Abstract
Three experiments addressed whether pronounced alterations in the circadian system yielded concomitant changes in ultradian timing. Female Siberian hamsters were housed in a 16L:8D photoperiod after being subjected to a disruptive phase-shifting protocol that produced 3 distinct permanent circadian phenotypes: some hamsters entrained their circadian rhythms (CRs) with predominantly nocturnal locomotor activity (ENTR), others displayed free-running CRs (FR), and a third cohort was circadian arrhythmic (ARR). The period of the ultradian locomotor rhythm (UR) did not differ among the 3 circadian phenotypes; neuroendocrine generation of URs remains viable in the absence of coherent circadian organization and appears to be mediated by substrates functionally and anatomically distinct from those that generate CRs. Pronounced light-dark differences in several UR characteristics in ENTR hamsters were completely absent in circadian arrhythmic hamsters. The disruptive phase-shifting protocol may compromise direct visual input to ultradian oscillators but more likely indirectly affects URs by interrupting visual afference to the circadian system. Additional experiments documented that deuterium oxide and constant light, each of which substantially lengthened the period of free-running CRs, failed to change the period of concurrently monitored URs. The resistance of URs to deuteration contrasts with the slowing of virtually all other biological timing processes, including CRs. Considered together, the present results point to the existence of separable control mechanisms for generation of circadian and ultradian rhythms.
Keywords: locomotor activity, D2O, arrhythmia, SCN
Ultradian rhythms (URs) provide important temporal integration of the endocrine milieu (Veldhuis, 2008; Knobil, 1999), but fitness consequences of behavioral URs for the most part remain to be established (but see Daan and Slopesma, 1978). The period of URs ranges from seconds and minutes up to 8 h, with greater interindividual variability than for circadian rhythms (CRs; Aschoff, 1981). Environmental factors that entrain behavioral URs remain unspecified, with no evidence for direct synchronization by ultra-short light-dark cycles (Gerkema et al., 1993; Redlin and Mrosovsky, 1999). The light-dark cycle is not, however, without influence on URs. In male Syrian hamsters, the period of the wheel-running UR is longer during the light than the dark phase (Gattermann, 1985), and the amplitude of the body temperature UR is substantially greater in the dark than the light phase (Refinetti, 1994). In addition, the influence of reproductive hormones on female Syrian hamster URs is gated by the light-dark cycle: robust effects of the estrous cycle and of reproductive condition on URs are evident during the dark but not the light phase (Prendergast et al., 2012).
The precise relation of ultradian to circadian rhythms remains elusive. URs in behavior and physiology persist after surgical ablation of the suprachiasmatic nucleus (SCN) and elimination of coherent circadian organization in rats, Syrian hamsters, common voles, and Siberian hamsters (Eastman et al., 1984; Rusak, 1977; Refinetti, 1994; Gerkema et al., 1990, 1993; Ruby and Zucker, 1992; but see Wollnik and Turek, 1989). This does not imply that SCN activity and circadian rhythms normally are without influence on URs: An increase in the number of significant URs is positively correlated with the power of Syrian hamster free-running circadian rhythms (Refinetti, 1994), and hamsters bearing the tau mutation have shorter UR periods in feeding (Oklejewicz et al., 2001) and locomotor activity (Refinetti, 1996) relative to wild-type hamsters.
Ablation of the SCN typically destroys adjacent brain tissue and eliminates retinohypothalamic projections that provide visual afference to the neuroendocrine system that may interdict pathways that mediate masking effects of light on URs. Siberian hamsters provide a favorable model system to probe interactions of circadian and ultradian timing systems. Appropriately timed phase shifts of the LD cycle induce permanent circadian arrhythmicity in locomotor activity, body temperature, sleep, and cognitive rhythms in a substantial proportion of Siberian hamsters (Ruby et al., 2004; Larkin et al., 2004; Ruby et al., 2008), without the undesirable sequelae of brain lesions. Circadian arrhythmicity in this model system is sustained for many months despite maintenance of hamsters in a fixed 16L:8D light-dark cycle; the SCN of arrhythmic hamsters retains robust c-fos and per1 mRNA responses to acute light signals (Barakat et al., 2005) but does not support masking of locomotor activity by light (Barakat et al., 2005). Behavioral arrhythmicity induced by this disrupting phase-shift protocol is associated with arrhythmic SCN expression of the clock genes per1, per2, and bmal1 (Grone et al., 2011). A related deficit has been reported in a subset of male Siberian hamsters transferred from long to short day lengths (Puchalski and Lynch, 1988) that exhibit noncircadian locomotor activity in constant darkness and failure to modify activity in response to light pulses.
The goal of the present study was to assess the impact of circadian organization on ultradian rhythms of neurologically intact Siberian hamsters utilizing the circadian-disrupting phase-shift protocol originally described by Ruby et al. (1998) and since refined (Steinlechner et al., 2002; Ruby et al., 2004). One additional manipulation with the potential to discriminate circadian from ultradian control mechanisms utilizes heavy water (deuterium oxide; D2O), which lengthens the period of circadian rhythms in a dose-dependent manner in many species and taxa (Enright, 1971). D2O lengthened the high-frequency ultradian licking rhythm in a preliminary study of rats (Logothetis et al., 1984) and very high frequency URs in fish and crustaceans (reviewed in Enright, 1971) but had no impact on 2-h ultradian feeding rhythms of voles (Gerkema et al., 1993), despite lengthening the period of the circadian activity rhythm by almost 2 h. Ingestive behavior rhythms may be uniquely resistant to D2O, as evidenced by the failure of the food-entrained circadian rhythms of rats to be affected by D2O treatment (Mistlberger et al., 2001). To test the generality of D2O effects on non–food-related URs, and to extend the analysis to female rodents (Beery and Zucker, 2011), we investigated URs of home cage locomotor activity in Siberian hamsters in response to deuteration. We also determined the impact of the transition from constant darkness to constant light on URs, a manipulation that substantially lengthens the period of circadian rhythms (Carpenter and Grossberg, 1984). Collectively, the present experiments suggest both shared and separate control systems for ultradian and circadian behavior rhythms.
Materials and Methods
Animals and Housing
Siberian hamsters (Phodopus sungorus) were obtained from a breeding colony maintained on a light:dark cycle of 15L:9D (15L; lights-off at 1800 h CST). Hamsters were housed in polypropylene cages (28 × 17 × 12 cm) on wood shaving bedding (Harlan Sani-Chips, Harlan Inc., Indianapolis, IN). Ambient temperature was 20 ± 0.5 °C, and relative humidity was 53 ± 2%. Food (Teklad Rodent Diet 8604, Harlan Inc.) and filtered tap water were provided ad libitum. Cotton nesting material was continuously available in the cages. All procedures conformed to the USDA Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Chicago.
Activity Measurements
Many studies of URs measure foraging or feeding behavior (Daan and Slopsema, 1978; Gerkema et al., 1990; 1993; van der Veen et al., 2006). We measured URs and CRs of spontaneous general locomotor activity—a non–food-specific behavior that correlates highly with daily rhythms of sleep—wakefulness, body temperature, and drinking behavior (Rusak & Zucker, 1979; Kriegsfeld et al., 2002); in the ultradian domain, locomotor activity correlates positively with feeding rhythms (Gerkema et al., 1993). Locomotor activity studies therefore address qualitative and quantitative aspects of underlying circadian and ultradian timing systems. Hereafter, when referring to “URs” and “CRs,” we are referencing locomotor behavior rhythms.
Locomotor activity data were collected in the home cage for a minimum of 10 consecutive days with passive infrared motion detectors (Coral Plus, Visonic, Bloomfield, CT) positioned 22 cm above the cage floor. Motion detectors registered activity when 3 of 27 zones were crossed. Activity triggered closure of an electronic relay, recorded by a computer running ClockLab software (Actimetrics, Evanston, IL). Cumulative activity counts were collected at 6-min intervals.
Experiment 1: Effects of Circadian Arrhythmia on Ultradian Rhythms
Adult female Siberian hamsters 60 to 90 days of age from the 15L breeding colony (n = 128) were housed 1 per cage and transferred to 16L (lights-off at 1800 h CST) for 4 weeks prior to the implementation of a circadian disrupting phase-shift (DPS) protocol. Briefly, after 4 weeks in the 16L photoperiod, a 2-h light pulse was administered during the 5th through 7th hours of the dark phase to a subset of hamsters (n = 104). The next day, the 16L photocycle was phase delayed 3 h by extending the light phase (lights-off at 2100 h CST; for further description of this procedure, see Ruby et al., 2004). This DPS protocol typically renders >50% of hamsters permanently circadian arrhythmic (“ARR”; Ruby et al., 2004). A second subset of control hamsters (n = 24) was subjected to the 3-h phase delay but without the 2-h light pulse on the preceding night; approximately 90% of such hamsters re-entrain to the new photocycle (“ENTR”; Ruby et al., 2004). CRs and URs in locomotor activity were monitored in all hamsters 1 to 3 months after the phase shift was administered.
Experiment 2: Effects of Deuterium Oxide on Ultradian Rhythms
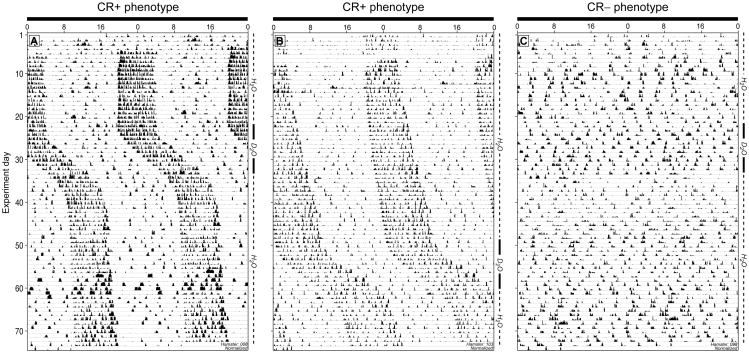
Circadian arrhythmic (ARR; n = 31) and circadian entrained (ENTR; n = 15) females from experiment 1 were transferred from the 16L photoperiod into constant darkness (DD), where they remained thereafter. A dim red light (<0.1 lux) remained on at all times to facilitate animal care. Locomotor activity of ENTR hamsters began free-running in DD; these hamsters were designated as CR+; ARR hamsters remained circadian arrhythmic in DD and were designated as CR–.
A minimum of 10 days after transfer to DD, deuterium oxide (D2O) at a concentration of 25% in filtered tap water (v/v) was provided in the home cage in graduated bottles; during control treatments, hamsters had access to filtered tap water (H2O treatment). D2O/H2O treatments lasted for 12 days, followed by a 14-day washout interval during which all hamsters received tap water only. D2O and H2O were administered to all hamsters in a randomized, counterbalanced design with continuous monitoring of CR and UR activity before and during D2O/H2O treatments. Data for 10 consecutive days of CR and UR activity were collected during the pretreatment (H2O baseline), and treatment intervals were assessed statistically.
Experiment 3: Effects of Constant Light on Ultradian Rhythms
A separate cohort of circadian entrained (ENTR; n = 6 females and n = 10 males) and circadian arrhythmic animals (ARR; n = 7 females and 5 males) was generated as described in experiment 1, and after confirmation of circadian phenotype in 16L, the cohort was transferred to DD for 16 days, followed by LL (∼450 lux at the level of the cage bottom) for 7 days. As in experiment 2, upon transfer to constant conditions, hamsters previously identified as ENTR were designated as CR+, as entrainment per se was no longer present. Hamsters previously designated as ARR were labeled as CR−. Home cage locomotor activity was collected continuously during each photoregimen, and activity data were analyzed as described below. Because there were no detectable sex differences in either group (F1,10-14 ≤ 2.7, p > 0.10, all comparisons), data for males and females were combined to create sample sizes of 16 and 12 for the ENTR and ARR groups, respectively.
Activity Analyses
Ultradian Rhythms
Experiment 1
For ENTR and ARR hamsters, activity data collected at 6-min intervals were parsed into light-phase activity (photophase: 0200–1800 h; 160 data points/24 h) and dark-phase activity (scotophase: 1800–0200 h; 80 data points/24 h) data files that were separately subjected to Lomb-Scargle periodogram (LSP) and cosinor periodogram analyses, as described in detail elsewhere (Prendergast et al., 2012). For each hamster, successive days of photophase activity data were concatenated into a single file from 10 consecutive nights or 5 consecutive days to generate records with equivalent numbers of sample data (800 points) to equalize statistical power in periodogram analyses performed on records from different photoperiods.
Locomotor activity of FR hamsters was parsed into files containing activity from subjective night (the circadian interval of relatively increased activity, α) and subjective day (the circadian interval of relative inactivity, σ) in a manner similar to that described above for ENTR and ARR hamsters. The onset and offset of free-running locomotor activity were calculated with Clocklab software (version 2.72; Actimetrics). Subjective night was defined as the interval between the onset and offset of activity in a given circadian cycle and subjective day as the interval between the offset and onset of activity. For each hamster, activity data from 9 to 10 consecutive subjective nights and 8 to 9 consecutive subjective days were concatenated into separate data files. These records were separately subjected to LSP and cosinor periodogram analyses. Finally, for a separate analysis, locomotor activity of FR hamsters was also parsed into (objective) light-phase and (objective) dark-phase activity, in a manner identical to that described above for ENTR and ARR hamsters.
Experiment 2
Unparsed files (240 data points/24 h), generated during 10 consecutive days of D2O or H2O treatment were subjected to LSP and cosinor periodogram analyses.
Experiment 3
Unparsed files generated during the final 6 days of DD treatment and during the first 6 days of LL treatment were subjected to LSP and cosinor periodogram analyses as in Experiment 2.
Circadian Rhythms
In all experiments, unparsed files (240 data points/24 h), 10 days in length, were first analyzed with the chi-square periodogram program (Clocklab, Actimetrics). Data from ENTR, FR, and ARR hamsters also were subjected to LSP and cosinor periodogram analyses to extract quantitative CR parameters. Hamsters classified as entrained (ENTR) exhibited significant circadian periods (p < 0.01) of ∼24 h (mean ± standard deviation = 24.06 ± 0.13 h), those classified as free-running (FR) had significant (p < 0.01) circadian periods ≠24 h (mean ± standard deviation = 24.8 ± 0.3 h), and others classified as arrhythmic (ARR) did not exhibit significant periodicity (p > 0.05) in the circadian range (22–26 h). These classifications were confirmed by visual inspection of actograms.
Statistical Analyses
LSP analyses (Lomb, 1976) were performed to identify the statistical presence/absence of URs and CRs and the complexity of the UR waveform (i.e., the number of significant peaks in the UR spectrum; range: 0.1–7.9 h). The level of statistical significance was set to 0.01. Cosinor analyses were employed to determine several quantitative measures of behavioral URs (range: 0.1–7.9 h) and CRs (range: 22–26 h): robustness (or “prominence,” the percentage of variance accounted for by the best-fit cosine model, which corresponds to the coefficient of determination R2 in regression analyses; Refinetti et al., 2007), mesor (rhythm-adjusted mean value around which the waveform oscillates), and amplitude (the difference between the peak or trough value and the mesor), expressed as absolute values (activity counts) and relative values referenced to the photophase-specific mesor value; the latter measure incorporates baseline activity levels during each photophase in determining rhythm amplitude. Lastly, acrophase was computed as the average time relative to the onset or offset of light at which the waveform peaks. The level of statistical significance was set to 0.05 with a Bonferroni correction for multiple comparisons.
The LSP is an all-purpose, robust procedure for detecting ultradian periodicities, well suited for measurement of data binned into separate scotophase/photophase files. It optimizes detection of URs by not displaying peaks at multiples of all rhythms detected (Ruf, 1999; van Dongen et al., 1999, 2001). Supplemental analyses after completion of LSP analysis were adopted as recommended by Refinetti et al. (2007). The cosinor periodogram (Bingham et al., 1982) is a reliable, preferred curve-fitting tool to quantify rhythm parameters (Refinetti et al., 2007).
ANOVAs and pairwise comparisons were performed on a computer with Statview 5.0 (SAS Institute, Cary NC) and LSP and cosinor analyses with soft ware written by R. Refinetti (available at http://www.circadian.org/softwar.html). The proportion of hamsters displaying URs and CRs was evaluated with chi-square tests. Effects of arrhythmia (experiment 1), D2O (experiment 2), and LL (experiment 3) on quantitative aspects of URs and CRs were examined with ANOVA; pairwise comparisons were performed using Fisher PLSD tests or unpaired, 2-tailed t tests. Differences were considered significant if p ≤ 0.05.
Results
Experiment 1: Effects of Circadian Disruption on URs
Circadian Rhythms
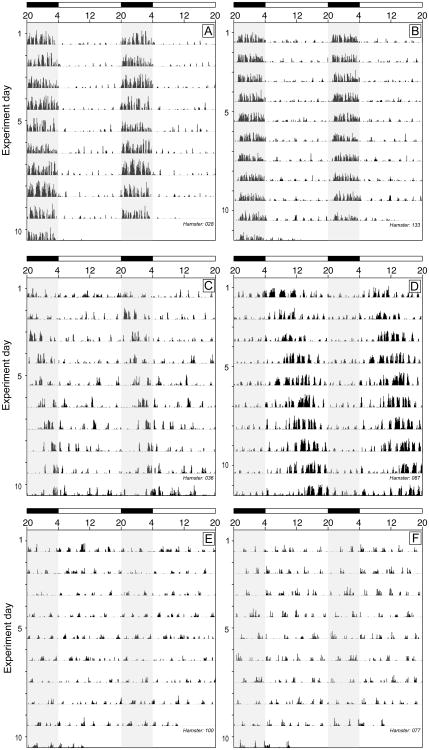
Of 104 hamsters subjected to the DPS treatment, 43 re-entrained to the shifted light-dark cycle (ENTR), 29 exhibited free-running locomotor activity (FR), and 32 were behaviorally arrhythmic (ARR). Of the 24 control hamsters subjected to a 3-h phase delay of the light-dark cycle, 2 were classified as ARR, 1 as FR, and 21 re-entrained. ENTR hamsters were combined into a single treatment group (representative actograms in Fig. 1A, 1B), as were FR (Fig. 1C, 1D) and ARR (Fig. 1E, 1F) hamsters. Data from 9 ENTR hamsters were excluded from analysis because their patterns of entrained locomotor activity were either diurnal (n = 3) or had split into multiple, distinct activity components (n = 6). Data from 11 FR hamsters failed to exhibit consistent, distinct circadian intervals of activity (α) and inactivity (σ) and were excluded from analyses.
Figure 1.
Locomotor activity of entrained, free running, and circadian arrhythmic hamsters. Representative double-plotted activity records of ENTR (A, B), FR (C, D), and ARR (E, F) hamsters in experiment 1. All actograms are drawn to the same scale (0–30 counts/bin). Clock time is indicated on the horizontal axis at the top of each actogram, along with light (white) and dark (black) phases of the shifted (post-DPS) 16 h light:8 h dark photocycle (lights off from 2000 h to 0400 h). Shading over the activity record denotes the daily dark phase.
Ultradian Rhythms
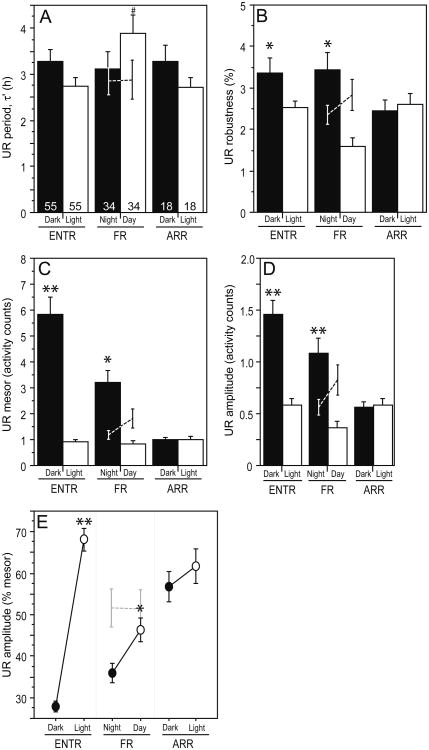
There was no main effect of circadian phenotype on UR period (τ′; p > 0.05; Fig. 2A); however, UR τ′ of FR hamsters during subjective day was significantly greater than UR τ′ of ENTR (p = 0.005) and ARR (p < 0.01) hamsters during the light phase (Fig. 2A). Phenotype and photo/circadian phase interacted to affect UR robustness (p < 0.05; Fig. 2B). Among ENTR hamsters, UR robustness was significantly greater in the dark than the light phase (p < 0.05; Fig. 2B). In FR hamsters, a significant increase in robustness was also evident during subjective night compared with subjective day (Fig. 2B; p < 0.005). Among ARR hamsters, however, UR robustness was similar in the light and dark phases (Fig. 2B; p > 0.70). UR complexity was unaffected by circadian phenotype (p > 0.70; data not illustrated).
Figure 2.
Effects of circadian arrhythmia on ultradian rhythms. Mean ± SEM (A) period (τ′), (B) robustness, (C) mesor, (D) absolute amplitude, and (E) relative amplitude (as % mesor) of ultradian waveforms in ENTR, FR, and ARR hamsters. For ENTR and ARR groups, bars indicate locomotor activity during the dark phase (Dark) and light phase (Light) of the 16L:8D photoperiod. For the FR groups, bars indicate locomotor activity occurring during the subjective night and subjective day of the free-running circadian activity waveform, and dashed lines indicate activity occurring during the objective night (dark phase) and objective day (light phase) of the 16L:8D photoperiod. Sample sizes are indicated along the abscissa in panel A.*p < 0.05 vs. light-phase (or subjective day) value, within circadian phenotype; **p < 0.001 vs. all other groups; #p < 0.05 vs. ENTR-light and ARR-light values.
Circadian arrhythmia (ARR) did not affect the percentage of hamsters displaying dark-phase or light-phase URs. In the dark phase, 60% of ENTR hamsters, 44% of ARR hamsters, and 66% of FR hamsters exhibited URs (see Supplementary Figure S1 online); in the light phase, URs were evident in 47% of ENTR hamsters, 44% of ARR hamsters, and 50% of FR hamsters (p > 0.10, all between-phenotype comparisons). UR expression was equally likely in the dark and light phases for all groups.
Phenotype and photo/circadian phase interacted to affect UR mesor activity levels (p < 0.001; Fig. 2C), which were significantly higher during the dark than light phase in ENTR hamsters (p < 0.001) but indistinguishable between the light and dark phases in ARR hamsters (p > 0.90). In FR hamsters, activity counts were significantly higher during subjective night than subjective day (p < 0.005).
Phenotype and photo/circadian phase also interacted to affect absolute (p < 0.001) and relative (p < 0.001) UR amplitude (Fig. 2D, 2E). Among ENTR and FR hamsters, absolute amplitude was higher and relative amplitude was lower in the objective/subjective night as compared with objective/subjective day (p < 0.05, all comparisons). Among ARR hamsters, amplitude values were comparable in the light and dark phases (p > 0.20, all comparisons).
Lastly, when activity data from FR hamsters were parsed according to (objective) light phase and (objective) dark phase, there were no significant differences in any quantitative measure of URs (τ′: p > 0.90; robustness: p > 0.20; mesor: p > 0.10; absolute amplitude: p > 0.10; relative amplitude: p > 0.90; dashed lines in Fig. 2A-2E).
Experiment 2: Effects of Deuterium Oxide on CRs and URs
Hamsters received D2O and H2O treatment in a randomized order. Treatment order did not affect any of the quantitative measures of CRs (p > 0.15, all comparisons) or URs (p > 0.20, all comparisons).
Circadian Rhythms
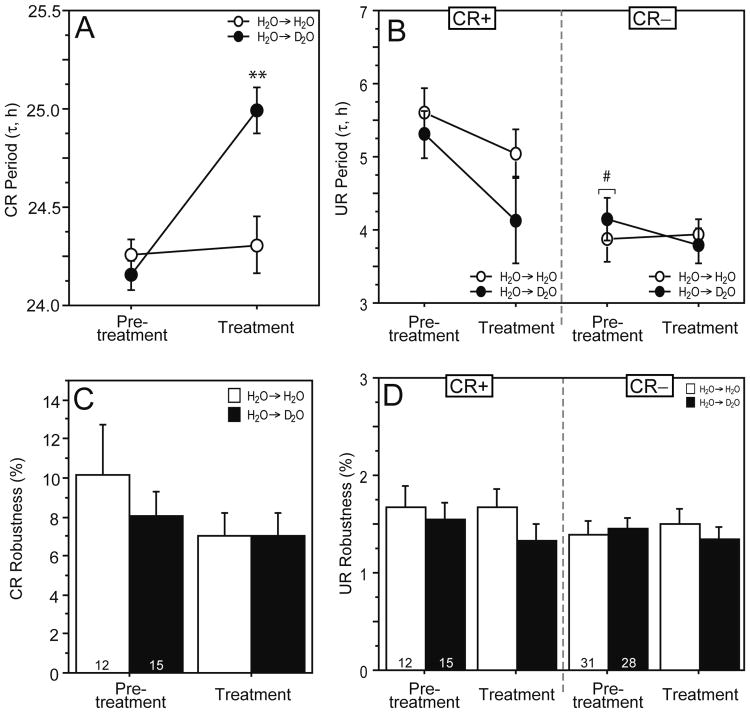
D2O treatments lengthened τ by 0.8 h in hamsters exhibiting CRs (p = 0.001; Fig. 3A, 3B, Fig. 4A) but did not influence circadian rhythm robustness (Fig. 4C), mesor, or amplitude (data not illustrated; p > 0.50, all comparisons).
Figure 3.
Effects of deuterium oxide (D2O) on circadian and ultradian locomotor activity. Representative double-plotted activity records of CR+ (A, B) and CR− (C) hamsters. Note free-running circadian activity with τ < 24 h (A) and τ > 24 h (B) and circadian arrhythmicity (C) prior to D2O treatment. Intervals of 25% D2O and H2O availability are indicated along the right ordinate. Clock time and a black bar designating constant darkness (DD) are at the top of each actogram.
Figure 4.
Effects of D2O on circadian and ultradian rhythms. Mean ± SEM period (A, B) and robustness (C, D) of circadian (CR) and ultradian (UR) rhythms in circadian-rhythmic (CR+) and circadian-arrhythmic (CR−) hamsters housed in DD and provided with H2O or D2O. Sample sizes within the figure key. **p < 0.001 vs. H2O group. #Significantly lower than corresponding pretreatment values for CR+ hamsters. ○: during both pretreatment and treatment phases, hamsters were maintained on H2O; ●: during pretreatment, hamsters were provided with H2O and during treatment with D2O.
Ultradian Rhythms
Ultradian τ′ was significantly longer in CR+ (mean ± SEM = 5.44 ± 0.23 h) than CR− hamsters (4.00 ± 0.21 h) prior to deuteration (pretreatment interval, p < 0.001; Fig. 4B). D2O did not significantly affect ultradian τ′ of CR+ hamsters, which decreased slightly more than did τ′ of the control group consuming tap water during the treatment interval (p > 0.10; Fig. 4B), nor were robustness (Fig. 4D), mesor, or amplitude (not illustrated) affected by deuteration (p > 0.10, all comparisons). D2O also was without effect on τ′ (Fig. 4B), robustness (Fig. 4D), mesor, and amplitude (not illustrated) of CR− hamsters (p > 0.40, all comparisons). The absolute and percentage changes in CRs and URs are summarized in Table 1.
Table 1. Effects of 25% D2O on circadian and ultradian periods.
| Circadian τ | Ultradian τ′ | Relative Change | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Pre | TRT | Pre | TRT | % ΔCR | % ΔUR | |
|
| ||||||
| CR+ hamsters | ||||||
| H2O → H2O | 24.3 (0.1) | 24.3 (0.2) | 5.6 (0.3) | 5.1 (0.3) | +0.2% | −10.0% |
| H2O → D2O | 24.2 (0.1) | 25.0 (0.1)a | 5.3 (0.3) | 4.1 (0.6) | +3.5% | −22.4% |
|
| ||||||
| CR− hamsters | ||||||
| H2O → H2O | — | — | 3.9 (0.3) | 3.9 (0.2) | — | +1.8% |
| H2O → D2O | — | — | 4.2 (0.3) | 3.8 (0.2) | — | −8.92% |
Values indicate mean period in hours (standard error). Values are rounded to 0.1 h. Pre = 10-day pretreatment interval; TRT = 10-day treatment interval.
p < 0.001 vs. H2O → H2O value
Experiment 3: Effects of Constant Light on CRs and URs
Circadian Rhythms
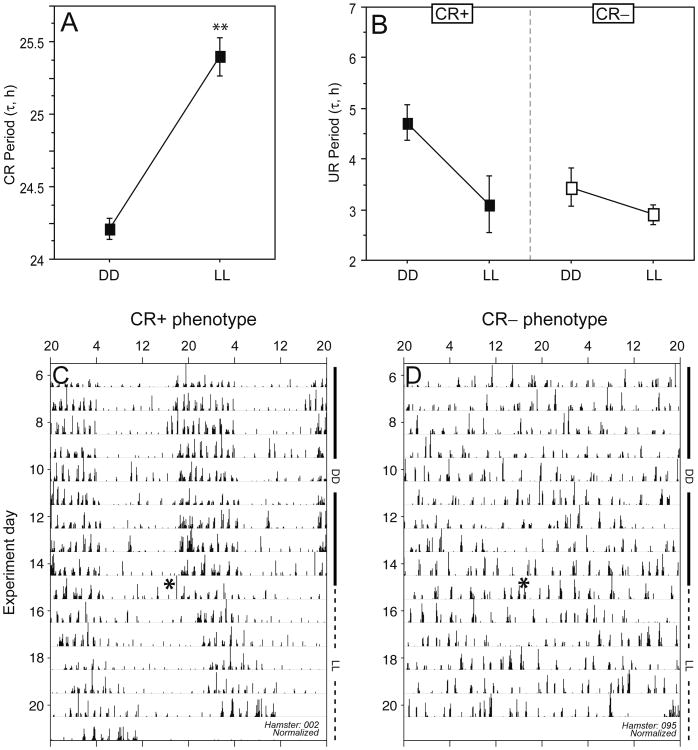
In CR+ hamsters, the free-running period was 24.2 ± 0.1 h in DD and 25.4 ± 0.1 h in LL (mean ± SEM; t15 = 7.34, p < 0.001; Fig. 5); circadian τ increased in 15 of 16 hamsters after the transition from DD to LL. The absence of coherent circadian rhythms in the CR− hamsters precluded assessment of the influence of the DD to LL transition. Other components of the circadian waveform (complexity, robustness, mesor, amplitude) of CR+ hamsters did not differ significantly in LL as compared with DD (p > 0.10, all comparisons; data not illustrated).
Figure 5.
Effects of constant light on circadian and ultradian rhythms. Mean ± SEM period of circadian (CR; panel A) and ultradian (UR; panel B) rhythms in circadian-rhythmic (CR+; n = 16) and circadian-arrhythmic (CR−; n = 12) hamsters that were housed in DD for 16 days and were then transferred to constant light. (C, D) Representative double-plotted activity records of a CR+ (C) and a CR− (D) hamster. Note free-running circadian activity (C) and circadian arrhythmicity (D) in both DD and LL. Intervals of exposure to DD and LL are indicated along the right ordinate. Clock time is at the top of each actogram. **p < 0.001 vs. DD value (within-group).
Ultradian Rhythms
The period of URs did not change significantly after the transition from DD to LL in CR+ or CR− hamsters (Fig. 5B). The increase in the circadian period of CR+ hamsters was accompanied by a decrease of ∼1.6 h in the ultradian period, although this change in τ′ was not statistically significant (p > 0.05). In CR+ hamsters, UR robustness and mesor also did not differ significantly in LL relative to DD (p > 0.1, both comparisons), but UR amplitude was significantly diminished in LL (p < 0.05; data not illustrated). Similarly, in CR− hamsters, transfer from DD to LL resulted in a nonsignificant decrease in UR period (from 3.44 ± 1.3 h to 2.90 ± 0.7 h; p > 0.10; Fig. 5B); UR complexity, robustness, mesor, and amplitude were unaffected by transfer to LL (p > 0.10, all comparisons; data not illustrated). Thus, τ′ was not affected by transfer to LL in either phenotype.
Discussion
Appropriately timed phase shifts of the light-dark cycle produced 3 distinct circadian phenotypes in Siberian hamsters housed in a 16L:8D photoperiod. One group entrained normally with predominantly nocturnal locomotor activity (ENTR), a second cohort displayed free-running (FR) circadian rhythms of locomotion, and a third phenotype lost all vestiges of circadian organization (arrhythmic; ARR), thereby resembling rodents with SCN lesions (cf. Ruby et al., 1998; Ruby et al.,2004). The latter 2 groups appear to be completely unresponsive to the masking or entraining effects of light (Barakat et al., 2005). In previous reports, light-induced locomotor arrhythmia has been accompanied by arrhythmic patterns of sleep-wake cycles (Larkin et al., 2004) and body temperature (Ruby et al., 1998) and inhibition of nocturnal melatonin secretion (Ruby et al., 2000; Steinlechner et al., 2002), consistent with the interpretation that the circadian pacemaker in the SCN is rendered functionally arrhythmic in these individuals (Grone et al., 2011). Because ARR hamsters lack coherent circadian organization but retain functional light perception (Barakat et al., 2005), they afford the opportunity to investigate photic control of ultradian rhythms independent of circadian influences.
The period of the locomotor UR in the 16L photoperiod did not differ among the 3 circadian phenotypes, indicating that the substrates that generate URs remain functional in neurologically intact hamsters lacking circadian organization and in those that do not entrain their CRs to the 16L photoperiod; this confirms and extends studies of other rodent species (e.g., Gerkema et al., 1990; Refinetti, 1994; Eastman et al., 1984) in which ultradian rhythmicity persisted after ablation of the SCN. In the present model system, the SCN of ARR hamsters receives visual input from the retinohypothalamic tract (Barakat et al., 2005) and remains structurally intact, but it lacks normal circadian oscillations in the expression of several major clock genes (per1, per2, cry1, and bmal1; Grone et al., 2011). Arrhythmic expression of these 4 clock genes evidently does not interfere with behavioral UR generation.
The induction of circadian arrhythmicity did, however, substantially alter several quantitative aspects of URs. In hamsters with circadian rhythms that remained either entrained (ENTR) or became free running (FR), UR robustness, amplitude, and mesor were substantially greater in the dark phase and subjective night than in the light phase and subject day, respectively (Fig. 2). In sharp contrast, these light-dark differences were completely absent in circadian arrhythmic (ARR) hamsters. The disrupting light protocol used to generate circadian arrhythmicity either eliminates direct photic influences on URs or indirectly eliminates day-night differences in URs by disrupting circadian input to the ultradian system (cf. Gerkema et al., 1993). Other UR characteristics such as rhythm complexity and period were not affected by circadian disruption, suggesting a degree of independence of these UR components from circadian control.
Not all Siberian hamsters exhibited URs in the present study. This is in contrast with the common vole, in which behavioral URs are evident in most individuals (Gerkema et al., 1990, 1993). Common voles appear to reflect an extreme variant of UR robustness, in which URs are expressed by all individuals, and are dominated by the feeding rhythm. This is unlikely to obtain in most mammals in which both body temperature and locomotor activity rhythms are not dependent on feeding cycles (e.g., Refinetti, 1996; Prendergast et al., 2012). The absence of URs in many Siberian hamsters may reflect either labile expression of URs in these hamsters or the categorical absence of URs in these individuals.
In prior reports, the DPS procedure rendered more than 50% of hamsters arrhythmic (Ruby et al., 2004, 2008), whereas in the present study, approximately one-third of hamsters expressed the ARR phenotype. Identical procedural and statistical protocols were used in the present and earlier reports. Ruby et al. (2004) documented marked strain differences in the efficacy of the DPS protocol, which, along with differences in light intensity or animal husbandry, may contribute to the incidence of arrhythmia (Ruby et al., 2004). Arrhythmia may result from desynchrony of normal oscillations in clock gene expression in individual SCN neurons or from suppression in the amplitude of clock gene expression in many individual SCN cells. Both mechanisms would be accompanied by an absence of rhythms in clock gene expression at the tissue level, but normal median values of clock gene expression would be expected if the former mechanism were operant, whereas suppressed median values of clock gene expression would accompany the latter mechanism. Grone et al. (2011) observed that, at the whole-SCN level, the median amplitude of per1, per2, and bmal1 mRNA expression was significantly (20%-40%) lower following the DPS procedure, suggesting that DPS-induced arrhythmia in Siberian hamsters is likely a consequence of amplitude suppression.
Experiment 2 confirmed and extended the surprising observations of Gerkema et al. (1993) that 25% D2O, which markedly lengthens the free-running period of the circadian wheel-running rhythm of voles by 1.89 h (7.9 % increase), increased the ultradian feeding rhythm by only 0.03 h, a 1.24% increase that was not statistically significant, but requires replication given the small sample size (n = 4). In the same study, the UR feeding rhythm of SCN-lesioned, circadian arrhythmic voles (n = 5) was unchanged at 2.97 h during deuteration (Gerkema et al., 1993). In the present study, D2O significantly lengthened the period of the CR of hamster locomotor behavior by 0.8 h (3.5%), which was accompanied by a nonsignificant decrease of 1.2 h (22%) in the period of the UR. A similar, smaller decrease in ultradian τ′ was manifested by hamsters kept on tap water during the treatment interval. Deuterium produces a wide range of chemical and physical effects, including slowing of permeability and diffusion rates in cells and decreases in ion mobility (White et al., 1992); differential effects on 1 or more of these cellular activities could account for different effects of deuterium on the periods of ultradian and circadian rhythms. The isolated eye-stalks of crayfish exhibit a linear correlation between period lengthening of URs and CRs of electroretinogram activity over a range of D2O concentrations (Pardo and Sáenz, 1988), a relation strikingly different from the behavioral results in hamsters and voles. The present results corroborate the findings of Gerkema et al. (1993) that D2O treatments that substantially lengthen the circadian period exert no similar actions on the period of URs. The investigation of a non–food-related behavior suggests that this class of rhythms is categorically unresponsive to deuteration in rodents and differs from the profound slowing of most other biological timing processes during D2O treatment (Enright, 1971; Gerkema et al., 1993).
Experiment 3 established that substantial lengthening of the circadian period by constant light treatment was without significant effect on the period and most other components of the ultradian locomotor rhythm. Considered together, these findings provide convergent evidence in support of independence of UR period from circadian control.
Hamsters exhibited polyphenic responses to the disruptive light treatments. In FR hamsters, coherent circadian rhythms are maintained, but retinohypothalamic light input to the SCN is unable to entrain CRs, suggesting that the disrupting phase-shift protocol has in some respects desensitized the SCN to subsequent light input (Barakat et al., 2004); conversely, in ARR hamsters, coherent circadian rhythms in behavior and SCN clock gene expression are compromised, but RHT-to-SCN signaling is preserved (Barakat et al., 2005). Quantitative aspects of URs in hamsters exhibiting FR and ARR responses permit insights into the roles of light and the circadian system in the generation of day-night differences in URs. First, in FR hamsters, circadian alternations in UR robustness, mesor, and amplitude followed subjective time (subjective day and subjective night) despite the prevailing LD cycle. Indeed, URs of FR hamsters were unaffected by the light-dark cycle itself. This suggests that the free-running circadian system alone is sufficient to generate circadian oscillations in UR amplitude; masking effects of light are neither necessary nor sufficient in this regard. Additional evidence from circadian arrhythmic hamsters also suggests a critical role for the circadian system in the generation of day-night differences in URs as circadian modulation of URs was completely absent in these hamsters. ARR hamsters lack behavioral CRs and normal SCN molecular CRs, but light induces normal responses in the SCN of these hamsters (Barakat et al., 2005); nor are ARR hamsters perceptually blind in higher-order measures of visual processing (motion detection, object recognition; Ruby et al., 2008). Nevertheless, in the absence of a normal circadian system, light information registering in the SCN of ARR hamsters does not affect URs, which are functionally blind to light information. This underscores the importance of coherent circadian organization in mediating effects of LD cycles on URs; extra-RHT-SCN visual pathways are unable to drive light-dark rhythms in URs.
In summary, the present work probed interactions between the circadian and ultradian systems in Siberian hamsters, in which an exceptionally labile circadian pacemaker can be rendered unresponsive to light or arrhythmic. Robust circadian rhythms were evident in the amplitude of the ultradian waveform. Circadian modulation of ultradian rhythm amplitude occurred in a manner suggesting that light affects URs indirectly, via the circadian system through RHT-SCN–mediated mechanisms. Constant light- and deuteration-induced lengthening of the circadian period of the locomotor rhythm were not accompanied by parallel changes in the period of the ultradian locomotor rhythm, suggesting a degree of independence of ultradian rhythms from circadian control.
Supplementary Material
Acknowledgments
We thank Kenneth Onishi, Janine Kirin, and Dr. Betty Theriault for expert technical assistance. This research was supported by NIH grant AI-67406 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Conflict of Interest Statement: The authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Note: Supplementary material is available on the journal's website at http://jbr.sagepub.com/supplemental
References
- Aschoff J. A survey on biological rhythms. In: Aschoff J, editor. Handbook of Behavioral Neurobiology, Vol 4, Biological Rhythms. New York: Plenum Press; 1981. pp. 3–10. [Google Scholar]
- Barakat MT, O'Hara BF, Cao VH, Heller HC, Ruby NF. Light induces c-fos and per1 expression in the suprachiasmatic nucleus of arrhythmic hamsters. Am J Physiol. 2005;289:R1381–R1386. doi: 10.1152/ajpregu.00695.2004. [DOI] [PubMed] [Google Scholar]
- Barakat MT, O'Hara BF, Cao VH, Larkin JE, Heller HC, Ruby NF. Light pulses do not induce c-fos or per1 in the SCN of hamsters that fail to reentrain to the photocycle. J Biol Rhythms. 2004;19:287–297. doi: 10.1177/0748730404266771. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham C, Arbogast B, Guillaume GC, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- Carpenter GA, Grossberg S. A neural theory of circadian rhythms: Aschoff's rule in diurnal and nocturnal mammals. Am J Physiol. 1984;247:R1067–R1082. doi: 10.1152/ajpregu.1984.247.6.R1067. [DOI] [PubMed] [Google Scholar]
- Daan S, Slopsema S. Short-term rhythms in foraging behavior in the common vole, Microtus arvalis. J Comp Physiol A. 1978;127:215–227. [Google Scholar]
- Eastman CI, Mistlberger RE, Rechtschaffen A. Suprachiasmatic nuclei lesions eliminate circadian temperature and sleep rhythms in the rat. Physiol Behav. 1984;32:357–368. doi: 10.1016/0031-9384(84)90248-8. [DOI] [PubMed] [Google Scholar]
- Enright JT. Heavy water slows biological timing processes. Z Vergl Physiol. 1971;72:1–16. [Google Scholar]
- Gattermann R. Zur biorhthmik des goldhamsters (Mesocricetus auratus Waterhouse 1839) Zool Jb. 1985;89:107–113. [Google Scholar]
- Gerkema MP, Daan S, Wilbrink M, Hop MW, van der Leest F. Phase control of ultradian feeding rhythms in the common vole (Microtus arvalis): the roles of light and the circadian system. J Biol Rhythms. 1993;8:151–171. doi: 10.1177/074873049300800205. [DOI] [PubMed] [Google Scholar]
- Gerkema MP, Groos GA, Daan S. Differential elimination of circadian and ultradian rhythmicity by hypothalamic lesions in the common vole, Microtus arvalis. J Biol Rhythm. 1990;5:81–95. doi: 10.1177/074873049000500201. [DOI] [PubMed] [Google Scholar]
- Grone BP, Chang D, Bourgin P, Cao V, Fernald RD, Heller HC, Ruby NF. Acute light exposure suppresses circadian rhythms in clock gene expression. J Biol Rhythms. 2011;26:78–81. doi: 10.1177/0748730410388404. [DOI] [PubMed] [Google Scholar]
- Knobil E. The wisdom of the body revisited. News Physiol Sci. 1999;14:1–11. doi: 10.1152/physiologyonline.1999.14.1.1. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Le Sauter J, Hamada T, Pitts SM, Silver R. Circadian rhythms in the endocrine system. In: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, Rubin RT, editors. Hormones, Brain and Behavior. New York: Academic Press; 2002. pp. 33–91. [Google Scholar]
- Larkin JE, Yokogawa T, Heller HC, Franken P, Ruby NF. Homeostatic regulation of sleep in arrhythmic Siberian hamsters. Am J Physiol. 2004;287:R104–R111. doi: 10.1152/ajpregu.00676.2003. [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Boulos Z, Terman M. Lick rate and the circadian rhythm of water intake in the rat: effects of deuterium oxide. Ann N Y Acad Sci. 1984;423:614–617. [Google Scholar]
- Lomb N. Least-squares frequency analysis of unequally spaced data. Astrophys Space Sci. 1976;39:447–462. [Google Scholar]
- Mistlberger RE, Marchant EG, Kippin TE. Food-entrained circadian rhythms in rats are insensitive to deuterium oxide. Brain Res. 2001;919:283–291. doi: 10.1016/s0006-8993(01)03042-6. [DOI] [PubMed] [Google Scholar]
- Oklejewicz M, Overkamp GJ, Stirland JA, Daan S. Temporal organization of feeding in Syrian hamsters with a genetically altered circadian period. Chronobiol Int. 2001;18:657–664. doi: 10.1081/cbi-100106079. [DOI] [PubMed] [Google Scholar]
- Pardo BF, Sáenz EM. Action of deuterium oxide upon the ERG circadian rhythm in crayfish, Procambarus bouvieri. Comp Biochem Physiol. 1988;90A:435–440. [Google Scholar]
- Prendergast BJ, Beery AK, Paul MJ, Zucker I. Enhancement and suppression of ultradian and circadian rhythms across the female hamster reproductive cycle. J Biol Rhythms. 2012;27:246–256. doi: 10.1177/0748730412441315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchalski W, Lynch GR. Characterization of circadian function in Djungarian hamsters insensitive to short day photoperiod. J Comp Physiol A. 1988;162:309–316. doi: 10.1007/BF00606119. [DOI] [PubMed] [Google Scholar]
- Redlin U, Mrosovsky N. Masking of locomotor activity in hamsters. J Comp Physiol A. 1999;184:429–437. doi: 10.1007/s003590050342. [DOI] [PubMed] [Google Scholar]
- Refinetti R. Circadian modulation of ultradian oscillation in the body temperature of the golden hamster. J Therm Biol. 1994;19:269–275. [Google Scholar]
- Refinetti R. Ultradian rhythms of body temperature and locomotor activity in wild-type and tau-mutant hamsters. Anim Biol. 1996;5:111–115. [Google Scholar]
- Refinetti R, Cornélissen G, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38:275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Barakat MT, Heller HC. Phenotypic differences in reentrainment behavior and sensitivity to nighttime light pulses in Siberian hamsters. J Biol Rhythms. 2004;19:530–541. doi: 10.1177/0748730404268055. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Dubocovich ML, Heller HC. Siberian hamsters that fail to reentrain to the photocycle have suppressed melatonin levels. Am J Physiol. 2000;278:R757–R762. doi: 10.1152/ajpregu.2000.278.3.R757. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci USA. 2008;105:15593–15598. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Joshi N, Heller HC. Phase shift magnitude and direction determine whether Siberian hamsters reentrain to the photocycle. J Biol Rhythms. 1998;13:506–517. doi: 10.1177/074873049801300606. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Zucker I. Daily torpor in the absence of the suprachiasmatic nucleus in Siberian hamsters. Am J Physiol. 1992;263:R353–R362. doi: 10.1152/ajpregu.1992.263.2.R353. [DOI] [PubMed] [Google Scholar]
- Ruf T. The Lomb-Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biol Rhythm Res. 1999;30:178–201. [Google Scholar]
- Rusak B. The role of the suprachiasmatic nuclei in the generation of circadian rhythms in the golden hamster, Mesocricetus auratus. J Comp Physiol. 1977;118:145–164. [Google Scholar]
- Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Steinlechner S, Stieglitz A, Ruf T. Djungarian hamsters: a species with a labile circadian pacemaker? Arrhythmicity under a light-dark cycle induced by short light pulses. J Biol Rhythms. 2002;17:248–258. doi: 10.1177/074873040201700308. [DOI] [PubMed] [Google Scholar]
- van der Veen DR, Minh NL, Gos P, Arneric M, Gerkema MP, Schibler U. Impact of behavior on central and peripheral circadian clocks in the common vole Microtus arvalis, a mammal with ultradian rhythms. Proc Natl Acad Sci U S A. 2006;103:3393–3398. doi: 10.1073/pnas.0507825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen HPA, Olofsen E, Van Hartevelt JH, Kruyt EW. A procedure of multiple period searching in unequally spaced time series with the Lomb-Scargle method. Biol Rhythm Res. 1999;30:149–177. doi: 10.1076/brhm.30.2.149.1424. [DOI] [PubMed] [Google Scholar]
- van Dongen HPA, Ruf T, Olofsen E, van Hartevelt JH, Kruyt EW. Analysis of problematic time series with the Lomb–Scargle method, a reply to “Emphasizing difficulties in the detection of rhythms with Lomb–Scargle periodograms”. Biol Rhythm Res. 2001;32:347–354. doi: 10.1076/brhm.32.3.347.1348. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD. Pulsatile hormone secretion: mechanisms, significance and evaluation. In: Lloyd D, Rossi E, editors. Ultradian Rhythms from Molecules to Mind. Berlin: Springer Science & Business; 2008. pp. 229–247. [Google Scholar]
- White LA, Ringo JM, Dowse HB. Effects of deuterium oxide on temperature and heart rate in Drosophila melanogaster. J Comp Physiol B. 1992;162:278–283. doi: 10.1007/BF00357535. [DOI] [PubMed] [Google Scholar]
- Wollnik F, Turek FW. SCN lesions abolish ultradian and circadian components of activity rhythms in LEW/Ztm rats. Am J Physiol. 1989;256:R1027–R1039. doi: 10.1152/ajpregu.1989.256.5.R1027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.