Abstract
Understanding the variability of marine biodiversity is a central issue in microbiology. Current observational programs are based on in situ studies, but their implementation at the global scale is particularly challenging, owing to the ocean extent, its temporal variability and the heterogeneity of the data sources on which compilations are built. Here, we explore the possibility of identifying phytoplanktonic biodiversity hotspots from satellite. We define a Shannon entropy index based on patchiness in ocean color bio-optical anomalies. This index provides a high resolution (1 degree) global coverage. It shows a relation to temperature and mid-latitude maxima in accordance with those previously evidenced in microbiological biodiversity model and observational studies. Regional maxima are in remarkable agreement with several known biodiversity hotspots for plankton organisms and even for higher levels of the marine trophic chain, as well as with some in situ planktonic biodiversity estimates (from Atlantic Meridional Transect cruise). These results encourage to explore marine biodiversity with a coordinated effort of the molecular, ecological and remote sensing communities.
Keywords: biodiversity, biogeography, remote sensing, global oceans, stirring
The question of how the diversity of marine microorganisms is distributed over the global ocean is of primary ecological relevance, as well as a fundamental issue for understanding biogeochemical and climatic processes. In the open ocean, however, boundaries for marine biodiversity hotspots are difficult to trace because of the multiplicity of ecological and physical scales involved in shaping oceanic ecosystems (Levin et al., 1992). In particular, at the mesoscale (10–100 kms), environmental parameters and physical barriers change on the same time scale of the primary producer's demography (d′Ovidio et al., 2010).
Plankton biogeography and biodiversity is commonly addressed with in situ sampling. Species observed at a given location can be characterized at the morphological, functional, genetic and genomic levels, while the explanation for their presence can be searched in the local physico-chemical context (Jones et al., 2007). Even if an increasing number of campaigns and fixed station recordings apply a whole spectrum of up-to-date techniques to get a fine-tuned image of the microbial community (Rusch et al., 2007; Karsenti et al., 2011, Zinger et al., 2011), the extensive use of in situ measures to monitor biodiversity at the global scale appears overwhelming.
Remote sensing of ocean color is an established tool to observe the global distribution of phytoplankton. Beyond the estimation of chlorophyll concentration, different spectral analyses have been developed for characterizing phytoplankton biogeography at a resolution of few tens of kilometers and few days (Supplementary Information). Among those, the PHYSAT algorithm (Alvain et al., 2008; Inter Deposit Digital Number IDDN.FR.001.330003.000.S.P.2012.000.30300) clusters spectral anomalies into classes of bio-optical properties. These classes correlate with the dominant phytoplankton types (see Supplementary Information for details) and provide a simple means for identifying niches that sustain distinct planktonic communities (d'Ovidio et al., 2010).
Here we propose to combine information on biological heterogeneity and fluid dynamics in order to estimate putative biodiversity hotspots. We use patchiness in maps of bio-optical anomalies as an indicator of niche spatial heterogeneity at a given time, and assume that patches that are about 100 kms apart are typically mixed within a few weeks by oceanic turbulence (d'Ovidio et al., 2010). With these assumptions (see Supplementary Information for details), we suggest an index based on remote sensing as a proxy for diversity induced by horizontal stirring, and show that such an index is consistent with many features of in situ and model studies of global biodiversity patterns in the open ocean.
We consider 1-week global PHYSAT composites at 9 km resolution and define, for a focal point of the ocean surface, the index:
 |
The frequency pi is the fraction of pixels, within a disk centered in the focal point, falling in the i-th bio-optical class. This is an area-based Shannon entropy of the distribution of bio-optical classes. It is maximal when these are all present and evenly distributed inside the disk, and minimal when only one class is observed. We choose a radius of 100 kms because this is sufficiently large for generating a statistically meaningful frequency distribution, and sufficiently small for stirring to mix the disk content on the time scale of a planktonic bloom (see Supplementary Information for further discussion).
The global map of τ (Figure 1a) presents clear global patterns that extend across oceanic basins. High values of τ occur both in productive systems (upwellings; western boundary currents; islands' wakes; North Sea) and in moderately productive and oligotrophic regions (SW and NE Pacific; North Pacific transition zone; see Figure 1b).
Figure 1.
(a) Index τ for the global ocean: average of daily maps of τ computed over 7-day composites of PHYSAT data for the period 2003–2010. The hotspots emerging from this analysis characterize regions with a standing representation of several nearby communities, as identified by their dominant phytoplankton types (PHYSAT algorithm applied to SeaWiFS radiances). (b) Average of daily chlorophyll-a maps (SeaWiFS, 2003–2010).
Are hotspots of τ consistent with known global patterns of biodiversity? We outline here the comparison with previous studies on oceanic biodiversity, and refer to the Supplementary Information for a more detailed discussion, additional references and Supplementary Figures.
Regions of high values of τ largely overlap with biodiversity hotspots of primary producers (Barton et al., 2010) and even higher trophic levels (Tittensor et al., 2010; Block et al., 2011). When zonally averaged (Figure 1a), τ shows the intermediate latitude maxima that are consistently reported in observational and model biodiversity studies (Barton et al., 2010; Kaschner et al., 2011; Raes et al., 2011). Moreover, the comparison with in situ data from the 1996 AMT cruise (Irigoien et al., 2004; Cermeño et al., 2008) shows that nano- and microplankton biodiversity has, out of upwelling regions, a latitudinal band structure in agreement with the τ index computed at the sampling site locations (Supplementary Information).
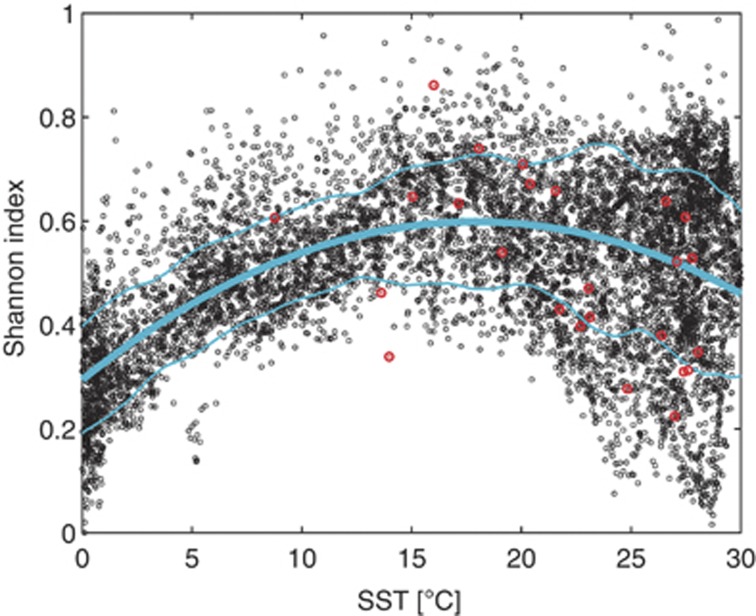
A common pattern of biodiversity measured along environmental gradients is the decrease in diversity with lowering temperature or increasing latitude (Hillebrand, 2004). The τ index displays a highly significant positive correlation with sea surface temperature (Figure 2, R2=0.26, P<10−4), with a unimodal relation consistent with what is previously documented (Rutherford et al., 1999; Worm et al., 2005; Tittensor et al., 2010). Such relationship also agrees with the predictions of evolutionary models based on metabolic theory (Stegen et al., 2012). See Supplementary Information, section 4 for details.
Figure 2.
Time-averaged index τ versus average sea surface temperature at the same location (AMSR-E, 2003–2010). The cyan lines are the best quadratic fit to the data (R2=0.26, P<10−4) and one s.d. of the residuals. The red points are the Shannon index for nano- and microplankton, from measures of the AMT-2 cruise and rescaled so that the two data sets have the same mean and s.d. (see Supplementary Information for further details).
Ocean color-derived products provide global-scale information with high spatiotemporal resolution and degree of synopticity. Such information can be potentially used to define other remotely sensed proxies of biodiversity. Together with the index we propose here, such proxies would contribute to optimize sampling strategies, and to lay out the ecological context of in situ observations. Moreover, 30 years of ocean color data offer the opportunity of studying trends in biodiversity patterns from a new, global perspective. This work shows that satellite products may contain untapped potential for biodiversity studies. However, only a concerted effort among the satellite and marine biology communities will allow to assess the power of satellite-based indexes in resolving biodiversity patterns.
Acknowledgments
We thank NASA and the GLOBCOULOUR project for access to ocean color data and AMSR-E SST data. This study uses data from the Atlantic Meridional Transect consortium (NER/0/5/2001/000680) provided by the British Oceanographic Data Centre, and supported by the Natural Environment Research Council. SDM was supported by the ANR blanc program Phytback and by the EU 7FP MicroB3. AS is supported by the EMERGENCE EME111 UPMC project. This work is a contribution to the OSTST ALTIMECO project.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Alvain S, Moulin C, Dandonneau Y, Loisel H. Seasonal distribution and succession of dominant phytoplankton groups in the global ocean: a satellite view. Global Biogeochem Cy. 2008;22:GB3001. [Google Scholar]
- Barton AD, Dutkiewicz S, Flierl G, Bragg J, Follows MJ. Patterns of diversity in marine phytoplankton. Science. 2010;327:1509–1511. doi: 10.1126/science.1184961. [DOI] [PubMed] [Google Scholar]
- Block BA, Jonsen ID, Jorgensen SJ, Winship AJ, Shaffer SA, Bograd SJ, et al. Tracking apex marine predator movements in a dynamic ocean. Nature. 2011;475:86–90. doi: 10.1038/nature10082. [DOI] [PubMed] [Google Scholar]
- Cermeño P, Marañón E, Harbour D, Figueiras GF, Crespo GB, Huete-Ortega M, et al. Resource levels, allometric scaling of population abundance, and marine phytoplankton diversity. Limnol Oceanogr. 2008;53:312–318. [Google Scholar]
- d′Ovidio F, De Monte S, Alvain S, Dandonneau Y, Lévy M. Fluid dynamical niches of phytoplankton types. PNAS. 2010;107:18366–18370. doi: 10.1073/pnas.1004620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrandt H. On the generality of the latitudinal diversity gradient. Am Nat. 2004;163:192–211. doi: 10.1086/381004. [DOI] [PubMed] [Google Scholar]
- Irigoien X, Huisman J, Harris RP. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature. 2004;429:863–867. doi: 10.1038/nature02593. [DOI] [PubMed] [Google Scholar]
- Jones SE. The marine microbial world: a voyage of discovery. ISME J. 2007;1:469–470. doi: 10.1038/ismej.2007.72. [DOI] [PubMed] [Google Scholar]
- Karsenti E, Acinas SG, Bork P, Bowler C, De Vargas C, Raes J, et al. A holistic approach to marine eco-systems biology. PLoS Biol. 2011;9:e1001177. doi: 10.1371/journal.pbio.1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschner K, Tittensor DP, Ready J, Gerrodette T, Worm B. Current and future patterns of global marine mammal biodiversity. PLoS ONE. 2011;6:e19653. doi: 10.1371/journal.pone.0019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin S. The problem of scale in biology. Ecology. 1992;73:1943–1967. [Google Scholar]
- Raes J, Letunic I, Yamada T, Jensen LJ, Bork P. Toward molecular trait-based ecology through integration of biogeochemical, geographical and metagenomic data. Mol Syst Biol. 2011;7:473. doi: 10.1038/msb.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch DB, Halpern AL, Sutton G, Heidelberg KB, Williamson S, Yooseph S, et al. The Sorcerer II global ocean sampling expedition: Northwest Atlantic through Eastern Tropical Pacific. PLoS Biol. 2007;5:e77. doi: 10.1371/journal.pbio.0050077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S, D'Hondt S, Prell W. Environmental controls on the geographic distribution of zooplankton diversity. Nature. 1999;400:749–753. [Google Scholar]
- Stegen JC, Ferriere R, Enquist BJ. Evolving ecological networks and the emergence of biodiversity patterns across temperature gradients. Proc R Soc B. 2012;279:1051–1060. doi: 10.1098/rspb.2011.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittensor DP, Mora C, Jetz W, Lotze HK, Ricard D, Berghe EV, et al. Global patterns and predictors of marine biodiversity across taxa. Nature. 2010;466:1098–1101. doi: 10.1038/nature09329. [DOI] [PubMed] [Google Scholar]
- Worm B, Sandow M, Oschlies A, Lotze HK, Myers RA. Global patterns of predator diversity in the open oceans. Science. 2005;309:1365–1369. doi: 10.1126/science.1113399. [DOI] [PubMed] [Google Scholar]
- Zinger L, Amaral-Zettler LA, Fuhrman JA, Horner-Devine MC, Huse SM, DBM Welch, et al. Global patterns of bacterial beta-diversity in seafloor and seawater ecosystems. PLoS One. 2011;6:e24570. doi: 10.1371/journal.pone.0024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.