Abstract
Repair of soft-tissue defects resulting from lumpectomy or mastectomy has become an important rehabilitation process for breast cancer patients. This study aimed to provide an adipose tissue engineering platform for soft-tissue defect repair by combining decellularized human adipose tissue extracellular matrix (hDAM) and human adipose-derived stem cells (hASCs). To derive hDAM, incised human adipose tissues underwent a decellularization process. Effective cell removal and lipid removal were proved by immunohistochemical analysis and DNA quantification. Scanning electron microscope examination showed three-dimensional nanofibrous architecture in hDAM. hDAM composition included collagen, sulfated glycosaminoglycan, and vascular endothelial growth factor but lacked major histocompatibility complex antigen I. hASC viability and proliferation on hDAM were proven in vitro. hDAM implanted subcutaneously in Fischer rats did not cause an immunogenic response, and it underwent remodeling as indicated by host cell infiltration, neovascularization, and adipose tissue formation. Fresh fat grafts (Coleman technique) and engineered fat grafts (hDAM combined with hASCs) were implanted subcutaneously in nude rats. The implanted engineered fat grafts maintained volume at week 8, and the hASCs contributed to adipose tissue formation. In summary, the combination of hDAM and hASCs provides not only a clinically translatable platform for adipose tissue engineering but also a vehicle for elucidating fat grafting mechanisms.
Keywords: Decellularization, Adipose tissue extracellular matrix, Adipose-derived stem cell, Fat grafting, Adipose tissue engineering
1. Introduction
Fat grafting has recently come to prominence as an important tool in breast reconstruction after lumpectomy or mastectomy, and it has even been promoted as a means to achieve complete breast reconstruction [1-4]. This approach not only adjusts the volume of the reconstructive site but also improves the tissues surrounding where the grafts are placed. In particular, fat grafting with adipose tissue harvested through a small incision by liposuction has enormous appeal to patients and surgeons alike because this technique is minimally invasive and has shown promise as a means of restoring varying degrees of soft-tissue loss throughout the body. However, the outcome of fat grafting is quite variable; the degree of volume loss after fat transfer has ranged from 10% to 90% [5, 6]. Factors that have been proposed to account for such discrepant outcomes include the superiority of certain harvest sites, recipient site quality, differences in fat harvest and injection techniques, trauma during graft preparation, and variability in patient stem cell quantity and quality [3, 5, 7-9]. It is also unclear how fat grafts survive and interact with the surrounding microenvironment [3].
Adipose cells, which constitute most of the adipose tissue, are the major cellular component of fat grafts. These cells regulate numerous cellular processes, including insulin action, energy homeostasis, inflammation, and cell growth. However, adipose cells are too fragile to sustain mechanical stress during the grafting process and the hypoxic conditions after grafting [10]. Adipose-derived stem cells (ASCs), another cellular component of adipose tissue, play an important role in fat grafting and adipose tissue regeneration and are considered an ideal autologous cell source for adipose tissue engineering [11-13]. ASCs could be easily derived from excised adipose tissue or liposuction samples, which are usually discarded after operation, and they maintain their ability to proliferate and their differentiation potential in culture. Clinical reports and basic research have suggested that ASCs play an important role in successful fat grafting. Repeated injection of ASCs instead of fat grafts has been used effectively in the clinic. However, how injected ASCs work in the grafting procedure is unclear.
Providing the microenvironment for cellular components to interact with, extracellular matrix (ECM) is another important component of adipose tissue. Natural and synthetic materials have been developed to provide three-dimensional (3D) scaffolding that mimics ECM properties for adipose tissue regeneration and reconstruction. In accordance with the plastic surgery rule of ‘replace with alike’, ECM from adipose tissue could function as a biomimetic scaffold. Decellularization, which is a helpful way of deriving native ECM from adipose tissues, would allow the study of native ECM properties and would guide tissue engineering to design a biomimetic scaffold [14, 15]. ECM from both porcine [16] and human [17-20] adipose tissue have been used for tissue engineering. Adipose tissue-derived matrix has also been made into gels for injection [19, 20]. However, native 3D structural and biochemical properties of adipose ECM are destroyed to various extents with the current protocols. The decellularization process needs to be perfected so that it not only effectively removes cellular components and oil but also maintains intact ECM properties.
This study aimed to provide an adipose tissue engineering platform and explore the fate of fat grafts from both the engineering and clinical perspectives. First, we developed a protocol to derive decellularized human adipose tissue ECM (hDAM) from incised fat tissues. By combining hDAM and human ASCs (hASCs), an engineered graft platform was created. Second, hDAM was subcutaneously implanted into Fischer rats to study the hDAM-induced immunoresponse, and we compared the cellular dynamics in fresh fat graft versus an engineered hDAM–hASC construct in a nude rat model. To gain a better clinical perspective, instead of culturing and differentiating hASCs in vitro, we mixed freshly harvested hASCs with hDAM to mimic the clinical fat grafting procedures.
2. Materials and methods
2.1. Human adipose tissue decellularization and injectable microparticle fabrication
All procedures were conducted under institutional review board approval and in accordance with research guidelines at the University of Texas MD Anderson Cancer Center. Patients had provided informed consent for the use of their tissues for basic research. Adipose tissue samples (subcutaneous adipose tissue in the abdominal wall area) were collected from patients undergoing reconstructive surgery, stored in saline on ice, and delivered to the lab for processing within 4 h after harvest. Samples were frozen at −80 °C, then cut into 4×2×1-cm pieces at −20 °C and processed following the protocol previously described[21]. 6 to 10 pieces were put into one flask for processing; 10 flasks could be used so 60 to100 pieces could be processed at the same time. Briefly, samples were re-frozen at −80 °C and thawed at room temperature for 3 cycles, and then washed in ultrapure water for 2 days at room temperature with agitation (120 rpm). These samples were then treated with 0.5 M NaCl for 4 h, which was followed by 1 M NaCl for 4 h, and washed in ultrapure water overnight; this saltwater wash procedure was repeated. After being treated with 0.25% trypsin/EDTA for 2 h and washed in deionized water for 1 h, samples were processed with isopropanol (IPA) overnight. The samples were then treated with 1% Triton X-100 for 3 days (1 change daily), washed in ultrapure water for 2 days (3 changes daily), and rinsed in phosphate-buffered saline (PBS) for 1 day. Samples were stored at 4 °C in PBS with 1% penicillin/streptomycinuntil.
To make hDAM injectable, hDAM scaffolds were freeze-dried and further processed into small pieces using an IKA A11 basic analytical mill (Sigma-Aldrich, St. Louis, MO). Mixed with saline, these microparticles were injectable with 16.5-gauge needles for engineered fat graft evaluation in vivo.
2.2. Histological and immunohistochemical (IHC) analysis
Native adipose tissues and hDAM were fixed in 10% formalin, embedded in paraffin, and sectioned into 5-μm slices. Slides cut from paraffin-embedded samples were processed for histological and IHC staining [21]. Slides underwent staining with hematoxylin and eosin (H&E), Oil Red O, and Masson's trichrome. Slides were also stained using antibodies against vascular endothelial growth factor (VEGF; Oncogene Science, Cambridge, MA) and major histocompatibility complex antigen class I (MHC-I; Abcam, Cambridge, MA). After staining, slides were dehydrated, mounted, and imaged using an Olympus IX71 microscope (Olympus, Center Valley, PA).
2.3. DNA assessment and quantification
Slides cut from paraffin-embedded samples underwent DAPI staining to show cell removal. Cell removal was quantified by measuring nucleic acid concentration with the Quant-iT PicoGreen dsDNA assay kit (Molecular Probes, Eugene, OR) as previously reported [21]. DNA quantity was normalized to the initial dry weight of the tissue.
2.4. Sulfated glycosaminoglycan (GAG) content
The sulfated GAG content of hDAM samples was quantified using an Alcian blue colorimetric assay kit (sGAG dye-binding assay, ALPCO, Salem, NH) as previously described [21]. GAG content was normalized to the initial dry weight of the tissue.
2.5. Scanning electron microscopy (SEM)
hDAM samples were frozen at −80 °C and dried through lyophilization. These dry samples were coated under vacuum using a Balzer MED 010 evaporator (Technotrade International, Manchester, NH) with platinum alloy for a thickness of 25 nm and were immediately flash carbon coated under vacuum. Samples were examined with a JSM-5910 scanning electron microscope (JEOL, Peabody, MA) at an accelerating voltage of 5 kV. Fiber size was measured with ImageJ software (National Institutes of Health, Bethesda, MD).
2.6. Porosity measurement
The porosity values of hDAM samples were measured by liquid displacement [22]. We used ethanol because it penetrates easily into pores and does not induce shrinkage or swelling.
2.7. Yield measurement
Adipose tissue samples (n = 3) were measured for weight and volume before and after the decellularization process. Yield was defined as the ratio of final weight or volume to initial weight or volume.
2.8. hASC culture and integration with hDAM in vitro
hASCs were isolated and cultured using our established protocol [24]. hASC identification was confirmed by flow cytometry analysis using antibodies against CD29 and CD90 (ASCs/stromal cells), CD11b (immune cells), and CD45 (hematopoietic cells). hASCs within 3 passages were harvested and plated onto hDAM samples at a density of 2.5×104 cells/cm2. Cell viability and proliferation on hDAM were studied by live cell staining using calcein AM (Biotium, Hayward, CA) as described previously [21]. Samples were examined with an Axiovert 200 fluorescence microscope (Zeiss, Thornwood, NY) on days 1, 3, and 7 after cell seeding. Cell morphologic features (perimeter, area, roundness, and elongation) were measured using Adobe Photoshop CS5.1 image-processing software (Adobe, San Jose, CA). Roundness was defined as 4*π*area/(perimeter)2 and elongation as length/width.
2.9. Examination of hDAM-host reaction in vivo
All procedures for which animals were used were approved by the Institutional Animal Care and Use Committee of the MD Anderson Cancer Center and met all requirements of the U.S. Animal Welfare Act. Four male 8- to 10-week-old Fischer 344 rats (Harlan Laboratories, Indianapolis, IN) were anesthetized and maintained with isoflurane (0.5–2%, 3–5 L/min) and oxygen. One hDAM sample (0.5×1 cm) was implanted subcutaneously on the back of each rat. The animals were monitored for clinical signs of inflammation or rejection for 30 days and then euthanized by applying CO2. Specimens were cut at the center of the explants and fixed in 10% formalin. Slides cut from paraffin-embedded samples underwent H&E staining, Masson's trichrome staining, and IHC staining with antibodies against CD31, CD68, CD80, CD163, CD4, and CD8 (all at 1:200, Abcam). CD 31 is an endothelial cell marker; CD68, CD80, and CD163 are macrophage markers; CD4 and CD8 are lymphocyte markers. Positively stained cells were counted for quantification.
2.10. Adipose tissue engineering by hDAM combined with hASC implantation in vivo
Male nude rats (8- to 10-week-old, National Institutes of Health, Bethesda, MD) were anesthetized and maintained with isoflurane (0.5–2%, 3–5 L/min) and oxygen. To prepare the hDAM-hASC construct, all cells isolated from 0.5 mL of fresh fat graft (Coleman technique) were suspended in 0.5 mL of saline and loaded on hDAM microparticles. A total of 2×105 live cells from 0.5 mL of fresh fat graft contained 4×104 hASCs (unpublished data). In one set of animals, the combination construct was subcutaneously injected on the back of the nude rats. In other sets of animals, 0.5 mL of fresh fat graft, or total live cells derived directly from 0.5 mL of fresh fat graft (2×105 cells) and resuspended in 0.5 mL of saline, was subcutaneously injected on the back (n = 4 per group). Samples of the injected material were harvested at weeks 1, 2, 4, and 8. Specimens were cut from the center of the explants and fixed in 10% formalin. Slides cut from paraffin-embedded samples underwent H&E staining as well as IHC staining with HuNu antibody (for human cell nuclei, 1:200, Millipore, Bedford, MA) and anti-CD31 antibody (for CD31, 1:200, Abcam). Positively stained cells were quantified for comparison.
2.11. Statistical analysis
Data are presented as means ± standard deviation (s.d.). Data were analyzed using one-way analysis of variance with SigmaStat software (Systat Software Inc., San Jose, CA). P values of less than 0.05 were considered significant.
3. Results
3.1. Characterization of hDAM
Human adipose tissue samples turned from yellow to white after decellularization. As confirmed by H&E and DAPI staining, cell nuclei were absent in hDAM compared with native tissues (Figure 1). A low level of DNA content was detected (2.1 ± 0.9 ng/mg DNA/dry sample weight). These results indicated the removal of the cellular component in hDAM. Oil Red O staining confirmed the removal of oil in hDAM (Figure 1).
Figure 1.
Sample images of native adipose tissue and hDAM, and evaluation of removal of cells (H&E and DAPI staining) and oil (Oil Red O staining) from adipose tissue.
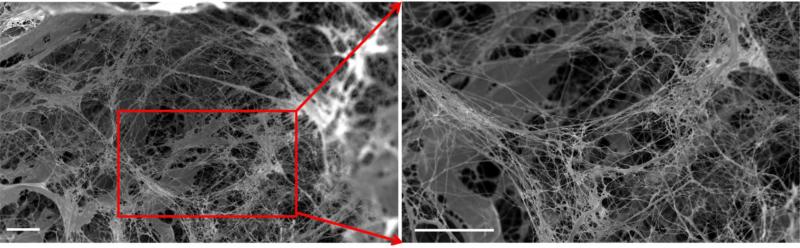
SEM images confirmed that cells were absent in hDAM, leaving 3D porous structures (porosity = 81.8 ± 9.5%) (Figure 2A). Nanofibrous structures of ECM were well maintained in hDAM (fiber size = 481.5 ± 96.9 nm) (Figure 2B). The decellularization process generated hDAM scaffolds with a yield of 10.6 ± 0.4% per weight or 12.0 ± 3.4% per volume.
Figure 2.
SEM image of hDAM samples. Freeze-dried hDAM scaffolds were highly porous. Scale bar = 50 μm.
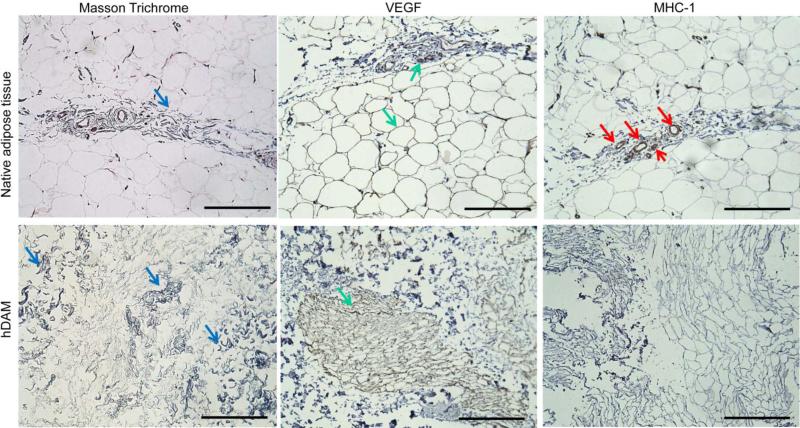
Masson's trichrome staining showed that collagen was a major component in native adipose tissue (Figure 3). After decellularization, the collagen component was maintained in hDAM (Figure 3). As indicated by assay results with Alcian blue, GAG was also retained in hDAM (1.72 ± 0.64 μg/mg GAG/dry sample weight). IHC analysis indicated that VEGF, which plays an important role in angiogenesis and neovascularization, was retained in hDAM. In native adipose tissue, VEGF was found mainly at the spots of blood vessels as well as surrounding adipose cells. After decellularization, VEGF was observed along fibrous structures (Figure 3), which corresponded to the nanofibrous structures observed with SEM (Figure 2). As determined by IHC analysis, MHC-I was found mainly at the spots of blood vessels in native adipose tissue. The absence of MHC-I in hDAM indicated the removal of alloantigenicity from hDAM (Figure 3).
Figure 3.
Characterization of hDAM components. Samples were stained with Masson's trichrome to detect collagen, and antibodies were used to detect VEGF and MHC-I. Corresponding staining for native adipose tissue was used for comparison. Positive staining is indicated by arrows. Scale bar = 200 μm.
3.2. hASC integration with hDAM
hASCs (CD29+CD90+CD45−CD11b−) were cultured on hDAM (Figure S1). Live cell staining with calcein AM proved that hDAM supported hASC survival and proliferation (Figure 4). hASCs attached well to hDAM at day 1 after plating. When cultured in vitro, hASCs proliferated on hDAM from day 1 (5541 cells/cm2) through day 7 (7405 cells/cm2) (P<0.05). Quantitative analysis of cell morphologic features confirmed significant differences between cells cultured on hDAM and cells cultured on 2D glass slides (Table 1). For example, hASCs on hDAM exhibited smaller spreading areas and perimeters and rounder shapes (P<0.05 for each comparison).
Figure 4.
Fluorescent images showing that hASCs (stained with calcein AM; green) proliferated on hDAM samples and on 2D glass on days 1, 3, and 7 of culture. Scale bar = 100 μm.
Table 1.
Summarization of cellular morphology on DAM samples and 2D glass slides on day 1, 3 and 7 in culture.
| hDAM | 2D glass | |||||||
|---|---|---|---|---|---|---|---|---|
| Area (μm2) | Perimeter (μm) | Roundness | Elongation | Area (μm2) | Perimeter (μm) | Roundness | Elongation | |
| Day 1 | 4.7×102±1.6±102 * (n=40) | 1.4×102±0.5×102 * (n=40) | 0.4±0.2 * (n=40) | 1.0±0.8 (n=40) | 3.9×103±1.4×103 (n=16) | 4.3×102±0.1×102 (n=16) | 0.3±0.1 (n=16) | 1.4±0.7 (n=16) |
| Day 3 | 4.0×102±1.5×102 # (n=46) | 1.1×102±0.4x102 #! (n=46) | 0.5±0.2 #% (n=46) | 1.5±1.0 & (n=46) | 1.2×103±0.4×103 $ (n=19) | 2.1×102±0.6×102 ^ (n=19) | 0.4±0.1 (n=19) | 1.5±0.9 (n=19) |
| Day 7 | 4.1×102±1.5×102 @ (n=21) | 1.0×102±0.3×102 @! (n=21) | 0.5±0.2 @% (n=21) | 1.6±1.0 @& (n=21) | 2.2×103±1.8×103 $ (n=36) | 2.4×102±1.0×102 ^ (n=36) | 0.5±0.1 ** (n=36) | 0.9±0.6 !! (n=36) |
Values are presented as mean±SD
P<0.05 compared with corresponding parameter from 2D at day 1
P<0.05 compared with corresponding parameter from 2D at day 3
P<0.05 compared with corresponding parameter from 2D at day 7
P<0.05 compared with DAM at day 1(perimeter)
P<0.05 compared with DAM at day 1(roundness)
P<0.05 compared with DAM at day 1(elongation)
P<0.05 compared with 2D at day 1(area)
P<0.05 compared with 2D at day 1(perimeter)
P<0.05 compared with 2D at day 1(roundness)
P<0.05 compared with 2D at day 3(elongation)
To make hDAM injectable for in vivo application, hDAM scaffolds were further processed into microparticles (Figure S2). The variance in hDAM microparticle was limited by the mill processing. The size of resultant hDAM microparticles was mostly below 200 μm. hASCs cultured on this particle system also formed cellular aggregates distributed in three dimensions in vitro (Supplementary Video 1).
3.3. Evaluation of hDAM–host interaction in vivo
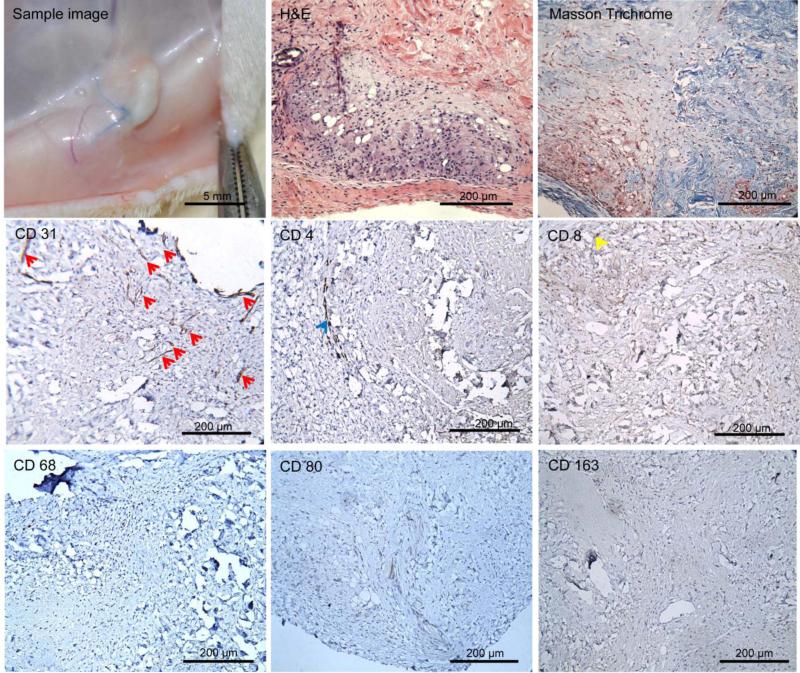
Implanted hDAM scaffolds were surrounded by a thin capsule (Figure 5). Uniform cell infiltration was observed within implanted samples from H&E staining. According to results from Masson's trichrome staining, most implants remained collagen-based scaffolds but exhibited local remodeling where they were close to the capsular layer. In the remodeled area, neovascularization (CD31+) was observed. All infiltrated cells in hDAM samples stained negatively for macrophage markers CD68, CD163, and CD80 (Figure 5). A few cells stained positively for lymphocyte markers CD4 and CD8 (cell density = 19.7 ± 19.6 and 32.0 ± 27.6 cells/mm2, respectively). Taken together, these results indicated that the hADM grafts were well vascularized and without obvious rejection or inflammation 30 days after subcutaneous implantation.
Figure 5.
hDAM scaffolds were embedded subcutaneously in Fischer rats for 4 weeks. Samples were harvested, imaged, processed for H&E and Masson's trichrome staining, and processed for IHC analysis with antibodies against CD31, CD68, CD163, CD80, CD4, and CD8. Infiltrated cells were negative for inflammatory cells (CD68−, CD163−, and CD80−). A few cells were observed for CD4+ (blue arrow) and CD8+ (yellow arrow). Blood vessels (red arrow) were distributed throughout implanted samples. Scale bar = 200 μm.
3.4. Evaluation of in vivo adipose tissue engineering
The engineered hDAM–hASC construct recapitulates the major components in native adipose tissue. The in vivo implanted fresh fat graft and engineered fat graft were harvested at week 2, 4, or 8. All graft samples were encapsulated by a thin and loose fibrous capsule layer and retained their 3D volume through 8 weeks post-implantation (Figure 6).
Figure 6.
Fresh fat graft (Coleman technique; 0.5 mL of fat graft/spot), hDAM combined with hASCs (0.5 mL of hDAM in PBS mixed with hASCs, cell density 4×105 cells/mL), and hASCs alone (0.5 mL of hASCs in PBS, cell density 4×105 cells/mL) were injected subcutaneously in nude rats. Samples were harvested at weeks 2, 4, and 8. Inserts show cross-section views of implants. Scale bar = 1 mm.
Cell infiltration was assessed by H&E staining, and the presence of injected hASCs was evaluated by IHC staining for HuNu antibody (Figure 7). In engineered fat graft, the density of ‘donor’ cells (i.e., original hASCs, HuNu+) did not change significantly from weeks 2 to 4 but did decrease significantly from weeks 4 to 8 (P<0.05) (Figure 7C, left). The density of ‘host’ cells (i.e., rat cells, HuNu−) was maintained from weeks 2 to 4 but increased significantly from weeks 4 to 8 (P<0.05). The percentage of donor cells in total observed cells decreased significantly from weeks 2 to 8 (P<0.05) (Figure 7C, right). Adipose tissue formation was observed at weeks 4 and 8 (Figures 7A and 7B). Cells at the adipose structures stained positively for HuNu, indicating that implanted hASCs developed into adipose tissue after 4 weeks.
Figure 7.
H&E staining of engineered (A) and fresh (D) fat graft after subcutaneous injection into nude rats. (B) and (E): Harvested samples from injected engineered (B) and fresh (E) fat graft were evaluated with HuNu antibody (brown, indicated by yellow arrows) and hematoxylin staining (blue, indicated by blue arrows). (C) and (F): Cell density (left) and percentage of HuNu+ cells in total observed cells (right) were quantified and compared. Scale bar = 200 μm.
In the fresh fat graft group, fat graft became remodeled from the outer layer toward the center, which was indicated by the observed cell infiltration and ECM remodeling (Figure 7D). From weeks 4 to 8, HuNu+ cells were observed at the center of the fat grafts, which were not remodeled. At the remodeled areas, most of these cells observed were HuNu−. The total number of cells observed within fat graft at the remodeling site significantly decreased from weeks 2 to 4 (P<0.05) but did not change significantly from weeks 4 to 8 (Figure 7F, left). The number of donor cells at the remodeling site kept decreasing from weeks 2 to 8 (P<0.05), while the number of host cells kept increasing (P<0.05). The percentage of donor cells at the remodeling site decreased significantly from weeks 2 to 8 (P<0.05) (Figure 7F, right). These results indicated that after injection of fresh fat graft, host cells played an important role in remodeling the implants.
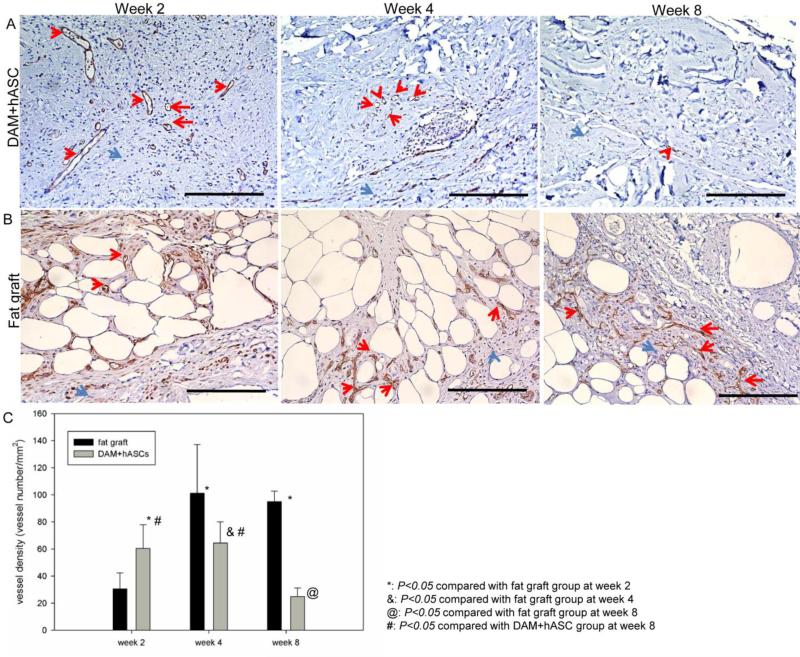
Vessel structure was confirmed by IHC staining for anti-CD31 antibody. Vascular structures were observed from weeks 2 to 8 in engineered fat grafts (Figure 8A); vessel density did not change from weeks 2 to 4, but decreased significantly from weeks 4 to 8 (Figure 8C). In the fresh fat graft group, vessel density increased significantly from weeks 2 to 4 and did not change significantly from weeks 4 to 8 (Figure 8C). Compared with the fresh group, the engineered group exhibited significantly higher vessel density at week 2 (P<0.05) but lower values at week 4 (P<0.05) and week 8 (P<0.05) (Figure 8C).
Figure 8.
At weeks 2, 4, and 8, harvested samples of engineered (A) and fresh (B) fat graft were studied with anti-CD31 antibody (red arrows) and hematoxylin staining (blue arrows). Scale bar = 200 μm. (C) Vessel density was semi-quantified and compared.
4. Discussion
In this study, we created a platform for engineering adipose tissues and exploring the fate of fat grafts from both the engineering and clinical perspectives. ECM provides the microenvironment for cells to interact with and cues for cellular function and activity. However, reports of the native structure of adipose tissue ECM have been highly variable [17, 19, 23-25]. To study ECM properties in adipose tissue, we developed a protocol for human adipose tissue decellularization. It is important that this model recapitulate not only the component properties of ECM after decellularization for tissue regeneration but also the 3D architecture to mimic the native niche or microenvironment. Incised abdominal wall adipose tissue was used for decellularization in this study. Compared with other sources, such as the placenta or liposuction samples [25-28], incised abdominal wall adipose tissue better maintains intact components and structures.
We demonstrated a lack of DAPI staining in hDAM, confirming the removal of the cellular component. DNA was barely detected in hDAM with a DNA assay. In contrast with the remaining DNA quantity of about 0.6 μg/mg reported by Young et al. [19] and about 100 nm/mg reported by Kim et al. [25], hDAM in our study showed a very low level of DNA quantity (2.1 ± 0.9 ng/mg DNA/dry sample weight), demonstrating highly effective cell removal. The oil component was effectively removed by IPA. Ethanol and enzymes such as lipase and colipase have been used to remove oil [18, 19], but IPA appears to be the most effective agent [18]. Our SEM images showed that hDAM maintained the native 3D architecture of ECM, as characterized by microscopic nanofibrous features. The remaining scaffolds were composed mainly of collagen and GAG. Laminin is another important component in ECM, which affects cell attachment and adhesion. However, hDAM derived using the current protocol did not show positive signal for laminin (data not shown). The presence of VEGF in hDAM indicated a pre-angiogenic property, whereas the absence of MHC-I implied that hDAM was free of implantation rejection. To make hDAM injectable for in vivo application, hDAM scaffolds were further processed into microparticles (Figure S2). The variance in hDAM microparticles was limited by the mill processing. Sieving could be applied to improve the size control of hDAM microparticles. This study provided a proof-of-concept of a soft-tissue repair platform for clinical application. In order to translate this platform for clinical application, further improvements to the protocol are needed to shorten the processing cycle while maintaining the native adipose tissue ECM properties (e.g., better maintenance of ECM components such as laminin). An automated high-throughput processing system will eventually be involved to increase the efficiency of the platform and meet the quantity needed in clinic.
Live-cell staining with calcein AM proved that hASCs were successfully integrated with hDAM in vitro. In addition, hDAM affected hASC morphology as compared with 2D cultured hASCs on glass slides. hASCs on hDAM exhibited in vivo-like morphologic features, as indicated by smaller areas and rounder shapes compared with hASCs cultured on 2D surfaces. The effect of hDAM on hASC morphological changes may be due to the distinctive architectural properties of hDAM: hDAM scaffolds presented porous structures with nanofibrous structures, which have been reported to affect cellular morphology and function [18, 20, 29-31]. Thus, our study of the interaction between hASC and hDAM supports the idea that hDAM with native ECM properties provides a suitable niche for hASC integration.
After allogeneic tissue transplantation, implants are recognized as foreign antigens by the immune system, which leads to implant degradation and graft loss [32, 33]. An immunogenicity evaluation of an allogeneic tissue-derived biomaterial is necessary before it can be clinically applied. We studied hDAM-induced implant–host interaction by examination of macrophage and T lymphocyte infiltration within grafts. As is well known, CD80 is a macrophage marker for the inflammation stage and CD163 is a macrophage marker for the remodeling stage[21]. CD4 and CD8 are markers for two populations in T lymphocytes: Lymphocytes that carry CD4 molecules mainly induce immune responses, whereas lymphocytes that carry CD8 molecules are predominantly cytotoxic[32]. Our results showed that few macrophages and a very low level of lymphocytes were noticed in implants, which implies that xenogeneic implantation of hDAM did not elicit an immunogenic or infectious response. In our previous report on decellularized musculofascial matrix, heterotopic implantation elicited a foreign-body response as shown by macrophage infiltration; further analysis suggested that these macrophages were mainly at the remodeling phage at week 4 of implantation [21]. In the present study, few macrophages were observed in the hDAM graft, suggesting that the hDAM-induced host response might have been past the macrophage infiltration and remodeling phase at week 4 of implantation. Instead of acting as a passive filling material, hDAM also underwent vascularization and adipose tissue regeneration at day 30 of implantation, which is consistent with other reports on adipose tissue-derived matrix [16, 17, 19]. Decellularized muscle or fascia have shown potential for muscle regeneration [21], while in our study hDAM showed potential for adipose tissue regeneration. These results support the strategy of ‘replace with alike’. Before our platform can be translated to clinic application, however, further research on sterilization and immune response in large-animal models is needed.
ASCs play an important role in adipose tissue regeneration and have been applied in the clinic for reconstruction. In addition, ASCs are known to secrete growth factors, such as VEGF, to improve vascularization or recruit cells at the implant site for vascularization and remodeling [34]. However, ASC injection alone is not sufficient to retain volume (Figure 6). Thus, scaffolds are needed to hold ASCs at the injection site over the long term. In our study, both engineered graft (combined hDAM and hASCs) and fresh fat graft retained implantation volume for 8 weeks. All implants underwent remodeling from the outer layer to the center. In both engineered graft and fresh fat graft, the total number of observed cells within implantations decreased from week 2 to week 8, with a decrease in donor cells and an increase in host cells, suggesting donor cell loss and host cell infiltration after implantation. In all, these two groups shared similar trends in cellular dynamics within implants. These results support the engineered graft, which recapitulates the major components of fresh fat graft, as a promising alternative for soft-tissue repair.
hASCs were detected with IHC staining for HuNu antibody in engineered fat graft at week 8, suggesting that adipose regeneration within engineered graft originated from implanted hASCs in our model. The strategy we presented in this study uses an in vivo scenario as the bioreactor and mimics procedures performed in operating rooms. It provides a proof-of-concept for translatable clinical application. In addition, it is a simple procedure that is easy to implement. Therefore, an hDAM scaffold combined with stem cell-based treatment is a promising development in adipose tissue engineering [20, 35, 36].
In engineered graft, vascularization decreased from weeks 2 to 8, whereas in fresh fat graft, vascularization increased from weeks 2 to 8. After the decellularization process, even though hDAM maintained the major adipose tissue ECM components and 3D structure, there was still a gap between native adipose tissue and hDAM. Adipose tissues consist of components other than cells, such as a complicate cytokines system. Thus, the next step for adipose tissue engineering may be the integration of cells plus multiple growth factors with hDAM implantation. In addition, the role of each cell type in the fat grafting mechanism needs to be explored.
5. Conclusion
We developed an effective decellularization protocol for processing human adipose tissue. The resultant hDAM led to effective removal of cellular components and oil and to well-maintained 3D architecture and biochemical composition. hDAM was non-cytotoxic to ASC culture, offering a natural niche for hASC integration and proliferation. In vivo testing demonstrated the biocompatibility, vascularization, and potential of adipose tissue regeneration of the hDAM–hASC construct. Hence, this study provides a platform and scaffold design standard for adipose tissue engineering.
Supplementary Material
Acknowledgements
We dedicate this study in memory of Dr. Elisabeth K. Beahm for her invaluable contribution. We thank Tejaswi S. Iyyanki for technical assistance, Dr. Edward Chang for adipose tissue support, the MD Anderson High Resolution Electron Microscopy Facility (NIH Cancer Center Core Grant CA16672) for SEM imaging, and the MD Anderson Flow Cytometry and Cellular Imaging Core Facility for confocal imaging. This study was supported by the Kyte Foundation through the Department of Plastic Surgery of MD Anderson.
References
- 1.Illouz YG, Sterodimas A. Autologous fat transplantation to the breast: A personal technique with 25 years of experience. Aesth Plast Surg. 2009;33:706–15. doi: 10.1007/s00266-009-9377-1. [DOI] [PubMed] [Google Scholar]
- 2.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: Safety and efficacy. Plast Reconstr Surg. 2007;119:775–85. doi: 10.1097/01.prs.0000252001.59162.c9. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman MR, Bradley JP, Dickinson B, Heller JB, Wasson K, O'Hara C, et al. Autologous fat transfer national consensus survey: Trends in techniques for harvest, preparation, and application, and perception of short- and long-term results. Plast Reconstr Surg. 2007;119:323–31. doi: 10.1097/01.prs.0000244903.51440.8c. [DOI] [PubMed] [Google Scholar]
- 4.Sommer B, Sattler G. Current concepts of fat graft survival: Histology of aspirated adipose tissue and review of the literature. Dermatol Surg. 2000;26:1159–66. [PubMed] [Google Scholar]
- 5.Choi M, Small K, Levovitz C, Lee C, Fadl A, Karp NS. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg. 2013;131:185–91. doi: 10.1097/PRS.0b013e3182789b13. [DOI] [PubMed] [Google Scholar]
- 6.Chung MT, Hyun JS, Lo DD, Montoro DT, Hasegawa M, Levi B, et al. Micro-computed tomography evaluation of human fat grafts in nude mice. Tissue Eng Part C Methods. 2013;19:227–32. doi: 10.1089/ten.tec.2012.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer B, Sattler G. Current concepts of fat graft survival: Histology of aspirated adipose tissue and review of the literature. Dermatol Surg. 2000;26:1159–66. [PubMed] [Google Scholar]
- 8.Khansa I, Momoh AO, Patel PP, Nguyen JT, Miller MJ, Lee BT. Fat necrosis in autologous abdomen-based breast reconstruction: a systematic review. Plast Reconstr Surg. 2013;131:443–52. doi: 10.1097/PRS.0b013e31827c6dc2. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman MA, Mirrafati S. Fat transfer techniques: The effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg. 2001;27:819–26. doi: 10.1046/j.1524-4725.2001.01062.x. [DOI] [PubMed] [Google Scholar]
- 10.von Heimburg D, Hemmrich K, Zachariah S, Staiger H, Pallua N. Oxygen consumption in undifferentiated versus differentiated adipogenic mesenchymal precursor cells. Respiratory Physiol Neurobiol. 2005;146:107–16. doi: 10.1016/j.resp.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Choi JH, Gimble JM, Lee K, Marra KG, Rubin JP, Yoo JJ, et al. Adipose tissue engineering for soft tissue regeneration. Tissue Eng Part B. 2010;16:413–26. doi: 10.1089/ten.teb.2009.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuk PA, Zhu M, Ashjian P, De Ugarte AD, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lequeux C, Oni G, Wong C, Damour O, Rohrich R, Mojallal A, et al. Subcutaneous fat tissue engineering using autologous adipose-derived stem cells seeded onto a collagen scaffold. Plast Reconstr Surg. 2012;130:1208–17. doi: 10.1097/PRS.0b013e31826d100e. [DOI] [PubMed] [Google Scholar]
- 14.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Choi YC, Choi JS, Kim BS, Kim JD, Yoon HI, Cho YW. Decellularized extracellular matrix derived from porcine adipose tissue as a xenogeneic biomaterial for tissue engineering. Tissue Eng Part C Methods. 2012;18:866–76. doi: 10.1089/ten.tec.2012.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JS, Kim BS, Kim JY, Kim JD, Choi YC, Yang HJ, et al. Decellularized extracellular matrix derived from human adipose tissue as a potential scaffold for allograft tissue engineering. J Biomed Mater Res A. 2011;97:292–9. doi: 10.1002/jbm.a.33056. [DOI] [PubMed] [Google Scholar]
- 18.Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715–24. doi: 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 19.Young DA, Ibrahim DO, Hu D, Christman KL. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 2011;7:1040–9. doi: 10.1016/j.actbio.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner AE, Yu C, Bianco J, Watkins JF, Flynn LE. The performance of decellularized adipose tissue microcarriers as an inductive substrate for human adipose-derived stem cells. Biomaterials. 2012;33:4490–9. doi: 10.1016/j.biomaterials.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Johnson JA, Chang DW, Zhang Q. Decellularized musculofascial extracellular matrix for tissue engineering. Biomaterials. 2013;34:2641–54. doi: 10.1016/j.biomaterials.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R, Ma PX. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J Biomed Mater Res. 1999;44:446–55. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Turner AE, Flynn LE. Design and characterization of tissue-specific extracellular matrix-derived microcarriers. Tissue Eng Part C Methods. 2012;18:186–97. doi: 10.1089/ten.TEC.2011.0246. [DOI] [PubMed] [Google Scholar]
- 24.Brown BN, Freund JM, Han L, Rubin JP, Reing JE, Jeffries EM, et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng Part C Methods. 2011;17:411–21. doi: 10.1089/ten.tec.2010.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim BS, Choi JS, Kim JD, Choi YC, Cho YW. Recellularization of decellularized human adipose-tissue-derived extracellular matrix sheets with other human cell types. Cell Tissue Res. 2012;348:559–67. doi: 10.1007/s00441-012-1391-y. [DOI] [PubMed] [Google Scholar]
- 26.Flynn L, Semple JL, Woodhouse KA. Decellularized placental matrices for adipose tissue engineering. J Biomed Mater Res A. 2006;79:359–69. doi: 10.1002/jbm.a.30762. [DOI] [PubMed] [Google Scholar]
- 27.Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials. 2007;28:3834–42. doi: 10.1016/j.biomaterials.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff JH. An injectable adipose matrix for soft-tissue reconstruction. Plast Reconstr Surg. 2012;129:1247–57. doi: 10.1097/PRS.0b013e31824ec3dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 30.Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering in vivo with adipose-derived stem cells on naturally derived scaffolds. J Biomed Mater Res A. 2009;89:929–41. doi: 10.1002/jbm.a.32044. [DOI] [PubMed] [Google Scholar]
- 31.Flynn LE, Prestwich GD, Semple JL, Woodhouse KA. Proliferation and differentiation of adipose-derived stem cells on naturally derived scaffolds. Biomaterials. 2008;29:1862–71. doi: 10.1016/j.biomaterials.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 32.Siemionow M, Klimczak A. Basics of immune responses in transplantation in preparation for application of composite tissue allografts in plastic and reconstructive surgery: Part I. Plast Reconstr Surg. 2008;121:4e–12e. doi: 10.1097/01.prs.0000299470.95855.ce. [DOI] [PubMed] [Google Scholar]
- 33.Zang M, Zhang Q, Chang EI, Mathur AB, Yu P. Decellularized tracheal matrix scaffold for tissue engineering. Plast Reconstr Surg. 2012;130:532–40. doi: 10.1097/PRS.0b013e31825dc084. [DOI] [PubMed] [Google Scholar]
- 34.Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr Opin Organ Tran. 2010;15:86–91. doi: 10.1097/MOT.0b013e328334f074. [DOI] [PubMed] [Google Scholar]
- 35.Yu C, Bianco J, Brown C, Fuetterer L, Watkins JF, Samani A, et al. Porous decellularized adipose tissue foams for soft tissue regeneration. Biomaterials. 2013;34:3290–302. doi: 10.1016/j.biomaterials.2013.01.056. [DOI] [PubMed] [Google Scholar]
- 36.Wang JQ, Fan J, Gao JH, Zhang C, Bai SL. Comparison of in vivo adipogenic capabilities of two different extracellular matrix microparticle scaffolds. Plast Reconstr Surg. 2013;131:174e–87e. doi: 10.1097/PRS.0b013e3182789bb2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.