Abstract
Human gastric carcinomas are among the most treatment refractory epithelial malignancies. Increased understanding of the underlying molecular aberrations in such tumors could provide insights leading to improved therapeutic approaches. In this study, we characterized diverse genetic aberrations leading to constitutive Wnt signaling activation in a series of human gastric carcinoma cell lines. Downregulation of TCF signaling by stable transduction of dominant negative TCF-4 (DNTCF4) resulted in inhibition of proliferation in Wnt activated AGS tumor cells. c-Myc downregulation and the associated upregulation of its repression target, p21 observed in these tumor cells, as well as the profound growth inhibition induced by c-Myc shRNA implied their c-Myc addiction. In striking contrast, Wnt activated MKN-28 and MKN-74 tumor cells appeared refractory to DNTCF4 inhibition of proliferation despite comparably decreased c-Myc expression levels. The resistance of these same tumor cells to growth inhibition by c-Myc shRNA established that their refractoriness to DNTCF was due to their independence from c-Myc for proliferation. There was no correlation between this resistance phenotype and the presence or absence of constitutive MAPK and/or AKT pathway activation, commonly observed in gastrointestinal tumors. However, in both DNTCF sensitive and resistant tumor cells with MAPK and/or AKT pathway activation, the ability of small molecule antagonists directed against either pathway to inhibit tumor cell growth was enhanced by Wnt pathway inhibition. These findings support the concept that while certain Wnt activated tumors may escape c-Myc dependence for proliferation, disruption of other oncogenic pathways can unmask cooperative antiproliferative effects for Wnt pathway downregulation.
Keywords: β-catenin, DNTCF4, c-Myc, gastric cancer
Introduction
Gastric cancer is among the deadliest epithelial malignancies with 5-year survival rates of approximately 24% (Van Cutsem et al., 2006). Its high mortality rates reflect the prevalence of advanced disease at presentation, aggressive biology despite the lack of symptoms at an early stage, and relatively poor response to current adjuvant and neo-adjuvant treatments (Van Cutsem et al., 2006). Surgical resection remains the only treatment that can lead to long-term survival, but it is feasible in less than 50% of gastric cancer patients (Van Cutsem et al., 2006).
The Ras/Raf/Erk (MAPK) and phosphatidylinositol 3-kinase/AKT (PI3K/AKT) signaling pathways are key oncogenic pathways that are frequently dysregulated in human gastric cancers due to genetic aberrations of key components, leading to their constitutive activation (Michl and Downward, 2005). Whereas RAS mutations occur at relatively low frequency in gastric cancers, the MAPK and PI3K/AKT pathways are frequently activated by mechanisms that signal through these pathways including amplification or overexpression of various receptor tyrosine-kinases and/or their ligands (EGF/EGFR, HGF/c-Met), frequent monoallelic inactivation of PTEN and amplification or mutations in AKT1/2 (Michl and Downward, 2005). Several studies have reported concomitant activation of Wnt/β-catenin and MAPK and/or PI3K/AKT in human tumors, suggesting that Wnt signaling activation cooperates with these signaling pathways (D'Cruz et al., 2001; Jang et al., 2006; Kim et al., 2007; Park et al., 2006). For example, conditional expression of oncogenic K-ras in intestinal epithelium of adult mice in the context of Apc deficient intestinal tumors resulted in a higher number of tumors with greatly accelerated tumor formation leading to reduced life span, in comparison to Apc tumors expressing wild type K-ras (Janssen et al., 2006).
More than 90% of colorectal tumors (CRCs) harbor activating mutations in APC or CTNNB1 (β-catenin) (Morin et al., 1997). Wnt pathway activation has also been studied in other gastrointestinal tumors, such as gastric, small intestinal, and esophageal adenocarcinomas. Small intestinal carcinoma is exceedingly rare in humans (Overman, 2009) and mutations in APC or CTNNB1 have been found infrequently in these patients (Overman, 2009) as well as in individuals with esophageal adenocarcinoma (Bas et al., 2000). Conversely, individuals with germ line mutations in APC have increased risk of developing gastric cancer (Giardiello et al., 1993), and frequent nuclear localization of β-catenin has been observed by immunostaining of such tumors. Some gastric tumors have been shown to contain CTNNB1 or APC mutations (Clements et al., 2002; Woo et al., 2001). However, the biological impact of Wnt signaling inhibition on the phenotypes of this tumor type is not well established (Caca et al., 1999; Clements et al., 2002; Grabsch et al., 2001; Tomita et al., 2007). In this study, we sought to extend understanding of mechanisms involved in Wnt pathway activation in human gastric tumor lines as well as biological effects of downregulating the constitutively active Wnt pathway.
Results
Characterization of Wnt pathway aberrations in human gastric cancer cell lines
To assess the frequency and magnitude of constitutive Wnt signaling upregulation in human gastric carcinomas, we screened a series of tumor lines derived from these malignancies for increased levels of uncomplexed β-catenin. An immortalized mammary epithelial cell line, B5/589 (Rubin et al., 1989) and either A427 human lung carcinoma or MEL-888 melanoma, which contain activating β-catenin mutations (Rubinfeld et al., 1997; Sunaga et al., 2001), served as negative and positive controls in this assay. Fig. 1A shows that 4 of 11 gastric tumor lines analyzed, exhibited obviously increased uncomplexed β-catenin levels. To confirm and extend these findings, we measured TCF dependent transcriptional activity using TOP containing seven TCF/LEF consensus binding sequence or FOP, a two base-pair mutant of this sequence, driving EGFP or luciferase as the reporters in a lentiviral based transduction system (Fig. 1B) (Akiri et al., 2009). A lentivirus expressing EGFP under the control of a basal PGK promoter served as an infection control. The ratio of TOP/FOP activity determined as mean fluorescence intensity utilizing FACS with each of the gastric cancer cell lines generally correlated well with their respective levels of uncomplexed β-catenin (Fig. 1B). An example of Wnt signaling activity visualized under the microscope is shown in Fig. 1C for a positive (AGS) and negative (SNU-1) tumor line. However, TCF reporter activities observed with one tumor line, N-87, was substantially higher than would have been expected based on the absence of any obvious increase in uncomplexed β-catenin levels in this line (Fig. 1A,B).
Figure 1. Wnt signaling upregulation in human gastric tumor cell lines.
(A) 1 mg of total cell lysates was subjected to GST-E-cadherin pull down assay (Bafico et al., 1998). Uncomplexed β-catenin and total cell lysates (0.1 mg) were immunobloted using an anti-β-catenin mAb. (B) TCF-GFP reporter activity in human gastric cell lines. Cells were infected with TOP or FOP GFP lentiviruses as described in materials and methods. Columns represent TOP/FOP Mean Fluorescence Intensity (MFI). Infections were performed in triplicates. (C) Phase contrast and fluorescence images of AGS and SNU-1 cells infected with TOP or FOP TCF-GFP reporter lentiviruses or with GFP expressing lentivirus. BF - Bright Field, FL - Fluorescence.
Previous reports have characterized some of these gastric tumor lines for Wnt pathway activation. For example, AGS harbors an activating mutation (Caca et al., 1999) (Fig. 2A) and KATO-III, an amplification (Suriano et al., 2005) (Fig. 2B) of CTNNB1 (β-catenin), while MKN-28 and MKN-74 contain inactivating mutations of APC (Ikenoue et al., 2002). To confirm the genetic lesion responsible for Wnt pathway activation in AGS gastric tumor cells, we sequenced CTNNB1 exon 3, which is known to contain activating mutations that prevent its encoded protein from phosphorylations that targets it for proteasome degradation (Morin et al., 1997). This analysis revealed the activating lesion at codon 34 (Fig. 2A). Amplification of CTNNB1 has been described for KATO-III (Suriano et al., 2005). Our RT-PCR analysis revealed that genomic DNA from KATO-III cells contained around 20 copies of this gene. This result is compatible with gene amplification as the mechanism responsible for activation of Wnt signaling in this tumor line (Fig. 2B).
Figure 2. Mechanisms involved in TCF activity in gastric tumor cell lines.
(A) Sequencing of CTNNB1 (β-catenin) gene in 293T and AGS cells. AGS cell line shows a missense mutation in codon 34. (B) Analysis of CTNNB1 gene copy number using real time PCR (RT-PCR) analysis. Results are depicted as gene copies relative to 293T cells. Error bars indicate S.D. (*)=p<0.05. (C) Effects of shRNA knockdown of either β or γ-catenin on TCF-Luciferase (Luc) activity in N-87 cells. ShRNA targeting keratinocyte growth factor receptor (KGFR) was used as control. Data represent the mean ± S.D. of three independent experiments. (D) Efficiency of lentiviral mediated shRNA knockdown of total β or γ-catenin in N-87 cells. ShRNA targeting KGFR was used as control. (E) Sequencing of exon 15 of APC gene in MKN-28 and MKN-74 cells. Both MKN-28 and MKN-74 cell lines show “C” to “T” base change leading conversion of R1450 to a stop codon.
Fearon and colleagues previously identified a CTNNG (γ-catenin) mutation in N-87 tumor cells and showed that this mutant activated the Wnt pathway when transfected into recipient cells (Caca et al., 1999). To confirm and extend these findings, we established N-87 stable TCF-Luciferase (TOP/FOP-Luciferase) reporter cell lines and tested the effects of shRNA mediated knockdown of either β or γ-catenin expression on its TCF reporter activity (Fig. 2C) along with expression levels of well-characterized TCF-target genes, c-Myc (He et al., 1998; van de Wetering et al., 2002), BMP-4 (Kim et al., 2002), and Axin-2 (Filali et al., 2002; Jho et al., 2002) by real time PCR (RT-PCR) (Supplementary Figure 1). The effectiveness of β and γ-catenin shRNAs was confirmed by immunoblot analysis (Fig. 2D). Knockdown of γ-catenin in N-87 cells markedly reduced TCF reporter activity as well as the expression levels of c-Myc, BMP-4, and Axin-2, known TCF target genes (p<0.05) in a specific manner, while β-catenin knockdown had little or no effect under the same conditions (Fig. 2C, Supplementary Figure 1). These results demonstrate that the CTNNG mutant identified by Caca et al. (Caca et al., 1999) must be responsible for constitutively activated Wnt signaling in N-87 tumor cells. Finally, we confirmed the APC mutations previously reported for MKN-28 and MKN-74 (Fig. 2E). A summary of Wnt pathway activating lesions, levels of uncomplexed β-catenin, and TCF reporter activity in Wnt activated human gastric tumor cell lines is provided in Table 1.
Table 1.
Summary of Wnt pathway activating lesions, levels of uncomplexed β-catenin, and TCF reporter activity in Wnt activated human gastric tumor cell lines.
| Cell line | Wnt activating lesion |
Levels of uncomplexed β-catenin |
Level of TCF activity |
|---|---|---|---|
| AGS | CTNNB1 mutation | +++ | +++ |
| KATO-III | CTNNB1 amplification | +++ | +++ |
| MKN-28 | APC mutation | +++ | +++ |
| MKN-74 | APC mutation | ++ | ++ |
| N-87 | CTNNG mutation | − | +++ |
Effects of TCF signaling inhibition on proliferation of Wnt activated gastric cancer cells
Previous studies have shown that inhibition of TCF signaling can inhibit proliferation of tumor cells with Wnt pathway activation (Akiri et al., 2009; van de Wetering et al., 2002). In order to assess the effects of dominant negative TCF-4 (DNTCF4) lentiviral mediated transduction on growth properties of gastric carcinoma cells with constitutive Wnt pathway activation, we established stable TCF-Luciferase (TOP/FOP-Luciferase) reporters in AGS, MKN-28, and MKN-74 gastric carcinoma lines, which each exhibited high levels of constitutive TCF activity (Table 1, Fig. 1B). Infection of mass cultures with a lentivirus expressing DNTCF4 resulted in a reduction of 4–7 fold in TCF reporter activity (Fig. 3A, Fig. 3B, and Supplementary Figure 2A). To investigate whether downregulation of constitutively active Wnt signaling in mass cultures was sufficient to induce the growth inhibitory effects observed previously in clonally selected DNTCF4 inducible colon cancer cells (CRC) (van de Wetering et al., 2002), we compared cell cycle distribution of PI stained cells by FACS analysis. A single cycle of DNTCF4 transduction led to a significant reduction in S-phase population in AGS (Fig. 3C), while there was little if any inhibition observed with MKN-28 and MKN-74 cells (Fig. 3D, Supplementary Figure 2B). To assess whether TCF signaling could be downregulated to a greater extent and if so, the effects on cell proliferation, we subjected AGS, MKN-28 and MKN-74 gastric tumor cells to two cycles of lentiviral mediated DNTCF4 transduction. With AGS cells, this resulted in further inhibition of TCF reporter activity (Fig. 3A) associated with more marked S-phase inhibition (Fig. 3C). We also observed a small increase in the subG1 population in this tumor line following two cycles of DNTCF4 transduction. Despite the reduction of TCF signaling in MKN-74 cells and an even more marked reduction in MKN-28 cells following two cycles of DNTCF4 transduction (Fig. 3B, Supplementary Figure 2A), there was still no detectable reduction in S-phase in these tumor cells (Fig. 3D, Supplementary Figure 2B). These results suggested that some Wnt activated tumors do not depend on this upregulated pathway for proliferation.
Figure 3. Effects of Wnt pathway inhibition on TCF reporter activity and cell cycle profile in AGS and MKN-28 cell lines.
Analysis of TOP/FOP TCF-Luciferase (Luc) activity in AGS (A) and MKN-28 (B) cells following one or two cycles of transduction using lentiviruses expressing VECTOR or DNTCF4. Data represent the mean ± S.D. of three independent experiments performed in duplicates. (*)=p<0.05, VECTOR vs. DNTCF4 (one cycle of transduction). (**)=p<0.05, VECTOR vs DNTCF4, (two cycles of transduction). Representative FACS analysis cell cycle profiles of propidium iodide (PI) stained AGS (C) and MKN-28 (D) cells following one or two cycles of transduction with either VECTOR or DNTCF4 expressing lentiviruses.
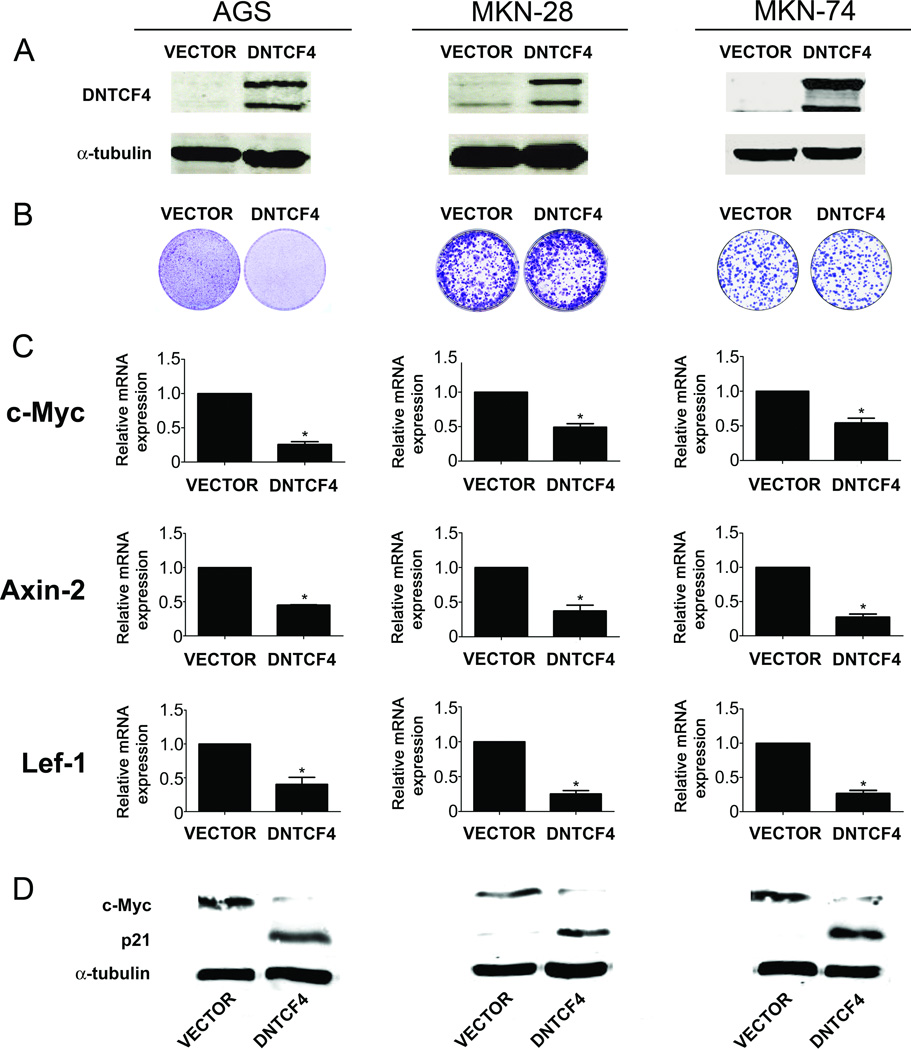
To investigate the effect of DNTCF4 on longer-term growth inhibition, we subjected mass cultures of AGS, MKN-28, and MKN-74 to a single cycle of DNTCF4 transduction. Although we observed comparable DNTCF4 expression levels in all cell lines (Fig. 4A), DNTCF4 exhibited significant growth inhibition on AGS cells, while no discernible inhibition was observed with MKN-28 or MKN-74 cells (Fig. 4B). We also observed no effects of DNTCF4 on cell cycle or cell proliferation with a A3008 gastric cell line, which lacked detectable Wnt pathway activation (data not shown).
Figure 4. Effects of TCF signaling inhibition on cell proliferation of gastric cell lines.
(A) Immunoblot analysis of DNTCF4 expression in AGS, MKN-28, and MKN-74 cells infected with either VECTOR or DNTCF4 expressing lentiviruses. DNTCF4 expression was detected using an antibody to TCF-4. (B) Effects of DNTCF4 on cell growth of AGS, MKN-28, and MKN-74 cells. For long term growth, cells were seeded in 60 mm dishes and cultures visualized by crystal violet staining after 14 days. (*)=p<0.05. (C) Effects of DNTCF4 on expression of TCF target genes. RT-PCR analysis of c-Myc, Axin-2, and Lef-1 mRNA levels in AGS, MKN-28, and MKN-74 cells transduced with VECTOR or DNTCF4 expressing lentiviruses. Error bars indicate S.D. (*)=p<0.05. (D) Effects of DNTCF4 on expression of c-Myc, and p21 protein levels in AGS, MKN-28, and MKN-74 cells transduced with VECTOR or DNTCF4 expressing lentiviruses.
Sensitivity to Wnt pathway inhibition depends on the level of c-Myc addiction for tumor cell proliferation
To investigate possible mechanisms for the inability of DNTCF4 to inhibit MKN-28 or MKN-74 cell proliferation, we compared expression levels of known TCF target genes in these and other Wnt-upregulated tumor lines. In particular, we analyzed c-Myc, which has been shown to exert a central role in the proliferation of CRC cells with Wnt pathway activation (He et al., 1998; van de Wetering et al., 2002). RT-PCR analysis revealed statistically significant reduction in mRNA expression levels (p<0.05) of c-Myc, Axin-2, and Lef-1, another well documented TCF target gene (Filali et al., 2002; Jho et al., 2002), in AGS tumor cells transduced with DNTCF4 (Fig. 4C). There were no obvious differences in the degree of inhibition observed for these target genes in DNTCF4-resistant MKN-28 and MKN-74 as compared to DNTCF4-sensitive tumor lines (Fig. 4C). Of note, basal expression levels of each of these Wnt target genes analyzed were similar in all cell lines tested, as assessed by their respective cycle threshold (Ct) values (data not shown). c-Myc RT-PCR data were confirmed by Western blotting, which showed comparable reduction of protein levels in all cell lines transduced with DNTCF4 (Fig. 4D). Axin-2 and Lef-1 protein levels were too low and/or the available anti-Axin-2 and anti-Lef-1 antibodies not sufficiently sensitive to compare their expression levels with those determined by RT-PCR.
Clevers and colleagues have shown that decreased expression of c-Myc led to increased expression of p21(CIP1/WAF1), which mediated cell-cycle arrest and differentiation of DLD-1 colon carcinoma cells (He et al., 1998; van de Wetering et al., 2002). Hence, we also analyzed the expression levels of p21 by immunoblot analysis in DNTCF4-sensitive AGS, and resistant MKN-28 and MKN-74 tumor cells expressing either VECTOR or DNTCF4. The increase in p21 expression associated with reduction in c-Myc (Fig. 4D) correlated well with the effects observed on cell growth (Fig. 4B) in AGS cells. However, despite comparable responses of c-Myc and p21 to DNTCF4 (Fig. 4D), MKN-28 and MKN-74 cells showed no detectable inhibition of proliferation (Fig. 4B).
To directly assess whether Wnt activated gastrointestinal tumor cells refractory to DNTCF4 inhibition were independent of c-Myc-mediated regulation of cell proliferation, we tested the effects of c-Myc downregulation by shRNA on cell-cycle profile and proliferation. While only modest inhibition was observed in DNTCF4-resistant MKN-28 cells, lentiviral-mediated c-Myc shRNA resulted in a striking G1-arrest in DNTCF4-sensitive AGS cells (Fig. 5A). Similarly, c-Myc shRNA induced little inhibition of colony formation by MKN-28 cells, while significantly inhibiting AGS cells under the same conditions (Fig. 5B). The effectiveness of c-Myc shRNA in inducing a comparable inhibition of c-Myc expression in each cell line was confirmed by immunoblot analysis (Fig. 5C). All of these findings established that the refractoriness to DNTCF4 of some Wnt activated tumors was due to their c-Myc independence for proliferation.
Figure 5. Effects of c-Myc shRNA signaling inhibition on proliferation of AGS and MKN-28 gastrointestinal tumor lines.
(A) Representative FACS analysis cell cycle profiles of propidium iodide (PI) stained AGS and MKN-28 cells following infection with lentiviral vectors expressing control or c-Myc-shRNA. (B) Effects of c-Myc-shRNA on cell growth of AGS, and MKN-28 cells. For colony formation, 2×103 cells were seeded in 6-well plates, marker selected with puromycin, and cultures visualized by crystal violet staining after 14 days. (*)=p<0.05. (C) Efficiency of lentiviral mediated shRNA knockdown of c-Myc in AGS and MKN-28 cells.
Cooperative effects of inhibition of constitutive Wnt, ERK and/or AKT activation on proliferation of gastric carcinoma lines
Constitutive activation of MAPK and/or PI3K/AKT signaling pathways plays a critical role in the pathogenesis of many tumors, including gastric carcinomas (Michl and Downward, 2005). Recent evidence suggests that Wnt signaling cooperates with these pathways in in vivo models of tumorigenesis (D'Cruz et al., 2001; Jang et al., 2006). Thus, we sought to assess ERK and AKT phosphorylation in cell lysates of these same tumor lines to determine whether differential activation of these pathways might explain the refractoriness of MKN-28 and MKN-74 tumor cells to growth inhibition resulting from Wnt pathway downregulation. Following overnight serum starvation, the background levels of pERK and pAKT detectable by immunoblot analysis in immortalized, non-tumorigenic NIH3T3 cells were low (Supplementary Fig. 3A, B). In contrast, both ERK and AKT phosphorylation were readily observed in AGS and MKN-28 tumor lines under the same conditions (Supplementary Fig. 3A, B), while MKN-74 tumor cells showed minimal if any AKT phosphorylation and very low ERK activation. Thus, these findings could not account for the sensitivity or resistance of Wnt activated tumor lines to DNTCF4 inhibition of proliferation.
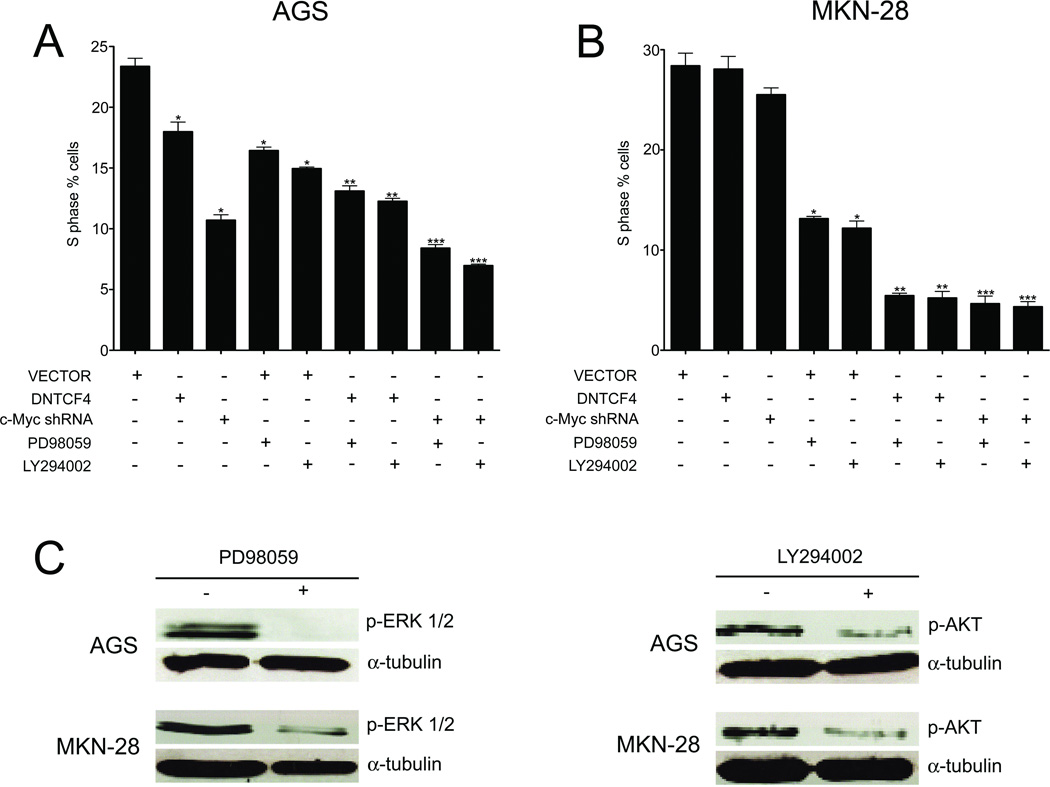
We next evaluated the effects of selective downregulation of Wnt signaling or c-Myc alone or in combination with MEK1, or AKT inhibitors in AGS and MKN-28 cells. AGS cells stably infected with one cycle of DNTCF4 or c-Myc shRNA showed significant reduction in proliferation (S-phase) (p<0.05) compared to vector control cells (Fig. 6A), while MKN-28 cells showed little if any growth inhibition in response to either transduction (p>0.05) (Fig. B). The lesser effectiveness of DNTCF4 compared to c-Myc shRNA in inhibiting proliferation of AGS cells likely reflected use of a single cycle of infection as even greater inhibition than that induced by c-Myc shRNA (Fig. 6A) was observed with two cycles of DNTCF4 in these cells (Fig. 3). Exposure to PD98059, a MEK1 inhibitor, or LY294002, a PI3K inhibitor alone, was growth inhibitory for each tumor line (p<0.05) (Fig. 6). When either PD98059 or LY294002 treatment was combined with DNTCF4 transduction in AGS cells, the effects on proliferation inhibition were additive at best (Fig. 6A). In striking contrast, combining treatment of either agent with transduction of DNTCF4 or c-Myc shRNA in MKN-28 cells led to comparable and more marked inhibition of proliferation than that observed with the sum of drug treatment and transduction by themselves (Fig. 6B). Synergism was confirmed by analysis of the interaction effects in a two-way ANOVA with Bonferroni’s correction (p<0.05) (Slinker, 1998) (data not shown). At the concentrations utilized, PD98059 and LY294002 markedly reduced pERK and pAKT, respectively, in each tumor line (Fig. 6C). Significant reduction in the proportion of cells in S-phase was associated with an increased fraction in G1, consistent with a G1 arrest. None of the combinations induced an increase in the subG1 or G2/M population, and we observed no cooperative effects of DNTCF4 with either inhibitor on cell cycle or cell proliferation in gastric cell lines without detectable Wnt pathway activation (data not shown). All of these findings support the concept that certain Wnt activated tumors can escape c-Myc dependence for proliferation through other oncogenic alterations, which in the case of MKN-28 tumor cells involve ERK and AKT activation.
Figure 6. Effects of combined downregulation of Wnt together with MAPK or PI3K/AKT pathways on gastric tumor cell growth.
(A, B) AGS and MKN-28 cells transduced with VECTOR, DNTCF4, or c-Myc shRNA were treated with either LY294002 (40 µM), PD98059 (40 µM), or DMSO for 36 h. Cells were analyzed for cell cycle profile by FACS analysis. (*)=p<0.05, VECTOR vs. DNTCF4 or c-Myc shRNA. (**)=p<0.05, DNTCF4 alone vs. DNTCF4 + LY294002 or PD98059. (***)=p<0.05, c-Myc shRNA alone vs. c-Myc shRNA + LY294002 or PD98059. Data represent the mean ± S.D. from three independent experiments. (C) Immunoblot analysis of pERK and pAKT protein levels in AGS, and MKN-28 cells treated with PD98059 or LY294002.
Discussion
In the present study, we characterized Wnt canonical pathway aberrations in human gastric carcinoma lines. In general, we observed a good correlation between pathway activation as detected by increased levels of uncomplexed β-catenin and increased TCF dependent transcriptional reporter activities in the different tumor lines, some of which have been previously documented as having CTNNB1 or APC mutations(Caca et al., 1999; Ikenoue et al., 2002). KATO-III gastric tumor cells, which overexpress wild type CTNNB1 associated with gene amplification (Suriano et al., 2005) also showed high levels of uncomplexed β-catenin and TCF reporter activity.
A mutation in the γ-catenin gene that moderately increases its transcriptional activity was previously identified in N-87 cells (Caca et al., 1999), suggesting that this could represent an alternative mechanism for Wnt activation in tumors. This hypothesis was supported by data that overexpression of γ-catenin stimulated TCF activity, c-Myc expression and cell transformation (Kolligs et al., 2000). However, later studies provided evidence that γ-catenin formed only weak interactions with TCF transcription factors, while it potently stabilized β-catenin and, therefore, acted indirectly to stimulate TCF signaling (Zhurinsky et al., 2000). Consistent with this model, recent in vitro and in vivo studies revealed that inactivation of the β-catenin gene strongly inhibited the effects of γ-catenin on reporter activity and tumor formation (Shimizu et al., 2008; Teuliere et al., 2004). In the present study, we found that knockdown of γ-catenin, but not β-catenin, dramatically inhibited TCF signaling in N-87 gastric cancer cells, demonstrating that, in these cells, aberrantly activated γ-catenin can stimulate the Wnt pathway in a β-catenin independent manner.
Thus, Wnt signaling activation in gastric carcinoma lines occurs at reasonably high frequency and involves a wide variety of mechanisms.
Previous studies have correlated reduction in levels of c-Myc, a TCF target gene, with growth inhibition of CRC cells (He et al., 1998; van de Wetering et al., 2002). Similarly, Wnt pathway inhibition by DNTCF4 impaired proliferation of AGS gastric tumor cells associated with a decrease in Myc RNA levels. AGS cells also showed decreased levels of Axin-2, and Lef-1, two other established TCF target genes (Filali et al., 2002; Jho et al., 2002) in response to DNTCF4. In contrast, MKN-28 and MKN-74 gastric tumor cells appeared not to retain any obvious dependence on Wnt/β-catenin signaling for proliferation, as we observed no detectable effects of DNTCF4 expression on this phenotype despite the ability of DNTCF4 to comparably inhibit TCF signaling. Of note, levels of c-Myc, a known TCF target gene previously shown to mediate the Wnt proliferation phenotype in colon tumor cells (Akiri et al., 2009; van de Wetering et al., 2002), were comparably inhibited in the resistant tumor cells associated with increased expression of p21(CIP1/WAF1), the growth-suppressive target of c-Myc repression (Akiri et al., 2009; van de Wetering et al., 2002). Additionally, c-Myc shRNA induced only modest growth inhibitory effects on DNTCF4-resistant MKN-28 cells, corroborating their independence from c-Myc for proliferation. DNTCF4 inhibition of DLD-1 colon cancer cell proliferation has been shown to be mediated by upregulation of the p21 cell cycle inhibitor (Akiri et al., 2009; van de Wetering et al., 2002). These findings argue that Wnt activated tumor cells resistant to DNTCF4 induced proliferation inhibition have evolved mechanisms to escape p21 induced growth inhibition.
Previous studies have shown that Wnt-1 or c-myc synergize with H-ras, or K-ras, respectively, in mouse models of mammary tumorigenesis (D'Cruz et al., 2001; Jang et al., 2006). Of note, ras driven MAPK activation has been reported to promote progression of around 30% of such tumors to independence of either Wnt-1 or c-myc oncogenes initially required for their growth (Jang et al., 2006). In our studies, we observed that the presence or absence of constitutive MAPK or PI3K/AKT activation did not predict Wnt pathway independence for tumor cell proliferation. As a number of gastric carcinomas including AGS cells possess Ras activating mutations (Morin et al., 1997), therapeutic interference with the PI3K/AKT or MAPK signaling pathways has been attempted in efforts to improve survival in patients with such tumors. Unfortunately, the effects of these approaches have been disappointing so far, given the modest improvement in survival (Van Cutsem et al., 2006). We observed that in DNTCF4 resistant MKN-28 tumor cells with MAPK and/or PI3K/AKT pathway activation, the ability of small molecule antagonists directed against either pathway to inhibit tumor cell proliferation synergized with Wnt pathway inhibition in a c-Myc dependent manner. These findings support the concept that some Wnt activated tumors escape c-Myc dependence for proliferation through activation of other oncogenic pathways, whose disruption can cooperate with the antiproliferative effects of Wnt pathway downregulation. In the case of MKN-28 tumor cells, our evidence strongly argues that these oncogenic alterations involve ERK and AKT signaling pathways. The extent that Wnt activation impacts on other biological properties of tumor cells such as invasiveness or pro-survival signaling as well as the contribution of known Wnt target genes such as c-Myc to such phenotypes should be possible to dissect by analogous approaches.
Materials and Methods
Cell culture
Human tumor cell lines including gastric (KATO-III, N-87, AGS, SNU-1, A3008, SNU-5, OKAJIMA, MKN-74, MKN-28, MKN-7, and KATO-II), lung (A427), melanoma (MEL-888), and the immortalized human renal epithelial cell line (293T) were maintained in either DMEM medium (Invitrogen) or RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) (20% for KATO-III cells) and 1% Penicillin/Streptomycin (GIBCO). The immortalized mammary epithelial cell line B5/589 (Stampfer and Bartley, 1985) was cultured in DMEM medium as above supplemented with 5 ng/mL hEGF (Peprotech). All cells were cultured at 37°C in 5% CO2.
Analysis of uncomplexed β-catenin and immunoblot analysis
GST-E-cadherin binding and immunoblot analysis was performed as previously described (Bafico et al., 1998). For immunoblot analysis the following primary antibodies were used: phospho and total AKT and ERK1/2 (Cell Signaling); α-tubulin (Sigma); TCF-4 (Upstate); c-Myc (Santa Cruz); p21, β and γ-catenin (BD Bioscience). Anti-mouse IgG or anti-rabbit IgG secondary antibodies conjugated to Alexa Fluor 680 were from Molecular Probes. Quantification of signal immunoreactivity was obtained using the Licor Odyssey infrared Imaging system (LI-COR).
Fluorescence Activated Cell Sorting (FACS) analysis of TCF mediated GFP reporter activity
Cells infected with TOP or FOP EGFP reporter lentiviruses or a lentivirus expressing GFP from a constitutive PGK promoter were trypsinized 4 days after infection, transferred to polystyrene tubes (Falcon) and subjected to FACS analysis using Cell Quest 3.2 software (Becton Dickinson).
Quantification of TCF mediated luciferase reporter activity
To determine TCF-Luciferase reporter activity cells were transduced with TOP or FOP TCF reporter lentiviruses expressing firefly luciferase together with Renilla luciferase lentivirus (1:20 ratio) used to normalize for infection efficiency. Four days after infection, cells were lysed and analyzed utilizing the dual luciferase reporter assay system (Promega), according to the manufacturer's instructions. Luciferase reporter activity was calculated by dividing the ratio TOP/RL by the FOP/RL ratio. Results were normalized to the results with vector transduced cultures. For experiments in which DNTCF4 was used, stable reporter cell lines expressing TOP or FOP TCF luciferase and renilla luciferase were plated in 6 well plates at 1×105 cells/well. The following day cells were infected with VECTOR or DNTCF4 lentiviruses, selected with puromycin (2 µg/mL) for 3 days and analyzed for luciferase activity as described above.
FACS analysis of cell cycle profile
For DNA content analysis, cells were trypsinized, combined with floating cells, washed with PBS and stained with propidium iodide (PI) using the CycleTEST Plus DNA reagent kit (Becton Dickinson). Cells were transferred to polystyrene tubes with cell-strainer caps (BD Biosciences) and subjected to FACS analysis using Cell Quest 3.2 software (Becton Dickinson).
Cell growth analysis assay
For colony growth assay, 3×104 MKN-28 or MKN-74 cells, and 1×105 AGS cells were transduced with VECTOR or DNTCF4 lentiviruses, selected with puromycin (2 µg/mL), and seeded in 60-mm plates. At 2 weeks, cells were washed with PBS, fixed in 10% methanol/acetic acid solution and stained with 1% crystal violet. For experiments in which c-Myc shRNA was used, 2×103 AGS or MKN-28 cells were seeded in 6-well plates. Experiments were performed at least twice. For some experiments the specific MEK1 inhibitor PD98059 (2'-amino-3'-methoxyflavone; Calbiochem) (Alessi et al., 1995) and the specific PI3 kinase inhibitor LY294002 (2-[chloro-4-iodo-phenylamino]-N-cyclopropylmethoxy-3,4-difluoro-benzamide; Cayman) (Vlahos et al., 1994) were dissolved in dimethyl sulfoxide (DMSO; Sigma) at a concentration of 100 mM. Cells were treated with the compounds at a concentration of 40 µM for 36 h and subjected to FACS analysis. We titrated each inhibitor on all cell lines utilizing a range from 1–150 µM and selected 40 µM, as previously reported (Bechard and Dalton, 2009; Pumiglia and Decker, 1997), as the lowest concentration of each that resulted in efficient downregulation of either pERK or pAKT.
Real time PCR analysis
Total RNAs were extracted from cell lines using Trizol (Invitrogen). 5 µg total RNA was reverse transcribed using Superscript II (Invitrogen) and RT-PCR was performed using SYBR Green Mix (Roche) on Stratagene MxPro3005 system. 50 ng cDNA were amplified and relative mRNA expression levels were quantified using the ΔΔC(t) method (Pfaffl, 2001). Each reaction was performed in duplicate, and results of 3 independent experiments were used for statistical analysis. Results were normalized to those for TATA Binding Protein (TBP). Primer sequences are given in Table S1.
Statistical analysis
Statistical analysis was performed using unpaired Student’s t-test and one-way-ANOVA with Bonferroni and Dunnet’ correction tests. A two-way-ANOVA with Bonferroni’s correction tests was performed to analyze synergism (Slinker, 1998), A p value <0.05 was considered statistically significant. All values are given as means ± S.D. of at least three independent experiments.
Supplementary Material
Acknowledgments
This work was supported by grant number 5R01CA071672 from the National Cancer Institute. We are grateful to Dr. Yoshio Yamaoka (Baylor College of Medicine, Houston, TX) for MKN-7 and MKN-28 cells; Dr. Gary Schwartz (Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY) for MKN-74 cells and Dr. Reuben Lotan (M.D. Anderson Cancer Center, Houston, TX) for KATO-II cells. SV is a recipient of a post-doctoral fellowship award from the American Urological Association.
Grant Support: This work was supported by grant number 5R01CA071672 from the National Cancer Institute.
Abbreviations
- MAPK
Ras/Raf/Erk
- PI3K/AKT
Phosphatidylinositol 3-kinase/AKT
- CRC
Colorectal cancer
- FACS
Fluorescence Activated Cell Sorting
Footnotes
Conflict of interest
No conflicts of interest to disclose.
References
- Akiri G, Cherian MM, Vijayakumar S, Liu G, Bafico A, Aaronson SA. Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene. 2009;28:2163–2172. doi: 10.1038/onc.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 Is a Specific Inhibitor of the Activation of Mitogen-activated Protein Kinase Kinase in Vitro and in Vivo. Journal of Biological Chemistry. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Bafico A, Gazit A, Wu-Morgan SS, Yaniv A, Aaronson SA. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene. 1998;16:2819–2825. doi: 10.1038/sj.onc.1201797. [DOI] [PubMed] [Google Scholar]
- Bas PLW, Friedel N, Nico JDB, Hugo WT, Winand NMD. Genetic alterations involving exon 3 of the beta-catenin gene do not play a role in adenocarcinomas of the esophagus. International Journal of Cancer. 2000;86:533–537. doi: 10.1002/(sici)1097-0215(20000515)86:4<533::aid-ijc15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bechard M, Dalton S. Subcellular Localization of Glycogen Synthase Kinase 3-beta Controls Embryonic Stem Cell Self-Renewal. Mol. Cell. Biol. 2009;29:2092–2104. doi: 10.1128/MCB.01405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caca K, Kolligs FT, Ji X, Hayes M, Qian J, Yahanda A, et al. Beta- and gamma-catenin mutations, but not E-cadherin inactivation, underlie T-cell factor/lymphoid enhancer factor transcriptional deregulation in gastric and pancreatic cancer. Cell Growth Differ. 1999;10:369–376. [PubMed] [Google Scholar]
- Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, et al. beta-Catenin Mutation Is a Frequent Cause of Wnt Pathway Activation in Gastric Cancer. Cancer Res. 2002;62:3503–3506. [PubMed] [Google Scholar]
- D'Cruz CM, Gunther EJ, Boxer RB, Hartman JL, Sintasath L, Moody SE, et al. c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nat Med. 2001;7:235–239. doi: 10.1038/84691. [DOI] [PubMed] [Google Scholar]
- Filali M, Cheng N, Abbott D, Leontiev V, Engelhardt JF. Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter. J Biol Chem. 2002;277:33398–33410. doi: 10.1074/jbc.M107977200. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Offerhaus GJ, Lee DH, Krush AJ, Tersmette AC, Booker SV, et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34:1394–1396. doi: 10.1136/gut.34.10.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabsch H, Takeno S, Noguchi T, Hommel G, Gabbert HE, Mueller W. Different patterns of beta-catenin expression in gastric carcinomas: relationship with clinicopathological parameters and prognostic outcome. Histopathology. 2001;39:141–149. doi: 10.1046/j.1365-2559.2001.01177.x. [DOI] [PubMed] [Google Scholar]
- He T-C, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a Target of the APC Pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Ikenoue T, Ijichi H, Kato N, Kanai F, Masaki T, Rengifo W, et al. Analysis of the beta-catenin/T cell factor signaling pathway in 36 gastrointestinal and liver cancer cells. Jpn J Cancer Res. 2002;93:1213–1220. doi: 10.1111/j.1349-7006.2002.tb01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JW, Boxer RB, Chodosh LA. Isoform-Specific Ras Activation and Oncogene Dependence during MYC- and Wnt-Induced Mammary Tumorigenesis. Mol. Cell. Biol. 2006;26:8109–8121. doi: 10.1128/MCB.00404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen K-P, Alberici P, Fsihi H, Gaspar C, Breukel C, Franken P, et al. APC and Oncogenic KRAS Are Synergistic in Enhancing Wnt Signaling in Intestinal Tumor Formation and Progression. Gastroenterology. 2006;131:1096–1109. doi: 10.1053/j.gastro.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-S, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, et al. Oncogenic beta-Catenin Is Required for Bone Morphogenetic Protein 4 Expression in Human Cancer Cells. Cancer Res. 2002;62:2744–2748. [PubMed] [Google Scholar]
- Kim S-E, Lee W-J, Choi K-Y. The PI3 kinase-Akt pathway mediates Wnt3a-induced proliferation. Cellular Signalling. 2007;19:511–518. doi: 10.1016/j.cellsig.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Kolligs FT, Kolligs B, Hajra KM, Hu G, Tani M, Cho KR, et al. gamma-catenin is regulated by the APC tumor suppressor and its oncogenic activity is distinct from that of beta-catenin. Genes Dev. 2000;14:1319–1331. [PMC free article] [PubMed] [Google Scholar]
- Michl P, Downward J. Mechanisms of Disease: PI3K/AKT Signaling in Gastrointestinal Cancers. Mechanisms of Disease: PI3/K/Akt-Signalweg in gastrointestinalen Tumoren. 2005:1133–1139. doi: 10.1055/s-2005-858638. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta -Catenin-Tcf Signaling in Colon Cancer by Mutations in beta -Catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Overman MJ. Recent advances in the management of adenocarcinoma of the small intestine. Gastrointest Cancer Res. 2009;3:90–96. [PMC free article] [PubMed] [Google Scholar]
- Park K-S, Jeon SH, Kim S-E, Bahk Y-Y, Holmen SL, Williams BO, et al. APC inhibits ERK pathway activation and cellular proliferation induced by RAS. J Cell Sci. 2006;119:819–827. doi: 10.1242/jcs.02779. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumiglia KM, Decker SJ. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:448–452. doi: 10.1073/pnas.94.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Fukunaga Y, Ikenouchi J, Nagafuchi A. Defining the roles of beta-catenin and plakoglobin in LEF/T-cell factor-dependent transcription using beta-catenin/plakoglobin-null F9 cells. Mol Cell Biol. 2008;28:825–835. doi: 10.1128/MCB.02375-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinker BK. The statistics of synergism. J Mol Cell Cardiol. 1998;30:723–731. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- Stampfer MR, Bartley JC. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci U S A. 1985;82:2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunaga N, Kohno T, Kolligs FT, Fearon ER, Saito R, Yokota J. Constitutive activation of the Wnt signaling pathway by CTNNB1 (beta-catenin) mutations in a subset of human lung adenocarcinoma. Genes Chromosomes Cancer. 2001;30:316–321. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1097>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Suriano G, Vrcelj N, Senz J, Ferreira P, Masoudi H, Cox K, et al. beta-catenin (CTNNB1) gene amplification: a new mechanism of protein overexpression in cancer. Genes Chromosomes Cancer. 2005;42:238–246. doi: 10.1002/gcc.20135. [DOI] [PubMed] [Google Scholar]
- Teuliere J, Faraldo MM, Shtutman M, Birchmeier W, Huelsken J, Thiery JP, et al. beta-catenin-dependent and -independent effects of DeltaN-plakoglobin on epidermal growth and differentiation. Mol Cell Biol. 2004;24:8649–8661. doi: 10.1128/MCB.24.19.8649-8661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Yamada Y, Oyama T, Hata K, Hirose Y, Hara A, et al. Development of Gastric Tumors in ApcMin/+ Mice by the Activation of the beta-Catenin/Tcf Signaling Pathway. Cancer Res. 2007;67:4079–4087. doi: 10.1158/0008-5472.CAN-06-4025. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Dicato M, Arber N, Benson A, Cunningham D, Diaz-Rubio E, et al. The neo-adjuvant, surgical and adjuvant treatment of gastric adenocarcinoma. Current expert opinion derived from the Seventh World Congress on Gastrointestinal Cancer, Barcelona, 2005. Ann Oncol. 2006;17:vi13–vi18. doi: 10.1093/annonc/mdl976. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, et al. The [beta]-Catenin/TCF-4 Complex Imposes a Crypt Progenitor Phenotype on Colorectal Cancer Cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) Journal of Biological Chemistry. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Woo DK, Kim HS, Lee HS, Kang YH, Yang HK, Kim WH. Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int J Cancer. 2001;95:108–113. doi: 10.1002/1097-0215(20010320)95:2<108::aid-ijc1019>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Zhurinsky J, Shtutman M, Ben-Ze'ev A. Differential Mechanisms of LEF/TCF Family-Dependent Transcriptional Activation by beta -Catenin and Plakoglobin. Mol. Cell. Biol. 2000;20:4238–4252. doi: 10.1128/mcb.20.12.4238-4252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.