Abstract
Contrary to expectation, populations of clonal organisms are often genetically highly diverse. In phytoplankton, this diversity is maintained throughout periods of high population growth (that is, blooms), even though competitive exclusion among genotypes should hypothetically lead to the dominance of a few superior genotypes. Genotype-specific parasitism may be one mechanism that helps maintain such high-genotypic diversity of clonal organisms. Here, we present a comparison of population genetic similarity by estimating the beta-dispersion among genotypes of early and peak bloom populations of the diatom Asterionella formosa for three spring-blooms under high or low parasite pressure. The Asterionella population showed greater beta-dispersion at peak bloom than early bloom in the 2 years with high parasite pressure, whereas the within group dispersion did not change under low parasite pressure. Our findings support that high prevalence parasitism can promote genetic diversification of natural populations of clonal hosts.
Keywords: beta-dispersion, chytrid, diatom, genetic diversity, parasitism
Populations of (predominantly) clonal organisms, such as diatoms, are often genetically highly diverse (Evans et al., 2009), even throughout blooms (Rynearson and Armbrust, 2005). Over the short periods of time within which diatom spring-blooms develop, abiotic variation is limited, and competition between genotypes should lead to numerical dominance of few superior genotypes driven by genotype sorting (De Meester, 1996). But biotic interactions, such as parasitism, may increase host population genetic dissimilarity through time-lagged, negative frequency-dependent selection (Bell, 1982), if parasites adapt to- and select against the most common genotypes (Hardin, 1968). This can lead to an increase in genotypic evenness of host populations in infected systems compared with noninfected ones (Wolinska and Spaak, 2009). To our knowledge, no field study has yet described an increase in genetic dissimilarity in asexual host populations as they are being invaded by a parasite epidemic.
Materials and methods
In Lake Maarsseveen (The Netherlands), the diatom Asterionella formosa Hassall regularly forms spring-blooms, which are followed by high prevalence parasite epidemics of its fungal parasite, the chytrid Zygorhizidium planktonicum Canter (Van Donk and Ringelberg, 1983; Ibelings et al., 2011). The Asterionella population is genetically very diverse (De Bruin et al., 2004) even though it is presumed to reproduce (predominantly) clonally. Sexual reproduction has been hypothesized, but never observed (Mann, 1988). Moreover, gene flow is expected to be negligible as Lake Maarsseveen is hydrologically isolated, and as the Asterionella population is genetically distinct from that of lakes close-by (De Bruin, 2006). Differential susceptibility of host genotypes to the chytrid was shown experimentally (De Bruin et al., 2004), suggesting that this parasite can act as selection agent.
The population development of host and parasites was monitored in weekly intervals during 3 years (2008–2010). Each spring, genetic diversity of the early and the peak bloom population was sampled at 5 m depth in the pelagic zone of Lake Maarsseveen. Single Asterionella colonies were isolated from these six subpopulations (3 years, early and peak bloom), cultured and amplified fragment length polymorphism (AFLP) fingerprinted (Vos et al., 1995), for more details on methods see Supplementary Material. A total of 65 isolates were fingerprinted (2008: n=25 (13 early, 12 peak), 2009: n=26 (13 early, 13peak) and 2010: n=14 (5 early, 9 peak)). To measure the changes in genetic similarity of the host population, we assessed beta-diversity among all subpopulations (Figure 2a) and the homogeneity of beta-dispersion for early and peak subpopulation of each year (Figure 2b) by permutational analysis of variance (Anderson et al., 2006) based on pair-wise Jaccard distances (Jaccard, 1901). Beta-dispersion calculates distances from observations to their group centroids and then compares the average of these distances among groups. P-values were obtained by 999 permutations of the raw data using R package vegan (R, 2011).
Results
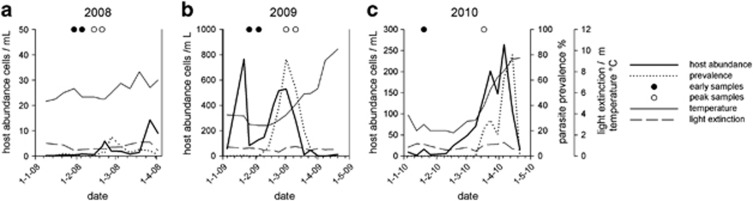
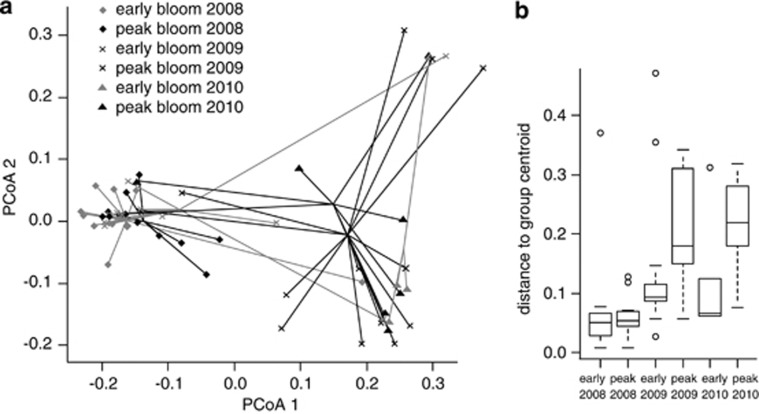
The spring of 2008 showed no clear host bloom or parasite epidemic (Figure 1a). Asterionella was present in low numbers (maximum 14 cells ml−1) and parasitized at low levels (maximum prevalence 15%). The 2009 and 2010 spring-blooms (Figures 1b and c) showed more typical population maxima (2009: 530 cells ml−1; and 2010: 264 cells ml−1) and were followed by high prevalence epidemics (maximum prevalence 2009: 77% and 2010: 80%) (Ibelings et al., 2011). The AFLP analysis yielded on average 32.0±2.2 marker bands per primer pair (128 marker bands; 87.5% polymorphism). Two genotypes occurred twice, all other isolates showed unique fingerprints. Although the six group centroids differed significantly (F5,64=6.47, P<0.001), temporally consecutive populations overlapped to large extent (Figure 2a), suggesting that the host population was changing over time but remained coherent. The beta-dispersion among all subpopulations was nonhomogeneous (F5,59=3.71, P=0.005). Within a year, analysis of beta-dispersion showed a significantly larger dispersion in the peak bloom subpopulation than in the early bloom subpopulations in the heavily parasitized years 2009 and 2010; 2009: F1,25=5.38, P=0.029; and 2010: F1,13=14.33, P=0.002 (Figure 2b). However, the beta-dispersion of early and peak bloom subpopulation of the low parasitized 2008 bloom showed no difference: F1,24=1.33, P=0.24 (Figure 2b).
Figure 1.
Host abundance (solid line) and parasite prevalence (dotted line) over the first 4 months of the years 2008–2010 at 5 m depth in Lake Maarsseveen. The sampling events for population genetic diversity samples (n=1 or 2 for each subpopulation) are indicated for early bloom (black circles) and peak bloom (empty circles). In 2008 (a), Asterionella population density and parasite prevalence in spring remain low, and winter and spring surface water temperatures remain relatively high. In 2009 and 2010 (b and c), the Asterionella spring-blooms show more typical time courses and are followed by high prevalence parasite epidemics. Winter and spring surface water temperatures start off cooler but increase over time. Light extinction shows no particular pattern over the observed time period.
Figure 2.
(a) Two-dimensional representation of the principal coordinates analysis based on Jaccard's similarities among AFLP fingerprints of genotypes: gray symbols represent members of the early bloom populations and black symbols represent members of the peak bloom populations. The plot shows how the subpopulation centroids shift over time, however, temporally subsequent subpopulations show considerable overlap. (b) Boxplot of beta-dispersion per subpopulation, based on the distances of each genotype to its respective group centroid. The thick line represents the median, the limits of the lower and upper box are 25 and 75 percentile, the bars extend to the most extreme values (excluding outliers), points indicate the position of putative outliers. The beta-dispersion of early and peak bloom subpopulation shows no significant differences in the low parasitized year 2008. However, the beta-dispersion of the peak bloom subpopulations in heavily parasitized years 2009 and 2010 is significantly larger than that of their respective early bloom subpopulation.
Conclusions
What mechanisms may maintain host genetic diversity is particularly relevant in spring-bloom phytoplankton species as they form the base of lake food webs (Sommer et al., 2012). Genetically, more diverse host populations are known to be more resistant to disease than genetically poor ones (Altermatt and Ebert, 2008; Whitehorn et al., 2011), as higher host diversity hampers adaptation of the parasite (De Bruin et al., 2008). Paradoxically, high-parasite pressure is also expected to increase host genetic diversity through frequency-dependent selection (Little, 2002). Parasite epidemics exert a short-lived albeit strong selective force, whereas hosts are under different selection forces during the rest of the year. However, our results support the idea that high prevalence parasitism can drive genetic diversification in natural host populations, which then again serves as raw material for selection processes throughout the rest of the year.
Acknowledgments
We thank Nico Helmsing and Koos Swart for fieldwork support and Suzanne Naus-Wiezer for laboratory assistance. Also, we thank three anonymous reviewers for their constructive and thoughtful comments. ASG is supported by grant 816.01.018 of the Dutch Organization for Scientific Research—Earth and Life Science Foundation (NWO-ALW) to BWI and EvD. LNdSD is supported by grant 817.01.007 of NWO-ALW.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Altermatt F, Ebert D. Genetic diversity of Daphnia magna populations enhances resistance to parasites. Ecol Letts. 2008;11:918–928. doi: 10.1111/j.1461-0248.2008.01203.x. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Ellingsen KE, McArdle BH. Multivariate dispersion as a measure of beta diversity. Ecol Lett. 2006;9:683–693. doi: 10.1111/j.1461-0248.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- De Bruin A, Ibelings BW, Kagami M, Mooij WM, Van Donk E. Adaptation of the fungal parasite Zygorhizidium planktonicum during 200 generations of growth on homogeneous and heterogeneous populations of its host, the diatom Asterionella formosa. J Eukaryot Microbiol. 2008;55:69–74. doi: 10.1111/j.1550-7408.2008.00306.x. [DOI] [PubMed] [Google Scholar]
- De Meester L. Local genetic differentiation and adaptation in freshwater zooplankton populations: Patterns and processes. Ecoscience. 1996;3:385–399. [Google Scholar]
- Bell G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. Croom Helm Ltd.: London, UK; 1982. [Google Scholar]
- De Bruin A.2006The potential for coevolution in a aquatic host-parasite systemPhD thesis, Aquatic Ecology. Radboud University: Nijmegen, The Netherlands [Google Scholar]
- Evans KM, Chepurnov VA, Sluiman HJ, Thomas SJ, Spears BM, Mann DG. Highly differentiated populations of the freshwater diatom Sellaphora capitata suggest limited dispersal and opportunities for allopatric speciation. Protist. 2009;160:386–396. doi: 10.1016/j.protis.2009.02.001. [DOI] [PubMed] [Google Scholar]
- De Bruin A, Ibelings BW, Rijkeboer M, Brehm M, van Donk E. Genetic variation in Asterionella formosa (Bacillariophyceae): is it linked to frequent epidemics of host-specific parasitic fungi. J Phycol. 2004;40:823–830. [Google Scholar]
- Hardin G. Tragedy of commons. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- Jaccard P. Étude comparative de la distribution florale dans une portion des Alpes et des Jura. Bull Soc Vaud Sci Nat. 1901;37:547–579. [Google Scholar]
- Ibelings BW, Gsell AS, Mooij WM, Van Donk E, Van Den Wyngaert S, De Senerpont Domis LN. Chytrid infections and diatom spring blooms: paradoxical effects of climate warming on fungal epidemics in lakes. Freshw Biol. 2011;56:754–766. [Google Scholar]
- Little TJ. The evolutionary significance of parasitism: do parasite-driven genetic dynamics occur ex silico. J Evol Biol. 2002;15:1–9. [Google Scholar]
- R Development Core Team R Foundation for Statistical Computing. Vienna, Austria. 2011.
- Mann DG, Round FE. Why didn't Lund see sex in Asterionella? A discussion of the diatom life cycle in nature. Algae and the aquatic environment. 1988;29:385–412. [Google Scholar]
- Rynearson TA, Armbrust EV. Maintenance of clonal diversity during a spring bloom of the centric diatom Ditylum brightwellii. Mol Ecol. 2005;14:1631–1640. doi: 10.1111/j.1365-294X.2005.02526.x. [DOI] [PubMed] [Google Scholar]
- Van Donk E, Ringelberg J. The effect of fungal parasitism in the succession of diatoms in Lake Maarsseveen 1 (The Netherlands) Freshw Biol. 1983;13:241–251. [Google Scholar]
- Sommer U, Adrian R, De Senerpont Domis L, Elser JJ, Gaedke U, Ibelings B, et al. Beyond the PEG-model: mechanisms driving plankton succession. Annu Rev Ecol Evol Syst. 2012;43:429–448. [Google Scholar]
- Vos P, Hogers R, Bleeker M, Reijans M, Vandelee T, Hornes M, et al. AFLP—a new technique for DNA-fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinska J, Spaak P. The cost of being common: evidence from natural Daphnia populations. Evolution. 2009;63:1893–1901. doi: 10.1111/j.1558-5646.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- Whitehorn PR, Tinsley MC, Brown MJF, Darvill B, Goulson D. Genetic diversity, parasite prevalence and immunity in wild bumblebees. Proc Biol Sci. 2011;278:1195–1202. doi: 10.1098/rspb.2010.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.