Abstract
Coronary artery disease and acute myocardial infarction (AMI) are the leading causes of death for both men and women. Serum cardiac-specific troponin level is now used for the “early” diagnosis of AMI. However, due to the “delayed” release of troponin, an earlier, more sensitive and specific biomarker is urgently demanded to further reduce AMI mortality. Recent studies have found that circulating microRNAs (miRNAs) are closely linked to myocardial injury. Due to the cell-specific physiological functions and the stability of miRNAs in plasma, serum, and urine, they are emerging as sensitive biomarkers of AMI. This review summarizes the latest insights into the identification and potential application of plasma and serum miRNAs as novel biomarkers for diagnosis and prognosis of AMI.
Keywords: MicroRNA, Biomarker, Circulating, Acute myocardial infarction
Introduction
Coronary artery disease (CAD) and acute myocardial infarction (AMI) are the leading causes of death in developed and developing countries [1]. According to the American Heart Association, mortality caused by CAD in United States of America was 406,351 in 2007, accounting for about 1 in every 6 deaths. It is estimated that approximately every minute, someone in the USA dies from a heart attack [2]. AMI accounts for most of the mortality due to CAD. However, the mortality from AMI in the USA has been declining, partly due to earlier recognition and effective revascularization therapy, including percutaneous coronary intervention (PCI) and coronary artery bypass surgery (CABG) [3]. Circulating biomarkers of myocardial damage, especially cardiac-specific troponin, have led to an early diagnosis of AMI, maximizing the benefits of revascularization therapy. In AMI patients, troponin levels rise as early as 3.5 hours (hrs) after the onset of chest pain. However, due to the relative “delayed” release time of troponin, earlier biomarkers with both high sensitivity and specificity are urgently demanded to further reduce the AMI mortality [4].
In addition to high sensitivity and specificity, the ideal biomarker for AMI should meet three criteria [5]. (1) Good accessibility: samples must be easily obtained by the least invasive methods, such as those from the body fluids, including plasma, serum and urine. (2) Predictability: the biomarkers should have a relative long half-life in the blood, which ensures the predictability of detection. The expression signatures and levels of biomarkers should closely correlate with the extent and healing of myocardial injury. (3) Robust reliability: the detecting methods should be rapid, accurate, sensitive, inexpensive, without special requirement for instrument or equipment, which is essential for point-of-care, and wide-spread popularization. Most of the currently available biomarkers with clinical applications are proteins and polypeptides [6]. Novel biomarkers, such as molecular and genetic biomarkers, are under investigation [7]. In recent years, miRNAs have emerged as novel biomarkers due to their diverse but tissue- or cell-specific biological and pathological functions [8–10].
In this review, we summarize recent information on biomarkers for AMI, focusing on the latest insights in the identification and potential use of miRNAs in the plasma and serum as novel specific and sensitive biomarkers for AMI.
The biogenesis of circulating miRNAs
MicroRNAs are short (17–25 nucleotides) non-coding RNAs that regulate gene expression at the post-transcriptional level, either by inhibiting mRNA translation or inducing its degradation [11]. It is estimated that the human genome encodes more than 1000 miRNAs [12,13]. Indeed, up to 1424 miRNAs are in the miRBase Database (release 17.0, April, 2011) [14,15]. Most of the microRNA genes are located in the introns of protein-coding genes or as independent entities in between the genes [16–19]. As shown in Fig. 1, the primary miRNA (primiRNA) is transcribed from the microRNA genes in the nucleus by RNA polymerase II [20]. The pri-miRNAs are processed by the Drosha–Dgcr8 enzyme complex, generating a ~70-nucleotide long hair-pin-folded precursor miRNA (pre-miRNA), which is subsequently exported into the cytoplasm by the nuclear export factor, exportin-5, in a Ran-GTP-dependent manner [21–23]. In the cytoplasm, the pre-miRNAs are cleaved by an RNAse III enzyme complex, Dicer, into 20 to 24 nucleotides mature miRNAs, and subsequently incorporated into the RNA-induced silencing complex (RISC) [24]. If the miRNAs bind perfectly or almost perfectly to the target mRNAs, the 3′- or 5′-untranslated regions of the mRNAs can be cleaved and degraded [25]. If the miRNAs bind imperfectly to the mRNAs, the translation of mRNA is suppressed without degrading the target mRNAs (Fig. 1). More than 30% of human genes are predicted to be miRNA target genes [26]. The miRNA gene network consists of thousands of miRNAs and their target genes. A single miRNA can regulate one or more distinct target genes, while a single protein-coding gene can be regulated by several different miRNAs [27]. Therefore, miRNAs have diverse functions in the regulation of cellular function, including proliferation, apoptosis, and necrosis [28–30].
Fig. 1.
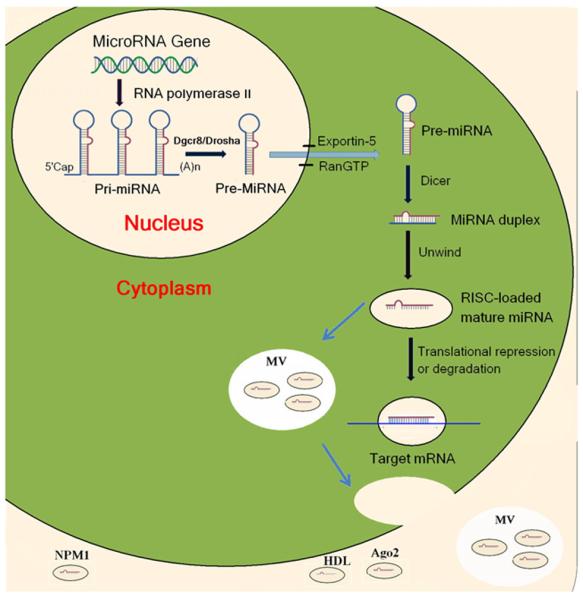
Biogenesis of miRNA and transfer to the circulation. Pri-miRNAs are transcribed from miRNA genes in the nucleus by RNA polymerase II and then processed by the Drosha-Dgcr8 enzyme complex to generate pre-miRNAs. Exportin-5 recognizes the pre-miRNAs and exports them to the cytoplasm in a Ran-GTP-dependent manner. In the cytoplasm, the pre-miRNAs are subsequently cleaved by Dicer to produce miRNA duplex. The duplex is separated and usually one strand is translated into the mature miRNA while the other strand is degraded. The mature miRNAs couple with a multiple protein nuclease complex to form RISC. The miRNAs bind to the target mRNAs to repress translation or induce degradation. MiRNAs in the cells can be incorporated into microvesicles (MV) or miRNA-binding proteins and secreted into the circulation. Argonaute (Ago2) complexes may be vesicle-independent carriers of circulating miRNA. Nucleophosmin 1 (NPM1) participates in the packing and exporting of circulating miRNA. High density lipoproteins (HDL) may act as endogenous transporters of miRNAs to the recipient cells.
The existence of miRNAs in the plasma and serum has been recently demonstrated and become the focus of translational research. More than 100 circulating miRNAs have been sequenced in healthy individuals [31]. The exact origins and biological functions of circulating miRNAs under physiological and pathological conditions remain to be elucidated. Normally, most of the circulating miRNAs are derived from the liver, lung, kidney, and blood cells. Dead and living cells may release miRNAs into circulation. Extracellular non-membrane binding circulating miRNAs may be the by-products of dead cells or cellular response after biological stimulation that is released into the extracellular space [32–34].
The miRNAs in the plasma or serum are resistant to RNAase digestion and remain stable in the RNAase-rich environment of blood. MiRNAs are stable even stabilize to extreme conditions, including boiling, very high or low pH, extended storage, and freeze-thaw cycles [32]. The circulating miRNAs per se are not intrinsically resistant to RNase digestion, their resistance with the formation of a protein–miRNA complex or by chemical modification. Circulating miRNAs tend to bond to lipid-based carriers and exist mainly in two forms: nonvesicle and vesicle-associated forms. The non-vesicle form accounts for 80% of total circulating miRNA. Argonaute 2 (Ago2) protein, a part of RNA-induced silencing complex, forms a highly stable complex with miRNA, remain stable as the by-products of dead cells, and can be detected in the circulation [33]. Nucleophosmin 1 (NPM1), primarily located in the nucleolus and involved in ribosomal processing, is also involved in the packing and exporting of circulating miRNAs [34]. High density lipoproteins (HDL), which are important reverse transporters of endogenous cholesterols [35,36], also transport miRNAs from the peripheral cells to the hepatocytes and act as mediators of cell-to-cell communication [37].
Microvesicles, also called exosomes and microparticles, contribute to contain a small portion of circulating miRNAs [38–40]. Neutral sphingomyelinase 2, which regulates the biogenesis of ceramide, is responsible for the secretion of exosomes to the extracellular matrix [41,42]. Microvesicles contain more than 1200 mRNAs and approximately 121 miRNAs and can be delivered from one cell to another. Circulating miRNA may be physiologically active and play a pivotal role in cell-to-cell communication [43].
Circulating miRNAs in acute myocardial infarction
More than 200 miRNAs exist in the heart [44–47]. The most abundant miRNAs in cardiac muscles are miR-1, let-7, miR-133, miR-126-3p, miR-30c and miR-26a; arterial smooth muscle cells have miR-145, let-7, miR-125a, miR-125b, miR-23 and miR-143 [48,49]. Some studies have reported that circulating miRNAs may be novel biomarkers for CAD and AMI [50,51]. For example, Fichtlscherer et al. [52] reported that in patients with stable CAD, plasma levels of miR-126, miR-17, miR-92a, and miR-155 are decreased, while miR-133a and miR-208a are increased. Hoekstra et al. [53] reported that stable and unstable angina pectoris could be distinguished from each other based on different miRNA profiles. Wang et al. [54] found that plasma miR-133 and miR-328 are increased 11- to 16-fold in Chinese with AMI relative to controls. Similar results were reported in Italian and Japanese; miRNA-133 is increased 12-fold (relative to values in controls) 156 minutes after the onset of AMI, a time course earlier than traditional AMI biomarkers, such as troponin I [55,56]; MiR-1 and miR-499-5p are also found to be increased while miR-122 and miR-375 are decreased relative to controls [55].
Animal studies have corroborated the elevated circulating miRNA levels found in humans with AMI. For example, the levels of miR-1, miR-133a, miR-208a, and miR-499 in the infarcted mouse myocardium are decreased; while their circulating levels are increased, indicating that the elevated circulating miRNAs are released from the infarcted heart. The elevated cardio-specific miRNAs are localized in the exosomes [56]. In adult pigs, circulating miR-1, miR-133a, miR-208b, miR-499-5p levels are rapidly increased by 70–4000-fold after induction of AMI by inflation of a balloon in the coronary artery [57]. The increased levels of circulating miRNAs after AMI are a reflection of myocardial injury, and parallel the extent of myocardial damage as measured by cardiac troponin [58] (Table 1).
Table 1.
Summary of circulating miRNA levels in AMI.
| miRNA | Source | Species | Patient number (n) | Control number (n) | Basal level (fold) | Time of measurable increase | Peak level (fold) | Peak Time (hr) | Duration (hr) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| miR-1 | Serum | Human | 31 | 20 | 1 | - | 100 | 6–72 | 168 | [61] |
| Plasma | Human | 33 | 17 | 1 | - | 15 | <3 | <5 | [55] | |
| Plasma | Human | 93 | 66 | - | - | - | - | - | [62] | |
| Plasma | Human | 33 | 30 | 1 | - | - | - | - | [54] | |
| Plasma | Human | 444 | - | - | - | - | - | - | [65] | |
| Plasma | Human | 25 | - | - | <12h | ~100 | <12 | >72 | [57] | |
| Plasma | Rat | - | - | 1 | 11h | ~800 | 3 | >24 | [54] | |
| Serum | Rat | - | - | 1 | 11h | 260 | 6 | 72 | [61] | |
| Plasma | Mice | - | - | 1 | 6h | 6 | 18 | 72–120 | [55] | |
| Plasma | Pig | 1 | 90 min | 1.6 | 2 | - | [57] | |||
| miR-499 | Plasma | Human | 14 | 10 | undetectable | <48h | - | 6–12 | Before discharge | [70] |
| Plasma | Rat | - | - | 1 | 11h | ~40–60 | 3 | >24 | [54] | |
| miR-499-5p | Plasma | Human | 33 | 17 | 1 | 9–11 h | 100 | 9–11 | >72 | [55] |
| Plasma | Mice | - | - | 1 | <15min | 70 | 24 | 72 | [55] | |
| Plasma | Pig | 25 | - | undetectable | 60 min | 1.6 | >2 | - | [57] | |
| miR-133a | Plasma | Human | 33 | 30 | 1 | - | - | - | - | [54] |
| Plasma | Human | 444 | - | - | - | - | - | - | [65] | |
| Plasma | Human | 51 | 28 | - | - | - | - | <168 | [10] | |
| Plasma | Rat | - | - | 1 | 11h | 1000 | 3 | >24 | [54] | |
| Plasma | Mice | 33 | 17 | 1 | 6h | 13 | 6 | >72 | [55] | |
| miR-133b | Plasma | Human | 33 | 17 | 1 | - | 60 | <3 | >72 | [55] |
| Plasma | Mice | - | - | 1 | 6h | 13 | 6 | 28–72 | [55] | |
| miR-208a | Plasma | Human | undetectable | - | - | - | - | [54] | ||
| Plasma | Rat | - | - | 1 | 11h | ~800–900 | 3 | 12 | [54] | |
| miR-208b | Plasma | Human | 33 | 30 | - | - | - | - | - | [66] |
| Plasma | Human | 25 | - | - | <12h | ~1,000 | <12 | >72 | [57] | |
| Plasma | Pig | - | - | 1 | 11h | 1.75 | 2 | - | [57] | |
| miR-30c | Whole blood | Human | 22 | 20 | - | - | - | - | - | [72] |
| miR-145 | Whole blood | Human | 22 | 20 | - | - | - | - | - | [72] |
miR-1
MiR-1 is primarily expressed in cardiac and skeletal muscles and consists of two subfamilies, miR1-1 and miR1-2. The two subfamilies have identical sequences, but are encoded by two distinct genes in chromosomes 18 and 20, respectively. MiR-1, involved in cardiogenesis and muscle differentiation, may regulate cardiac arrhythmogenicity by repressing the expression of the gap junction protein, connexin (GJA1) and the inward-rectifier potassium ion channel (KCNJ2) [59,60].
In the rat, the circulating miR-1 level is rapidly increased 1 hr after coronary artery ligation, and peaked at 200-fold higher than baseline 6 hrs after AMI. The elevated miR-1 level returned to basal levels 3 days after AMI, a time course earlier than traditional AMI biomarkers, such as troponin [54,61]. In the mouse, miR-1 began to rise at 6 hrs after coronary artery ligation, suggesting possible species-specific releasing time-course [55].
In a small cohort of 31 AMI patients and healthy controls, a nearly 100-fold elevation in serum miR-1 was detected 6 hrs after AMI [61]. In a larger cohort with 159 patients with or without AMI, plasma miR-1 level was found to be significantly higher in AMI patients, and the level returned to normal on discharge. The increased miR-1 level was not associated with age, gender, diabetes or the other well established AMI biomarkers. The area under the receiver operating characteristic (ROC) curve was 0.774 for separating AMI from non-AMI patients [62]. Cheng et al. [61] reported a positive relationship between the elevated miR-1 and serum creatine kinase-MB (CKMB). In Gidlöf et al. research, they found that miR-1 and miR-133a levels are strongly correlated with the renal glomerular filtration rate, indicating that the renal elimination might affect the plasma miRNA levels [57].
miR-133
MiR-133 is expressed in smooth, skeletal, and cardiac muscles, and consists of miR-133-a and miR-133-b [63]. MiR-133, a key regulator of vascular smooth muscle cell phenotypic switch, contributes to the progression of atherosclerosis [64].
In a rat AMI model, circulating miR-133a levels began to rise within 1 hr after AMI and peaked at 3 hrs to more than 1000-fold higher than baseline [54]. In contrast, in the mouse AMI model, the circulating miR-133a levels peaked at 6 hrs to approximately 13-fold above baseline [55]. Although miR-133 is also highly expressed in the skeletal muscles, acute limb ischemia does not result in increased circulating levels suggesting that the release of miRNA into the circulation after an insult is tissue-specific [65].
The above-mentioned results were also found in human studies. D'Alessandra et al. [55] investigated 33 patients with AMI and found that plasma miR-133a and miR-133b levels are at their peak about 156 minutes after the onset of symptoms. In a larger cohort comprised of 444 patients with acute coronary syndrome (ACS), the study design of which differs from others by using ACS subtypes as comparisons. The plasma miR-133a and miR-133b levels were independently associated with increased high sensitive cardiac troponin T (hsTnT) levels [65]. MiR-133a levels were significantly associated with the risk of death but lost the independent association with the all-cause mortality after adjusting for hsTnT levels, suggesting that the net reclassification improvement after incorporating the miRNA data to the traditional AMI marker, hsTnT, seemed to be minor [65]. Wang et al. [54] also reported a 4.4 fold increase of miR-133 in plasma from AMI patients and the area under ROC curve was 0.89. A positive correlation was also reported in the elevated miR-133 and cardiac troponin I. This report also indicated that miR-133 may be superior to cardiac troponin I due to the some confounding factors that may affect troponin I levels [66]. For example, in the end-stage renal disease, the lower glomerular filtration would increase the troponin I level in the plasma [67].
MiR-208
MiR-208, a miRNA mainly expressed in the heart, is encoded by an intron of the α-myosin heavy chain gene [68]. The miR-208 family includes two subfamilies: miR-208a and miR-208b. MiR-208 is involved in cardiomyocyte hypertrophy, fibrosis, and regulation of other cardiac muscle gene expression and function [69].
In a rat AMI model, plasma miR-208a began to increase 1 hr after coronary artery occlusion, peaked at 3 hrs to approximately 1000-fold above baseline, and returned to basal levels within 24 hrs [54].
Circulating miR-208 is immeasurable in healthy humans. Wang et al. [54] reported that miR-1, miR-133a, miR-499, and miR-208a are increased after AMI. Among these four miRNAs, miR-208a displayed the highest sensitivity and specificity for AMI. Widera et al. [65] confirmed the association between elevated miR-208b and AMI in a large cohort with 444 patients with ACS, and further indicated that miR-208b levels were predictive of 6 month mortality. The associations remained significant after adjustment for age and gender, but not after the adjustment for admission hsTnT levels, indicating that incorporating the circulating miRNA information to the traditional prognostic factors failed to increase the net reclassification improvements. Other similar studies failed to confirm the utility of circulating miR-208b levels in AMI patients, partly due to the rapid clearance and low concentrations of miR-208b in the circulation [70].
MiR-499 and other miRNAs
MiR-499 is also a cardiac-specific miRNA [71]. The increased levels of circulating miR-499 in patients with AMI were corroborated in the rat and mouse models of AMI [54,70]. Similar to miR-208a, plasma miR-499 concentrations are below the lower limit of detection in healthy subjects but are elevated in patients with AMI [70]. Other circulating miRNAs have also been reported to be increased in patients with AMI; miR-1291 and miR-663b are claimed to have the highest sensitivity and specificity in discriminating cases from controls [72]. These authors also reported that circulating miR-30c and miR-145 levels correlated with infarct size, estimated by troponin T release [72].
Current limitations and future directions
In recent years, miRNAs have been suggested as biomarkers for AMI diagnosis and prognosis. However, some critical issues have to be resolved before they can be applied into clinical practice. First, all the above-mentioned studies were done in a small number of cases. Therefore, global and large-scale clinical studies are required to confirm the correlation with and establish the time-related circulating miRNA levels after AMI. Second, the values of circulating miRNAs should be reevaluated when incorporating the traditional biomarkers including troponin into the current diagnostic and prognostic model. The multiple biomarker strategy might be an effective way for assessing the improvements of risk stratification beyond the risk assessment based on established risk factors [73,74]. Third, the cost involved in measuring circulating miRNAs is also very important consideration before for incorporating new biomarkers to clinical management. Fourth, some technical issues should be taken into consideration. Comparing circulating miRNA levels across studies can be confounded by multiple technical factors in the pre-analytical and analytical procedures [75,76]. A unified standard analytical method should be formulated to minimize inter-procedure bias. The current golden standard for quantifying circulating miRNA is real-time PCR. However, the procedure is specific to a specific miRNA and the analysis can take 2–3 hrs. This is a critical limitation because the time needed for quantifying circulating miRNA far exceeds the reperfusion time recommended by the latest global AMI management guidelines [77]. Therefore, the development of fast, highly sensitive, and specific methods for the detection and quantification of ultra-low levels (femtomolar) of miRNA in blood samples is critical for the clinical application of the circulating miRNAs as biomarkers for AMI diagnosis and prognosis.
Conclusions
Circulating miRNAs are emerging as novel biomarkers for AMI. However, more large-scale multi-centered clinical studies are needed before they can be used in clinical practice. The development of fast, highly sensitive, specific, and effective methods for the detection of the circulating miRNA at ultra-low levels may accelerate the pace of the application of circulating mRNAs biomarkers in guiding the diagnostic, therapeutic, and prognostic strategies of AMI.
Acknowledgement
Dr. Zeng's laboratory is supported by grants from the National Natural Science Foundation of China (30925018, 31130029, 81070559, 81100190),National Basic Research Program of China (973 Program, 2008CB517308, 2012CB517801), and Natural Science Foundation Project of CQ CSTC (CSTC, 2009BA5044); Dr. Duan's laboratory is supported by the National Center for Research Resources P-20 RR-15581, National Heart, Lung, and Blood Institute Grants HL106256 and HL63914, American Diabetes Association #07-8-IN-08, and American Heart Association Western States Affiliate 11GRNT7610161.
References
- [1].He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–34. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- [2].Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–65. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- [4].Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–45. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- [5].Moe KT, Wong P. Current trends in diagnostic biomarkers of acute coronary syndrome. Ann Acad Med Singapore. 2010;39:210–5. [PubMed] [Google Scholar]
- [6].Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC Med. 2010;8:34. doi: 10.1186/1741-7015-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Iribarren C, Phelps BH, Darbinian JA, McCluskey ER, Quesenberry CP, Hytopoulos E, et al. Circulating angiopoietins-1 and -2, angiopoietin receptor Tie-2 and vascular endothelial growth factor-A as biomarkers of acute myocardial infarction: a prospective nested case-control study. BMC Cardiovasc Disord. 2011;11:31. doi: 10.1186/1471-2261-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRTPCR) Methods. 2010;50:298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fichtlscherer S, Rosa SD, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–84. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- [10].Wang R, Li N, Zhang Y, Ran Y, Pu J. Circulating microRNAs are promising novel biomarkers of acute myocardial infarction. Intern Med. 2011;50:1789–95. doi: 10.2169/internalmedicine.50.5129. [DOI] [PubMed] [Google Scholar]
- [11].Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- [12].Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function genomics: the miRNA genes. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- [13].Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, et al. Identification of hundreds of conserved and non conserved human microRNAs. Nat Genet. 2005;37:766–70. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- [14].Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. MiRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–8. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kozomara A, Griffiths-Jones S. MiRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–7. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gschwend AR, Weingartner LA, Moore RC, Ming R. The sex-specific region of sex chromosomes in animals and plants. Chromosome Res. 2012;20:57–69. doi: 10.1007/s10577-011-9255-y. [DOI] [PubMed] [Google Scholar]
- [17].Yin Z, Li C, Han X, Shen F. Identification of conserved microRNAs and their target genes in tomato (Lycopersicon esculentum) Gene. 2008;414:60–6. doi: 10.1016/j.gene.2008.02.007. [DOI] [PubMed] [Google Scholar]
- [18].Dhandapani V, Ramchiary N, Paul P, Kim J, Choi SH, Lee J, et al. Identification of potential microRNAs and their targets in Brassica rapa L. Mol Cells. 2011;32:21–37. doi: 10.1007/s10059-011-2313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sibley CR, Seow Y, Saayman S, Dijkstra KK, El Andaloussi S, Weinberg MS, et al. The biogenesis and characterization of mammalian microRNAs of mirtron origin. Nucleic Acids Res. 2012;40:438–48. doi: 10.1093/nar/gkr722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Starega-Roslan J, Koscianska E, Kozlowski P, Krzyzosiak WJ. The role of the precursor structure in the biogenesis of microRNA. Cell Mol Life Sci. 2011;68:2859–71. doi: 10.1007/s00018-011-0726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha–DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–27. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–6. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang X, Xu X, Ma Z, Huo Y, Xiao Z, Li Y, et al. Dynamic mechanisms for pre-miRNA binding and export by Exportin-5. RNA. 2011;17:1511–28. doi: 10.1261/rna.2732611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Madina BR, Kuppan G, Vashisht AA, Liang YH, Downey KM, Wohlschlegel JA, et al. Guide RNA biogenesis involves a novel RNase III family endoribonuclease in Trypanosoma brucei. RNA. 2011;17:1821–30. doi: 10.1261/rna.2815911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Park JE, Hel I, Tian Y, Simanshu DK, Chang H, Jee D, et al. Dicer recognizes the 5′ end of RNA for efficient and accurate processing. Nature. 2011;475:201–5. doi: 10.1038/nature10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;12:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- [27].Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–9. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu CD, Kuo YS, Wu HC, Lin CT. Microrna-1 induces apoptosis by targeting prothymosin alpha in nasopharyngeal carcinoma cells. J Biomed Sci. 2011;18:80. doi: 10.1186/1423-0127-18-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].El Gazzar M, Church A, Liu T, McCall CE. Microrna-146a regulates both transcription silencing and translation disruption of TNF-a during TLR4-induced gene reprogramming. J Leukoc Biol. 2011;90:509–19. doi: 10.1189/jlb.0211074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- [32].Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–33. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–59. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kennedy MA, Barrera GC, Nakamura K, Baldán Á , Tarr P, Fishbein MC, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–31. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- [36].Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–3. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- [37].Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–95. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- [41].Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- [43].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- [44].Wang Z, Luo X, Lu Y, Yang B. MiRNAs at the heart of the matter. J Mol Med (Berl) 2008;86:771–83. doi: 10.1007/s00109-008-0341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chinchilla A, Lozano E, Daimi H, Esteban FJ, Crist C, Aranega AE, et al. MicroRNA profiling during mouse ventricular maturation: a role for miR-27 modulating Mef2c expression. Cardiovasc Res. 2011;89:98–108. doi: 10.1093/cvr/cvq264. [DOI] [PubMed] [Google Scholar]
- [46].Shen E, Diao X, Wei C, Wu Z, Zhang L, Hu B. MicroRNAs target gene and signaling pathway by bioinformatics analysis in the cardiac hypertrophy. Biochem Biophys Res Commun. 2010;397:380–5. doi: 10.1016/j.bbrc.2010.05.116. [DOI] [PubMed] [Google Scholar]
- [47].Fukushima Y, Nakanishi M, Nonogi H, Goto Y, Iwai N. Micro-managing myocyte mitosis. Circ Res. 2011;109:611–3. doi: 10.1161/CIRCRESAHA.111.252627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bauersachs J, Thum T. Biogenesis and regulation of cardiovascular microRNAs. Circ Res. 2011;109:334–47. doi: 10.1161/CIRCRESAHA.110.228676. [DOI] [PubMed] [Google Scholar]
- [49].Hartmann D, Thum T. MicroRNAs and vascular (dys)function. Vascul Pharmacol. 2011;55:92–105. doi: 10.1016/j.vph.2011.07.005. [DOI] [PubMed] [Google Scholar]
- [50].Di Stefano V, Zaccagnini G, Capogrossi MC, Martelli F. microRNAs as peripheral blood biomarkers of cardiovascular disease. Vascul Pharmacol. 2011;55:111–8. doi: 10.1016/j.vph.2011.08.001. [DOI] [PubMed] [Google Scholar]
- [51].Hoekstra M, van der Lans CA, Halvorsen B, Gullestad L, Kuiper J, Aukrust P. The peripheral blood mononuclear cell microRNA signature of coronary artery disease. Biochem Biophys Res Commun. 2010;394:792–7. doi: 10.1016/j.bbrc.2010.03.075. [DOI] [PubMed] [Google Scholar]
- [52].Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–84. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- [53].Hoekstra M, van der Lans CA, Halvorsen B, Gullestad L, Kuiper J, Aukrust P, et al. The peripheral blood mononuclear cell microRNA signature of coronary artery disease. Biochem Biophys Res Commun. 2010;394:792–7. doi: 10.1016/j.bbrc.2010.03.075. [DOI] [PubMed] [Google Scholar]
- [54].Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659–66. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- [55].D'Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31:2765–73. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011;4:446–54. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- [57].Gidlöf O, Andersson P, van der Pals J, Götberg M, Erlinge D. CardiospecificmicroRNA plasma levels correlate with troponin and cardiac function in patients with ST elevation myocardial infarction, are selectively dependent on renal elimination, and can be detected in urine samples. Cardiology. 2011;118:217–26. doi: 10.1159/000328869. [DOI] [PubMed] [Google Scholar]
- [58].De Rosa S, Fichtlscherer S, Lehmann R, Assmus B, Dimmeler S, Zeiher AM. Trans-coronary concentration gradients of circulating microRNAs. Circulation. 2011;124:1936–44. doi: 10.1161/CIRCULATIONAHA.111.037572. [DOI] [PubMed] [Google Scholar]
- [59].Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–91. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- [60].Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H, et al. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci. 2007;120:3045–52. doi: 10.1242/jcs.010728. [DOI] [PubMed] [Google Scholar]
- [61].Cheng Y, Tan N, Yang J, Liu X, Cao X, He P, et al. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin Sci (Lond) 2010;119:87–95. doi: 10.1042/CS20090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun. 2010;391:73–7. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- [63].Han M, Toli J, Abdellatif M. MicroRNAs in the cardiovascular system. Curr Opin Cardiol. 2011;26:181–9. doi: 10.1097/HCO.0b013e328345983d. [DOI] [PubMed] [Google Scholar]
- [64].Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, et al. Micro-RNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109:880–93. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- [65].Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, et al. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011;51:872–5. doi: 10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- [66].Freda BJ, Tang WH, Van Lente F, Peacock WF, Francis GS. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol. 2002;40:2065–71. doi: 10.1016/s0735-1097(02)02608-6. [DOI] [PubMed] [Google Scholar]
- [67].Korkmaz H, Saşak G, Celik A, Kurtoğlu E, Gürger M, Bursalı KB, et al. The comparison of cardiac biomarkers positivities in hemodialysis patients without acute coronary syndrome. Ren Fail. 2011;33:578–81. doi: 10.3109/0886022X.2011.585264. [DOI] [PubMed] [Google Scholar]
- [68].van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–9. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- [69].Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest. 2009;119:2772–86. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, et al. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem. 2010;56:1183–5. doi: 10.1373/clinchem.2010.144121. [DOI] [PubMed] [Google Scholar]
- [71].Shieh JT, Huang Y, Gilmore J, Srivastava D. Elevated miR-499 levels blunt the cardiac stress response. PLoS One. 2011;6:e19481. doi: 10.1371/journal.pone.0019481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Meder B, Keller A, Vogel B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, et al. MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic Res Cardiol. 2011;106:13–23. doi: 10.1007/s00395-010-0123-2. [DOI] [PubMed] [Google Scholar]
- [73].Kim HC, Greenland P, Rossouw JE, Manson JE, Cochrane BB, Lasser NL, et al. Multimarker prediction of coronary heart disease risk: the Women's Health Initiative. J Am Coll Cardiol. 2010;55:2080–91. doi: 10.1016/j.jacc.2009.12.047. [DOI] [PubMed] [Google Scholar]
- [74].Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- [75].Duttagupta R, Jiang R, Gollub J, Getts RC, Jones KW. Impact of cellular miRNAs on circulating miRNA biomarker signatures. PLoS One. 2011;6:e20769. doi: 10.1371/journal.pone.0020769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem. 2011;57:833–40. doi: 10.1373/clinchem.2010.157198. [DOI] [PubMed] [Google Scholar]
- [77].Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]


