Abstract
A Workshop entitled “Lessons Learned from Radiation Oncology Trials” was held on December 7–8th, 2011 in Bethesda, MD, to present and discuss some of the recently conducted Radiation Oncology clinical trials with a focus on those that failed to refute the null hypothesis. The objectives of this Workshop were to summarize and examine the questions that these trials provoked, to assess the quality and limitations of the pre-clinical data that supported the hypotheses underlying these trials, and to consider possible solutions to these challenges for the design of future clinical trials.
Several themes emerged from the discussions, including the: a) opportunities to learn from null-hypothesis trials through tissue and imaging studies; b) value of pre-clinical data supporting the design of combinatorial therapies; c) significance of validated biomarkers; d) necessity of quality assurance in radiotherapy delivery; e) conduct of sufficiently-powered studies to address the central hypothesis; and f) importance of publishing results of the trials regardless of the outcome.
The fact that well-designed hypothesis-driven clinical trials produce null or negative results is expected given the limitations of trial design, and complexities of cancer biology. It is important to understand the reasons underlying such null results however, in order to effectively merge the technological innovations with the rapidly evolving biology for maximal patient benefit, through the design of future clinical trials.
Keywords: Cancer, Null hypothesis, Radiation oncology, Radiation therapy, Randomized clinical trial
INTRODUCTION
Clinical trials involving RT for cancer are initiated to identify novel technological and biological approaches that can improve local tumor control, DFS, OS, reduce toxicity, and/or improve quality of life. The design of these trials should be based on solid preclinical evidence supporting such approaches; however, oftentimes, patients participating in the experimental arm fare no better than control subjects (1). To identify possible reasons for these negative outcomes, and to propose pathways to increase the likelihood of “success”, a Workshop entitled “Lessons Learned from Radiation Oncology Trials” was held on December 7–8th, 2011 in Bethesda, MD, sponsored by the Radiation Research Program of the NCI. The objectives of the Workshop were to assess the quality, quantity, and limitations of the pre-clinical data that supported the hypotheses underlying a few recently completed trials, and to consider possible solutions. Attendees included radiation and medical oncology clinical trialists, radiation biologists, clinician-scientists, radiation physicists, statisticians, and representatives from the pharmaceutical industry. To provide common ground for dialogue, results from ten Phase III RCTs from several different malignancies were discussed (Table 1), which included the spectrum of positive, negative, and null outcomes.
Table 1.
Radiation Oncology Phase III randomized clinical trials on central nervous system, head and neck, lung, gastrointestinal, and genitourinary malignancies presented and discussed by the Workshop participants
| Trial | Target Tumor Site | Primary Objective (& Results) | Accrual Period | Patients Accrued (completed or randomized) |
Notable Secondary Findings |
|---|---|---|---|---|---|
|
RTOG 0525 EORTC 26052-22053 |
GBM | Does dose-intensifying adjuvant TMZ improve OS? (No evidence for improvement) |
01/2006 to 06/2008 | 1173 (833) |
|
| RTOG 0211 | GBM | Phase I/II study of EGFR TK inhibition (Iressa™) with RT (No OS benefit for patients treated with gefitinib + RT vs. RT alone) |
06/2003 to 01/2012 | Phase I: 31 Phase II: 147 (119 successfully completed therapy) |
|
| RTOG-0129 | HNSCC | Does accelerated RT combined with cisplatin improve survival of patients with LA HNSCC? (No evidence for improvement) |
07/2002 to 05/2005 | 743 patients |
|
| TROG 02.02 | HNSCC | Does adding a hypoxic toxin (Tirapazamine) to RT-cisplatin regimen improve survival for patients with LA HNSCC? (No evidence for improvement) |
09/2002 to 04/2005 | 861 patients |
|
| RTOG 0522 | HNSCC | Does adding Cetuximab to the RT-cisplatin regimen improve PFS for patients with LA HNSCC? (No evidence for improvement) |
11/2005 to 03/2009 | 940 enrolled (895 evaluable) |
|
| RTOG 0617 | NSCLC | Does higher RT dose (60 Gy vs. 74 Gy with CRT ± Cetuximab) confer a treatment response benefit? (No evidence for improvement) |
11/2007 to 04/2011 | 423 enrolled |
|
| RTOG 9811 | Anal Canal | Is efficacy of cisplatin-based (experimental) therapy better than mitomycin-based (standard) therapy in treatment of anal canal carcinoma? 5FU/CDDP + RT vs. 5FU/MMC + RT (OS & DFS better with 5FU/MMC) |
10/1998 to 06/2005 | 682 randomized (644 included in outcomes analysis) |
|
| RTOG 0020 | Pancreatic Cancer | Does addition of maintenance with a farnesyltransferase inhibitor (FTI) improve gemcitabine/Taxol chemo-RT? Weekly Gemcitabine, Paclitaxel and External Irradiation (50.4 Gy) followed by the FTI R115777 (Addition of FTI demonstrated no improvement in clinical outcome, yet was associated with increased toxicities) |
11/2001 to 09/2003 | 195 accrued (174 in analysis) |
|
| RTOG 94-13 | High-risk Prostate Cancer | Does pelvic RT improve progression-free survival compared with prostate-only RT among patients with a chance of lymph node involvement? (No evidence for improvement) | 04/1995 to 06/1999 | 1323 patients accrued (1292 enrolled) |
|
| EORTC 22961 | High-risk Prostate Cancer | 6 months androgen-suppression followed by RT, then either observed or additional 2.5 years of androgen-suppression. (Marginal improvement in long-term outcome) |
04/1997 to 11/2001 | 1113 patients |
|
SUMMARY OF CLINICAL TRIALS
Central Nervous System Tumors
Two studies focused on GBMs were presented and discussed. The RTOG 0525/EORTC 26052-22053 was an international Phase III RCT determining whether dose-intensifying adjuvant TMZ could improve OS. The overall conclusion was “no evidence for improvement”, although the prognostic value of MGMT promoter methylation status was confirmed.
The second Phase I/II RTOG 0211 trial examined the addition of an EGFR TKI (Gefitinib, Iressa™) to RT for GBM patients, which failed to demonstrate any OS benefit with the combinatorial approach. In fact, tumors with elevated SRC or PTEN expression fared worse with the TKI, illustrating the complex signalling cascades underlying most GBMs.
Head and Neck Squamous Cell Carcinoma
Despite the success of the landmark Cetuximab plus RT for patients with LA HNSCC (2, 3), more recent trials have been disappointing. The RTOG 0129 asked whether AFX plus CDDP will improve OS for LA HNSCC patients (4); in fact, no difference was observed between the standard vs. AFX group, suggesting that CDDP likely offsets tumor cell repopulation during fractionated RT.
The TROG 02.02 trial examined the value of adding a hypoxic cytotoxic agent TPZ to CDDP-RT for LA HNSCC patients (5). Disappointingly, this study also demonstrated no difference in outcome, but underscored the importance of QA in RT delivery (6), as well as questioning the clinical importance of tumor hypoxia (7). A third trial (RTOG 0522) asked whether the addition of Cetuximab to CDDP-RT could improve progression-free survival (8); this study not only failed to demonstrated an advantage to the triple-modality, but observed greater acute toxicities. Furthermore, Cetuximab and CDDP appeared to have overlapping mechanisms of action; hence, utilizing complementary tumoricidal agents would likely be more effective.
Lung
The four-arm RTOG 0617 trial compared OS differences between high-vs. standard-dose conformal RT with concurrent chemotherapy (Carboplatin and Paclitaxel), with or without Cetuximab for patients with stage IIIA/IIIB NSCLC. The results demonstrated no difference in OS between the high- (74 Gy) vs. standard-dose (60 Gy) patients (9), even suggesting an inferior survival with the high-dose arm, possibly related to treatment-related deaths, which again underscores the importance of QA in RT planning and delivery (10).
Gastrointestinal
The RTOG 9811 Phase III RCT addressed the efficacy of substituting CDDP for MMC, in the standard 5-FU/MMC/RT regimen for anal canal carcinoma. The results demonstrated no difference in DFS between the two treatment arms, but the CDDP group experienced a significantly higher colostomy rate (11). The major design flaw related to two new hypotheses of drug and sequence, both being addressed simultaneously; the new drug being CDDP, delivered in an induction manner. Consequently, it remained unclear if the negative results were related to an ineffective drug, an ineffective sequence, or both.
The RTOG 0020 Phase II randomized trial of Gemcitabine/Paclitaxel/RT, followed by an FTI (R115777) for unresectable pancreatic cancer demonstrated that maintenance FTI failed to improve clinical outcome, yet was associated with increased toxicities, highlighting the challenges to inhibiting K-ras, an established oncogenic target in this disease (12).
Genitourinary
The RTOG 94-13 trial, a complex four-arm randomization of whole pelvis vs. prostate only RT, with secondary randomization of neo-adjuvant vs. concurrent hormone scheduling (13, 14) reported no significant difference in progression-free survival for any group. This was an under-powered 4-arm trial, and failed to address the issues of field size, or timing of androgen suppression. There might also have been an unpredicted biological interaction between concurrent androgen suppression with RT, arguing for the importance of companion translational studies to acquire biological insights.
The EORTC 22961 trial demonstrated that longer-term (total of 3 years) was marginally superior to shorter-term (6 months) androgen suppression when patients were also treated with RT (15). The effect size was small; 5-year cumulative prostate-specific mortality differed by only 2.5%, plus the majority of patients had low Gleason scores. Hence, it still remained unclear if androgen ablation is beneficial for most patients.
EMERGING THEMES
I. Pre-clinical Studies
Many reasons could account for the success of the Cetuximab plus RT RCT for HNSCC (2, 3), including: a) the universally reported prognostic value for EGFR over-expression (16–18); b) the role of EGFR in mediating radiation resistance (19–21); c) the demonstration of efficacy of EGFR inhibitors in several different pre-clinical cancer models (22–24); d) a well-designed drug (25) which was highly efficacious and well-tolerated (26); and e) a well-constructed and efficiently-executed clinical trial (2).
Based on the above success, and corroborating the framework for preclinical studies as outlined by the UK group (27), it is recommended that before any combinatorial treatments are considered with RT, a minimal expectation would be an in vitro clonogenic assay of novel drug-of-interest plus RT in relevant pre-clinical cancer models. The MTT and apoptotic assays are simple, but are poor substitutes for the more quantitative clonogenic survival assays, which until demonstrated otherwise, will remain the gold standard for the evaluation of any radiation sensitizer, DNA repair modification, or combinations of RT with drug.
The Molecular Radiation Therapeutics Branch within the Radiation Research Program of the NCI (rrp.cancer.gov/aboutRRP/mrtb.htm) has already generated extensive data for multiple targeted agents combined with RT in panels of human cancer cell lines; therefore, this resource should be the first point of contact before embarking on any combinatorial therapies. Next is the generation of in vivo data using different human cancer xenograft models, which have their limitations by only partially reflecting human tumor heterogeneity; furthermore, the tumor micro-environment (e.g. hypoxia), stromal factors, or the human metastatic patterns are not completed recapitulated. Some orthotopic models might address such limitations (28, 29), as well as early-passaged human tumor xenografts. An alternative is the utilization of GEMMs of human cancers (30), which could be useful for lung cancer (31, 32), and soft tissue sarcomas (33).
Many of these xenograft models are readily available within the Radiation Oncology community including CNS (34); lung (35, 36), breast (37), head and neck (38), pancreas (29, 39), and cervix (28). Funding for these studies remains challenging, although some pharmaceutical companies could be interested since such data will inform the design of early-phase clinical trials. Finally, another potential solution could be the utilization of a panel of molecularly annotated first generation xenografts harbouring high and low levels of the putative target (40); this could guide clinically realistic RT and drug doses for subsequent clinical trials.
II. Biomarker Studies
Biomarkers are germane to categorizing patients into distinct risk groups for prognostic or predictive value, enriching cohorts for clinical trials, and tracking longitudinal response to therapies. With the emergence of data derived from the ICGC (www.icgc.org/) and TCGA (cancergenome.nih.gov) deep-sequencing projects, this is an opportune moment to capitalize on such resources to triage patients into genetically- or proteomically-defined groups, to identify novel targets, and actionable mutations for RT-combinatorial trials, although tumor heterogeneity will remain challenging (41). Many of the ICGC/TCGA clinical data are not yet sufficiently mature to identify robust prognostic markers; the role of RT might also be difficult to discern, if such treatment details are lacking. Consequently, the value of well-annotated biospecimens linked to RT RCTs cannot be overstated.
The landmark observation of the benefit of TMZ to RT for GBM (42) changed practice, and led to the evaluation of TMZ dose intensification (RTOG 0525), corroborating the prognostic value of MGMT methylation status. A translational study evaluating primary GBM tissues from participants in multiple clinical trials demonstrated a potential 2-gene signature (ΔNF-kBIA plus MGMT methylation), as well as suggesting a biological explanation for the lack of efficacy of Erlotinib (43), since NF-kBIA deletion and EGFR amplification emerged to be mutually exclusive aberrations in GBM. Similar important insights have been derived from RCT tissue studies for HNSCC, not only corroborating the superior outcome for HPV-associated HNSCC (4), but also their limited benefit by hypoxic modifiers (44), which might in part account for the negative TROG 02.02 trial (5, 7). These data clearly illustrate the value of correlative tissue studies in providing biological insights, and informing the design of future trials.
Another approach is the utilization of an adaptive design (45), which requires the analysis of multiple known mutations such as KRAS, EGFR, and EML4-ALK in the context of lung cancer tissues derived from RT RCTs. This is a very promising area of investigation that should influence the design of future RT-drug trials for lung cancer. Yet another critically important consideration is the utilization of “clinical-ready” PD read-outs. Stable and validated PD assays of DNA damage such as γH2AX in tumor tissues (46), or quantifying PAR levels in PBMCs (47) might be highly applicable for RT clinical studies, as opposed to P-Akt, which is notoriously unstable. This is an area of active investigation by the Frederick National Laboratory for Cancer Research; an important resource for the Radiation Oncology research community.
III. Imaging Biomarker Studies
Tumor response assessment in clinical trials has typically been derived from longitudinal assessments of anatomically-based diagnostic images (CT, MRI), using RECIST, which could be subject to observer bias, differences in scanning techniques, or lack of quantitative rigor. In an effort to address these shortcomings, an NCI-led Quantitative Imaging Network was established, to develop robust automated and semi-automated methods for tumor identification, segmentation and characterization. Each institution in this Network has engaged teams of clinicians and researchers to develop enhanced QA methods for image acquisition and data analysis, and to improve inter-institutional reproducibility.
The ability to quantify a metabolic tumor volume on PET/CT scans across institutions will be critical, particularly for RTOG trials, to achieve an additional level of consistency. This will also expand the use of molecular imaging via an array of novel PET tracers, as well as application of advanced MRI methods including spectroscopy, DCE, and DWI. The synergy between the QIN and cooperative groups will be crucial for the future of RT research.
IV. Microenvironment as a Target
Over 60 years of research on hypoxia and RT tumor response can be summarized as: a) rodent and human tumors contain hypoxic cells; b) rodent tumors are more hypoxic than human tumors; thus, will model only the most hypoxic of human tumors; c) hypoxic human tumors are RT-resistant; d) methods to overcome hypoxia in human tumors are less than perfect but are beneficial (48); and e) the ideal methods to identify or treat hypoxic tumors do not yet exist.
Three limitations of the TROG 02.02 trial (5) relate to: administration of TPZ, QA of RT plans, and HPV status. The TPZ dose was sufficiently high to potentiate CDDP; however, it was administered with only 9 of 35 fractions, which could have compromised the anticipated benefit. Tumors were not selected for hypoxia, and 12% of these patients had non-compliant RT plans that adversely affected tumor control (6), which was mal-distributed to the TPZ arm. Finally, TROG 02.02 was designed before the full appreciation of HPV-associated OPC, which appear not to benefit from hypoxic modifications (44), thereby diluting the potential benefit of TPZ.
Other tumor microenvironment properties such as extracellular pH, angiogenesis, and interstitial fluid pressure, might also influence tumor response to RT, as well as targeting stromal cells, cytokines, and oxidative stress. To date however, other than hypoxia, no Phase III RCTs have evaluated such strategies with RT outcome.
In summary, hypoxia is a negative predictor in some tumors treated with RT. Despite clear benefits in multiple trials of hypoxia modifiers with RT, the results have not been sufficiently dramatic to uniformly change clinical practice (49). Improved agents are being developed (50), and will be evaluated with hypoxia imaging in order to better select the appropriate patients.
V. Importance of Radiation Therapy Quality Assurance
The critical importance of QA in RT was succinctly illustrated in the aforementioned TPZ trial, wherein deficient RT plans caused a 20% reduction in OS (6), which far outweighed any potential benefits from biologically-targeted agents. The fundamental principle is that if the tumor is not irradiated, it will not be controlled. Many international efforts have been undertaken to conduct pre-reviews of IMRT plans (51), plus QA programs for IGRT protocols (52). These are critically important endeavors to ensure patient safety, treatment fidelity, and quality of RT.
The recently completed RTOG 0617 trial for NSCLC was a null trial, failing to demonstrate a benefit for the higher-dose arm. Multiple reasons might explain this observation, but there was definitely a higher incidence of treatment-related deaths in the latter arm; posing dosimetric considerations as one possible explanation. Similarly, a review of RTOG gastrointestinal trials uncovered a significant minority of unacceptable RT plans which might also in part, account for their null results. Of note, such trials wherein unacceptable RT plans were corrected resulted in positive observations (53). By harnessing the capabilities of digital technology, pre-treatment reviews of RT plans could be undertaken in an expeditious and resource-efficient manner.
VI. Data Sharing and Publication Biases
A current challenge in our biomedical research community is a tendency towards publication bias of positive results, documented decades ago wherein meta-analyses of published data would overestimate the treatment benefit vs. all registered clinical trials (54). This tendency continues today, wherein more than 20% of Phase III clinical trial abstracts presented at ASCO remain unpublished after 6.5 years, or took longer than 5 years to be published (55).
The requirement to reproduce published data is a fundamental tenet to achieving true medical advances. The lack of data reproducibility is a major problem for drug development, wherein two-thirds of these studies have significant inconsistencies (56, 57). One example relates to Motexafin Gadolinium that proceeded to Phase III testing (58), despite laboratory evidence documenting its lack of radio-sensitization (59). The lack of reproducibility costs both patients, for participating in treatments which are unlikely to be beneficial, and society. Pharmaceutical companies lose time and money on pursuing academic discoveries which remain difficult to reproduce (60, 61), which can be further compounded by off-target effects with siRNAs (62, 63).
In the current era of genomic medicine, this situation becomes even more challenging (64); wherein data from only 2 of 18 micro-array publications in Nature Genetics could be replicated; the major problem being inaccessibility to the original raw data files (65), with potentially dire consequences for patients (64). Science devoted its entire Dec 2nd, 2011 issue to this very topic (66), and recommended 6 steps: 1) analytical validity (different platforms); 2) repeatability (different scientists); 3) replication (meta-analyses of different data sets); 4) external validation (consistent large-scale datasets); 5) clinical validity (can predict clinical outcome); and 6) clinical utility (actually improves clinical outcome), before any –omic data be utilized in clinical medicine. Similar guidelines have been suggested for predictive or prognostic biomarkers based on 5 levels of evidence, ranging from under-powered observational reports to prospectively-designed clinical trials examining a biomarker (67).
These recommendations have been developed to temper human nature which prefers celebratory vs. sobering news, the competition in science and academia, and the explosive quadrupling growth in the number of scientific Journals from 1970 to 2011. E-Journals such as BMC Research Notes encourage the publication of negative data and replication of previously-reported results. Recognizing the academic and societal value of well-conducted but null or negative publications would enhance the likelihood of such studies becoming publicly available.
VII. Designs of Clinical Trials
In designing complex clinical trials, there needs to be a deep appreciation of the characteristics of the targeted population, and competing risks. For example, if the proportion of patients in a hypothetical “hypoxic cytotoxic” trial is only 15%, depending on the anticipated benefit of the intervention, up to 1000 patients might be required to demonstrate such a difference in outcome. Similarly, if the targeted population has competing risks (e.g. lung or HNSCC patients); the sample size needs to be increased significantly, if OS was the primary end-point.
Alternatively, if the design of clinical trials is complex (e.g. RTOG 94-13 had a complex 2×2 design), and if the interaction between the modalities is not fully appreciated, then this could lead to a potentially under-powered study. In the RTOG 94-13 trial, at the time of its design, the interaction of hormonal therapy with RT for prostate cancer was not yet fully elucidated (68), underscoring the importance of pre-clinical evaluations to better understand such potentially complex biology.
VIII. Consideration of an International Consortium
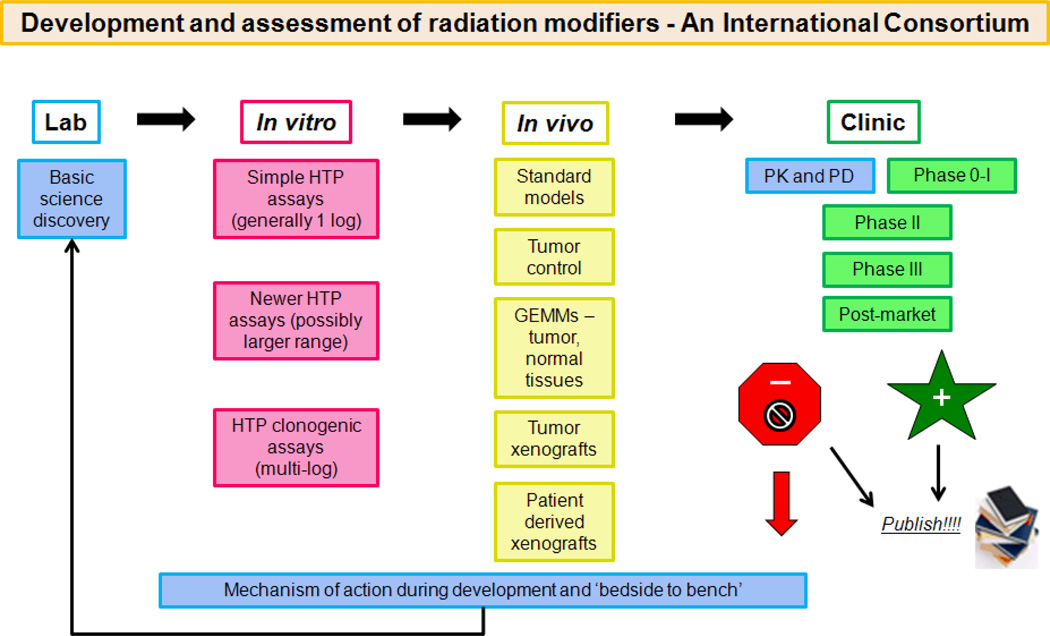
The clinical development of radiation modifiers is frequently a secondary path, spin-off or occasional afterthought to drug development by industry, academia or government (Fig 1). Basic discovery defines a tumor molecular target, and if the developer considers this to be useful for RT, it will be included in the developmental plans (Fig 1). In this context, the formation of an International Consortium for the Evaluation of Radiation Modifiers could be considered with pooling of resources, developed in a collaborative manner, to expedite the discovery and translation of effective agents which will enhance the curative outcomes of RT for cancer patients.
Figure 1.
Pathway of in vitro to in vivo to Phase I/II/III clinical trials. Proposed model and activities of an International Consortium whereby potential drugs can be provided from academia, industry and government, and prioritized for evaluation through a ‘Steering Committee’.
As shown (Fig 1), there could be a step-wise progression of examining molecular targets combined with RT, prioritized through a Steering Committee, with assignation of specific assays to different groups with such expertise. This will result in a pipeline of potential therapeutic candidates advancing through in vitro, in vivo, PK/PD, and Phase 0/I to II, and even RCTs, if such targets fulfill the pre-defined criteria for progression. Furthermore, the prompt publication of null, negative or positive results can be of great benefit in avoiding patient toxicity as well as the needless expense in developing a less-than-adequate drug.
CONCLUSION
Several recently-conducted Radiation Oncology clinical trials were presented and discussed at an NCI-US sponsored Workshop. By nature, clinical trials, which are resource-intensive, can often lead to null observations; hence, it behooves us to capitalize upon each opportunity, in order to maximize the derived information. To that end, important themes emerged from this Workshop, including: a) deriving robust pre-clinical data; b) conducting companion translational studies; c) designing appropriately-powered clinical trials; and d) performing expeditious real-time QA of RT plans.
The resources available through the NCI-US Molecular Radiation Therapeutics Branch, the QIN, and the Frederick National Laboratory for Cancer Research should be harnessed by the Radiation Oncology biomedical research community before embarking on designing future RT clinical trials, particularly when combined with novel targeted agents. Exploring the establishment of an International Consortium for the Evaluation of Radiation Modifiers should be undertaken to pool resources in this important pursuit. Finally, we must all remember that the focus of all of our research efforts is the patient; our obligations are first and foremost, to them.
STATEMENT OF TRANSLATIONAL RELEVANCE.
Clinical trials are conducted to advance clinical outcome, by examining new technologies and novel treatments, to improve survival and quality of life for our cancer patients. Such trials are resource-intensive, for both patients and investigators; hence, it behooves us to ensure that all such studies are supported and based upon solid evidence informing the underlying hypothesis and subsequent design.
A Workshop entitled “Lessons Learned from Radiation Oncology Trials” underscored several issues, including: the importance of pre-clinical data supporting the combination of a novel molecular agent plus radiation; the value of companion translational studies; the significance of quality assurance in radiation planning and delivery; and the need for academia to acknowledge the value of publishing all results, including those with negative data.
This is the era of rapidly-advancing technological and biological platforms; we need to harness such innovations optimally, for maximal benefit for our cancer patients.
ACKNOWLEDGEMENT
This work was been supported by funds from the NCI.
Grant Support: None
ABBREVIATIONS
- AFX
Accelerated fractionated radiation therapy
- Akt
Serine/Threonine specific protein kinase and oncogene (protein kinase B)
- ASCO
America Society of Clinical Oncology
- CNS
Central nervous system
- CDDP
Cisplatin
- Cre
Causes Recombination
- CT
Computed tomography
- DCE
Dynamic contrast enhanced
- DFS
Disease-free survival
- DWI
Diffusion-weighted imaging
- EGFR
Epidermal growth factor receptor
- EML4-ALK
Echinoderm microtubule-associated protein-like 4 – ALK (anaplastic lymphoma kinase)
- EORTC
European Organization for Research and Treatment of Cancer
- FTI
Farnesyltransferase inhibitor
- GBM
Glioblastoma multiforme
- GEMM
Genetically-engineered mouse model
- γ-H2AX
Gamma-histone 2AX
- HNSCC
Head and neck squamous cell carcinoma
- HPV
Human papilloma virus
- ICGC
International Cancer Genome Consortium
- IDH1
Isocitrate dehydrogenase 1
- IGF1R
Insulin-like growth factor-1 receptor
- IGRT
Image-guided radiation therapy
- IMRT
Intensity-modulated radiation therapy
- K-ras
Kirsten rat sarcoma viral oncogene
- LA HNSCC
Locally-advanced head and neck squamous cell carcinoma
- MRI
Magnetic resonance imaging
- MGMT
O-6-Methylguanine-DNA-Methyltransferase
- MMC
Mitomycin-C
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NCCTG
North Central Cancer Treatment Group
- NCI
National Cancer Institute
- NF1
Neurofibromin 1
- NF-kBIA
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- NSCLC
Non-small cell lung cancer
- OPC
Oropharyngeal cancer
- OS
Overall survival
- P-Akt
Phosphorylated Akt
- PAR
Poly-ADP-ribose
- PBMC
Peripheral blood mononuclear cell
- PD
Pharmacodynamic
- PK
Pharmacokinetics
- PDGFRA
Platelet-derived growth factor receptor, alpha polypeptide
- PET
Positron emission tomography
- PTEN
Phosphatase and tensin homolog
- QA
Quality assurance
- QIN
Quantitative Imaging Network
- RT
Radiation therapy
- RCT
Randomized clinical trial
- RECIST
Response evaluation criteria in solid tumors
- RTOG
Radiotherapy and Oncology Group
- siRNA
Short-interfering RNA
- SRC
Tyrosine kinase proto-oncogene “sarcoma”
- STK33
Serine/threonine-protein kinase 33
- TCGA
The Cancer Genome Atlas
- TKI
Tyrosine kinase inhibitor
- TMZ
Temozolomide
- TPZ
Tirapazamine
APPENDIX 1
Workshop participants
Abrams, Jeffrey - NIH, Bethesda, MD
Ang, Kian - MD Anderson Cancer Center, Houston, TX
Ataman, Ozlem - AstraZeneca Corporation, Manchester, U.K.
Bailey, Paul - Pfizer Corporation, Ney York, NY
Ben-Josef, Edgar - University of Pennsylvania, Philadelphia, PA
Bentzen, Soren - University of Wisconsin, Madison, WI
Bradley, Jeffrey - Washington University, St. Louis, MO
Bristow, Robert - Princess Margaret Cancer Centre, Toronto, Canada
Brown, J. Martin - Stanford University, Stanford, CA
Buatti, John - University of Iowa, Iowa City, IA
Camphausen, Kevin - NIH, Bethesda, MD
Chakravarti, Arnab - Ohio State University-James Cancer Hospital, Columbus, OH
Choyke, Peter - NIH, Bethesda, MD
Chung, Christine - Johns Hopkins Medical Institute, Baltimore, MD
Curran, Walter - Emory University, Atlanta, GA
Dewesse, Theodore - Johns Hopkins Medical Institute, Baltimore, MD
Dewhirst, Mark - Duke University Medical Center, Durham, NC
Dicker, Adam - Thomas Jefferson University Hospitals, Philadelphia, PA
Doroshow, James - NIH, Bethesda, MD
Efstathiou, Jason - Massachusetts General Hospital, Boston, MA
Galvin, James - Thomas Jefferson University Hospitals, Philadelphia, PA
Garcia-Vargas, Jose - Bayer HealthCare, USA
Guha, Udayan - NIH, Bethesda, MD
Ha, Chul - University of Texas Health Science Center at San Antonio, TX
Hahn, Steve - University of Pennsylvania, Philadelphia, PA
Hill, Richard - Princess Margaret Cancer Centre, Toronto, Canada
Kirsch, David - Duke University Medical Center, Durham, NC
Krishnan, Sunil - MD Anderson Cancer Center, Houston, TX
Le, Quynh-Thu - Stanford University, Stanford, CA
Langer, Corey - University of Pennsylvania,, Philadelphia, PA
Liao, Zhongxiang - MD Anderson Cancer Center, Houston, TX
Mendonca, Marc - Indiana University, Indianapolis, IN
Machtay, Mitchell - University Hospitals Case Medical Center, Cleveland, OH
Mehta, Minesh - Northwestern University, Chicago, Ill
Miskel, Robin - Sanofi-Aventis Corporation, Boston, MA
Mitchell, James - NIH, Bethesda, MD
Pollack, Alan - University of Miami Miami,, FL
Prasanna, Pataje - NIH, Bethesda, MD
Teicher, Beverly - NIH, Bethesda, MD
van der Kogel, Albert - University of Wisconsin, Madison, WI
Wang, Dian - Medical College of Wisconsin, Milwaukee, WI
White, Julia - Medical College of Wisconsin, Milwaukee, WI
Willett, Christopher - Duke University Medical Center, Durham, NC
Williams, Jackie - Rochester Medical Center, Rochester, NY
Winter, Kathryn - American College of Radiology, Reston, VA
Zwiebel, James - NIH, Bethesda, MD
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Soares HP, Kumar A, Daniels S, Swann S, Cantor A, Hozo I, et al. Evaluation of new treatments in radiation oncology: are they better than standard treatments? JAMA. 2005;293:970–978. doi: 10.1001/jama.293.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 3.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rischin D, Peters LJ, O'Sullivan B, Giralt J, Fisher R, Yuen K, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28:2989–2995. doi: 10.1200/JCO.2009.27.4449. [DOI] [PubMed] [Google Scholar]
- 6.Peters LJ, O'Sullivan B, Giralt J, Fitzgerald TJ, Trotti A, Bernier J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol. 2010;28:2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK. More lessons learned from the suffocation of hypoxia. J Clin Oncol. 2010;28:2941–2943. doi: 10.1200/JCO.2010.28.3085. [DOI] [PubMed] [Google Scholar]
- 8.Ang K. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III–IV head and neck squamous cell carcinomas (HNC) ASCO. 2011 doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley J, Paulus R, Komaki R. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy +/− cetuximab for stage IIIa/IIIb non-small cell lung cancer: Preliminary findings on radiation dose in RTOG 0617. 53rd Annual Meeting of the American Society of Radiation Oncology; 2011; Miami, FL. [Google Scholar]
- 10.Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys. 2012;82:1042–1044. doi: 10.1016/j.ijrobp.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, 3rd, Thomas CR, Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 12.Rich TA, Winter K, Safran H, Hoffman JP, Erickson B, Anne PR, et al. Weekly paclitaxel, gemcitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer. Onco Targets Ther. 2012;5:161–170. doi: 10.2147/OTT.S33560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roach M, 3rd, DeSilvio M, Lawton C, Uhl V, Machtay M, Seider MJ, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21:1904–1911. doi: 10.1200/JCO.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Lawton CA, DeSilvio M, Roach M, 3rd, Uhl V, Kirsch R, Seider M, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 16.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 17.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 18.Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 19.Akimoto T, Hunter NR, Buchmiller L, Mason K, Ang KK, Milas L. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res. 1999;5:2884–2890. [PubMed] [Google Scholar]
- 20.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 21.Sheridan MT, O'Dwyer T, Seymour CB, Mothersill CE. Potential indicators of radiosensitivity in squamous cell carcinoma of the head and neck. Radiat Oncol Investig. 1997;5:180–186. doi: 10.1002/(SICI)1520-6823(1997)5:4<180::AID-ROI3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Rubin Grandis J, Chakraborty A, Melhem MF, Zeng Q, Tweardy DJ. Inhibition of epidermal growth factor receptor gene expression and function decreases proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. Oncogene. 1997;15:409–416. doi: 10.1038/sj.onc.1201188. [DOI] [PubMed] [Google Scholar]
- 23.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 24.Milas L, Mason K, Hunter N, Petersen S, Yamakawa M, Ang K, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6:701–708. [PubMed] [Google Scholar]
- 25.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 26.Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, et al. Phase I study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–3243. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 27.Harrington KJ, Billingham LJ, Brunner TB, Burnet NG, Chan CS, Hoskin P, et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br J Cancer. 2011;105:628–639. doi: 10.1038/bjc.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lunt SJ, Kalliomaki TM, Brown A, Yang VX, Milosevic M, Hill RP. Interstitial fluid pressure, vascularity and metastasis in ectopic, orthotopic and spontaneous tumours. BMC Cancer. 2008;8:2. doi: 10.1186/1471-2407-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Q, Jurisica I, Do T, Hedley DW. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res. 2011;71:3110–3120. doi: 10.1158/0008-5472.CAN-10-4049. [DOI] [PubMed] [Google Scholar]
- 30.Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol. 2010;28:585–593. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- 31.Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, et al. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- 32.Kirsch DG, Grimm J, Guimaraes AR, Wojtkiewicz GR, Perez BA, Santiago PM, et al. Imaging primary lung cancers in mice to study radiation biology. Int J Radiat Oncol Biol Phys. 2010;76:973–977. doi: 10.1016/j.ijrobp.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirsch DG, Dinulescu DM, Miller JB, Grimm J, Santiago PM, Young NP, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13:992–997. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]
- 34.Russo AL, Kwon HC, Burgan WE, Carter D, Beam K, Weizheng X, et al. In vitro and in vivo radiosensitization of glioblastoma cells by the poly (ADP-ribose) polymerase inhibitor E7016. Clin Cancer Res. 2009;15:607–612. doi: 10.1158/1078-0432.CCR-08-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graves EE, Vilalta M, Cecic IK, Erler JT, Tran PT, Felsher D, et al. Hypoxia in models of lung cancer: implications for targeted therapeutics. Clin Cancer Res. 2010;16:4843–4852. doi: 10.1158/1078-0432.CCR-10-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao C, Mu Y, Hallahan DE, Lu B. XIAP and survivin as therapeutic targets for radiation sensitization in preclinical models of lung cancer. Oncogene. 2004;23:7047–7052. doi: 10.1038/sj.onc.1207929. [DOI] [PubMed] [Google Scholar]
- 37.Feng Z, Scott SP, Bussen W, Sharma GG, Guo G, Pandita TK, et al. Rad52 inactivation is synthetically lethal with BRCA2 deficiency. Proc Natl Acad Sci U S A. 2010;108:686–691. doi: 10.1073/pnas.1010959107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bornstein S, White R, Malkoski S, Oka M, Han G, Cleaver T, et al. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J Clin Invest. 2009;119:3408–3419. doi: 10.1172/JCI38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CJ, Spalding AC, Ben-Josef E, Wang L, Simeone DM. In vivo bioluminescent imaging of irradiated orthotopic pancreatic cancer xenografts in nonobese diabetic-severe combined immunodeficient mice: a novel method for targeting and assaying efficacy of ionizing radiation. Transl Oncol. 2010;3:153–159. doi: 10.1593/tlo.09184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, et al. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1:508–523. doi: 10.1158/2159-8290.CD-11-0109. [DOI] [PubMed] [Google Scholar]
- 41.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 43.Bredel M, Scholtens DM, Yadav AK, Alvarez AA, Renfrow JJ, Chandler JP, et al. NFKBIA deletion in glioblastomas. N Engl J Med. 2011;364:627–637. doi: 10.1056/NEJMoa1006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother Oncol. 2010;94:30–35. doi: 10.1016/j.radonc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Nelson NJ. Adaptive clinical trial design: has its time come? J Natl Cancer Inst. 2010;102:1217–1218. doi: 10.1093/jnci/djq319. [DOI] [PubMed] [Google Scholar]
- 46.Kinders RJ, Hollingshead M, Lawrence S, Ji J, Tabb B, Bonner WM, et al. Development of a validated immunofluorescence assay for gammaH2AX as a pharmacodynamic marker of topoisomerase I inhibitor activity. Clin Cancer Res. 2010;16:5447–5457. doi: 10.1158/1078-0432.CCR-09-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji J, Kinders RJ, Zhang Y, Rubinstein L, Kummar S, Parchment RE, et al. Modeling pharmacodynamic response to the poly(ADP-Ribose) polymerase inhibitor ABT-888 in human peripheral blood mononuclear cells. PLoS One. 2011;6:e26152. doi: 10.1371/journal.pone.0026152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck--a systematic review and meta-analysis. Radiother Oncol. 2011;100:22–32. doi: 10.1016/j.radonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25:4066–4074. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
- 50.Hicks KO, Siim BG, Jaiswal JK, Pruijn FB, Fraser AM, Patel R, et al. Pharmacokinetic/pharmacodynamic modeling identifies SN30000 and SN29751 as tirapazamine analogues with improved tissue penetration and hypoxic cell killing in tumors. Clin Cancer Res. 2010;16:4946–4957. doi: 10.1158/1078-0432.CCR-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark CH, Miles EA, Urbano MT, Bhide SA, Bidmead AM, Harrington KJ, et al. Pre-trial quality assurance processes for an intensity-modulated radiation therapy (IMRT) trial: PARSPORT, a UK multicentre Phase III trial comparing conventional radiotherapy and parotid-sparing IMRT for locally advanced head and neck cancer. Br J Radiol. 2009;82:585–594. doi: 10.1259/bjr/31966505. [DOI] [PubMed] [Google Scholar]
- 52.Bissonnette JP, Balter PA, Dong L, Langen KM, Lovelock DM, Miften M, et al. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: a report of the AAPM TG-179. Med Phys. 2012;39:1946–1963. doi: 10.1118/1.3690466. [DOI] [PubMed] [Google Scholar]
- 53.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 54.Simes RJ. Publication bias: the case for an international registry of clinical trials. J Clin Oncol. 1986;4:1529–1541. doi: 10.1200/JCO.1986.4.10.1529. [DOI] [PubMed] [Google Scholar]
- 55.Tam VC, Tannock IF, Massey C, Rauw J, Krzyzanowska MK. Compendium of unpublished phase III trials in oncology: characteristics and impact on clinical practice. J Clin Oncol. 2011;29:3133–3139. doi: 10.1200/JCO.2010.33.3922. [DOI] [PubMed] [Google Scholar]
- 56.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483:531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 57.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10:712. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- 58.Mehta MP, Shapiro WR, Phan SC, Gervais R, Carrie C, Chabot P, et al. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: results of a phase III trial. Int J Radiat Oncol Biol Phys. 2009;73:1069–1076. doi: 10.1016/j.ijrobp.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 59.Bernhard EJ, Mitchell JB, Deen D, Cardell M, Rosenthal DI, Brown JM. Re-evaluating gadolinium(III) texaphyrin as a radiosensitizing agent. Cancer Res. 2000;60:86–91. [PubMed] [Google Scholar]
- 60.Scholl C, Frohling S, Dunn IF, Schinzel AC, Barbie DA, Kim SY, et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell. 2009;137:821–834. doi: 10.1016/j.cell.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 61.Babij C, Zhang Y, Kurzeja RJ, Munzli A, Shehabeldin A, Fernando M, et al. STK33 kinase activity is nonessential in KRAS-dependent cancer cells. Cancer Res. 2011;71:5818–5826. doi: 10.1158/0008-5472.CAN-11-0778. [DOI] [PubMed] [Google Scholar]
- 62.Frohling S, Scholl C. STK33 kinase is not essential in KRAS-dependent cells--letter. Cancer Res. 2011;71:7716. doi: 10.1158/0008-5472.CAN-11-2495. author reply 7717. [DOI] [PubMed] [Google Scholar]
- 63.Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 64.Baggerly K. Disclose all data in publications. Nature. 2010;467:401. doi: 10.1038/467401b. [DOI] [PubMed] [Google Scholar]
- 65.Ioannidis JP, Allison DB, Ball CA, Coulibaly I, Cui X, Culhane AC, et al. Repeatability of published microarray gene expression analyses. Nat Genet. 2009;41:149–155. doi: 10.1038/ng.295. [DOI] [PubMed] [Google Scholar]
- 66.Ioannidis JP, Khoury MJ. Improving validation practices in "omics" research. Science. 2011;334:1230–1232. doi: 10.1126/science.1211811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–1452. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]