Abstract
Our genome is the blueprint for our bodies. A number of sophisticated mechanisms help protect our genome from life-threatening cellular mistakes and environmental insults. Much current research focuses on understanding these mechanisms, how they prevent disease, and whether they can be targeted for therapeutic purposes. Here, we review the main mechanisms maintaining genome integrity, how their malfunctioning results in disease, and the exciting progress toward targeting these mechanisms for cancer treatments.
Genome Instability
Maintaining the stability and the correct sequence composition of the three million bases that form our genome is critical for a correct transmission of genomic information. Unfortunately, our genome is under constant attack by agents that arise from either normal metabolism or exposures to natural or artificial products in the environment.1 As many as 105 lesions in DNA can occur per cell per day. DNA damage can result from side products of our normal metabolic activities, such as free radicals and reactive oxygen and nitrogen species, as well as from environmental factors such as UV radiation, X-rays, and chemical compounds.2 Our cells have evolved elegant mechanisms to cope with all these threats.3 Improper or inefficient repair of DNA damage causes mutations, abnormal chromosome structures, or loss of genetic information that could ultimately lead to a large number of human syndromes, including premature aging, various cancer predispositions, and genetic abnormalities.2,3 A large body of evidence has also implicated alterations in nuclear architecture and chromosome structure (so-called “epigenetic changes”) in genomic instability. Furthermore, defects in the structure of chromosome ends and in the integrity of the energy factories of our cells, the mitochondria, profoundly impact the stability of our genome. The degree of genomic instability caused by alterations in any of these mechanisms will determine whether the cell survives, undergoes a permanent growth arrest known as senescence, or dies.
The DNA Damage Response
The cell has evolved a complex pathway, termed the DNA damage response, to sense, signal, and ultimately repair DNA lesions arising from endogenous or exogenous insults.3 A variety of DNA repair mechanisms, with specificity towards different categories of DNA damage, have been identified. These include mechanisms devoted to repair single-stranded DNA lesions, such as those caused by UV light and certain chemicals, and mechanisms responsible for the repair of DNA double-strand breaks, such as those caused by X-rays and anti-tumor agents like cisplatin and mitomycin C. Homologous recombination (HR) and non-homologous end joining (NHEJ) are the two main pathways required to repair double-strand breaks. These two pathways have been extensively characterized due to the fact that these lesions are particularly dangerous to the cell.4 While NHEJ is an error-prone mechanism for DNA double-strand break repair, HR repairs the damage with great fidelity by utilizing sister chromatids as templates for recombination (See Figures 1 and 2).
Figure 1.
Top) Common endogenous and exogenous agents which generate different types of DNA lesions throughout the genome. Bottom) Mechanisms required for the repair of the different types of DNA lesions. Base-excision repair (BER) and nucleotide-excision repair (NER) are two mechanisms used to repair DNA single-strand breaks. HR, Homologous Recombination; NHEJ, non-homologous end-joining. 14-mer duplex DNA structure (pdb: 2M2C).
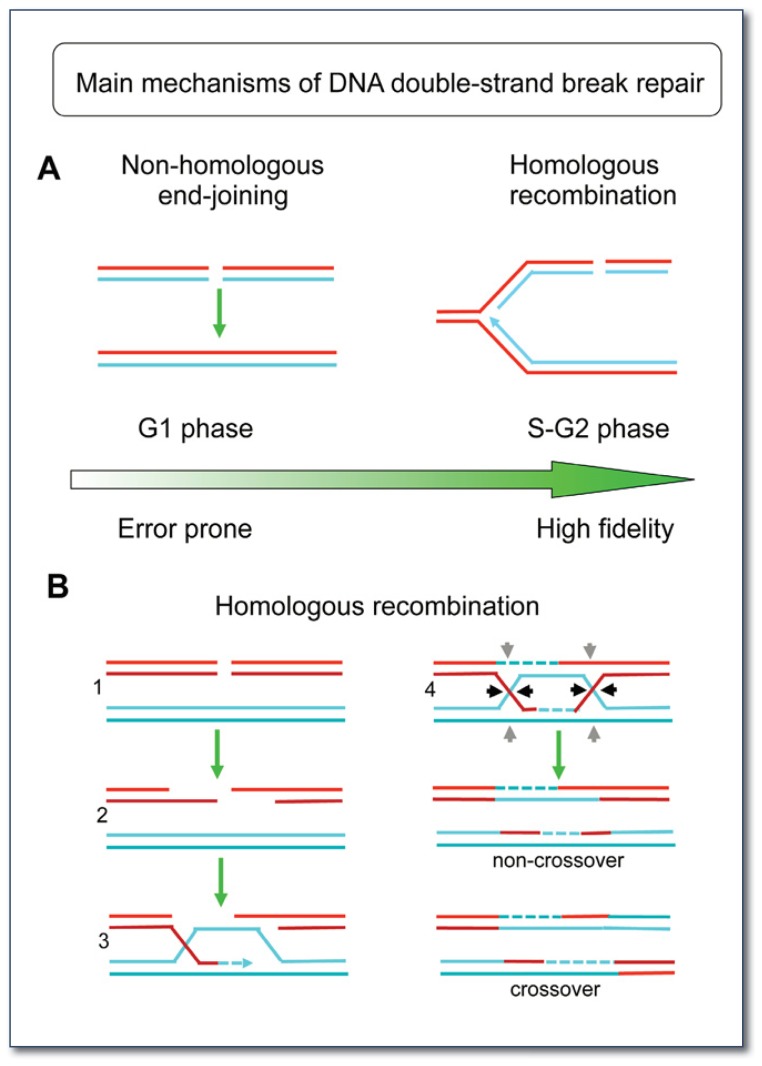
Figure 2.
A) Main double-strand break repair pathways.
NHEJ directly seals the broken DNA ends and occurs primarily during the G1 phase of the cell cycle. HR is a high-fidelity repair mechanism that occurs primarily during the S and G2 phases of the cell cycle and requires the information contained in the sister chromatid. The figure on the right schematically shows a replication fork with a double-strand break on one of the strands.
B) Schematic model for the HR mechanism.
1) A double-strand break is present on the DNA template.
2) HR is initiated by resection of a double-strand break to provide 3′ single-stranded DNA overhangs.
3) Strand invasion by these 3′ single-stranded DNA overhangs into a homologous sequence is followed by DNA synthesis at the invading end.
4) The second double-strand break end is captured to form an intermediate with two Holliday junctions (HJs). The structure is resolved at the HJs in non-crossover or crossover products by specific HJ resolvases (black and grey arrow heads).
DNA damage response is a complex pathway that mobilizes and recruits a variety of nuclear proteins to sites of DNA damage. Activation of this pathway can halt cell-cycle progression until the damage is restored, or initiate mechanisms of growth arrest or cell death if the damage is beyond repair.5 A growing list of factors has been clearly implicated in DNA damage response. These factors include sensors of the lesions that recruit and activate the ATM and ATR kinases at sites of damage. ATM is a master regulator that activates a variety of factors involved in DNA damage response and in cell cycle control. This process also facilitates changes in chromosome regions surrounding the break. These changes in turn permit the recruitment of the machinery required to repair DNA double-strand breaks. Two effectors have recently been in the spotlight due to their key roles in regulating the balance between the main pathways of double-strand break repair: the breast cancer type 1 protein (BRCA1), which promotes HR, and the tumor suppressor p53-binding protein 1 (53BP1), which facilitates NHEJ.6,7
Disease Associated With Genomic Instability
Almost invariably, mutations in DNA repair factors cause syndromes linked to genomic instability.8 For example, loss of ATM function causes the genome instability syndrome Ataxia-telangiectasia (A-T). A-T is a devastating disorder characterized by progressive neuromotor dysfunction, thymic atrophy and immunodeficiency, predisposition to lymphoid malignancies, and high sensitivity to DNA damage-inducing agents such as X-rays. Three other diseases, Xeroderma pigmentosa (XP), Cockayne syndrome (CS), and Trichothiodystrophy, are associated with defects in a specific single-strand break repair pathway.9 In addition, alterations in other factors with key roles in DNA replication stress response are linked to genomic instability syndromes, such as Fanconi anemia and Schimke immunoosseous dysplasia.10 RecQ helicases are an important family of DNA unwinding enzymes that play a key role in the maintenance of genome stability. Mutations in the genes of three human RecQ helicases are linked to defined genetic disorders associated with genomic instability, cancer predisposition, and features of premature aging; namely, Bloom syndrome (BLM gene mutations), Werner syndrome (WRN gene mutations), as well as Rothmund–Thomson, Rapadilino, and Baller-Gerold syndromes (all caused by mutation of RecQL4).11
In recent years, the generation of mouse models deficient in different DNA damage response pathways has provided important insight into the requirements for DNA repair pathways.8 In many instances, mice lacking different DNA repair factors die during embryonic development, and conditionally deficient mouse models have been developed to test the effects later in life. Mouse models with DNA repair deficiencies often develop premature aging phenotypes and cancer. One of the best-characterized models is the histone H2AX deficient mouse. H2AX is a key coordinator of DNA damage response and mice that lack this protein exhibit radiation sensitivity, growth retardation, immunodeficiency, and male infertility. These defects are associated with an impaired ability to assemble DNA repair factors at damaged sites.12 Similarly, mice that lack 53BP1 show growth retardation, chromosomal instability, increased radiosensitivity, immunodeficiency, and cancer susceptibility.13 Mice deficient in BRCA1 are embryonic lethal,14 whereas conditional BRCA1-deficient mouse models have consistently increased risk of developing cancer, especially when combined with mutations in other tumor suppressor proteins such as p53.
Genomic Instability and Cancer
Genomic instability has been most extensively studied in the context of cancer.9 Hereditary or acquired mutations targeting DNA repair genes are often associated with aberrant DNA replication and increased genomic instability, leading to a neoplastic phenotype. In particular, mutations or chromosome rearrangements can contribute to the loss of tumor suppressor functions, or abnormal activation of oncogenes, which in turn result in uncontrolled cell growth, the hallmark of cancer. BRCA1 is one of the best examples illustrating a direct link between DNA repair activities and tumor suppression: women born with a mutation in BRCA1 have a high risk of developing breast and ovarian cancer. Like BRCA1, loss of other DNA repair factors increase the risk of tumorigenesis, as well as the sensitivity to DNA damaging strategies such as radiation or chemotherapy. In addition, deregulation of mechanisms of chromosome segregation leads to an abnormal dosage of chromosomes or aneuploidy, often triggering growth arrest or cell death, or providing the cells with a proliferative advantage.
Genomic Instability and Cancer Treatment
The presence of defects in specific DNA repair pathways in most cancers has been exploited for the design of commonly used chemotherapy and radiotherapy regimens in the attempt to selectively target actively proliferating cancer cells. For example, platinum salts (carboplatin or cisplatin) are frequently given in combination with taxane paclitaxel to patients with advanced ovarian cancer. Platinum salts cause DNA inter- and intrastrand crosslinks that are repaired by a combination of mechanisms, including HR. These agents are particularly effective in patients with ovarian cancer because these tumors are commonly characterized by defects in key HR genes.15
An alternative approach based on inhibitors that directly target DNA damage response at the level of cell cycle regulation or DNA repair mechanisms has recently emerged as a new paradigm in cancer therapy.16 These inhibitors often enhance the effectiveness of radiotherapy and chemotherapy in cells, and are thus currently being tested in preclinical and clinical trials. Understanding the mechanism of action of these drugs is of high clinical relevance, and has allowed the identification of genetic defects in DNA metabolism pathways that can either confer resistance or sensitivity to certain chemotherapeutic agents. Knowing which DNA repair pathway is defective in a tumor will facilitate the design of the appropriate therapeutic regimen. However, inactivation of a specific DNA repair pathway is often compensated by the activation of an alternative pathway, which becomes essential for tumor cell survival. This dependence on an alternative mode of repair can be exploited therapeutically by “synthetic lethality” approaches. Synthetic lethality is a genetic concept that refers to the combined inactivation of two pathways to induce cell death when inactivation of each individual pathway is not effective.17 These new approaches have shown efficacy and reduced toxicity in preclinical studies, and hold great promise for cancer treatment as long as the defective pathways in a specific tumor can be identified. Thus, it is envisioned that these synthetic lethality approaches will need to be complemented by the discovery of robust biomarkers that allow the stratification of patients based on the type of DNA repair defects, so that the proper combination therapy can be delivered to the specific patient. These types of discoveries will bring us closer to individualized medicine.
New Frontiers for Cancer Treatment
Current research in our Department aims to understand the mechanisms of action of DNA replication and repair inhibitors with the ultimate goal of developing new drug combinations for cancer therapy. DNA topoisomerase I inhibitors, such as irinotecan and topotecan, could be regarded as the first generation of anticancer agents that target DNA replication. DNA topoisomerase I inhibitors are widely used for chemotherapy, but they are also highly toxic to many normal cell types. The mechanism of tumor response to DNA topoisomerase I inhibitors and the combination of DNA topoisomerase I inhibitors with other drugs for more effective tumor treatments is an area of vibrant investigation. Their cytotoxicity has been associated with their ability to generate single-strand breaks, which are eventually converted to double-strand breaks when the replication forks collide with the single-strand break.18 Recent studies provided new insight into the molecular basis of DNA topoisomerase I inhibitor cytotoxicity by showing that clinically relevant doses of DNA topoisomerase I poisons trigger a process called “fork reversal” where the replication forks back up, thus preventing the collision of the fork with the single-strand break generated by the DNA topoisomerase I inhibitor (See Figure 3).19 Following this discover y, our studies provided the first mechanistic insight into how this process occurs by showing that two DNA repair factors, the poly(ADP-ribose) polymerase 1 (PARP1) and the human RECQ1 helicase, play central roles in the accumulation and restart of reversed forks after DNA topoisomerase I inhibition.20 These studies provide a new rationale for the development of novel targeted inhibitors to sensitize cancer cells to lower DNA topoisomerase I inhibitor dosages that are not toxic to normal cells.20 Inducing fork reversal (DNA topoisomerase I inhibitors) and targeting the factors required for proper processing and restart of the reversed forks (PARP1 and RECQ1) should in principle synergize, thus increasing the DNA topoisomerase I inhibitor-sensitivity of cancer cells.
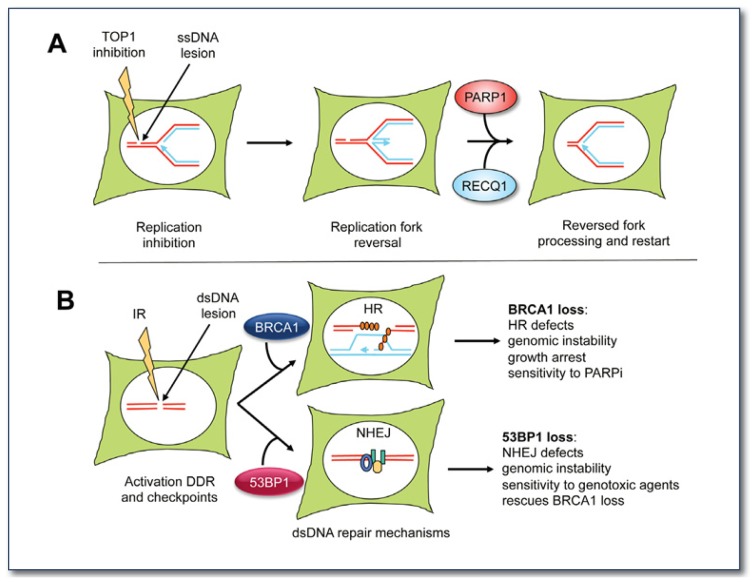
Figure 3.
A) Schematic model for the mechanism of replication fork reversal and restart upon TOP1 inhibition. The DNA replication fork is schematically shown in the white circle that represents the cell nucleus. TOP1 inhibitors trap TOP1 on the nicked DNA intermediate, thereby preventing repair of the ssDNA lesion, and causing the replication forks to reverse. RECQ1 is required to restart the replication forks reversed by TOP1 inhibition. PARP1 prevents premature restart of the reversed forks by inhibiting RECQ1 activity (for more details see 20).
B) Balance between DNA repair mechanisms of homologous recombination and non-homologous end-joining is important for genome stability. BRCA1 promotes HR and 53BP1 facilitates NHEJ. Loss of either BRCA1 or 53BP1 causes genomic instability and cancer predisposition, while loss of both ameliorates the phenotype. Recent studies showed that cancer cells deficient in BRCA1 lose 53BP1 to survive and resist therapy.
PARP inhibitors (olaparib and veliparib are the most extensively studied) have also received much attention as a promising therapeutic strategy for tumors that are deficient in the HR mechanism of DNA repair.21 A major breakthrough in the treatment of breast cancers with the poorest prognosis, BRCA1-deficient and triple-negative breast cancers, was the finding that these tumors are exquisitely sensitive to PARP inhibitors.22 Phase II studies with PARP inhibitors have shown a significant response rate in women carrying BRCA1 mutations with tolerable side effects.23 Furthermore, PARP inhibitors potentiate the antitumor activity of current therapeutic strategies, such as genotoxic agents and ionizing radiation. A significant fraction of advanced BRCA1-related cancers acquire resistance to PARP inhibitors. Landmark studies have demonstrated that loss of 53BP1 is “synthetically viable” with the loss of BRCA1 and induces resistance of BRCA1-deficient cells to PARP inhibitors.24 Targeting the degradation of 53BP1 represents a new strategy for breast cancer therapy. Interestingly, our recent studies identified a mechanism contributing to the loss of 53BP1 in breast cancers. In particular, activation of the protease cathepsin L was found to contribute to 53BP1 degradation in breast cancer cells.25 These findings provide a novel paradigm for the treatment of breast tumors that are resistant to PARP inhibitors due to the loss of 53BP1, such as the case of triple-negative and BRCA1 related cancers. In particular, inhibition of cathepsin L activity via treatment with vitamin D or specific cathepsin L inhibitors is expected to increase the levels of 53BP1, reduce proliferation, and restore the sensitivity of these tumors to PARP inhibitors and other DNA damaging strategies. These novel therapeutic strategies hold great promise for diagnosis, prognosis, and customization of breast cancer treatment.
Acknowledgments
We are grateful to Dr. Sergey Korolev for his help in the preparation of Figure 1 and to Dr. Joel Eissenberg for critical reading of the manuscript.
Biography
Alessandro Vindigni, PhD, (left) is an Associate Professor and Susan Gonzalo, PhD, (right) is an Assistant Professor in the Edward A. Doisy Department of Biochemistry and Molecular Biology at Saint Louis University School of Medicine.
Contact: avindign@slu.edu; sgonzalo@slu.edu


Footnotes
Disclosure
None reported.
References
- 1.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–15. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 2.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 3.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyman C, Kanaar R. DNA double-strand break repair: all’s well that ends well. Annu Rev Genet. 2006;40:363–83. doi: 10.1146/annurev.genet.40.110405.090451. [DOI] [PubMed] [Google Scholar]
- 5.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–54. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Matsuoka S, Carpenter PB, Elledge SJ. 53BP1, a mediator of the DNA damage checkpoint. Science. 2002;298:1435–8. doi: 10.1126/science.1076182. [DOI] [PubMed] [Google Scholar]
- 7.Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–75. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 8.Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–9. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- 9.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–85. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 10.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11:467–80. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickson ID. RecQ helicases: caretakers of the genome. Nat Rev Cancer. 2003;3:169–78. doi: 10.1038/nrc1012. [DOI] [PubMed] [Google Scholar]
- 12.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, et al. Genomic instability in mice lacking histone H2AX. Science (New York, NY. 2002;296:922–7. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward IM, Minn K, van Deursen J, Chen J. p53 Binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol Cell Biol. 2003;23:2556–63. doi: 10.1128/MCB.23.7.2556-2563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evers B, Jonkers J. Mouse models of BRCA1 and BRCA2 deficiency: past lessons, current understanding and future prospects. Oncogene. 2006;25:5885–97. doi: 10.1038/sj.onc.1209871. [DOI] [PubMed] [Google Scholar]
- 15.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–9. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 16.Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer. 2012;12:801–17. doi: 10.1038/nrc3399. [DOI] [PubMed] [Google Scholar]
- 17.Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–98. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 18.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 19.Ray Chaudhuri A, Hashimoto Y, Herrador R, Neelsen KJ, Fachinetti D, Bermejo R, et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat Struct Mol Biol. 2012;19:417–23. doi: 10.1038/nsmb.2258. [DOI] [PubMed] [Google Scholar]
- 20.Berti M, Ray Chaudhuri A, Thangavel S, Gomathinayagam S, Kenig S, Vujanovic M, et al. Human RECQ 1 promotes restart of replication forks reversed by DNA topoisomerase I inhibition. Nat Struct Mol Biol. 2013 doi: 10.1038/nsmb.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 22.Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785–90. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- 23.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–51. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 24.Cao L, Xu X, Bunting SF, Liu J, Wang RH, Cao LL, et al. A selective requirement for 53BP1 in the biological response to genomic instability induced by Brca1 deficiency. Mol Cell. 2009;35:534–41. doi: 10.1016/j.molcel.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grotsky DA, Gonzalez-Suarez I, Novell A, Neumann MA, Yaddanapudi SC, Croke M, et al. BRCA1 loss activates cathepsin L-mediated degradation of 53BP1 in breast cancer cells. J Cell Biol. 2013;200:187–202. doi: 10.1083/jcb.201204053. [DOI] [PMC free article] [PubMed] [Google Scholar]