Abstract
Since the groundbreaking hypothesis of X chromosome inactivation (XCI) proposed by Mary Lyon over 50 years ago, a great amount of knowledge has been gained regarding this essential dosage compensation mechanism in female cells. For the mammalian system, most of the mechanistic studies of XCI have so far been investigated in the mouse model system, but recently, a number of interesting XCI studies have been extended to human pluripotent stem cells, including both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Emerging data indicate that XCI in hESCs and hiPSCs is much more complicated than that of their mouse counterparts. XCI in human pluripotent stem cells is not as stable and is subject to environmental influences and epi-genetic regulation in vitro. This mini-review highlights the key differences in XCI between mouse and human stem cells with a greater emphasis placed on the understanding of the epigenetic regulation of XCI in human stem cells.
Introduction
X chromosome inactivation (XCI) is the process by which one of the two X chromosomes in female mammalian development is inactivated through a series of epigenetic modifications. This process allows for dosage compensation of the X-linked genes in female cells as proposed elegantly by Mary Lyon over 50 years ago (Lyon 1961, 1962). Since then, XCI has become a beautiful model system of molecular, cell, and developmental biology. One of the milestone discoveries in XCI is the isolation and cloning of the X-inactive specific transcript (XIST) gene, which is transcribed by the inactivated X chromosome (Xi) (Brown et al. 1991). As reviewed by many other excellent papers in this issue, we have gained insight into the mechanistic action of XIST and its associated epigenetic regulators including small non-coding RNAs, specific his-tone modifications, and DNA methylation in the initiation, choice, and maintenance of XCI. Much of the knowledge regarding the mechanism of XCI in mammals has largely been derived from the mouse model through the study of both mouse embryonic stem cells (mESCs) and embryos. Developmental studies of XCI in humans lag behind that of the murine model due to the obvious difficulty of working with human embryogenesis. Nevertheless, the advent of hESCs and hiPSCs provides an alternative avenue to understanding human XCI regulation in vitro (Dhara and Benvenisty 2004; Takahashi et al. 2007; Tchieu et al. 2010). Unexpectedly, several recent studies of hESCs and hiPSCs have indicated that XCI exhibits variations and switches from one state to another in culture, suggesting that there are significant differences in XCI regulation between human and mouse stem cells (Dvash and Fan 2009; Makhlouf and Rougeulle 2011).
Similarities and differences in XCI between human and mouse embryogenesis and embryonic stem cells (ESCs)
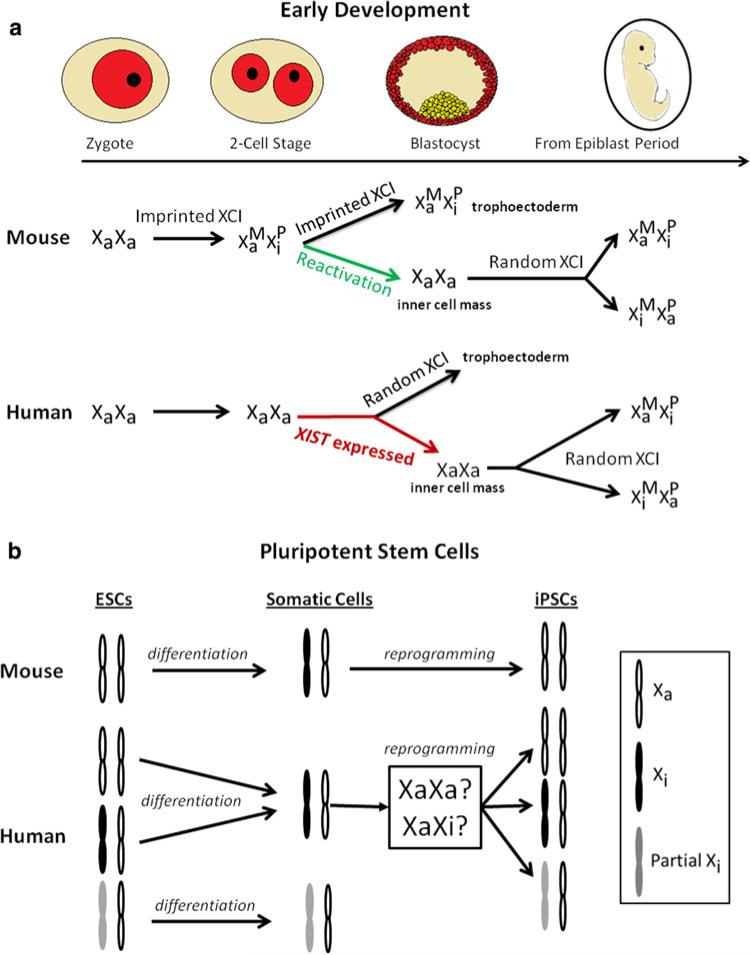
During mouse embryogenesis, the zygote contains two active X chromosomes (XaXa); however, upon the first cleavages, XCI establishes in two discrete events. At the two-cell stage, the first event of XCI initiates when the imprinted paternal X chromosome is exclusively inactivated (Okamoto et al. 2004; Huynh and Lee 2005) (Fig. 1). During blastocyst formation, cells of the inner cell mass (ICM) reactivate their paternal X chromosomes, while the trophectoderm and primitive endoderm preserve their imprinted XCI (Okamoto et al. 2004; for review, see Heard and Disteche 2006). The second stage of XCI occurs upon differentiation when epiblast cells randomly inactivate one of their X chromosomes (Heard and Disteche 2006). Therefore, each somatic cell will express either a maternal or paternal X chromosome (Heard and Disteche 2006; Pablo and Philip 2009) (Fig. 1).
Fig. 1.
Comparison of mouse and human XCI during embryogenesis and upon stem cell differentiation and reprogramming. a A schematic drawing of XCI status in the early stages of mouse and human development. XCI establishes in two discrete events in mouse, while only random XCI appears to occur in human embryonic development. XIST expression in human preimplantation embryos can be detected, but no XCI takes place yet in ICM. b XCI status in mouse and human pluripotent stem cells, upon differentiation, and after reprogramming. XCI in hESCs and hiPSCs show more variation with three distinct classes: pre-XCI, full XCI, and partial XCI. XCI status after the reprogramming of human somatic cells into hiPSCs also presents three states. It is still debated if XCI in human somatic cells are subjected to reactivation in human iPSCs as the case of mouse iPSCs upon cell reprogramming
Whereas XCI has been extensively studied in the mouse model, XCI in early human development is still inconclusive and more difficult to study. It is still unclear whether the biphasic nature of XCI establishment observed in mouse embryos also takes place in human preimplantation embryos. The presence of several key differences between murine and primate development may contribute to the variation of XCI in mammalian species. For example, the mouse embryo transitions from maternal to zygotic gene expression during the two-cell stage, earlier than does the human embryo, which shifts gene expression from maternal to zygotic at the four- to eight-cell stage. The primate embryo also devotes its first 2 weeks after implantation to develop an extraembryonic membrane (Enders and Schlafke 1981; Enders et al. 1986; Luckett 1978), while the mouse embryo has already passed midgestation and has initiated germ layer formation by the end of the second week. In extraembryonic tissues such as the placenta, mouse placental cells exclusively exhibit imprinted paternal X inactivation whereas human placental cells present random XCI (Moreira de Mello et al. 2010). A very recent study also suggests that human embryonic cells initiate XCI later than their mouse counterpart although XIST expression can already be detected during the morula stage in human preimplantation embryos (Okamoto et al. 2011) (Fig. 1). This finding is in contrast to the situation in hESCs because XIST is not expressed in pre-XCI hESCs in culture as will be discussed later in this review. It would be of interest to address whether the pre-XCI state in hESCs corresponds to a state in which XIST is not yet expressed in preimplantation embryos, or alternatively a result of silencing the biallelic XIST expression of human ICM in the derivation process of hESCs. In any case, the differences in the initiation and maintenance of XCI during embryonic development in humans, mice, and rabbits suggest that the mouse is not a sufficient model to understand XCI in human development. The differences in the initiation and maintenance of XCI during development in humans, mice, and rabbits suggest that the mouse is not a sufficient model to understand XCI in human development.
The study of XCI in the mouse model has been greatly advanced by the discovery that female mESCs, derived from ICM, exhibit XaXa in the pluripotent state, but initiate random XCI upon in vitro differentiation. The in vitro system of recapitulating XCI through mESCs differentiation has prompted a number of mechanistic studies of XCI at the molecular and cellular levels. As seen with mouse embryos, once XCI initiates in differentiated mESCs, the Xist gene is transcribed at the X inactivation center (Xic) of the X chromosome that was randomly selected to be inactivated. Only the X chromosome that is predestined to undergo XCI will express the Xist gene. The resulting RNA transcripts that are transcribed from the Xist gene will coat the X chromosome (Sado et al. 2005; Wutz 2007). Occurring simultaneously with Xist expression, Polymerase II is excluded on the Xi and followed by a series of repressive chromatin marks (Payer and Lee 2008).
A series of histone modifications induce the soon to be Xi into a heterochromatin structure, thereby silencing the genes localized on the chromosome (Wutz 2007; Wutz and Gribnau 2007). Meanwhile, the X chromosome whose expression remains active (Xa) expresses the Tisx gene, which prevents the expression of its respective Xist gene, thus preventing its own inactivation (Navarro et al. 2005; Sado et al. 2005).
Because human ESCs are also derived from the ICM, it was initially postulated that hESCs are the counterpart to mESCs in the study of human XCI (Dhara and Benvenisty 2004). However, subsequent studies found that hESC cultures do not exhibit a consistent XCI status. The status of XCI in hESCs has displayed three distinct classes: class I hESCs resemble mESCs in that they do not yet have an Xi, but when exposed to conditions favorable for differentiation, the hESCs undergo XCI; class II hESCs display an Xi and XIST expression although the cells remain in the undifferentiated and pluripotent state; class III hESCs exhibit partial XCI although XIST expression and other markers of XCI are absent (Dvash and Fan 2009; Makhlouf and Rougeulle 2011). Culture conditions and the length of passaging appear to influence the class in which XCI belongs (Hall et al. 2008; Shen et al. 2008; Silva et al. 2008). In our recent work, we minimized in vitro effects by studying XCI in hESCs of very early passages (P5-10) (Dvash et al. 2010). Nevertheless, we still found that XCI status had already displayed three distinct states (Dvash et al. 2010). Furthermore, even within subcultures of several female hESC lines, there is variation in XCI (Shen et al. 2008). We also demonstrated that class III female hESCs can lose Xist expression and exhibit partial reactivation of the originally silenced X chromosome (Shen et al. 2008).
Differences in XCI between mESCs and hESCs may be attributed to the innate property of cells. Global gene expression studies have suggested that hESCs are similar to mouse epiblast stem cells (EpiSCs) in terms of developmental stage. XCI is fully established and highly stable in mouse EpiSCs (Guo et al. 2009). In fact, genome-wide gene expression analysis confirms that hESCs are more similar to mouse EpiSCs than mESCs (Guo et al. 2009). This had led to the concept of deriving hESCs with a naïve mESC-like state of pluripotency, which has the potential of exhibiting XaXa with a pre-XCI pattern. Indeed, Hanna et al. (2010) was able to convert hESCs with XCI into a meta-stable state of hESCs that display XaXa and a mESC-like morphology. This was achieved by ectopic activation of signaling pathways involved in pluripotency and enforcing a culturing condition suitable for mESC growth in hESCs (Hanna et al. 2010). These results lead to the hypothesis that hESCs are initially in a naïve pluripotent and pre-XCI state; however, normal culture conditions are unable to preserve this pre-XCI state (Hanna et al. 2010).
Consistent with this notion that the naïve pre-XCI state is difficult to be preserved in normal culture conditions, Lengner et al. (2010) found that the level of O2 during the derivation of hESCs influences the status of XCI. When hESC derivation is done under physiological concentration of oxygen (5% O2 instead of 20% O2), hESC lines derived and cultured in this hypoxic condition displayed a naïve pre-XCI state (Lengner et al. 2010). These pre-XCI hESCs display two active X chromosomes and randomly inactivate one of the X chromosomes upon exposure to 20% O2 culture conditions or upon in vitro differentiation (Lengner et al. 2010). The introduction of antioxidants into culture media to reduce the levels of reactive oxidative species from atmospheric oxygen is also able to prevent the onset of XCI (Lengner et al. 2010). The authors conclude that XCI is highly sensitive to cellular stress, including stress from atmospheric O2 (20% O2), which is able to irreversibly induce XCI. This result is consistent with previous postulation that the XCI status in hESCs can be altered under different culture conditions (Hall et al. 2008; Shen et al. 2008; Silva et al. 2008; Dvash et al. 2010).
Similarities and differences in XCI between human and mouse induced pluripotent stem cells (iPSCs)
iPSCs avoid much of the ethical controversy associated with the derivation of human embryonic stem cells and can also become a potential source of patient-specific cells in regenerative medicine. This has generated significant interest in the study of iPSCs. Mouse and human iPSCs (miPSCs and hiPSCs, respectively) can be derived from either mouse or human somatic cells by the forced expression of transcription factors associated with pluripotency, including Oct4, Sox2, Klf4, and c-Myc (Takahashi and Yamanaka 2006; Takahashi et al. 2007; Park et al. 2008; Hochedlinger and Plath 2009). In the mouse model system, reprogramming of somatic fibroblasts into pluripotent stem cells is tightly associated with the switch of full XCI into the pre-XCI state (Maherali et al. 2007; Stadtfeld et al. 2008). Based on this relationship, a reversal of XCI in miPSCs during the reprogramming process from the somatic state can serve as an indicator of pluripotency and the return to the naïve pluripotent state (Maherali et al. 2007) (Fig. 1). Like mESCs, the differentiation of mouse iPSCs also results in random XCI in iPSC derivatives (Maherali et al. 2007; Stadtfeld et al. 2008).
The status of XCI in hiPSCs has only just begun to be studied. One study observed that female hiPSCs retain an inactivate X chromosome (Tchieu et al. 2010). XIST gene detection and allele specific expression assays suggest that hiPSCs maintain XCI during reprogramming (Tchieu et al. 2010). However, several other studies have also found that a portion of hiPSCs do exhibit XaXa, and upon in vitro differentiation, random XCI is established in the progeny cells (Marchetto et al. 2010; Bruck and Benvenisty 2011) (Fig. 1b). Bruck and Benvenisty (2011) systemically classified XCI status in a number of hiPSCs lines through a meta-analysis of the expression of the genes on the X chromosome. Like the aforementioned three classes of XCI in hESCs (reviewed by Dvash and Fan 2009; Makhlouf and Rougeulle 2011), the authors have also identified three classes of XCI in hiPSCs: those displaying complete XCI, partial XCI (X chromosome is partially inactivated regardless of the presence or absence of Xist expression), and pre-XCI (Bruck and Benvenisty 2011). The authors emphasize that XIST expression assays alone are not enough to characterize XCI in hiPSCs, especially those that display partial XCI. All these studies have so far indicated that the state of XCI in established lines of hiPSCs is variable, similar to that of female hESCs (Fig. 1b).
There is an ongoing debate on whether XCI in human somatic cells is subjected to reprogramming as in mouse iPSCs. Using inducible vectors expressing OCT4, SOX2, KLF4, and C-MYC together with chemical compounds and culture conditions normally used for mESCs, Hanna et al. (2010) have also generated hiPSCs with a mESC-like morphology. These mESC-like hiPSCs appear to be in the naïve pre-XCI with two active X-chromosomes (XaXa). However, when the naïve hiPSCs were transferred to hESC-culture medium containing bFGF treatment, hiPSCs began to exhibit XIST expression and the XCI phenotype (Hanna et al. 2010). This study further suggests that human somatic cells can be reprogrammed into the pre-XCI state as observed in miPSCs only under optimal culture conditions. Taken all these findings together, we suggest that XCI in human somatic cells is subject to reprogramming in hiPSCs as in miPSCs. However, due to the selection pressure and meta-stability of pre-XCI in human pluripotent stem cells, hiPSCs also exhibit variable XCI status in different experiments and different laboratories as currently reported in the literature.
The implications of XCI variations in studying X-linked genetic disorder using human stem cells
The understanding of XCI variations in female human stem cells may not only improve knowledge regarding epigenetic regulation of XCI in human biology, but also help establish appropriate cell models to study many X-linked human diseases. For example, a neurodevelopmental autism spectrum disorder, Rett syndrome (RTT) in females, is caused primarily by heterozygous mutations in the X-linked gene encoding methyl-CpG binding protein 2 (MECP2). Due to random XCI, RTT patients exhibit mosaic expression of mutant MECP2. A few hemizygous mutations of RTT in males cause a much earlier lethal phenotype. Recently, two independent groups have succeeded in deriving RTT-hiPSCs from female patients. One group was able to produce iPSCs that are at a pre-XCI state (Marchetto et al. 2010). Because random XCI is coupled with in vitro neural differentiation of RTT iPSCs, investigators are able to obtain neurons that express either wild-type or mutant MECP2. The other group was able to obtain iPSCs that already exhibit XCI at the undifferentiated stem cell stage (Cheung et al. 2011). Therefore, it allowed this group to compare the phenotypes of the isogenic control and RTT-hiPSCs from the same RTT patient. Both groups found interesting phenotypes of RTT neurons from RTT-iPSCs. These two studies provide an excellent example of using the proper human stem cells to study the pathogenesis of X-linked human genetic disorders by taking advantage of iPSCs technology and XCI variations in human stem cells.
It is also worth noting that the deregulation of XCI has also been associated with the altered expression of X-linked genes in pluripotent stem cells (Shen et al. 2008). Furthermore, altered X-inactivation has an impact on tumorigenesis and other genetic disorders (Agrelo and Wutz 2010). We suggest that when using female pluripotent stem cells to study human genetic disorders, one should carefully monitor the status of XCI in both pluripotent stem cells and their differentiated progenitor cells to assess the influence of a particular XCI state on the expression of X-linked genes and the pathogenesis of diseases.
Concluding remarks
The successful derivation of human and mouse ESCs and iPSCs has provided useful systems that can be used to further the understanding of XCI regulation in differentiation and reprogramming. While murine stem cells can recapitulate XCI regulation in vivo, XCI in human ESCs and iPSCs appears in multiple states: (a) a naïve pre-XCI state as seen in mouse ESCs and iPSCs; (b) the presence of full XCI; or (c) partial XCI due to epigenetic influences. Thus, for many of X-linked genetic disorders, random XCI in human pluripotent stem cells and differentiating derivatives allow investigators to isolate isogenetic controls and mutant cells from female somatic cells carrying heterozygous mutations. Many important questions remain to be resolved concerning XCI in stem cells. For example, what is the molecular change in human stem cells that underlies variations of XCI states in different culture conditions or upon reprogramming? How different is XCI regulation between human stem cells and embryos? Do we have optimal experimental conditions to consistently obtain hESCs and hiPSCs in the naïve pluripotent and pre-XCI state with two active X chromosomes? We expect that X-inactivation study and stem cell research will continue to intersect with each other and yield more exciting findings in the near future.
References
- Agrelo R, Wutz A. X inactivation and disease. Semin Cell Dev Biol. 2010;21:194–200. doi: 10.1016/j.semcdb.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, Willard HF. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349:38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- Bruck T, Benvenisty N. Meta-analysis of the heterogeneity of X chromosome inactivation in human pluripotent stem cells. Stem Cell Res. 2011;6:187–193. doi: 10.1016/j.scr.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Horvath LM, Grafodatskaya D, Grafodatskaya D, Pasceri P, Weksberg R, Hotta A, Carrel L, Ellis J. Isolation of MECP2-null Rett Syndrome patient hiPS cells and isogenic controls through X-chromosome inactivation. Hum Mol Genet. 2011;20:2103–2115. doi: 10.1093/hmg/ddr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhara SK, Benvenisty N. Gene trap as a tool for genome annotation and analysis of X chromosome inactivation in human embryonic stem cells. Nucleic Acids Res. 2004;32:3995–4002. doi: 10.1093/nar/gkh746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvash T, Fan G. Epigenetic regulation of X-inactivation in human embryonic stem cells. Epigenetics. 2009;4:19–22. doi: 10.4161/epi.4.1.7438. [DOI] [PubMed] [Google Scholar]
- Dvash T, Lavon N, Fan G. Variations of X chromosome inactivation occur in early passages of female human embryonic stem cells. PLoS One. 2010;5:e11330. doi: 10.1371/journal.pone.0011330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders AC, Schlafke S. Differentiation of the blastocyst of the rhesus monkey. Am J Anat. 1981;162:1–21. doi: 10.1002/aja.1001620102. [DOI] [PubMed] [Google Scholar]
- Enders AC, Schlafke S, Hendrickx AG. Differentiation of the embryonic disc, amnion, and yolk sac in the rhesus monkey. Am J Anat. 1986;177:161–185. doi: 10.1002/aja.1001770205. [DOI] [PubMed] [Google Scholar]
- Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LL, Byron M, Butler J, Becker KA, Nelson A, Amit M, Itskovitz-Eldor J, Stein J, Stein G, Ware C, Lawrence JB. X-inactivation reveals epigenetic anomalies in most hESC but identifies sublines that initiate as expected. J Cell Physiol. 2008;216:445–452. doi: 10.1002/jcp.21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard E, Disteche CM. Dosage compensation in mammals: fine-tuning the expression of the X chromosome. Genes Dev. 2006;20:1848–1867. doi: 10.1101/gad.1422906. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh KD, Lee JT. X-chromosome inactivation: a hypothesis linking ontogeny and phylogeny. Nat Rev Genet. 2005;6:410–418. doi: 10.1038/nrg1604. [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Gimelbrant AA, Erwin JA, Cheng AW, Guenther MG, Welstead GG, Alagappan R, Frampton GM, Xu P, Muffat J, Santagata S, Powers D, Barrett CB, Young RA, Lee JT, Jaenisch R, Mitalipova M. Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Cell. 2010;141:872–883. doi: 10.1016/j.cell.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Luckett WP. Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am J Anat. 1978;152:59–97. doi: 10.1002/aja.1001520106. doi:10.1002/aja.1001520106. [DOI] [PubMed] [Google Scholar]
- Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Lyon MF. Sex chromatin and gene action in the mammalian X chromosome. Am J Hum Genet. 1962;14:135–148. [PMC free article] [PubMed] [Google Scholar]
- Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Makhlouf M, Rougeulle C. Linking X chromosome inactivation to pluripotency: necessity or fate? Trends Mol Med. 2011 doi: 10.1016/j.molmed.2011.02.001. doi: 10.1016/j.molmed.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira de Mello JC, Araújo ÉSSd, Stabellini R, Fraga AM, Souza JESd, Sumita DR, Camargo AA, Pereira LV. Random X inactivation and extensive mosaicism in human placenta revealed by analysis of allele-specific gene expression along the X chromosome. PLoS One. 2010;5:e10947. doi: 10.1371/journal.pone.0010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro P, Pichard S, Ciaudo C, Avner P, Rougeulle C. Tsix transcription across the Xist gene alters chromatin conformation without affecting Xist transcription: implications for X-chromo-some inactivation. Genes Dev. 2005;19:1474–1484. doi: 10.1101/gad.341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science. 2004;303:644–649. doi: 10.1126/science.1092727. [DOI] [PubMed] [Google Scholar]
- Okamoto I, Patrat C, Thepot D, Peynot N, Fauque P, Daniel N, Diabangouaya P, Wolf JP, Renard JP, Duranthon V, Heard E. Eutherian mammals use diverse strategies to initiate X-chromosome inactivation during development. Nature. 2011;472:370–374. doi: 10.1038/nature09872. [DOI] [PubMed] [Google Scholar]
- Pablo N, Philip A. When X-inactivation meets pluripotency: an intimate rendezvous. FEBS Lett. 2009;583:1721–1727. doi: 10.1016/j.febslet.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Payer B, Lee JT. X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet. 2008;42:733–772. doi: 10.1146/annurev.genet.42.110807.091711. [DOI] [PubMed] [Google Scholar]
- Sado T, Hoki Y, Sasaki H. Tsix silences Xist through modification of chromatin structure. Dev Cell. 2005;9:159–165. doi: 10.1016/j.devcel.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Shen Y, Matsuno Y, Fouse SD, Rao N, Root S, Xu R, Pellegrini M, Riggs AD, Fan G. X-inactivation in female human embryonic stem cells is in a nonrandom pattern and prone to epigenetic alterations. Proc Natl Acad Sci USA. 2008;105:4709–4714. doi: 10.1073/pnas.0712018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SS, Rowntree RK, Mekhoubad S, Lee JT. X-chromosome inactivation and epigenetic fluidity in human embryonic stem cells. Proc Natl Acad Sci. 2008;105:4820–4825. doi: 10.1073/pnas.0712136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tchieu J, Kuoy E, Chin MH, Trinh H, Patterson M, Sherman SP, Aimiuwu O, Lindgren A, Hakimian S, Zack JA, Clark AT, Pyle AD, Lowry WE, Plath K. Female human iPSCs retain an inactive X chromosome. Cell Stem Cell. 2010;7:329–342. doi: 10.1016/j.stem.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A. Xist function: bridging chromatin and stem cells. Trends Genet. 2007;23:457–464. doi: 10.1016/j.tig.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Wutz A, Gribnau J. X inactivation Xplained. Curr Opin Genet Dev. 2007;17:387–393. doi: 10.1016/j.gde.2007.08.001. [DOI] [PubMed] [Google Scholar]