiblast cells migrate through a transient structure termed the primitive streak and form an epithelial layer. This sheet of DE then folds to form the primitive gut tube that comprises three major domains along the anterior posterior axis: (1) the foregut, eventually giving rise to esophagus, trachea, lungs, thyroid, parathyroid, thymus, stomach, liver, biliary system and pancreas; (2) the midgut, eventually forming intestines; and (3) the hindgut, which forms the colon. These domains are further patterned into specific regions that contain organ-specific progenitor populations, from which the rudiments of various endoderm organs are formed (reviewed in [4,5]).
Various endodermal tissue-specific stem cell populations that are expandable ex vivo have been identified in adult animals, including: (1) adult hepatic stem cells [6]; (2) lung multi-potent bronchioalveolar stem cells [7]; (3) basal cells for airway epithelium ([8], and reviewed in [9]); (4) mammary gland stem cells [10-12]; (5) gastric Lgr5+ pyloric stem cells ([13], and reviewed in [14]); (6) Lgr5+ intestinal stem cells [15]; and (7) clonal multi-potent endoderm stem cells isolated from mouse liver [16]. These populations will not be discussed as they have been reviewed elsewhere.
Endodermal Derivatives from PSCs: Promises and Obstacles
The generation of endoderm-derived tissues in vitro, including lung, liver, pancreas, and intestine, represent an incredibly powerful system for studying basic biology, disease modeling, drug testing, and as a future source of tissue for cellular therapies. It is possible to generate DE and its derivative lineages from PSCs in vitro through sequential exposure to growth factors that induce this developmental maturation [17-21]. In this manner, lung, hepatic, pancreatic and intestinal cells can be produced from ESCs and iPSCs that have differentiated to the DE stage [22-38]. While these studies highlight the promise of PSC-derived endodermal tissues, several obstacles remain. Endodermal cells generated from PSCs tend to display immature phenotypes and in many instances are not fully functional. For example, pancreatic beta cells generated directly from human ESCs express other endocrine hormones in addition to insulin and are not responsive to glucose stimulation in vitro [28,38]. In addition, the pluripotent nature of ESCs and iPSCs results in the production of multiple cell types from different germ layers in most differentiation protocols. Thus, it is problematic to produce pure cultures of a desired cell type [2,39], making it difficult to dissect the interactions between germ layers during differentiation. Finally, undifferentiated ESCs and iPSCs are tumorigenic and therefore must be completely removed from their derivative tissues to be used for transplantation studies [39].
To address these issues, it is desirable to generate germ layer/tissue-specific stem cells from PSCs, which proliferate in vitro and are capable of differentiating into mature lineages (see figure 1). For example, neuronal stem cells and mesenchymal stem cells have both been generated from human PSCs (reviewed in [40] and [41]). Such cells have several advantageous over using PSCs directly. First, because these cells have more restricted differentiation potentials, they should be devoid of teratoma forming ability. Second, being developmentally “closer” to the desired mature cell type than PSCs, differentiation should be more efficient. Third, their restricted developmental potential enables experimentation with pure cell populations that are not contaminated with cells from other germ layers. This in turn provides a system to study various cell-cell interactions during differentiation; for example, mesoderm derived mesenchyme patterning of the gut tube.
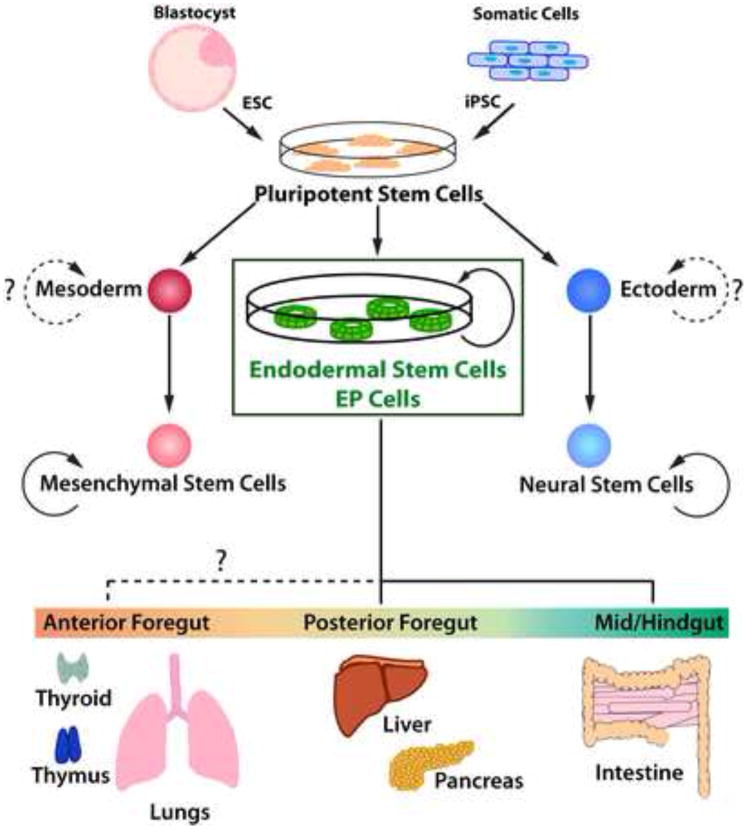
Figure 1. Expandable Stem Cell Populations Derived from Pluripotent Stem Cells.
A schematic illustration of the generation of germ layer specific stem cell populations from pluripotent stem cells is shown. While neural stem cells and mesenchymal stem cells have a relatively limited differentiation capacity, EP cells are capable of differentiating into cells of many endodermal organs including liver, pancreas and intestine that in vivo are derived from the fore-, mid- and hindgut. It is currently unknown if EP cells have the potential to give rise to all endodermal derivatives including the thymus, thyroid and lungs. (ESC: embryonic stem cell, iPSC: induced pluripotent stem cell; EP cell: Endodermal progenitor cell)
Expandable PSC-derived Endodermal Populations
An early study done by Nishikawa and colleagues [19] utilized a Gsc-GFP reporter mouse ESC line to purify DE cells that could be expanded in serum containing media for more than 6 months while maintaining the expression of endodermal markers. When transplanted into immune-deficient mice, these cells did not give rise to tumors. However, differentiation of the expanded endoderm to more mature lineages was not observed either in vivo or in vitro. In an attempt to isolate and maintain foregut endodermal cells, Morrison et al [42] used a Hex reporter mouse ESC line to purify endodermal cells that could be maintained and expanded in serum free conditions for an expansion of ~2000 fold. These cells expressed a series of anterior DE markers and could be induced into Pdx1+ pancreatic or AFP+ Albumin+ hepatocyte-like cells in vitro. When transplanted into adult mice, they did not generate teratomas, and endodermal ductal epithelial structures were observed in the transplants at low frequency. These two sets of studies, using distinct reporter mouse ESC lines to purify DE, demonstrated that the in vitro expansion of DE was possible. A more recent study performed by Kahan et al [43] demonstrated the generation of a Pdx1+ population that was able to expand continuously for over 7 months and expressed a repertoire of genes indicative of posterior foregut endoderm. To generate this population, purified DE cells derived from mouse ESCs were transplanted into immune-deficient mice and the resulting nodules explanted in culture. The differentiation potential of these putative posterior foregut progenitors is still to be determined.
In the human system, studies have also identified endodermal populations with proliferative potential. In an attempt to generate DE directly, Rossant and colleagues [44] ectopically expressed SOX17, a critical regulator of endoderm development [45], in human ESCs. The constitutive expression of SOX17 in human ESCs resulted in the generation of a population of cells that expressed a panel of DE markers and could be expanded for more than 50 passages. The fact that these cells expressed ESC markers, including OCT4 and NANOG, suggested that they were not endoderm committed, which was confirmed by their ability to give rise to both endodermal and mesodermal but not neuro-ectodermal lineages. Human DE derived populations with more restricted differentiation potential have also been identified. Deng and coworkers [46] have shown that an N-cadherin+ endodermal population can be purified from hepatocyte differentiation cultures of human ESCs, and these cells could be expanded and passaged for more than 3 months when co-cultured with murine embryonic stromal cells. These cells were able to further differentiate into hepatocyte-like cells as well as cholangiocyte-like cells, suggesting that they represented a hepatic stem/progenitor cell population. In addition, Spence et al [22] demonstrated that intestinal organoids derived from human ESCs could be expanded ~100,000 fold over 140 days. These organoids contained LGR5+ progenitor cells along with many differentiated progeny including enterocytes, goblet cells, enteroendocrine cells, and Paneth cells.
Self-renewing PSC-derived Endoderm Stem Cells
Recently, the establishment of endoderm-committed stem cell lines generated from human ESCs and iPSCs has been described [47] and figure 1. These cells, termed endodermal progenitor (EP) cells, display extensive self-renewal in culture (1016 expansion) using a defined serum-free culture media containing VEGF, EGF, FGF2, and BMP4 and plated on matrigel with a low density mouse embryonic fibroblast (MEF) feeder layer. EP cells do not generate mesodermal or ectodermal derivatives in vitro or in vivo and do not form teratomas when transplanted into immune-deficient mice. These cells maintain the ability to differentiate into endodermal lineages including liver, pancreas, and intestine, though it is unknown if these cells also have the potential to form more anterior endoderm lineages such as the thymus, thyroid and lungs (figure 1). An important finding is the ability of EP cells to generate mono-hormonal and glucose responsive pancreatic beta-like cells in vitro. Single cell derived sub-clones displayed a similar proliferative and developmental potential.
More recently, a second report demonstrated the ability to expand DE cells derived from both mouse and human ESCs by using a co-culture system with mouse mesenchymal cells in serum-containing media [48]. This study showed that these cells had extensive proliferative capacity (108) while maintaining the ability to generate pancreatic cells that upon transplantation in vivo could spontaneously mature into glucose responsive insulin+ cells. In addition, mouse ESC derived Ngn3+ cells could also be expanded several fold utilizing a similar co-culture system but with a different set of mesenchymal cells. The full developmental potential and the ability for clonal expansion of these populations remain to be tested.
A comparison of these studies may uncover common themes important for the appropriate development of endodermal derivatives. First, both of these studies required the use of a feeder layer for expansion of the DE cells, MEF cells in the case of Cheng et al [47] and mouse mesenchyme for the case of Sneddon et al [48]. MEF cells were unable to support expansion in the Sneddon report, suggesting that other culture components including matrigel and growth factors used in the Cheng report may act in concert with the supporting cells. It is interesting to note that in an earlier study limited expansion of mouse ESC derived DE used cultures lacking a feeder cell population [42]. It will be essential to determine the signals provided by these supporting cells to enable expansion in defined serum free culture systems. It will also be important to compare these expandable DE cells and ask if they have the same developmental potential? For example, EP cells when differentiated into pancreatic endocrine cells are able to generate mono-hormonal insulin+ cells that are glucose responsive in vitro. Using similar differentiation protocols with ESCs and iPSCs has consistently resulted in the generation of poly-hormonal, non-functional cells [28,38] and required in vivo transplantation of progenitors to mature into glucose responsive cells [25]. One hypothesis for this difference is that the expansion of cells at the endoderm stage of development is critical for establishing transcriptional and epigenetic networks necessary for appropriate differentiation. Examination of DE cells expanded using different methodologies will enable this hypothesis to be tested. It is also possible that these populations may represent distinct developmental intermediaries as exemplified by mouse ESCs and epiblast stem cells. ESCs and epiblast stem cells are two closely related stem cell types with similar developmental potentials, but require very different culture conditions for maintenance of the undifferentiated state and display some functional differences (reviewed in [49]). For example, ESCs when injected into a host blastocyst will contribute to a chimera while epiblast stem cells do not.
Future Directions: Direct Reprogramming to Generate Endodermal Stem Cells
Cellular reprogramming offers a complementary method to generate endodermal derived tissues both in vivo and in vitro (reviewed in [50,51]). This technology has proven very successful to generate a number of cellular lineages directly from mouse fibroblasts in vitro, including endodermal lineages such as hepatocytes [52,53] and insulin+ cells have been induced from mouse pancreatic exocrine cells in vivo [54]. While this technology has the potential to be used to generate many endodermal derivative cell types, there are challenges that need to be overcome. The first issue is the efficiency of reprogramming, as terminally differentiated cells have limited expansion potential. Human fibroblasts have a finite proliferative capacity requiring the procurement of additional primary cells. This can be extremely difficult in the case of patient specific samples, limiting the usefulness of direct reprogramming in disease modeling with patient material. Next is the issue of cellular memory that has been described in iPSCs. It has been shown that early passage mouse and human iPSCs can have differences in gene expression, epigenetic marks and in the ability to differentiate into particular lineages, depending on the reprogrammed cell of origin [55-57]. While these issues may be resolved in some clones by passaging the iPSC lines [56,57], this is not an option in the direct reprogramming field where a non-proliferative terminally differentiated cell type is generated. Work examining the reprogramming of hepatocytes into neurons did find low-level expression of some hepatocyte genes in the induced neurons, suggesting that epigenetic memory may be an issue in direct reprogramming as well [58].
Many of the problems associated with direct reprogramming could be overcome if reprogramming was performed not to a terminally differentiated cell type, but to a more restricted stem or progenitor cell population with proliferative capacity. The progenitor cell would be developmentally closer to terminally differentiated lineages of interest, and as such should be easier to differentiate than starting from iPSCs. The efficiency of reprogramming would also not be critical as rare clones could be expanded, as is the case in iPSC generation. Induced progenitor cells could be passaged in culture, hopefully bypassing memory effects. Recent studies performed by several laboratories have demonstrated that this is feasible with the generation of induced neuronal stem cells in both mice and humans [59-62]. EP cells and intestinal stem cells are two endodermal populations amendable to such a strategy, both of which can be expanded robustly in culture [47,63].
The ability to generate endodermal stem cell types that can be expanded in culture either from PSCs or through direct reprogramming provide new tools to study endoderm biology. Studies reporting the ability to expand endodermal cell types derived from PSCs are summarized in table 1. These cell populations provide a powerful system to study and model human diseases in vitro, as well as generating a source of cells for transplantation.
Table 1.
Expandable Endodermal Cells Derived from Pluripotent Stem Cells:
| Progenitor Cell Type (Reference) | Differentiation Potential | Expandability (In vitro) | Culture Conditions | Clonality | Species | ||
|---|---|---|---|---|---|---|---|
| Endoderm | DE [19••] | None | ++++ | Serum | ND | Mouse | |
| SOX17 Over-expression [44] | Endoderm & Mesoderm | ++++ (?) | Serum Free & MEFs | Yes | Human | ||
| Anterior DE [42••] | Pancreatic & Hepatic | ++ | Serum Free | ND | Mouse | ||
| PDX1+ EpCAM+ Posterior Foregut Cells [43] | ND (Putative Posterior Foregut Progenitor) | ++++ (?) | Serum Free & MEFs (Ex-vivo Culture of EpCAM+ Transplants) | Yes | Mouse | ||
| EP Cells [47••] | Pancreatic, Hepatic, and Intestinal | ++++ | Serum Free & MEFs | Yes | Human | ||
| SOX17+ DE [48••] | Pancreatic (Other DE Lineages ND) | +++ | Serum & Mouse Mesenchymal Cells | ND | Mouse & Human | ||
| Organ-Specific | Liver | Hepatic Progenitor Cells [46•] | Hepatocyte & Cholangiocyte | ++ | Serum Free & Mouse Embryonic Stromal Feeders | ND | Human |
| Pancreas | Ngn3+ Cells [48••] | ND | + | Serum & Mouse Mesenchymal Cells | ND | Mouse | |
| Intestine | Intestinal Organoids [22••] | Intestinal Lineages (Enterocytes, Goblet Cells, Enteroendocrine Cells, Paneth Cells) | ++ | Serum Free | ND | Human | |
Key: + ≤ 102 fold, ++ ≤ 105 fold, +++ ≤ 108, ++++ > 108; ?= Estimate as value not explicitly stated in publication; ND= not determined.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Tam PP, Behringer RR. Mouse gastrulation: the formation of a mammalian body plan. Mech Dev. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 4.Lu CC, Brennan J, Robertson EJ. From fertilization to gastrulation: axis formation in the mouse embryo. Curr Opin Genet Dev. 2001;11:384–392. doi: 10.1016/s0959-437x(00)00208-2. [DOI] [PubMed] [Google Scholar]
- 5.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 7.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotton DN. Next-generation regeneration: the hope and hype of lung stem cell research. Am J Respir Crit Care Med. 2012;185:1255–1260. doi: 10.1164/rccm.201202-0228PP. [DOI] [PubMed] [Google Scholar]
- 10.Zeng YA, Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6:568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 12.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 13.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–424. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol. 2002;156:173–184. doi: 10.1083/jcb.200108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. This manuscript along with [19••] demonstrates that DE can be induced from mouse ESCs by activation of the TGFbeta pathway using a serum free culture system with high levels of Activin A, mimicking the signal nodal provides in vivo [3, 4]. A similar protocol was also successful to induce DE from human ESCs [18•] [DOI] [PubMed] [Google Scholar]
- 18•.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. See annotation for [17••] [DOI] [PubMed] [Google Scholar]
- 19••.Tada S, Era T, Furusawa C, Sakurai H, Nishikawa S, Kinoshita M, Nakao K, Chiba T. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. See annotation for [17••]. In addition, this report demonstrates the expansion of endodermal cells derived from mouse ESCs. [DOI] [PubMed] [Google Scholar]
- 20.Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 21.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. This manuscript is the first to demonstrate efficient posterior endoderm differentiation from human ESCs, using Wnt3a and FGF4 as inducing signals. In addition, these authors show efficient expansion of intestinal organoids with an ~100,000 fold expansion potential utilizing an organoid culture system established previously [63]. These organoids could go on to differentiate into the various intestinal cell types, including enterocytes, goblet cells, enteroendocrine cells, and Paneth cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezania A, Riedel MJ, Wideman RD, Karanu F, Ao Z, Warnock GL, Kieffer TJ. Production of functional glucagon-secreting alpha-cells from human embryonic stem cells. Diabetes. 2011;60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 25.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 26.Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J, Qing T, Sun X, Zhang P, Ding M, et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333–344. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- 27.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 28.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Liu P, Liu C, Xiang D, Deng L, Li W, Wangensteen K, Song J, Ma Y, Hui L, et al. Hepatoblast-like progenitor cells derived from embryonic stem cells can repopulate livers of mice. Gastroenterology. 2010;139:2158–2169. e2158. doi: 10.1053/j.gastro.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 30.Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H, Pedersen R, Di Santo J, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51:1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- 31.Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, Gambhir SS, Zern MA. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
- 32.Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler CI, Kubo A, Shafritz DA, Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- 34.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol. 2012 doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB, et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009;2:198–210. doi: 10.1016/j.scr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Elkabetz Y, Studer L. Human ESC-derived neural rosettes and neural stem cell progression. Cold Spring Harbor symposia on quantitative biology. 2008;73:377–387. doi: 10.1101/sqb.2008.73.052. [DOI] [PubMed] [Google Scholar]
- 41••.Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. This report demonstrates the generation of mesoderm derived mesenchymal stem cells from human embryonic stem cells. Clonal MSC linesdemonstrated significant expansion ability (1010-1020) while mantaining osteogenic, chondrogenic, and adipogenic potential. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Morrison GM, Oikonomopoulou I, Migueles RP, Soneji S, Livigni A, Enver T, Brickman JM. Anterior definitive endoderm from ESCs reveals a role for FGF signaling. Cell Stem Cell. 2008;3:402–415. doi: 10.1016/j.stem.2008.07.021. This report demonstrates the expansion of endodermal cells (~2,000 fold) derived from mouse ESCs. Unlike the earlier report of Tada et al [19••], this cell population maintained the ability to differentiate into pancreatic and hepatic cell types. [DOI] [PubMed] [Google Scholar]
- 43.Kahan B, Magliocca J, Merriam F, Treff N, Budde M, Nelson J, Browning V, Ziehr B, Odorico J. Elimination of tumorigenic stem cells from differentiated progeny and selection of definitive endoderm reveals a Pdx1+ foregut endoderm stem cell lineage. Stem Cell Res. 2011;6:143–157. doi: 10.1016/j.scr.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seguin CA, Draper JS, Nagy A, Rossant J. Establishment of endoderm progenitors by SOX transcription factor expression in human embryonic stem cells. Cell Stem Cell. 2008;3:182–195. doi: 10.1016/j.stem.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 46•.Zhao D, Chen S, Cai J, Guo Y, Song Z, Che J, Liu C, Wu C, Ding M, Deng H. Derivation and characterization of hepatic progenitor cells from human embryonic stem cells. PLoS One. 2009;4:e6468. doi: 10.1371/journal.pone.0006468. This report demonstrates the expansion of hepatic cells (~10,000 fold) derived from human ESCs. This cell population maintained the ability to differentiate into cell expressing markers of hepatocytes and cholangiocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Cheng X, Ying L, Lu L, Galvao AM, Mills JA, Lin HC, Kotton DN, Shen SS, Nostro MC, Choi JK, et al. Selfrenewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell. 2012;10:371–384. doi: 10.1016/j.stem.2012.02.024. This report describes the generation of DE stem cell population termed EP cells from human ESCs or iPSCs. This population can be sub-cloned and expanded >1016 fold while maintaining the ability to differentiate into pancreatic, hepatic, and intestinal lineages. In addition, pancreatic beta-like cells derived from EP cells are mono-hormonal and glucose responsive in vitro in contrast to the poly-hormonal and nonfunctional cells generated directly from ESCs [28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Sneddon JB, Borowiak M, Melton DA. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature. 2012 doi: 10.1038/nature11463. This report describes the expansion of DE from mouse or human ESCs using a co-culture system with mouse mesenchyme. This population can be expanded >108 fold, while maintaining the ability to differentiate into pancreatic cells in vitro and in vivo. Other DE cell types where not examined. In addition, mouse ESC derived presumptive pancreatic progenitors (Ngn3+) could be expanded by different mesenchyme cell lines (~10 fold). The differentiation potential of the Ngn3+ population is still to be determined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Los Angeles A, Loh Y-H, Tesar P, Daley GQ. Accessing naïve human pluripotency. Curr Opin Genet Dev. 2012;22:272–282. doi: 10.1016/j.gde.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9:504–516. doi: 10.1016/j.stem.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Vierbuchen T, Wernig M. Molecular roadblocks for cellular reprogramming. Mol Cell. 2012;47:827–838. doi: 10.1016/j.molcel.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011 doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 53.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011 doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LIR, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Hongguang H, Loh Y-H, Aryee MJ, Lensch MW, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011 doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marro S, Pang ZP, Yang N, Tsai M-C, Qu K, Chang HY, Südhof TC, Wernig M. Direct Lineage Conversion of Terminally Differentiated Hepatocytes to Functional Neurons. Cell Stem Cell. 2011 doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Thier M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li VSW, Clevers H. In vitro expansion and transplantation of intestinal crypt stem cells. Gastroenterology. 2012;143:30–34. doi: 10.1053/j.gastro.2012.05.017. [DOI] [PubMed] [Google Scholar]