Abstract
Non-genetic maternal effects are widespread across taxa and challenge our traditional understanding of inheritance. Maternal experience with predators, for example, can have lifelong consequences for offspring traits, including fitness. Previous work in threespine sticklebacks showed that females exposed to simulated predation risk produced eggs with higher cortisol content and offspring with altered anti-predator behavior. However, it is unknown whether this maternal effect is mediated via the offspring glucocorticoid stress response and if it is retained over the entire lifetime of offspring. Therefore, we tested the hypothesis that maternal exposure to simulated predation risk has long-lasting effects on the cortisol response to simulated predation risk in stickleback offspring. We measured circulating concentrations of cortisol before (baseline), 15 min after, and 60 min after exposure to a simulated predation risk. We compared adult offspring of predator-exposed mothers and control mothers in two different social environments (alone or in a group). Relative to baseline, offspring plasma cortisol was highest 15 min after exposure to simulated predation risk and decreased after 60 min. Offspring of predator-exposed mothers differed in the cortisol response to simulated predation risk compared to offspring of control mothers. In general, females had higher cortisol than males, and fish in a group had lower cortisol than fish that were by themselves. The buffering effect of the social environment did not differ between maternal treatments or between males and females. Altogether the results show that while a mother’s experience with simulated predation risk might affect the physiological response of her adult offspring to a predator, sex and social isolation have much larger effects on the stress response to predation risk in sticklebacks.
Keywords: Parental effect, Glucocorticoid, HPA axis, Predators, Maternal programming
1. Introduction
Non-genetic maternal effects occur when conditions experienced by mothers influence the phenotype of their offspring [1]. Maternal effects occur in diverse taxa (plants [2]; insects [3]; amphibians [4]; and mammals [5]) and their effect on offspring fitness can be maladaptive [4,6,7] or adaptive [3,8]. There is growing evidence that some maternal effects are mediated by maternal steroid hormones (sex steroids [9], and glucocorticoids [10]). For example high levels of circulating glucocorticoids in stressed mothers are transferred to developing eggs in birds, affecting offspring phenotype [11].
Studies in diverse taxa have shown that maternal stress can have long-lasting effects on offspring traits including survival [5,12-14], growth [5,15,16], morphology [5,12,13], learning [17], and behavior [17-20]. In addition, some studies have shown long-lasting effects of maternal stress on the offspring glucocorticoid response to stressors [21,22], suggesting that maternal steroids have organizational effects [23] on the development of the offspring hypothalamus–pituitary–adrenal (HPA) or, in fishes, hypothalamus–pituitary–interrenal (HPI) axis that persist throughout an offspring’s lifetime. Therefore it is possible that mothers might ‘program’ their offspring for the types of environments they are likely to experience later in life [24,25].
Predators are one of the most important naturally occurring stressors for animals in natural populations [26,27]. Predator-induced maternal effects have been documented in diverse taxa, with effects on multiple offspring traits [5,8,20,28-30]. A previous study on threespine stickleback fish found that predator-exposed mothers produced offspring that exhibited higher levels of shoaling behavior than offspring of control mothers [31]. Shoaling involves swimming in close proximity to conspecifics, resulting in the formation of groups [32], and is an effective antipredator defense in small fishes [33]. Female sticklebacks exposed to simulated predator attacks also produced eggs with higher concentrations of cortisol [31]. Predator exposure triggers the release of cortisol in sticklebacks [34], therefore a plausible explanation for the maternal effect observed by Giesing et al. [31] is that maternally derived cortisol diffused into eggs and induced an organizational effect on the HPI axis during offspring development. If this is the case, then offspring of predator-exposed mothers might have an altered cortisol response to a stressor compared to offspring of control mothers.
Other studies have shown a buffering effect of the social environment on the cortisol response to stressors (cows [35], pigs [36], guinea pigs [37], and gorillas [38]). Therefore we hypothesized that a maternal effect on the stickleback cortisol response might differ according to the social environment. For example, offspring of predator-exposed stickleback mothers might shoal together in order to cope with simulated predation risk, and might perceive simulated predation risk as more threatening if they do not have the opportunity to shoal.
In this study we tested the maternal programming hypothesis in sticklebacks. Specifically, we examined whether maternal experience with a simulated predation risk by Northern pike (Esox lucius), a natural predator of sticklebacks, influences offspring cortisol response to simulated predation risk by pike. We measured circulating plasma cortisol of adult offspring of predator-exposed and unexposed mothers before (baseline), 15 min after and 60 min after exposure to simulated predation risk by pike. We predicted that offspring of predator-exposed mothers have higher baseline and predator-induced cortisol than offspring of control mothers. To test the hypothesis that the presence of a social group during exposure to simulated predation risk buffers the glucocorticoid response of offspring, we compared adult offspring of predator-exposed and unexposed mothers that were either alone or in a group at the time of exposure to simulated predation risk. Finally, hormonally mediated maternal effects are often sex-specific [26,27,39,40]. For example, pregnant mice exposed to stressors during the first week of gestation gave birth to male offspring that as adults exhibited a larger glucocorticoid response than male offspring of control mothers, an effect not seen in female offspring [25]. Therefore we also investigated the influence of sex on the cortisol response to predation risk.
2. Materials and methods
2.1. Maternal predator exposure
Adult threespine sticklebacks were collected from Putah Creek, CA in May 2010 and were acclimated to the laboratory for at least one month before experiments began. Throughout, all fish were maintained in a flow-through system with UV, charcoal, particulate and biological filters that remove olfactory cues and a photoperiod that mimics seasonal changes. Fish were fed ad libitum once daily frozen bloodworms, brine shrimp, mysis shrimp, and cyclopeez, and uneaten food removed at the end of the day.
Females were exposed to simulated predation risk by exposing them to a model pike as in McGhee et al. [14]. Briefly, they were housed in 38 L tanks (53 L × 33 W × 24 H cm) with artificial plant refuges, gravel bottom and opaque external shading. Females were randomly assigned to one of six 38 L tanks (‘treatment tanks’) at a density of five females per tank and either exposed to simulated predation risk (‘predator-exposed’ mothers) or left undisturbed (‘control’ mothers) until they became gravid. Predator-exposed females were chased for 30 s/day at a randomly chosen time with a painted clay model of Northern pike (23 cm standard length) to simulate exposure to predation risk [31]. Predator-exposed mothers were chased once daily for 56 ± 21 days (mean ± standard deviation, range 22 to 85 days) before being stripped of eggs. Consistent with a previous study (Giesing et al. 2011), there was no effect of the number of days in the maternal treatment on offspring cortisol (results not shown). Live Northern pike have been shown to elicit a cortisol response in sticklebacks [34] and simulated attack by a model pike has been shown to elicit antipredator behaviors as compared to a non-threatening stimulus [41]. Pike do not inhabit the section of Putah Creek where sticklebacks were caught; therefore, we know that mothers did not have experience with pike before the experiment. When females became gravid, they were stripped of eggs by hand and were replaced by a marked female in order to maintain the same density.
Testes were dissected from wild-caught males from the same population, and macerated to release sperm, and the sperm was used to fertilize eggs. Sperm was used from nine different males: sperm from three males fertilized eggs from control mothers only (n = 5 clutches), sperm from three other males fertilized eggs from predator-exposed mothers only (n = 5 clutches), and sperm from three additional males fertilized eggs from both control and predator-exposed mothers (n = 10 clutches). Altogether, clutches were collected from n = 10 predator-exposed mothers and n = 10 control mothers. After contributing one clutch of eggs, females were no longer used in the experiment.
2.2. Rearing offspring
Offspring rearing for this experiment was carried out as described in McGhee et al. [14]. Briefly, fertilized eggs were artificially incubated in plastic cups with air bubblers. Juveniles were transferred to 38 L tanks (‘tank of origin’, 53 L × 33 W × 24 H cm) surrounded by opaque shading and artificial plants for refuge in either single family groups (n = 11 families) or mixed family groups (n = 9 families) within each maternal treatment. Fry were fed newly hatched brine shrimp nauplii. Offspring were reared in their tank of origin until they entered the simulated predation risk experiment (see below) in August 2011, when they were approximately one year old (standard length 3.83 ± 0.39 cm mean ± standard deviation). On average, the number of offspring surviving to adulthood per mother did not differ between maternal treatments: control mothers had 60.5 ± 15.9 surviving offspring (mean ± standard error) per mother and predator-exposed mothers had 61.2 ± 9.9 surviving offspring per mother.
The number of offspring used in the experiment from each family housed in single family tanks did not differ between maternal treatments. A total of 14.33 ± 5.37 offspring (mean ± standard error) per mother were used from control mothers and 14.83 ± 3.54 offspring per mother used from predator-exposed mothers. Mixed and single family groups of offspring were equally represented across all treatments. Since individuals in mixed family tanks were not identified by parentage, it is possible that some families were overrepresented in the mixed family tanks.
2.3. Simulated predation risk experiment
Twelve 38 L observation tanks (53 L × 33 W × 24 H cm) with artificial plant refugia and surrounded on the sides with opaque material to minimize the effect of external visual stimuli were used for the offspring simulated predation risk experiment. Tanks were assigned to one of three different ‘time’ treatments for sampling: baseline, 15 min and 60 min, with n = 4 replicate tanks per treatment. Focal fish in the ‘baseline’ tanks were not exposed to simulated predation risk, focal fish in the ‘15 min’ tanks were exposed to simulated predation risk and sacrificed for cortisol 15 min later and focal fish in the ‘60 min’ tanks were exposed to simulated predation risk and sacrificed for cortisol 60 min later (described further, below).
Half of the 12 tanks were randomly assigned to one of two different social environment treatments (alone vs. group) with the constraint that the two social environments were equally distributed between the different ‘times’. The ‘group’ tanks contained four ‘background’ individuals from a pool of approximately 30 fish that were maintained in two 38 L holding tanks when not being used in the experiment. The background fish were spine-clipped one week prior to the experiment in order to distinguish them from the focal fish, which were unmarked. The ‘alone’ tanks did not contain background individuals.
Twelve focal sticklebacks were randomly assigned to the 12 observation tanks with one focal fish per observation tank, with the constraint that maternal treatments were equally represented across social environments and time and that no more than one individual was drawn from each tank of origin per day. In order to avoid pseudoreplication, there was only one focal fish per observation tank in the ‘group’ treatment. Focal fish were acclimated to the observation tanks for 2 h, an amount of time previously found to be sufficient for stickleback whole body cortisol to reach and maintain a stable 24-h level after handling and transfer [42]. Individuals in the ‘baseline’ tanks were sacrificed immediately after the 2-h acclimation period. Fish in the ‘15 min’ and ‘60 min’ tanks were exposed to simulated predation risk. A deceased and frozen Northern pike (Spirit Lake Fish Hatchery, Spirit Lake, IA; SL = 18.5 cm, mass = 68.8 g) was thawed, suspended from a dowel with fishing line, and used to simulate the predation risk, which consisted of repeated rounds of orientation, slow approach, and lunging at the stickleback for 60 s. Although different procedures were used to apply simulated predation risk to mothers (model pike) and their offspring (frozen pike), we assume that the model pike and the frozen pike were both perceived as a threatening pike predator by the sticklebacks [41]. The pike was frozen and thawed between successive days of the experiment.
Either 15 or 60 min after applying simulated predation risk, the opaque shading was removed from the observation tank in order to distinguish focal from background individuals; the focal fish was netted from the observation tank and immediately sacrificed with a fatal concentration (>0.2 mM) of MS-222 anesthetic. Time to opercular arrest was <30 s and the time from shade removal to opercular arrest was <60 s. After opercular arrest, the fish was blotted with paper towel, standard length was measured, and the caudal peduncle was severed posterior to the cloaca. Blood was drawn by capillary action from the caudal vein into a 75 mm heparinized microhematocrit tube (Statspin, Westwood, MA). Tubes were centrifuged on a microhematocrit rotor (Statspin, Westwood, MA) to pellet circulating cells, and plasma supernatant aspirated to a microfuge tube and stored at −20 °C. A small sample of muscle tissue was taken from the tail for determination of genetic sex using a sex-specific genetic marker [43].
This procedure (random assignment of observation tanks, addition of n = 12 focal animals to the observation tanks) was repeated 15 times between 2 and 17 August 2011. On some days (n = 6 days), the procedure was repeated twice, with at least 2 h between procedures. In 2 h, the water in the observation tanks was replaced at least four times; therefore there was no carryover of olfactory cues between procedures (82.2 ± 6.0 L/h mean ± SEM flow rate). Altogether we sampled n = 15 focal individuals per maternal treatment * time * social environment combination.
Individual offspring that entered the simulated predation risk experiment were not replaced, so the density of offspring in tanks of origin decreased over time as the experiment progressed. The density of offspring in the tanks of origin at the start of the offspring experiment was 10.0 ± 0.94 fish (mean ± standard error) and at the end of the experiment was 3.36 ± 0.65 fish. Feeding rates were adjusted according to density. Mortality of adult offspring during the course of the offspring simulated predation risk experiment was very low (number of deaths: offspring of control mothers = 0.25 ± 0.16 adults per mother (mean ± standard error); there were no deaths of adult offspring of predator-exposed mothers).
2.4. Cortisol assay
Plasma samples were thawed and their cortisol concentration measured in duplicate by competitive enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (Enzo Life Sciences, Plymouth Meeting, PA). The antibody used in this assay is reported by the manufacturer to have a sensitivity of 56.72 pg/ml and a 27.7% cross reactivity with corticosterone, 4.0% with 11-deoxycortisol, 3.6% with progesterone, and 0.1% or less with testosterone, androstenedione, cortisone, and estradiol. Samples of adequate volume (~4 μl) were treated with 1:10 dilution of steroid-displacement reagent to free cortisol from its binding proteins, diluted to 1:50 sample:assay buffer ratio, and divided in half to be measured as duplicates. To extend the manufacturer’s recommended standard curve for the ELISA an 8th standard of cortisol (78 pg/ml) was included on each plate. Following the manufacturer’s protocol, the plates were prepared and absorbance read at 405 nm for each sample in duplicate in a 96-well ELISA plate (Enzo Life Sciences, Plymouth Meeting, PA) on a FilterMax F3 microplate reader (Molecular Devices, Sunnyvale, CA) and absorbance data averaged across duplicate samples with Multi-Mode Analysis software (Molecular Devices version 3.4.0.25).
Blank-corrected, total-activity-corrected optical densities were converted to ‘percent bound’ for each sample by applying an equation derived from each plate’s standard curve. Both linear and four-factor polynomial standard curves were produced for each ELISA plate and tested for accuracy in calculating sample cortisol concentration from ‘percent bound’ value of the known standards. The polynomial method (which is recommended by the manufacturer) fit the expected concentration of the standards more closely than the linear method, and so was employed for the final analysis. Values were multiplied by their dilution factor to obtain plasma cortisol concentration in ng/ml of the original, undiluted plasma sample.
Plasma samples across all levels of maternal treatment (predator-exposed and control), social environment (alone and group), and time (0, 15, and 60) were represented on each of the six plates. The inter-assay coefficient of variation (CV) between all ELISA plates (n = 6 plates) was 2.40%; intra-assay CVs averaged 5.02 ± 2.37% (mean ± standard deviation). Six individuals either gave very little blood or the plasma was lost from the capillary tube during isolation; therefore the final sample size was 174 focal individuals.
2.5. Statistical analysis
All analyses were performed using SAS version 9.3. The cortisol data were ln-transformed to meet the assumptions of parametric tests. Cortisol data were analyzed with a general linear model using the “proc GLM” command in SAS. We started with a full model that included sex, social environment, time, and maternal treatment as fixed effects, date as a covariate and all the two- and three-way interactions. ‘Date’ was the day in August 2011 when a sample was collected. Terms with p-value > 0.05 were step-wise removed from the model, unless their interaction with another variable was statistically significant (p < 0.05). We plotted lncort against date for each level of time, and also for each level of maternal treatment * social environment * time, and we found no significant interactions between the covariate and these variables (data not shown).
Although the analyses were carried out on ln-transformed data, for ease of comparison with other studies we show the raw (untransformed) data in figures. Post-hoc tests were performed on a pairwise basis on all terms deemed significant by the statistical model. Least squares estimates of the mean of each term were obtained using the LSMEANS statement in SAS, and were compared between levels within each term.
3. Results
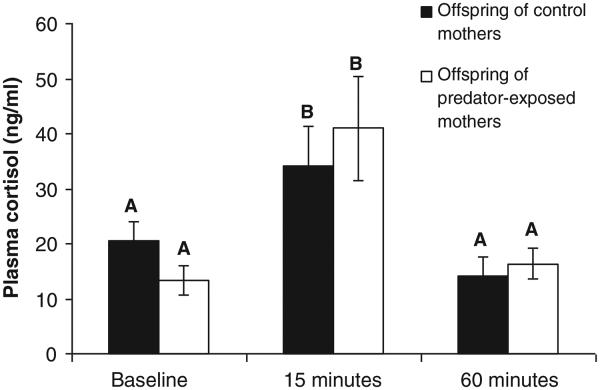
Relative to baseline, levels of plasma cortisol increased 15 min after exposure to simulated predation risk and dropped by 60 min (Table 1, main effect of time). We did not detect a main effect of maternal treatment on concentrations of cortisol. However, maternal exposure to simulated predation risk influenced the time course of the cortisol response of offspring (Table 1, maternal treatment * time interaction). That is, offspring of predator-exposed mothers mounted a different cortisol response to simulated predation risk than the offspring of control mothers (Fig. 1). Post-hoc tests comparing least squares means between levels of maternal treatment * time were non-significant (p-values > 0.05).
Table 1.
GLM model testing the effect of maternal treatment, social environment, sex, and exposure to predation risk over time on plasma cortisol in the threespine stickleback.
| Source | DF | Type I SS | Mean square | F value | p |
|---|---|---|---|---|---|
| Maternal treatment | 1 | 0.07183249 | 0.07183249 | 0.10 | 0.7515 |
| Time | 2 | 22.41042515 | 11.20521258 | 15.69 | <.0001 |
| Social environment | 1 | 22.04691896 | 22.04691896 | 30.87 | <.0001 |
| Sex | 1 | 10.24221408 | 10.24221408 | 14.34 | 0.0002 |
| Date | 1 | 7.17508595 | 7.17508595 | 10.05 | 0.0018 |
| Maternal treatment * Time | 2 | 4.38936628 | 2.19468314 | 3.07 | 0.0490 |
Date was included in the model as a covariate and did not significantly interact with any of the class variables (i.e. p > 0.05 for all interactions). Interactions that were not significant (i.e. p > 0.05) in the full model were stepwise-removed until only significant interactions remained.
Fig. 1.
The cortisol response of offspring of mothers exposed to simulated predation risk compared to offspring of control mothers. There was a significant interaction between maternal treatment and time (Table 1), indicating that the time course of the plasma cortisol response differs between offspring of predator-exposed mothers and control mothers. However, the post-hoc comparison of least squares means between maternal treatments at each time point was not significant (p > 0.05). n = 28–30 per bar. Data represent least squares (LS) means ± 1 standard error of raw (not transformed) data. LS-means with the same letter are not significantly different.
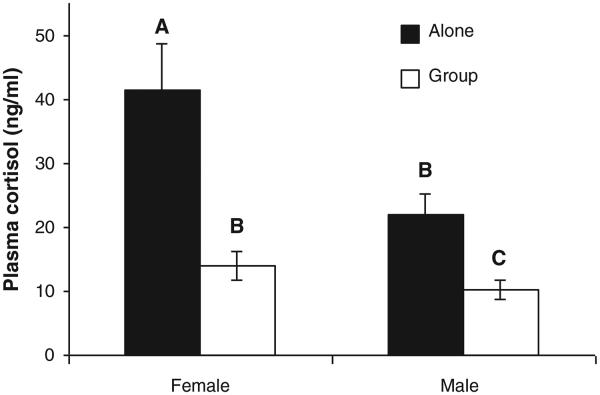
By comparison there was a relatively large effect of sex on plasma cortisol. Females had higher plasma cortisol than males (29.4 ± 4.3 ng/ml vs. 14.3 ± 1.7 ng/ml, Table 1, main effect of sex). We did not detect an interaction between sex and social environment; females had higher plasma cortisol in both the ‘alone’ and ‘group’ treatments (Fig. 2).
Fig. 2.
Sticklebacks that were alone had higher circulating concentrations of cortisol than sticklebacks that were in a group, and females had higher cortisol than males. Data within each level of sex * social environment represent all three time periods combined. n = 43–51 per bar. Data represent least squares (LS) means ± 1 standard error of raw (not transformed) data. LS-means with the same letter are not significantly different.
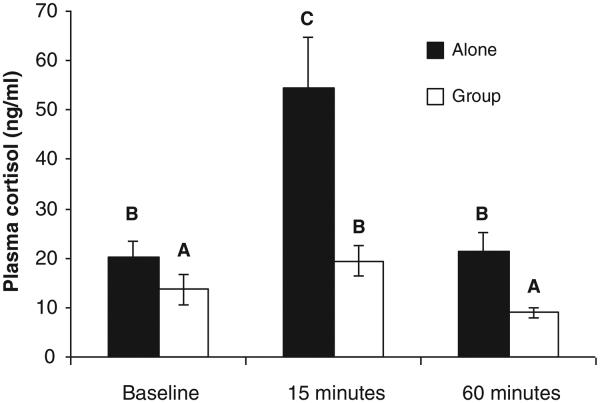
There was a strong buffering effect of the presence of a social group on the cortisol response to simulated predation risk; fish that were in a group had lower levels of plasma cortisol than fish that were by themselves (Fig. 3, Table 1, main effect of social environment). However there was no evidence that the social environment had more of a buffering effect on the response to a stressor of offspring of predator-exposed mothers: there was not a statistically significant interaction between the social environment and maternal treatment (Table 1).
Fig. 3.
Levels of plasma cortisol increased 15 min after exposure to simulated predation risk and subsided by 60 min after exposure. Fish that were in a group consistently had lower plasma cortisol than fish that were alone. n = 28–30 individuals per bar. Data represent least squares (LS) means ± 1 standard error of raw (not transformed) data. LS-means with the same letter are not significantly different.
There was an effect of date on plasma cortisol (Table 1): fish that were sampled later in the experiment had lower concentrations of cortisol than fish that were sampled at an earlier date. There were no significant interactions between date and maternal treatment, social environment, sex, or time (all p-values > 0.05).
Mean effect size for several studies that measured the glucocorticoid response to stressors in offspring exposed to either real or simulated maternal stress and compared to control offspring was 0.895 (range 0.317 to 2.189) [44-48]. Power analysis using our sample size confirmed that our study had high power (mean = 0.866) to detect the effect sizes of published studies.
The range of plasma cortisol levels observed in this study (22.00 ± 2.23 ng/ml SEM) is similar to whole body cortisol measured in sticklebacks [34,42], and was similar to the natural range between baseline and predator-induced levels observed in the plasma of other teleost species (25–110 ng/ml in rainbow trout [49],and 20–110 ng/ml in Atlantic cod [50]).
4. Discussion
These results show that sticklebacks mounted a cortisol response to simulated predation risk: plasma cortisol increased 15 min and then dropped 60 min after exposure to simulated predation risk. This pattern differs from another study which found that whole body cortisol continued to increase up to 60 min post exposure to simulated predation risk [34], but is consistent with studies in other animals which have found that the plasma cortisol response to an acute stressor declines by 60 min after a stressor [21,22]. The high cortisol levels 60 min after exposure to a predator observed in Bell et al. [34] likely reflect additional intracellular cortisol and cortisol byproducts in whole body extracts. Our results add to the growing literature on the cortisol response to predators, a naturally occurring, ecologically relevant stressor.
We tested the hypothesis that maternal experience with a pike predator influences offspring cortisol response to pike in sticklebacks. The hypothesis was partially supported in that there was a significant interaction between maternal treatment and time. The pattern in Fig. 1 suggests that offspring of predator-exposed mothers mounted a stronger cortisol response to predation risk (compare offspring of control and predator-exposed mothers at baseline versus at 15 min). However, post-hoc comparisons of least squares means between levels of maternal treatment and time were not significant.
We detected a significant sex difference in circulating plasma cortisol: females had higher cortisol than males. This result is similar to findings in other fish including sockeye salmon [51] and the poeciliid B. episcopi [52], but differs from the roach R. rutilus, in which males of the species had higher cortisol during spawning [53]. Offspring measured in this experiment were reproductive adults, so it is possible that cortisol levels we observed reflect the naturally occurring sex differences during spawning in sticklebacks. The lack of interaction between sex and maternal treatment in our study suggests that this maternal effect in sticklebacks does not depend on sex (unlike [26,27,39,40]).
We also found that the presence of a social group buffered the cortisol response for all offspring, independent of maternal treatment. A likely explanation for lower levels of cortisol in a group is the dilution effect: the risk of predation is lower in a group and therefore an individual’s perceived vulnerability is lower [33]. Although the maternal effect on the cortisol response did not depend on the presence of a social group in this experiment, it is possible that in natural populations where individuals are free to choose their own social environments (as opposed to an experimentally imposed environment), offspring of predator-exposed mothers might be more likely to actively seek out social groups. Indeed, Giesing et al. [31] found that offspring of predator-exposed stickleback mothers swam closer to their neighbors than offspring of control mothers.
We detected a significant effect of ‘date’ on plasma cortisol: fish that were sampled later in the experiment had lower cortisol than fish that were sampled earlier in the experiment. There are at least three non-exclusive explanations for this finding. First, a frozen and thawed pike was repeatedly used to chase offspring. It is possible that olfactory cues present in the pike’s skin degraded over the course of the experiment such that sticklebacks exposed to the pike towards the end of the experiment experienced fewer olfactory cues than sticklebacks exposed to the pike towards the beginning of the experiment. Second, individual offspring were not replaced in the tanks of origin; therefore sticklebacks measured towards the end of the experiment came from tanks at lower density than focal sticklebacks measured towards the beginning of the experiment. Given our finding that individuals housed with a social group had lower cortisol than individuals that were isolated, it is possible that fish tested later in the experiment which had experienced lower density were more stressed. Finally, it is possible that decreasing concentrations of cortisol over time reflect seasonal changes.
5. Conclusions
We found that sex, exposure to predation risk, the presence or absence of a social group, and a mother’s experience with predators all influence the cortisol content of an adult stickleback’s plasma. While sex and the social environment have a clear and strong influence, our data show a more subtle contribution of maternal experience with predation risk. However, these results suggest that if a female stickleback encounters a predator during her lifetime, the experience might influence the lifelong responses of her offspring to predators. The mechanism whereby information about a stickleback mother’s experience with predators is encoded and passed to her offspring to alter their cortisol response to stressors remains to be determined, as does its adaptive significance.
HIGHLIGHTS.
Sticklebacks mounted a cortisol response to predation risk.
Maternal experience with a predator influenced offspring response to a predator.
The maternal effect on offspring cortisol depended on when cortisol was measured.
Female sticklebacks had higher cortisol than males.
There was a buffering effect of the social environment on cortisol.
Acknowledgements
We thank Katie McGhee and Elissa Suhr for their work on the maternal predator exposure treatment, Ryan Paitz and Molly Kent for useful conversations, members of the Bell laboratory for help proofreading the manuscript, and the Department of Animal Biology, School of Integrative Biology at University of Illinois for their support. This work was supported by NSF (grant IOS 1121980) and was conducted in accordance with national standards on animal welfare (IACUC# 09204).
References
- 1.Mousseau T, Fox CW. The adaptive significance of maternal effects. Trends Ecol Evol. 1998;13:403–7. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal AA. Herbivory and maternal effects: mechanisms and consequences of transgenerational induced plant resistance. Ecology. 2002;83:3408–15. [Google Scholar]
- 3.Fox CW, Czesak ME, Mousseau TA, Roff DA. The evolutionary genetics of an adaptive maternal effect: egg size plasticity in a seed beetle. Evolution. 1999;53(2):552–60. doi: 10.1111/j.1558-5646.1999.tb03790.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RH. Greater maternal investment can decrease offspring survival in the frog Bombina orientalis. Ecology. 1992;73(1):280–8. [Google Scholar]
- 5.Sheriff MJ, Krebs CJ, Boonstra R. The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J Anim Ecol. 2009;78:1249–58. doi: 10.1111/j.1365-2656.2009.01552.x. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJP. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–4. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sie KKY, Medline A, Van Weel J, Sohn KJ, Choi SW, Croxford R, et al. Effect of maternal and postweaning folic acid supplementation on colorectal cancer risk in the offspring. Gut. 2011;60(12):1687–94. doi: 10.1136/gut.2011.238782. [DOI] [PubMed] [Google Scholar]
- 8.Storm JJ, Lima SL. Mothers forewarn offspring about predators: a transgenerational maternal effect on behavior. Am Nat. 2010;175:382–90. doi: 10.1086/650443. [DOI] [PubMed] [Google Scholar]
- 9.Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev. 2005;29:329–52. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Uller T, Hollander J, Astheimer G, Olssen M. Sex-specific developmental plasticity in response to yolk corticosterone in an oviparous lizard. J Exp Biol. 2009;212:1087–91. doi: 10.1242/jeb.024257. [DOI] [PubMed] [Google Scholar]
- 11.Groothuis TGG, Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Philos Trans R Soc Lond B Biol Sci. 2008;363:1647–61. doi: 10.1098/rstb.2007.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagliano M, McCormick MI. Hormonally mediated maternal effects shape off-spring survival potential in stressful environments. Oecologia. 2009;160:657–65. doi: 10.1007/s00442-009-1335-8. [DOI] [PubMed] [Google Scholar]
- 13.Saino N, Romano M, Ferrari RP, Martinelli R, Møller AP. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J Exp Zool. 2005;303A:998–1006. doi: 10.1002/jez.a.224. [DOI] [PubMed] [Google Scholar]
- 14.McGhee KE, Pintor LM, Suhr EL, Bell AM. Maternal exposure to predation risk decreases offspring antipredator behavior and survival in threespined stickleback. Funct Ecol. 2012;26(4):932–40. doi: 10.1111/j.1365-2435.2012.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormick MI. Mothers matter: crowding leads to stressed mothers and smaller offspring in marine fish. Ecology. 2006;87:1104–9. doi: 10.1890/0012-9658(2006)87[1104:mmclts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.McCormick MI. Indirect effects of heterospecific interactions on progeny size through maternal stress. Oikos. 2009;118:744–52. [Google Scholar]
- 17.Roche DP, McGhee KE, Bell AM. Maternal predator-exposure has lifelong consequences for offspring learning in threespined sticklebacks. Biol Lett. 2012 doi: 10.1098/rsbl.2012.0685. http://dx.doi.org/10.1098/rsbl.2012.0685 [Published online before print September 19, 2012] [DOI] [PMC free article] [PubMed]
- 18.Clarke AS, Schneider ML. Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Dev Psychobiol. 1993;26:293–304. doi: 10.1002/dev.420260506. [DOI] [PubMed] [Google Scholar]
- 19.Nishio H, Kasuga S, Ushijima M, Harada Y. Prenatal stress and postnatal development of neonatal rats—sex-dependent effects on emotional behavior and learning ability of neonatal rats. Int J Dev Neurosci. 2001;19:37–45. doi: 10.1016/s0736-5748(00)00070-8. [DOI] [PubMed] [Google Scholar]
- 20.Shine R, Downes SJ. Can pregnant lizards adjust their offspring phenotypes to environmental conditions? Oecologia. 1999;119:1–8. doi: 10.1007/s004420050754. [DOI] [PubMed] [Google Scholar]
- 21.Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–9. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu F, Crespi EJ, Denver RJ. Programming neuroendocrine stress axis activity by exposure to glucocorticoids during postembryonic development of the frog, Xenopus laevis. J Endocrinol. 2008;149:5470–81. doi: 10.1210/en.2008-0767. [DOI] [PubMed] [Google Scholar]
- 23.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. J Endocrinol. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 24.Darnaudéry M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57:571–85. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–9. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- 27.Canoine V, Hayden TJ, Rowe K, Goymann W. The stress response of European stonechats depends on the type of stressor. Behaviour. 2002;139:1303–11. [Google Scholar]
- 28.Agrawal AA, Laforsch C, Tollrian R. Transgenerational induction of defenses in animals and plants. Nature. 1999;401:60–3. [Google Scholar]
- 29.Gilbert JJ. Rotifer ecology and embryological induction. Science. 1966;151:1234–7. doi: 10.1126/science.151.3715.1234. [DOI] [PubMed] [Google Scholar]
- 30.Weisser WW, Braendle C, Minoretti N. Predator-induced morphological shift in the pea aphid. Proc R Soc Lond B Biol Sci. 1999;266:1175–81. [Google Scholar]
- 31.Giesing ER, Suski CD, Warner RE, Bell AM. Female sticklebacks transfer information via eggs: effects of maternal experience with predators on offspring. Proc R Soc Lond B Biol Sci. 2011;278:1753–9. doi: 10.1098/rspb.2010.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masuda R, Shoji J, Nakayama S, Tanaka M. Development of schooling behavior in Spanish mackerel Scomberomorus niphonius during early ontogeny. Fish Sci. 2003;69:772–6. [Google Scholar]
- 33.Magurran AE. The inheritance and development of minnow anti-predator behaviour. Anim Behav. 1990;39:834–42. [Google Scholar]
- 34.Bell AM, Backström T, Huntingford FA, Pottinger TG, Winberg S. Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol Behav. 2007;91:15–25. doi: 10.1016/j.physbeh.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Boissy A, Le Neindre P. Behavioral, cardiac and cortisol responses to brief peer separation and reunion in cattle. Physiol Behav. 1997;61:693–9. doi: 10.1016/s0031-9384(96)00521-5. [DOI] [PubMed] [Google Scholar]
- 36.Kanitz E, Puppe B, Tuchscherer M, Heberer M, Viergutz T, Tuchscherer A. A single exposure to social isolation in domestic piglets activates behavioural arousal, neuroendocrine stress hormones, and stress-related gene expression in the brain. Physiol Behav. 2009;98:176–85. doi: 10.1016/j.physbeh.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Machatschke IH, Wallner B, Schams D, Dittami J. Social environment affects peripheral oxytocin and cortisol during stress responses in guinea-pigs. Ethology. 2004;110:161–76. [Google Scholar]
- 38.Stoinski TS, Czekala N, Lukas KE, Maple TL. Urinary androgen and corticoid levels in captive, male Western lowland gorillas (Gorilla g. gorilla): age- and social group-related differences. Am J Primatol. 2002;56:73–87. doi: 10.1002/ajp.1065. [DOI] [PubMed] [Google Scholar]
- 39.McMullen S, Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;288:R85–90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- 40.Zohar I, Weinstock M. Differential effect of prenatal stress on the expression of corticotrophin-releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. J Neuroendocrinol. 2011;23:320–8. doi: 10.1111/j.1365-2826.2011.02117.x. [DOI] [PubMed] [Google Scholar]
- 41.Grobis MM, Pearish SP, Bell AM. A test of two hypotheses for the function of schooling behaviour in three-spined sticklebacks (Gasterosteus aculeatus) Anim Behav. 2013;85:187–94. doi: 10.1016/j.anbehav.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pottinger TG, Carrick TR, Yeomans WE. The three-spine stickleback as an environmental sentinel: effects of stressors on whole-body physiological indices. J Fish Biol. 2002;2002(61):207–29. [Google Scholar]
- 43.Peichel CL, Ross JA, Matson CK, Dickson M, Grimwood J, Schmutz J, et al. The master sex-determination locus in threespine sticklebacks is on a nascent Y chromosome. Curr Biol. 2001;14:1416–24. doi: 10.1016/j.cub.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 44.Marasco V, Robinson J, Herzyk P, Spencer KA. Pre- and post-natal stress in context: effects on the stress physiology in a precocial bird. J Exp Biol. 2012;215:3955–64. doi: 10.1242/jeb.071423. [DOI] [PubMed] [Google Scholar]
- 45.Haussmann MF, Longnecker AS, Marchetto NM, Juliano SA, Bowden RM. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc R Soc Lond B Biol Sci. 2012;279:1447–56. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park MK, Hoang TA, Belluzzi JD, Leslie FM. Gender specific effect of neonatal handling on stress reactivity of adolescent rats. J Neuroendocrinol. 2003;15:289–95. doi: 10.1046/j.1365-2826.2003.01010.x. [DOI] [PubMed] [Google Scholar]
- 47.Hayward LS, Richardson JB, Grogan MN, Wingfield JC. Sex differences in the organizational effects of corticosterone in egg yolk of quail. Comp Endocrinol. 2006;146:144–8. doi: 10.1016/j.ygcen.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Love OP, Williams TD. Plasticity in the adrenocortical response of a free-living vertebrate: the role of pre- and post-natal developmental stress. Horm Behav. 2008;54:496–505. doi: 10.1016/j.yhbeh.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Flores AM, Shrimpton JM. Differential physiological and endocrine responses of rainbow trout, Oncorhynchus mykiss, transferred from fresh water to ion-poor or salt water. Gen Comp Endocrinol. 2012;175:244–50. doi: 10.1016/j.ygcen.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Hori TS, Rise ML, Johnson SC, Afonso LOB, Gamperl AK. The mRNA expression of cortisol axis related genes differs in Atlantic cod (Gadus morhua) categorized as high or low responders. Gen Comp Endocrinol. 2012;175:311–20. doi: 10.1016/j.ygcen.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 51.Kubokawa K, Watanabe T, Toshioka M, Iwata M. Effects of acute stress on plasma cortisol, sex steroid hormone and glucose levels in male and female sockeye salmon during the breeding season. Aquaculture. 1999;172(3–4):335–49. [Google Scholar]
- 52.Archard GA, Early RL, Hanninen AF, Braithwaite VA. Correlated behaviour and stress physiology in fish exposed to different levels of predation pressure. Funct Ecol. 2012;26(3):637–45. [Google Scholar]
- 53.Vainikka A, Kortet R, Taskinen J. Epizootic cutaneous papillomatosis, cortisol and male ornamentation during and after breeding in the roach Rutilus rutilus. Dis Aquat Organ. 2004;60:189–95. doi: 10.3354/dao060189. [DOI] [PubMed] [Google Scholar]