Abstract
Intracerebral hemorrhage (ICH, or macrobleeds) and cerebral microbleeds—smaller foci of hemosiderin deposits commonly detected by magnetic resonance imaging (MRI) of older adults with or without ICH—are both associated with an increased risk of future ICH. These hemorrhagic pathologies also share risk factors with ischemic thromboembolic conditions that may require antithrombotic therapy, requiring specialists in cardiology, internal medicine and neurology to weigh the benefits versus hemorrhagic risks of antithrombotics in individual patients. This paper will review recent advances in our understanding of hemorrhage prone cerebrovascular pathologies with a particular emphasis on use of these markers in decision making for antithrombotic use.
Keywords: intracerebral hemorrhage, cerebral, microbleed, macrobleed, anticoagulation, antithrombotic therapy, stroke prevention, leukoaraiosis, sulcal siderosis
INTRODUCTION
Intracranial hemorrhages are classified based on the primarily affected intracranial compartment and they include intraparenchymal (IPH), intraventricular (IVH), subarachnoid (SAH), subdural and epidural hemorrhages. Subdural and epidural hemorrhages are most commonly related to head trauma whereas SAH generally arise from ruptured cerebral aneurysms. This review will primarily focus on spontaneous intracerebral hemorrhage (ICH), a common type of stroke including IPH and IVH, that occurs in the absence of gross vascular pathology or trauma. ICH makes up 8-18% of all strokes based on published registries.1,2 Bleeding within the brain parenchyma is classified as a macrobleed if it is greater than 5-10 mm in largest diameter as seen on head CT or MRI [Figure 1A].3 IPH and IVH are usually symptomatic with the acute onset of headache, altered consciousness and focal neurologic deficits. Most recent population based estimates suggest an overall ICH incidence of 24.6 per 100,000 person-years.4 Intracerebral hemorrhage is a devastating condition, as it carries a one-month case fatality rate of 40%4 one-year fatality of more than 50%.2 With only 20% of patients independent at six months,2 ICH creates a heavy financial burden as well. Recent studies show that initial hospital costs for ICH average $28,360 with another $16,035 first year post-discharge costs.5 With such devastating effects, it is important to monitor and manage the modifiable risk factors such as hypertension and lifestyle choices of smoking, cocaine, and excessive use of alcohol. Small vessel diseases related to cerebral amyloid angiopathy (CAA) and hypertension (HTN) are the most common etiologies of non-traumatic ICH, and other manifestations of these pathologies such as leukoaraiosis and sulcal siderosis should be included in a patient’s risk profile.
Figure 1.
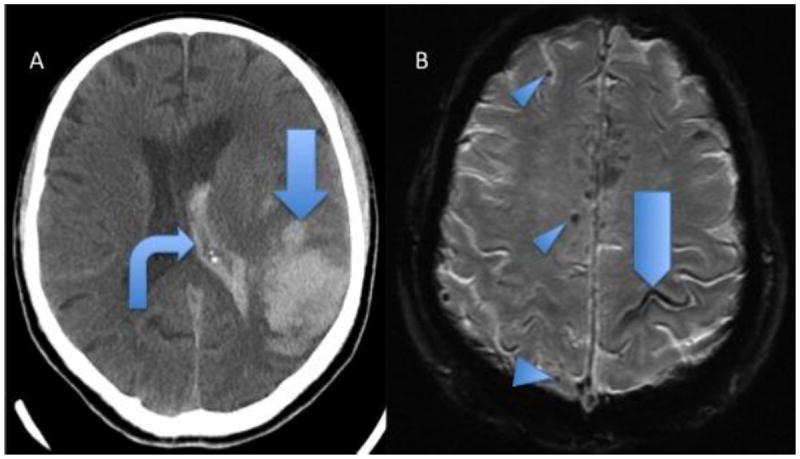
Hemorrhagic findings of 2 patients with pathologically proven cerebral amyloid angiopathy. A, Head CT showing acute lobar parenchymal macrobleed (arrow) and intraventricular hemorrhage (bent arrow). B, GRE MRI shows lobar microbleeds (arrowheads) and sulcal siderosis (pentagon).
Antithrombotic therapies commonly used in cardiovascular risk management increase the risk of ICH.6 While their benefit is substantial, the incidence of anticoagulant-associated ICH has quintupled with warfarin use for non-valvular atrial fibrillation7, 8 and mortality is increased due to a higher rate of hematoma expansion.9 Magnetic resonance imaging (MRI) evidence of cerebral microbleeds [Figure 1B], small (≤ 5-10 mm diameter) hemosiderin deposits detected on T2*-weighted gradient-recalled echo (GRE) sequences, are associated with cerebral small vessel disease.3,10 and an increased risk of anticoagulant-related ICH,11-14 This article will address the available data on imaging and clinical markers of increased ICH risk to guide clinicians on antithrombotic therapy recommendations. We will first define the major etiological categories of ICH and review the evidence on use of antithrombotics in patients who had macrobleeds caused by specific pathologies. We will then review evidence on microbleeds and other imaging markers of increased hemorrhagic risk with a focus on their usefulness in patients without macrobleeds.
CAUSES OF INTRACEREBRAL HEMORRHAGE
Understanding the cause of ICH guides treatment of potentially correctable lesions and helps stratify the risk of recurrent bleeding as they have intrinsically different risks of recurrent hemorrhage. The primary imaging modalities are CT, typically used to diagnose acute ICH [Figure 1A] and MRI to evaluate for underlying vascular malformations or tumor or support a diagnosis of CAA [Figure 1B]. Vascular imaging with MR angiography (MRA) and CT angiography (CTA) are often used and cerebral angiography is recommended patients under 50 years of age, after IVH, when MRI is suspicious for an underlying pathology or does not support a diagnosis of CAA.2 Repeat brain imaging may be performed after several months to reevaluate for any pathology that may be obscured by the initial bleed or edema. When appropriate imaging does not identify a gross structural or vascular pathology in older adults, the ICH is most commonly attributed hypertension or cerebral amyloid angiopathy.
CEREBRAL AMYLOID ANGIOPATHY-RELATED ICH
Cerebral amyloid angiopathy is commonly found in the elderly and affects the small vessels by the accumulation of amyloid beta-peptides in cortical and leptomeningeal vessel walls.15 The accumulation causes weakening of vessel walls resulting in hemorrhage or infarction.16-18 and is also an important cause of cognitive impairment through ischemic and hemorrhagic mechanisms.19,20 Cerebral microbleeds (CMB) are commonly found in lobar white and gray matter in patients with CAA. Longitudinal studies show that the risk of recurrent ICH in patients with CAA is increased with higher number of microbleeds making CMB an important risk predictor in this context.21 A definite diagnosis of CAA can only be made upon postmortem examination, but guidelines for diagnosis of CAA in the living are established in the modified Boston Criteria. ch define Probable CAA is defined as the presence of multiple hemorrhages restricted to the cortical or cortico-subcortical regions or a single lobar cortical or cortico-subcortical hemorrhage with either lobar microbleeds and/or disseminated superficial siderosis as detected by CT or MRI in patients ≥ 55 years.22-24 Lobar microbleeds detected by T2* GRE MRI thus both contribute to the diagnosis of CAA and predict the occurrence of future ICHs. While lobar hemorrhages might be associated with better long-term functional outcome compared to hemorrhages in the basal ganglia or thalamus25 the risk of lobar ICH recurrence is significantly higher in CAA patients.11,13 A study of fifty-nine patients with warfarin-related lobar hemorrhages showed that CAA was an important contributor to anticoagulant-related hemorrhage, even in context of a well-controlled international normalized ratio.26 There currently is no direct treatment for CAA but adequate control of hypertension is likely to provide protection against all types of ICH including CAA.27
HYPERTENSIVE INTRACEREBRAL HEMORRHAGE
Hypertensive small vessel disease is the most frequent cause of ICH.28, 29 resulting in hemorrhages in deep brain regions, such as the thalamus or basal ganglia.14 Hypertension is also a major risk factor for ischemic events, and both prior ischemic events and markers of ischemic injury such as white matter hyperintensities, or leukoaraiosis, increase the risk of ICH. 30, 31 The severity of leukoaraiosis correlates with both CAA and hypertensive microvasculopathy and is recognized as a strong risk factor for warfarin-related ICH in both lobar and deep brain locations.18,32
Antithrombotic Use in ICH Survivors
CAA-Related ICH
The risk of recurrent ICH in patients with past CAA-related ICH who are not taking antithrombotic therapies is approximately 10%; antithrombotic therapy increases the risk of and severity and poor outcome of the ICH.14,36 A decision analysis based on a 69-year-old man with a history of ICH and newly diagnosed nonvalvular atrial fibrillation suggested survivors of lobar ICH should not be anticoagulated with warfarin.14 When case scenarios with different thromboembolic and hemorrhagic risk were reviewed, the superiority of “do not anticoagulate” for lobar ICH survivors remained constant for nonvalvular atrial fibrillation. Although the recently approved newer anticoagulants (dabigatran, rivaroxaban, apixaban) have all shown lower ICH risk when compared to warfarin in phase III trials, 37-39 it remains unclear whether they will tip the risk-to-benefit balance in favor of their use.
Surgical or catheter-based alternatives to long-term anticoagulation in patients with non-valvular atrial fibrillation could be preferred for patients at high risk for ICH.41,42, 40
Antiplatelet agents were not associated with an increased risk of ICH recurrence in 27 patients of 127 survivors of lobar ICH (hazard ratio [HR] 0.8, 95% CI 0.3 to 2.3, p=0.73).43 However, an increased risk of ICH has been seen with aspirin use in patients with CAA-related lobar ICH and higher numbers of cerebral lobar.36 Aspirin and other antiplatelet use should be considered cautiously and avoided in those at low risk of thromboembolic events, such as in primary prevention.14,43
Hypertensive ICH
Antiplatelet treatment was not associated with higher risk of ICH recurrence in survivors of deep hypertensive ICH (HR 1.2, 95% CI 0.1 to 14.3, p=0.88) in an observational follow up study.43 Anticoagulation after hypertensive ICH might carry a lower hemorrhagic risk than after lobar hemorrhage, but currently should not be recommended unless the risk of an ischemic stroke is very high. Since aspirin carries a smaller risk of bleeding than warfarin, it may be an option for patients with an intermediate risk of ischemia.14 Similar to the case of lobar ICH, the role of the newer anticoagulants and mechanical approaches in prevention of cardioembolism in patients with non-valvular atrial fibrillation remain to be determined in survivors of deep hypertensive ICH. Other than avoidance of antithrombotics whenever possible, the best prevention of hypertensive ICH is control of hypertension and reduction of other modifiable risk factors, such as moderate-heavy alcohol consumption.27,44
Arteriovenous Malformation and Aneurysm-Related ICH
Recent reviews have not indicated increased risks of bleeding after successful treatment of vascular malformations,45 but there are no data testing the safety of antithrombotic medications in these patients and it is possible for aneurysms or AVMs to recur following treatment. In the absence of good data, a consultation with a vascular neurologist/ surgeon/ interventionalist is appropriate to participate in decision-making and longitudinal follow up.
Assessing Risks of First-Time ICH
With the development of higher-quality imaging techniques and rapid increase in advanced imaging-based research over the past decades, new radiologic markers have been emerged with the potential to identify people at high risk of first ICH. Among them, cerebral microbleeds have become relatively common incidental findings on MRI scans obtained for other indications.
Clinical Factors
The small vessel pathologies that result in ICH often manifest with their ischemic consequences as cognitive and gait impairments in older adults. There is an overlap between Vascular dementia and Alzheimer’s disease,46 but Alzheimer’s disease itself is not a strong risk factor for cerebral hemorrhage and should not be viewed as a contraindication for antithrombotic therapy In vascular dementia, the imaging features discussed below can help make a determination of the prevailing pathology and estimating the hemorrhage risk.
Neuroimaging Findings
In the 1980s, MRI findings of white matter changes, or leukoaraiosis, were commonly identified in older adults and were variously interpreted as Binswanger’s disease or as being “non-specific” or “incidental”. Leukoaraiosis has proved to be a strong marker of the severity of underlying small vessel disease whereas microbleeds and sulcal siderosis are markers of bleeding prone microvasculopathy.
Cerebral Microbleeds
CMBs [Figure 1B] in the absence of ICH are highly prevalent in the elderly, observed in approximately 15.3% of community-dwelling adults age 45 and older and 35.7% for people 80 years and older.12 The location of CMBs appears to reflect the type of microvasculopathy; deep CMBs are associated with hypertensive vascular pathology and strictly lobar CMBs with CAA-related small vessel disease.48 Microbleeds may be indicative of more bleeding-prone areas since the presence of CMB are also associated with ICH49 and higher risk of mortality for patients with more than 5 CMB.11 In a cross-sectional matched cohort analysis, the presence of microbleeds was more commonly found in patients with warfarin-related ICH when compared to anticoagulated patients without ICH.50 Cerebral microbleeds are also markers of progression in cerebral amyloid angiopathy, as CAA patients with higher numbers of microbleeds at baseline experience higher rates of future hemorrhages.3,21 The strong association of CMB with age, CAA, and warfarin-related ICH make them important features for hemorrhagic risk assessment in older patients.
It remains unclear whether the presence of CMBs without a history of ICH confers sufficient risk of future ICH to tip clinical decisions towards or away from antithrombotic treatment. A Markov decision model suggested, for example, that a risk factor would need to increase the risk of ICH approximately 13-fold to balance the known benefits of warfarin for preventing atrial fibrillation-related stroke. To date, only one study has compared incident risk of symptomatic ICH in patients who presented with lobar microbleeds without ICH to patients who presented with CAA-related lobar ICH.51 Cox regression analysis showed no statistically significant difference in incident ICH despite a trend for lower risk in microbleed-only (4.9 per 100 person years) versus the lobar ICH cohorts (8.3 per 100 person-years, p>0.05). Patients with lobar microbleeds had a risk of ICH that was not trivial. The use of warfarin in a small number of patients in the microbleed only group was a significant predictor of occurrence of ICH (p<0.05) whereas the use of aspirin was not associated with increased risk (p>0.5). 51 Further studies are needed to help establish the relationship between the presence of microbleeds and risk of incident ICH and how such risk is modulated by antithrombotic use, especially in community dwelling older people without history of ICH or symptoms.
Cerebral microbleeds have also been implicated as lesions contributing to neurologic dysfunction, as they could directly affect surrounding tissue causing functional or cognitive disabilities. One community based study showed that patients with numerous CMBs performed worse on neuropsychological tests than those without microbleeds.52 The significance and implications of CMB are important areas of ongoing research efforts.
White Matter Disease (Leukoaraiosis)
White matter disease is common in CAA as well as hemorrhage-prone hypertensive small vessel disease and currently there is no way to distinguish the underlying pathology from imaging characteristics (55,56). A cross sectional study showed that patients with white matter disease taking anticoagulants were at a higher risk for ICH, which increased with severity of leukoaraiosis, even when INR was within target therapeutic limits.32 Studies indicate that higher white matter disease burden, even without anticoagulants, predicts risk of lobar hemorrhage possibly due to CAA.53,54 One study found that white matter disease severity correlated with cognitive impairment before occurrence of first lobar intracerebral hemorrhage.53 Leukoaraiosis patterns have been associated with specific risk factors, such as gender, hypertension and smoking, but further data are needed to determine patterns associated with different neuropathologies.57 At the present time, mere presence or severity of leukoaraiosis should not be seen as an absolute contraindication for antithrombotic use but the adverse implications of both potential underlying pathologies (CAA and hypertensive small vessel disease) and the commonly co-existing clinical problems such as cognitive impairment and fall risk should all be considered before recommending antiplatelet or anticoagulant medications.
Sulcal (Superficial) Siderosis
Sulcal siderosis [Figure 1B] is caused by focal subarachnoid hemorrhages and may also signal risk for future ICH.58,59 Case studies have reported the presence of sulcal siderosis concurrent with CAA pathology.60 A retrospective analysis of T2*-weighted MRIs of patients with histopathologically diagnosed CAA found that sulcal siderosis was present in 23 of 38 CAA cases but none of 22 control patients with histopathologically proven non-CAA ICH.24 This finding led to the development of the modified Boston Criteria, which now considers presence of focal or disseminated superficial siderosis as a criterion similar to presence of lobar microbleeds.24 MRI imaging that includes GRE or SWI sequences are optimal to identify sulcal siderosis, micro- and macrobleeds but the meaning and impact of these markers are under investigation.
Future Directions: Genetics and PET Imaging
The Apolipoprotein E (APOE) presents a genetic polymorphism that is relatively common in CAA, determined by three alleles APOE ε2, APOE ε3, APOE ε4. Patients carrying APOE ε2 and APOE ε4 alleles are at greater risk of CAA-related ICH than those with only the common APOE ε3.61-64 If these markers were shown to predict ICH risk, then genetic screening could be a useful tool for guiding antithrombotic therapy for patients at risk for vascular events. 65
Another promising area of imaging research is in the use of noninvasive amyloid imaging with PET agents such as Pittsburg compound B (PiB)66 to detect the early stages of cerebral amyloid angiopathy.67 Recent studies have shown that CAA-related hemorrhages occur at sites of high baseline amyloid deposition that can be detected using PiB-PET imaging.68 Brain amyloid imaging is still an area of active research, but could be a very promising tool for assessing hemorrhage risk associated with CAA.
CONCLUSION
Cerebral macrobleeds and microbleeds originate from different small vessel pathologies that have distinct prognostic significance and management strategies. These lesions, in context with other clinical, imaging and laboratory findings, are helpful in predicting the risk of future intracerebral hemorrhage and guiding antithrombotic therapy recommendations. The current data do not support routine neuroimaging before starting antithrombotics in patients who are otherwise at low risk of ICH, but more research is needed to incorporate this data into a thorough hemorrhagic risk. Warfarin should not be used for non-valvular atrial fibrillation in survivors of CAA-related lobar ICH.
Acknowledgments
Dr. Greenberg and Dr. Gurol have research grant funding from National Institute of Health (NIH 5RO1NS070834-03, 5R01AG026484, NIH 5 P50NS051343-08).
Footnotes
Conflict of Interest
Kellen E. Haley, Steven M. Greenberg, and M. Edip Gurol declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Kellen E. Haley, Email: kellen.haley@gmail.com.
Steven M. Greenberg, Email: sgreenberg@partners.org.
M. Edip Gurol, Email: edip@mail.harvard.edu.
References
- 1.Massaro AR, Sacco RL, Mohr JP, et al. Clinical discriminators of lobar and deep hemorrhages: the Stroke Data Bank. Neurology. 1991 Dec;41(12):1881–1885. doi: 10.1212/wnl.41.12.1881. [DOI] [PubMed] [Google Scholar]
- 2.Gurol ME, Greenberg SM. Management of intracerebral hemorrhage. Curr Atheroscler Rep. 2008 Aug;10(4):324–331. doi: 10.1007/s11883-008-0050-y. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg SM, Nandigam RN, Delgado P, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009 Jul;40(7):2382–2386. doi: 10.1161/STROKEAHA.109.548974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.van Asch CJJ, Luitse MJA, Rinkel GE, van der Tweel I, Algra A, Klijn CJM. Incidence, case fatality, and functional outcome of intracerebral haemorrhage overtime, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurology. 2010 Feb;9(2):167–176. doi: 10.1016/S1474-4422(09)70340-0. Recent good quality systematic review of ICH epidemiology and prognosis. [DOI] [PubMed] [Google Scholar]
- 5.Russell MW, Boulanger L, Joshi AV, Neumann PJ, Menzin J. The economic burden of intracerebral hemorrhage: evidence from managed care. Manag Care Interface. 2006 Jun;19(6):24–28. 34. [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007 Jun 19;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007 Jan;68(2):116–121. doi: 10.1212/01.wnl.0000250340.05202.8b. [DOI] [PubMed] [Google Scholar]
- 8.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr;164(8):880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 9.Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004 Sep;63(6):1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology. 1996 Jun;46(6):1751–1754. doi: 10.1212/wnl.46.6.1751. [DOI] [PubMed] [Google Scholar]
- 11.Soo YO, Yang SR, Lam WW, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008 Nov;255(11):1679–1686. doi: 10.1007/s00415-008-0967-7. [DOI] [PubMed] [Google Scholar]
- 12.Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010 Oct;41(10 Suppl):103–106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- 13.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010 Jun;41(6):1222–1228. doi: 10.1161/STROKEAHA.109.572594. [DOI] [PubMed] [Google Scholar]
- 14.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003 Jul;34(7):1710–1716. doi: 10.1161/01.STR.0000078311.18928.16. [DOI] [PubMed] [Google Scholar]
- 15.Gurol ME. Cerebral Amyloid Angiopathy. Tur J Neurol. 2009;15(1):1–9. [Google Scholar]
- 16.Smith EE, Vijayappa M, Lima F, et al. Impaired visual evoked flow velocity response in cerebral amyloid angiopathy. Neurology. 2008 Oct;71(18):1424–1430. doi: 10.1212/01.wnl.0000327887.64299.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumas A, Dierksen GA, Gurol ME, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012 Jul;72(1):76–81. doi: 10.1002/ana.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurol ME, Viswanathan A, Gidicsin C, et al. Cerebral amyloid angiopathy burden associated with leukoaraiosis: A positron emission tomography/magnetic resonance imaging study. Ann Neurol. 2013 Apr;73(4):529–536. doi: 10.1002/ana.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keage HA, Carare RO, Friedland RP, et al. Population studies of sporadic cerebral amyloid angiopathy and dementia: a systematic review. BMC neurology. 2009;9:3. doi: 10.1186/1471-2377-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011 Feb;69(2):320–327. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004 Jun;35(6):1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 22.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001 Feb 27;56(4):537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 23.Smith EE, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Curr Atheroscler Rep. 2003 Jul;5(4):260–266. doi: 10.1007/s11883-003-0048-4. [DOI] [PubMed] [Google Scholar]
- 24.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010 Apr;74(17):1346–1350. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellanos M, Leira R, Tejada J, Gil-Peralta A, Davalos A, Castillo J. Predictors of good outcome in medium to large spontaneous supratentorial intracerebral haemorrhages. Journal of neurology, neurosurgery, and psychiatry. 2005 May;76(5):691–695. doi: 10.1136/jnnp.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000 Oct;55(7):947–951. doi: 10.1212/wnl.55.7.947. [DOI] [PubMed] [Google Scholar]
- 27.Arima H, Tzourio C, Anderson C, et al. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke. 2010 Feb;41(2):394–396. doi: 10.1161/STROKEAHA.109.563932. [DOI] [PubMed] [Google Scholar]
- 28.Thrift AG, McNeil JJ, Forbes A, Donnan GA. Risk factors for cerebral hemorrhage in the era of well-controlled hypertension. Melbourne Risk Factor Study (MERFS) Group. Stroke. 1996 Nov;27(11):2020–2025. doi: 10.1161/01.str.27.11.2020. [DOI] [PubMed] [Google Scholar]
- 29.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013 Jan;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. The American journal of medicine. 1989 Aug;87(2):144–152. doi: 10.1016/s0002-9343(89)80689-8. [DOI] [PubMed] [Google Scholar]
- 31.Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Stroke Prevention In Reversible Ischemia Trial (SPIRIT). European Atrial Fibrillation Trial (EAFT) study groups. Neurology. 1999 Oct;53(6):1319–1327. doi: 10.1212/wnl.53.6.1319. [DOI] [PubMed] [Google Scholar]
- 32.Smith EE, Rosand J, Knudsen KA, Hylek EM, Greenberg SM. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology. 2002 Jul;59(2):193–197. doi: 10.1212/wnl.59.2.193. [DOI] [PubMed] [Google Scholar]
- 33.Wiebers DO, Whisnant JP, Huston J, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003 Jul;362(9378):103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 34.Gross BA, Du R. Natural history of cerebral arteriovenous malformations: a meta-analysis. J Neurosurg. 2013 Feb;118(2):437–443. doi: 10.3171/2012.10.JNS121280. [DOI] [PubMed] [Google Scholar]
- 35.Mohr JP. A randomized trial of unruptured brain arteriovenous malformations (ARUBA) Acta Neurochir Suppl. 2008;103:3–4. doi: 10.1007/978-3-211-76589-0_1. [DOI] [PubMed] [Google Scholar]
- 36.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010 Aug;75(8):693–698. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2009 Sep 17;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 38.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. The New England journal of medicine. 2011 Sep 8;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 39.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2011 Sep 15;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 40•.Lubitz SA, Bauer KA, Benjamin EJ, et al. Stroke Prevention in Atrial Fibrillation in Older Adults: Existing Knowledge Gaps and Areas for Innovation. A Summary of an American Federation of Aging Research Seminar. J Am Geriatr Soc. 2013 doi: 10.1111/jgs.12456. in press. Good overview of both established and emerging stroke prevention strategies in patients with atrial fibrillation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy VY, Mobius-Winkler S, Miller MA, et al. Left Atrial Appendage Closure with the Watchman Device in Patients with a Contraindication for Oral Anticoagulation: ASA Plavix Feasibility Study with Watchman Left Atrial Appendage Closure Technology (ASAP Study) Journal of the American College of Cardiology. 2013 Apr 10; doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 42.Massumi A, Chelu MG, Nazeri A, et al. Initial experience with a novel percutaneous left atrial appendage exclusion device in patients with atrial fibrillation, increased stroke risk, and contraindications to anticoagulation. The American journal of cardiology. 2013 Mar 15;111(6):869–873. doi: 10.1016/j.amjcard.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 43.Viswanathan A, Rakich SM, Engel C, et al. Antiplatelet use after intracerebral hemorrhage. Neurology. 2006 Jan 24;66(2):206–209. doi: 10.1212/01.wnl.0000194267.09060.77. [DOI] [PubMed] [Google Scholar]
- 44.Juvela S, Hillbom M, Palomäki H. Risk factors for spontaneous intracerebral hemorrhage. Stroke. 1995 Sep;26(9):1558–1564. doi: 10.1161/01.str.26.9.1558. [DOI] [PubMed] [Google Scholar]
- 45.Zacharia BE, Vaughan KA, Jacoby A, Hickman ZL, Bodmer D, Connolly ES. Management of ruptured brain arteriovenous malformations. Curr Atheroscler Rep. 2012 Aug;14(4):335–342. doi: 10.1007/s11883-012-0257-9. [DOI] [PubMed] [Google Scholar]
- 46.Jellinger KA, Mitter-Ferstl E. The impact of cerebrovascular lesions in Alzheimer disease--a comparative autopsy study. J Neurol. 2003 Sep;250(9):1050–1055. doi: 10.1007/s00415-003-0142-0. [DOI] [PubMed] [Google Scholar]
- 47.Chi NF, Chien LN, Ku HL, Hu CJ, Chiou HY. Alzheimer disease and risk of stroke: a population-based cohort study. Neurology. 2013 Feb;80(8):705–711. doi: 10.1212/WNL.0b013e31828250af. [DOI] [PubMed] [Google Scholar]
- 48.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009 Feb;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SH, Bae HJ, Kwon SJ, et al. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology. 2004 Jan;62(1):72–76. doi: 10.1212/01.wnl.0000101463.50798.0d. [DOI] [PubMed] [Google Scholar]
- 50.Lee SH, Ryu WS, Roh JK. Cerebral microbleeds are a risk factor for warfarinrelated intracerebral hemorrhage. Neurology. 2009 Jan;72(2):171–176. doi: 10.1212/01.wnl.0000339060.11702.dd. [DOI] [PubMed] [Google Scholar]
- 51.van Etten ES, Auriel E, Haley KE, et al. Warfarin Increases Risk of Future Intracerebral Hemorrhage in Patients Presenting with Isolated Lobar Microbleeds on MRI. Stroke; International Stroke Conference; Honolulu: LWW; 2013. [Google Scholar]
- 52.Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012 Jan 31;78(5):326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 53.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004 Nov 9;63(9):1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 54.Chen YW, Gurol ME, Rosand J, et al. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006 Jul;67(1):83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith EE, Nandigam KR, Chen YW, et al. MRI markers of small vessel disease in lobar and deep hemispheric intracerebral hemorrhage. Stroke. 2010 Sep;41(9):1933–1938. doi: 10.1161/STROKEAHA.110.579078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holland CM, Smith EE, Csapo I, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008 Apr;39(4):1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rostrup E, Gouw AA, Vrenken H, et al. The spatial distribution of age-related white matter changes as a function of vascular risk factors--results from the LADIS study. Neuroimage. 2012 Apr;60(3):1597–1607. doi: 10.1016/j.neuroimage.2012.01.106. [DOI] [PubMed] [Google Scholar]
- 58.Linn J, Wollenweber FA, Lummel N, et al. Superficial siderosis is a warning sign for future intracranial hemorrhage. J Neurol. 2013 Jan;260(1):176–181. doi: 10.1007/s00415-012-6610-7. [DOI] [PubMed] [Google Scholar]
- 59••.Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke. 2012 Sep;43(9):2324–2330. doi: 10.1161/STROKEAHA.112.657759. A nice review of transient focal deficits in CAA patients including their correlations with imaging findings and prognostic significance. [DOI] [PubMed] [Google Scholar]
- 60.Barreto RD, Ruano L, Cruz VT, Veira C, Coutinho P. Superficial siderosis and anticoagulation therapy: different presentations, different outcomes. Case Rep Neurol Med. 2012;2012:745430. doi: 10.1155/2012/745430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greenberg SM, Briggs ME, Hyman BT, et al. Apolipoprotein E epsilon 4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Stroke. 1996 Aug;27(8):1333–1337. doi: 10.1161/01.str.27.8.1333. [DOI] [PubMed] [Google Scholar]
- 62.Greenberg SM, Vonsattel JP, Segal AZ, et al. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology. 1998 Apr;50(4):961–965. doi: 10.1212/wnl.50.4.961. [DOI] [PubMed] [Google Scholar]
- 63.O’Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. The New England journal of medicine. 2000 Jan 27;342(4):240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- 64.Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010 Dec;68(6):934–943. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eckman MH, Wong LK, Soo YO, et al. Patient-specific decision-making for warfarin therapy in nonvalvular atrial fibrillation: how will screening with genetics and imaging help? Stroke. 2008 Dec;39(12):3308–3315. doi: 10.1161/STROKEAHA.108.523159. [DOI] [PubMed] [Google Scholar]
- 66.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007 Sep;62(3):229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 67.Greenberg SM, Grabowski T, Gurol ME, et al. Detection of isolated cerebrovascular beta-amyloid with Pittsburgh compound B. Ann Neurol. 2008 Nov;64(5):587–591. doi: 10.1002/ana.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68••.Gurol ME, Dierksen G, Betensky R, et al. Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology. 2012 Jul;79(4):320–326. doi: 10.1212/WNL.0b013e31826043a9. Longitudinal study that suggests a potential role of amyloid imaging to predict risk of future ICH in CAA patients. [DOI] [PMC free article] [PubMed] [Google Scholar]


