Contents
Our objective was to examine the influences of differing media, protein supplementation and the microenvironment on cat vs dog primordial follicle viability in vitro. Ovarian cortical slices were cultured for 3, 9 or 15 days in α-minimum essential medium (α-MEM) or MEM supplemented with 10% fetal bovine serum (FBS), 10% knock-out serum replacement (KSR) or 0.1% polyvinyl alcohol (protein free). In a separate study, cat and dog ovarian tissues were cultured in protein-free α-MEM and MEM, respectively, in cell culture inserts, on 1.5% agarose gel or in 24-well cell culture plates (control). Follicle viability was assessed in both studies using calcein AM/ethidium homodimer and histological evaluation with haematoxylin/eosin staining. No cat follicle sustained viability beyond 9 days of in vitro culture in α-MEM compared to 37.5% of those incubated for 15 days in MEM in protein-free condition (p < 0.05). In contrast, α-MEM was superior (p < 0.05) to MEM in maintaining dog follicle viability (32.7% vs 8.1%) in protein-free condition at 15 days. Serum was detrimental (p < 0.05) to follicle survival in both species. Knock-out serum replacement supplementation and a protein-free condition supported cat follicle viability, whereas the latter was superior (p < 0.05) to the former for sustaining dog follicle survival. Likewise, dog follicle viability was enhanced (p < 0.05) by the agarose gel compared to the cell culture insert and control groups after 3 and 9 days of culture. For the cat, the agarose gel better (p < 0.05) supported follicle viability compared to the control, but was equivalent to the cell culture insert. Therefore, sustaining primordial follicle survival from intracortical ovarian slices requires a different in vitro microenvironment for the cat vs the dog. A key factor to enhancing survival of these early stage follicles in culture appears to be the use of agarose gel, which enhances follicle viability, perhaps by promoting gas exchange.
Introduction
Mammalian ovaries, including those of the cat and dog, contain thousands of follicles/oocytes that never mature, eventually degenerate or die with the host and, thus, are a valuable, but wasted resource. The ability to exploit this germplasm would offer endless opportunities for preserving the fertility and genetic diversity of valuable individuals, populations or entire species, including those that are endangered. More oocytes could be made available if there were consistently reliable means to provoke follicle development and ovulation, especially in the dog (Songsasen and Wildt 2007). However, an alternative would be to develop an approach for artificially growing in vitro a proportion of the thousands of primordial follicles available per ovary. It then would be possible to create a vast resource of oocytes that could be used for in vitro fertilization or stored until needed. Another benefit would be the applicability to animals of diverse ages, including pre-pubertal individuals that die before having the opportunity to reproduce.
The biological feasibility of this approach has been demonstrated in the mouse (Eppig and O’Brien 1996). In that study, primordial follicles in newborn mouse ovarian cortical tissue were grown by organ culture for 8 days and then isolated follicles further matured to produce mature oocytes that were fertilizable in vitro. Of the 190 two-cell embryos produced, two resulted in pups. Although offspring have not been produced from in vitro-grown oocytes in larger-size animals, including in the cat (Jewgenow 1998) and dog (Songsasen and Wildt 2007), a wealth of information has emerged on the sensitivity of such immature follicles to the microenvironment. For example, it is now well known that constituents of the culture medium significantly influence growth and viability of ovarian follicles (Hovatta et al. 1997; Jewgenow 1998). More specifically, Earle’s balanced salt solution is superior to α-minimum essential medium (α-MEM) in maintaining in vitro viability of human primordial follicles (Hovatta et al. 1997). Historically, MEM has been one of the most widely used media for culturing cat ovarian follicles and oocytes (Johnston et al. 1993). Eagle’s α-MEM, which is a rich resource for non-essential amino acids, has been used with some success to advance pre-antral follicle culture in the dog (Serafim et al. 2010; Songsasen et al. 2011).
Blood serum also has been commonly included as a protein supplement in follicle or oocyte culture systems (Eppig and O’Brien 1996; Lane and Gardner 2007). Serum as a source of albumin balances osmolarity and removes precursors of free-oxygen radicals (Freshney 2005). But, serum is comprised of many undefined factors, some of which can be detrimental to gamete and embryo development. For example, serum supplementation in culture has been shown to alter oocyte metabolism and gene expression profiles as well as cause excessive fetal size and pregnancy complications after embryo transfer in some species (Lane and Gardner 2007). One protein supplement substitute, a knock-out serum replacement (KSR, proprietary formulation), has been found to be superior for boosting the proportion of cattle blastocysts produced in vitro as well as increasing pregnancy success after embryo transfer (Moore et al. 2007). KSR was originally developed for culturing mouse embryonic stem cells and, more recently, has been determined to be superior to fetal calf serum in establishing embryonic germ cells from mouse primordial germ cells while effectively preventing differentiation (Horii et al. 2003).
In terms of the actual culture system, ovarian tissue traditionally has been incubated in multi-well plates (Matos et al. 2007) or in a cell culture insert system (Eppig and O’Brien 1996). Compared to the former, the latter prevents tissues from attaching to the dish surface while increasing the surface area available for nutrient exchange in medium (Freshney 2005). Recently, an agarose gel system has been developed for culturing mouse testicular tissue, which maintains normal cellular structure while promoting spermatogenesis in vitro (Gohbara et al. 2010). The advantage of this approach is that tissues are placed on an agarose gel saturated with culture medium, thereby offering a liquid/gas interface that apparently facilitates oxygen and nutrient exchange. Interestingly, this same agarose gel tactic was demonstrated more than 50 years ago to maintain immature rat ovarian tissue structure for 9 days (Trowell 1959). Since that early experiment, this method has not been re-applied to culturing ovarian tissue from other mammals.
The overall goal of the present study was to understand in vitro culture conditions that could sustain cat and dog primordial follicle survival in vitro. Although both are carnivores, these species differ markedly in fundamental reproductive physiology. Most of this variation has been demonstrated at the macro-level, especially in terms of oestrous cyclicity and stimulus, timing and endocrine control of ovulation (Wildt et al. 1981). There is little information available on the specifics of intraovarian follicular (and oocyte) sensitivity to ex situ culture, especially in pre-antral stages. Therefore, we were keen to conduct a comparative study in the cat vs the dog to determine similarities, and differences, in how primordial follicles within the ovarian cortex react to varied in vitro culture conditions. Our specific objectives were to determine the influences of (i) culture medium, (ii) protein supplementation and (iii) the microenvironment itself on subsequent in vitro viability of primordial follicles from freshly excised tissue slices.
Materials and Methods
Chemicals
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise indicated.
Sources
Ovaries (n = 20 pairs/species) were excised from domestic cats and dogs (age 5 month–1.5 years) undergoing routine ovariohysterectomy at local veterinary and spay clinics. Freshly collected ovaries were kept at 4°C in L-15 medium (Kreeger et al. 2005) with 25 mM HEPES, 100 μg/ml penicillin G sodium and 100 μg/ml streptomycin sulphate and transported to the laboratory within 1–6 h of surgery.
In vitro ovarian tissue culture
Ovarian cortical slices (0.5–1 mm thick) were dissected from the surface of each ovary and then sectioned in equal pieces (1–1.5 mm width). Tissues were incubated at 38.5°C in 5% CO2 in humidified air in different culture conditions (see below) for up to 15 days. Half of the volume of culture medium was changed every 3 days throughout the entire study interval.
Assessment of follicle viability
Follicle viability within the ovarian cortical tissues was evaluated after 0 (fresh control; day of tissue excision and initial incubation), 3, 9 and 15 days of in vitro culture using calcein AM/ethidium homodimer-1 staining (Invitrogen, Carlsbad, CA, USA) (Songsasen et al. 2011) and observation under a fluorescent microscope (Olympus BX40; Olympus America Inc., Central Valley, PA, USA). Follicles were considered viable when the oocyte and surrounding granulosa cells fluoresced green by calcein. Forty to 100 primordial follicles were observed for each ovarian piece. For all treatments and for the entire study, 8943 and 5450 follicles were examined in cat and dog, respectively.
Histological analysis and classification of primordial follicle structure
Pieces of fresh or cultured ovarian tissues (Days 3 and 15; n ≥ 3 cortical pieces/group/culture period) were fixed in Bouin’s solution, maintained at 4°C overnight, dehydrated in a graded series of ethanol solutions, clarified with d-Limonene (EK Industries, Joliet, IL, USA) and then embedded in paraffin. Serial sections (thickness, 5 μm) of each cortical piece were cut and stained with haematoxylin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and eosin.
Three serial sections from the largest cross section through the centre of each cortical piece in each culture group were assessed for follicle ‘class’. Primordial follicles within the tissue were morphologically characterized as ‘normal’, where the nucleus of the oocyte and surrounding granulosa cells were structurally intact, or ‘abnormal’, where the oocyte and/or granulosa cells contained a pyknotic, fragmented or shrunken nucleus. Numbers of normal and abnormal follicles were determined by counting within at least three non-serial sections for each piece of cultured tissue. Number of normal follicles was divided by total number of follicles assessed to produce a proportion of follicular viability per treatment.
Experimental design
Study 1: Influence of in vitro culture medium and protein supplementation on follicle viability
Ovarian cortical pieces were placed in 24-well cell culture plates (Corning Incorporated, Corning, NY, USA) and cultured in 1) Eagle’s α-MEM (Irvine Scientific, Santa Ana, CA, USA) or 2) MEM. Both were supplemented with 5.5 μg/ml insulin, 5.5 μg/ml transferrin, 6.7 ng/ml selenium, 2 mM L-glutamine, 100 μg/ml penicillin G sodium, 100 μg/ml streptomycin sulphate, 0.05 mM ascorbic acid and 10 ng/ml FSH (Folltropin-V; Bioniche Animal Health, Belleville, ON, Canada). For examining the effect of protein, each culture medium also was supplemented with either 1) 10% fetal bovine serum (FBS), 2) 10% KSR (Invitrogen) or 3) 0.1% (w/v) polyvinyl alcohol (PVA, protein free). Ovarian follicles within the tissues were assessed for viability after 0 (fresh control), 3, 9 and 15 days of in vitro incubation (as described above). At least three and four cortical pieces/group/culture period were used in the cat and dog, respectively.
Study 2: Influence of in vitro culture method on follicle viability and morphology
Cat and dog ovarian tissues were cultured for 3, 9 or 15 days in protein-free MEM and α-MEM, respectively, under one of following conditions: (i) in a cell culture insert (Millipore, Billerica, MA, USA), (ii) on an agarose gel block or (iii) in a 24-well cell culture plate (control). The agarose gel block was prepared as described by Gohbara et al. (2010). Briefly, 1.5% (w/ v) of agarose gel solution was allowed to set in a 6-cm dish for 20 min. The gel then was cut into a hexahedron of approximately 8 × 8 × 4 mm in size and soaked in the test culture medium for at least 24 h. Each gel was placed in a well of a 24-cell culture plate and appropriate medium added to the bottom edge of the gel. Ovarian follicles within the fresh and cultured tissues were assessed for the viability (as above) and tissues fixed and stained for morphological analysis (as above). At least six and four cortical pieces/group/culture period were used in the cat and dog, respectively.
Statistical analysis
Data are presented as the percentages of viable and structurally normal follicles. Comparisons between follicle viability and morphology among groups and culture periods were performed using chi-square test (GraphPad Prism ver. 4.00; GraphPad Software, La Jolla, CA, USA). Significance was set at 95%.
Results
Study 1: Influence of in vitro culture medium and protein supplementation on follicle viability
Histological analysis revealed that virtually all follicles (>98%) in cat and dog cortical pieces were at the primordial stage, most of these (>86%, Figs 1 and 2) being viable at the onset of collection based on calcein AM/ethidium homodimer staining. Follicular viability decreased (p < 0.05) after in vitro culture regardless of type of culture medium or presence/absence/type of protein supplement treatment. The species varied in medium preference. No cat primordial follicle survived beyond 9 days in α-MEM (Fig. 1a), whereas 37.5% still maintained viability after 15 days in MEM (Fig. 1b). In contrast, 32.7% of dog follicles cultured in α-MEM remained alive after 15 days of in vitro culture (Fig. 2a) compared to only 8.9% of cat counterparts (Fig. 2b).
Fig. 1.
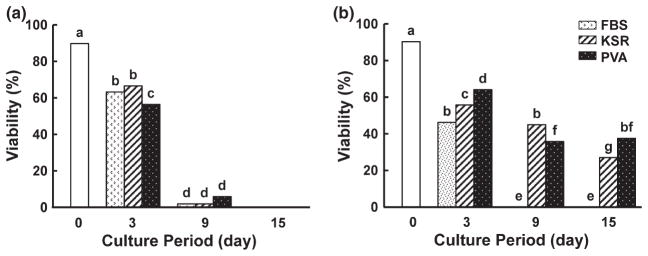
Follicle viability in cat ovarian tissue cultured for 0, 3, 9 or 15 days in α-MEM (a) or MEM (b) with FBS, KSR or polyvinyl alcohol (PVA; Protein free). Different letters indicate significant differences (p < 0.05)
Fig. 2.
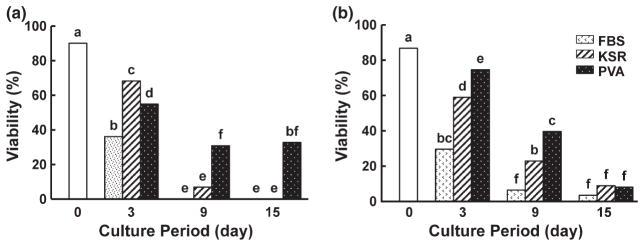
Follicle viability in dog ovarian tissue cultured for 0, 3, 9 or 15 days in α-MEM (a) or MEM (b) with FBS, KSR or polyvinyl alcohol (PVA; Protein free). Different letters indicate significant differences (p < 0.05)
Cat ovarian tissue cultured in protein-free conditions had the highest proportion of viable follicles (37.5%) at 15 days compared to those incubated in KSR (27.0%) (Fig. 1b). Follicle viability in the KSR-supplemented (27.0%) or protein-free (37.5%) medium was higher (p < 0.05) than achieved with FBS (0%) (Fig. 1b). For the dog, more (p < 0.05) follicles survived after 3 days in KSR-supplemented medium (68.3%) than when FBS-added (36.1%) or protein-free (54.9%) medium was used (Fig. 2a). However, highest follicle survival after 15 days of in vitro culture (32.7%; p < 0.05) occurred in protein-free α-MEM, in contrast to no survival (0%) in the presence of FBS or KSR (0%) (Fig. 2a).
Study 2: Influence of in vitro culture method on follicle viability
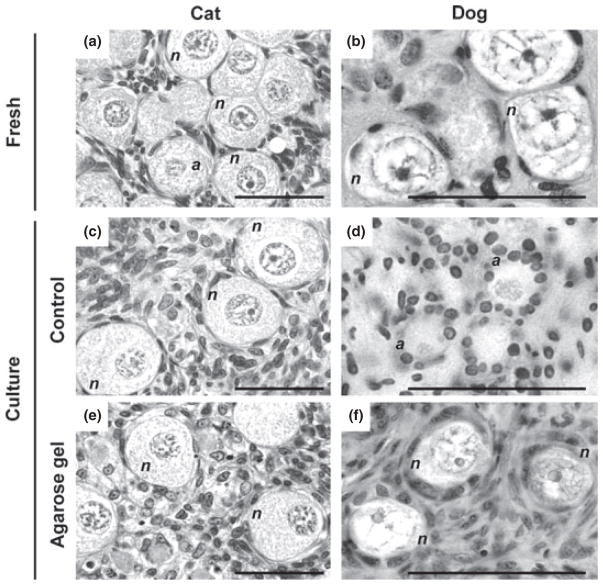
Culture method affected follicle viability in both species. Specifically, overall primordial follicle survival in the cat was promoted (p < 0.05) in the agarose gel culture system (55.0%, Fig. 3a) compared to 24-well dish control (40.8%, Fig. 3a). In contrast, follicle viability did not differ within this species at each time point between the cell culture insert and the agarose gel system (Fig. 3a). Normality of primordial follicles was affirmed histologically in the cat after 15 days of incubation in all culture systems (Figs 4 and 5). Although there were no obvious differences in follicle survival among methods after 15 days, percentage of morphologically normal follicles was higher (p < 0.05) on the agarose gel after 3 days (81.6%) than tissues cultured in the cell culture insert (36.6%) or control (58.2%) (Fig. 5a). Therefore, although calcein/AM-ethidium homodimer staining failed to reveal a difference in follicle viability between the agarose gel and control group at 3 days (Fig. 3a), histology indicated the superiority of agarose gel culture in sustaining normal primordial follicle structural integrity.
Fig. 3.
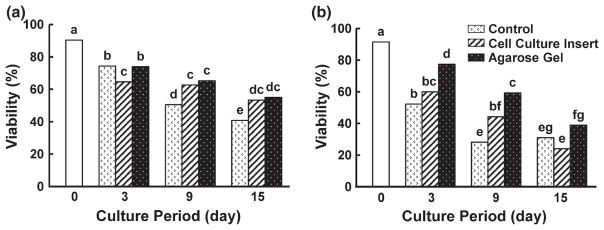
Follicle viability of cat (a) and dog (b) ovarian tissue cultured for 0, 3, 9 or 15 days in a 24-well dish (control), cell culture insert or agarose gel. Cat ovarian tissue was cultured in MEM + polyvinyl alcohol (PVA; Protein free), and dog tissues were cultured in α- MEM + polyvinyl alcohol (PVA; Protein free). Different letters indicate significant differences (p < 0.05)
Fig. 4.
Histographs of fresh (a, b) and cultured ovarian tissues in control (c, d) and in agarose gel (e, f) in the cat (a, c, e) and dog (b, d, f) at 15 days and 3 days of culture, respectively. The letters ‘n’ and ‘a’ indicate primordial follicles classified as follows normal (n) with both oocyte and granulosa cells having normal nuclei or abnormal (a) with the oocyte and/ or granulosa cells containing pyknotic, fragmented or shrunken nuclei. Bar indicates 50 μm
Fig. 5.
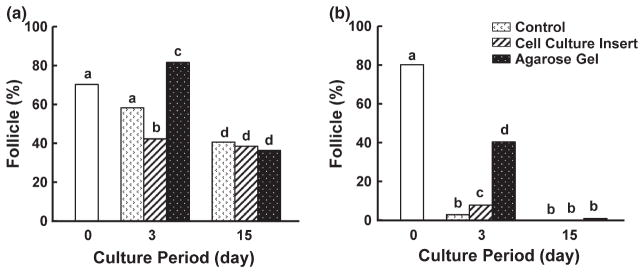
Mean percentages of morphologically normal primordial follicles in cat (a) and dog (b) ovarian tissues in fresh, 3 and 15 days cultures in protein-free medium. Primordial follicles were classified as normal, with both oocyte and granulosa cells containing normal nuclei, or abnormal, with the oocyte and/or granulosa cells containing pyknotic, fragmented or shrunken nuclei. Different letters indicate significant differences (p < 0.05)
The agarose gel system improved (p < 0.05) dog primordial follicle survival after 3 (77.5%) and 9 (59.3%) days of incubation compared to the control (Day 3, 52.2%; Day 9, 28.2%; Fig. 3b). But there was no difference at 15 days (Fig. 3b). The agarose gel system also was advantageous (p < 0.05) for follicle viability in dog ovarian tissue cultured for 3 (77.5%) and 15 (38.9%) days compared to using the cell culture insert (Day 3, 60.0%; Day 15, 24.0%; Fig. 3b). Histological normality in dog follicles was observed only in the agarose gel group and solely after 3 days of culture (Figs 4 and 5b). Although viable follicles (based on calcein AM/ethidium homodimer staining) were observed in all groups after 15 days (Fig. 3b), histology demonstrated that many follicles in cultured cortical pieces, and regardless of treatment, had decreased in size after 3 days with damaged or degenerate ovarian stroma compared to fresh control tissue (Fig. 4b,d). Nevertheless, tissues cultured for 3 days in the agarose gel system had more than a fivefold increase (p < 0.05) in the proportion of morphologically normal follicles (40.4%) compared to the cell culture insert (7.8%) or control (2.9%) groups (Figs 4f and 5b).
Discussion
Under the conditions described in this manuscript, it was possible to maintain viability of a significant portion of cat and dog primordial follicles within cortical ovarian slices for 15 and 3 days, respectively. Furthermore, it was clear that there are distinct species variations in basic nutrient requirement supporting the survival of these early stage follicles, with the main difference being the amount of non-essential amino acids present in the culture medium. Additionally, protein supplementation is not vital, and the agarose gel culture system is superior to the cell culture insert in supporting the viability of cultured cat and dog follicles.
It is conventional wisdom that one of the most important factors governing maintenance of mammalian tissue structure and function in vitro is composition of the culture medium, providing nutrient requirements simulating, as is reasonably possible, what occurs in vivo. Mammalian tissue and cell culture media generally are comprised of macronutrients, carbohydrates, nucleotides, vitamins, amino acids, metal ions and/or supplemental protein, for example, what often is provided by serum (Lane and Gardner 2007). The influence of culture medium type on in vitro developmental capacity of ovarian follicles and oocytes has been demonstrated for multiple species, including the domestic cat (Johnston et al. 1993; Jewgenow 1998) and dog (Songsasen et al. 2002).
We demonstrated in the present study that these two species varied markedly in the basic nutrient requirements supporting survival of primordial follicles within the ovarian cortex. Our test media, α-MEM and MEM, have been used successfully to culture ovarian tissues of the goat (Silva et al. 2004), sheep (Peng et al. 2010) and human (Hovatta et al. 1997). Specifically, 26% of early stage human follicles cultured in ovarian slices in α-MEM medium maintain normal structure after 14–21 days (Hovatta et al. 1997), a proportion that was lower less what we observed in the cat, but higher in the dog at 15 days. The α-MEM and MEM differ largely because the former contains higher concentrations of the non-essential amino acids L-alanine, L-asparagine, L-aspartic acid, L-cysteine, L-glutamic acid, glycine, L-proline and L-serine (http://www.sigmaaldrich.com/life-science/cell-culture/classical-media-salts/mem-media.html). Compared to little information on folliculogenesis, the role of these amino acids is better understood for the later stages of oocyte maturation and early embryo development. For example, these amino acids offer as an energy source, precursors for protein synthesis as well as regulating intracellular pH and osmotic pressure (Lane and Gardner 2007). More specifically, glutamine, taurine and glycine are known to stimulate the advancement of hamster embryos from the one-cell to blastocyst stage (McKiernan et al. 1995). By contrast, these same investigators have determined that leucine, tyrosine, valine, isoleucine, phenylalanine, arginine, methionine or cysteine can inhibit zygote development in the hamster (McKiernan et al. 1995). It has also been shown that the ‘stimulatory’ or ‘inhibitory’ impact of amino acid groups on embryonic development is linked to their abundance in the oviductal fluid of mouse and hamster (Gardner and Lane 1993). Thus, we speculate that the differences in the basic nitrogen requirement between cat and dog ovarian tissues may be due to the variations in endogenous amino acid pool within the follicle or oocyte. Furthermore, previous studies from our laboratory have revealed that the patterns of glutamine metabolism differ between cat (Spindler et al. 2000) and dog (Songsasen et al. 2007) oocytes. Thus, there also may be species-specific differences in amino acid metabolism of primordial follicles and/or oocytes reflecting a variation in amino acid requirements to sustain primordial follicle viability in vitro. Finally, the inhibitory effect of high amino acid content in α-MEM may be due to the accumulation of ammonium, a by-product of amino acid metabolism during in vitro culture (Gardner and Lane 1993). Ammonium is known to be toxic to mammalian cells for many reason including activation of the enzyme phosphofructokinase (Gardner and Lane 1993), which can perturb glycolytic activity, a pathway that is critical to the oocyte and embryonic development (Spindler et al. 2000).
Although blood serum is commonly used in primordial follicle incubations, as well as for oocyte maturation and embryo culture (Lane and Gardner 2007), there is growing evidence that supplemental serum in culture medium can compromise gamete and embryo development in vitro (Lane and Gardner 2007). In the present study, we noted subjectively that both cat and dog ovarian tissue incubated in serum-containing medium readily attached to the culture dish. We suspect this occurred because of serum-induced proliferation and adhesion of somatic cells (Freshney 2005) that, in turn, led to disrupted cell-to-cell interaction of follicles and ovarian tissue structure/integrity. KSR was developed as a serum substitute and has been found beneficial for sustaining proper early embryonic development, at least in cattle (Moore et al. 2007). Interestingly, although advantageous for early embryogenesis, KSR has not been useful as a protein supplement for enhancing oocyte maturation (Moore et al. 2007). Furthermore, our study revealed that KSR-supplemented medium did not support primordial follicle survival, which suggested that there were different protein requirements for maturing oocytes vs embryos. Even so, the most intriguing observation was that ovarian pieces containing primordial follicles survived 15 days in the cat and dog with no supplemental protein. PVA has been used for protein-free culture of oocytes in several species, including the cat (Johnston et al. 1993) and in a bovine study that indicated superiority over serum supplementation in promoting oocyte maturation (Fukui et al. 2000). Therefore, the rather simple media now available (i.e. α-MEM or MEM without complex additives) seem adequate for designing subsequent studies to more thoroughly understand what controls primordial follicle advancement and endurance.
Certainly, one of the most influential factors identified in our study was the microenvironment itself. While basic follicular viability was retained in standard 24-well culture or cell culture inserts, proportional and durational survival was markedly enhanced by utilizing an agarose gel system. Compared to the former two approaches (where tissue is immersed in medium), the latter permits the ovarian cortices to be continuously exposed to the ambient gas environment, thereby facilitating oxygen absorption. By contrast, when tissues are completely submerged in culture medium, oxygen absorption from the atmosphere is impaired, thereby causing central necrosis, a main cause of cellular damage (Freshney 2005). Furthermore, a high concentration (100%) of oxygen is known to improve primordial follicle development in cultured human ovarian tissue, including maintaining follicular ultrastructure and conserving the ability to secrete hormones (Morimoto et al. 2007). We suspect a similar benefit, especially the promotion of oxygen absorption, was occurring in our agarose-incubated cat and dog ovarian tissue, which led to enhanced follicle survival.
Compared to cat follicles, the lower viability of dog counterparts after 15 days suggested that gas exchange or another nutritional component was inadequate to sustain somatic cell survival or connectivity between the oocyte and surrounding granulosa cells. Owing to their unique reproductive physiology, the efficiency of in vitro oocyte maturation is naturally low in the domestic dog, and embryo production using in vitro matured oocytes has been unsuccessful so far in this species (Songsasen and Wildt 2007). This incubation challenge certainly holds for the maintenance of dog (compared to cat) primordial follicles in vitro. Besides this species difference, we also affirmed the value of using both the viability assay and a histological analysis with haematoxylin/ eosin staining to confirm follicular survival. This was especially notable from our dog assessments where histology often revealed evidence of perturbed follicle structure prior to the calcein AM/ethidium homodimer staining indications of cell death. Thus, future studies should continue to rely on both evaluative approaches.
In conclusion, a simultaneous comparative study of the cat and dog has disclosed important similarities and differences in the in vitro microenvironment required to sustain living primordial follicles within cultured ovarian cortices. The species contrast in that the dog requires more non-essential amino acids, whereas the cat does not, which we suspect reflects inherent differences between species in amino acid metabolism. Interestingly, both cat and dog primordial follicles tolerated and even preferred a protein-free culture medium. Without the confounding, ubiquitous elements of protein supplements, it will be easier in future studies to target explicit factors that regulate both follicle and oocyte development in these two species. Finally, our findings revealed the substantial advantages of using the recently developed agarose gel system for promoting cat and dog primordial follicle survival, mostly likely from improving oxygen absorption to enhance cell survival. These findings offer early insights into the potential and challenges of rescuing a least a portion of the vast amounts of germplasm encapsulated and wasted over a life time and at death in the cat and dog ovary.
Acknowledgments
The authors thank veterinary hospitals in Front Royal, Stephens City, Harrisonburg and Winchester, VA areas for providing cat and dog ovaries. The authors also acknowledge Dr. Budhan Pukazhenthi for technical advice. This research was supported by a grant from the Yamada Science Foundation and a gift from Dr. Clint and Missy Kelly to M. F.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Freshney RI. Culture of Animal Cells: A Manual of Basic Technique. John Wiley and Sons; New York: 2005. [Google Scholar]
- Fukui Y, Kikuchi Y, Kondo H, Mizushima S. Fertilizability and developmental capacity of individually cultured bovine oocytes. Theriogenology. 2000;53:1553–1565. doi: 10.1016/S0093-691X(00)00297-1. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod. 1993;48:377–385. doi: 10.1095/biolreprod48.2.377. [DOI] [PubMed] [Google Scholar]
- Gohbara A, Katagiri K, Sato T, Kubota Y, Kagechika H, Araki Y, Ogawa T. In vitro murine spermatogenesis in an organ culture system. Biol Reprod. 2010;83:261–267. doi: 10.1095/biolreprod.110.083899. [DOI] [PubMed] [Google Scholar]
- Horii T, Nagao Y, Tokunaga T, Imai H. Serum-free culture of murine primordial germ cells and embryonic germ cells. Theriogenology. 2003;59:1257–1264. doi: 10.1016/s0093-691x(02)01166-4. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- Jewgenow K. Role of media, protein and energy supplements on maintenance of morphology and DNA-synthesis of small preantral domestic cat follicles during short-term culture. Theriogenology. 1998;49:1567–1577. doi: 10.1016/s0093-691x(98)00102-2. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Donoghue AM, O’Brien SJ, Wildt DE. Influence of culture medium and protein supplementation on in vitro oocyte maturation and fertilization in the domestic cat. Theriogenology. 1993;40:829–839. doi: 10.1016/0093-691x(93)90218-t. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942– 950. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Embryo culture medium: which is the best? Best Pract Res Clin Obstet Gynaecol. 2007;21:83–100. doi: 10.1016/j.bpobgyn.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Matos MH, Lima-Verde IB, Luque MC, Maia JE, Jr, Silva JR, Celestino JJ, Martins FS, Bao SN, Lucci CM, Figueiredo JR. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote. 2007;15:173–182. doi: 10.1017/S0967199407004169. [DOI] [PubMed] [Google Scholar]
- McKiernan SH, Clayton MK, Bavister BD. Analysis of stimulatory and inhibitory amino acids for development of hamster one-cell embryos in vitro. Mol Reprod Dev. 1995;42:188–199. doi: 10.1002/mrd.1080420208. [DOI] [PubMed] [Google Scholar]
- Moore K, Rodriguez-Sallaberry CJ, Kramer JM, Johnson S, Wroclawska E, Goicoa S, Niasari-Naslaji A. In vitro production of bovine embryos in medium supplemented with a serum replacer: effects on blastocyst development, cryotolerance and survival to term. Theriogenology. 2007;68:1316–1325. doi: 10.1016/j.theriogenology.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Oku Y, Sonoda M, Haruki A, Ito K, Hashimoto S, Fukuda A. High oxygen atmosphere improves human follicle development in organ cultures of ovarian cortical tissues in vitro. Hum Reprod. 2007;22:3170–3177. doi: 10.1093/humrep/dem314. [DOI] [PubMed] [Google Scholar]
- Peng X, Yang M, Wang L, Tong C, Guo Z. In vitro culture of sheep lamb ovarian cortical tissue in a sequential culture medium. J Assist Reprod Genet. 2010;27:247–257. doi: 10.1007/s10815-010-9415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafim MK, Araujo VR, Silva GM, Duarte AB, Almeida AP, Chaves RN, Campello CC, Lopes CA, De Figueiredo JR, Da Silva LD. Canine preantral follicles cultured with various concentrations of follicle-stimulating hormone (FSH) Theriogenology. 2010;74:749–755. doi: 10.1016/j.theriogenology.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Silva JR, Van den Hurk R, Costa SH, Andrade ER, Nunes AP, Ferreira FV, Lobo RN, Figueiredo JR. Survival and growth of goat primordial follicles after in vitro culture of ovarian cortical slices in media containing coconut water. Anim Reprod Sci. 2004;81:273–286. doi: 10.1016/j.anireprosci.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Wildt DE. Oocyte biology and challenges in developing in vitro maturation systems in the domestic dog. Anim Reprod Sci. 2007;98:2–22. doi: 10.1016/j.anireprosci.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songsasen N, Yu I, Leibo SP. Nuclear maturation of canine oocytes cultured in protein-free media. Mol Reprod Dev. 2002;62:407–415. doi: 10.1002/mrd.10130. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Spindler RE, Wildt DE. Requirement for, and patterns of, pyruvate and glutamine metabolism in the domestic dog oocyte in vitro. Mol Reprod Dev. 2007;74:870–877. doi: 10.1002/mrd.20667. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Woodruff TK, Wildt DE. In vitro growth and steroidogenesis of dog follicles are influenced by the physical and hormonal microenvironment. Reproduction. 2011;142:113–122. doi: 10.1530/REP-10-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler RE, Pukazhenthi BS, Wildt DE. Oocyte metabolism predicts the development of cat embryos to blastocyst in vitro. Mol Reprod Dev. 2000;56:163–171. doi: 10.1002/(SICI)1098-2795(200006)56:2<163::AID-MRD7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Trowell OA. The culture of mature organs in a synthetic medium. Exp Cell Res. 1959;16:118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]
- Wildt DE, Chan SY, Seager SW, Chakraborty PK. Ovarian activity, circulating hormones and sexual behavior in the cat. I. Relationships during the coitus-induced luteal phase and the estrous period without mating. Biol Reprod. 1981;25:15–28. doi: 10.1095/biolreprod25.1.15. [DOI] [PubMed] [Google Scholar]