Abstract
How predators and prey interact has important consequences for population dynamics and community stability. Here we explored how predator-prey interactions are simultaneously affected by reciprocal behavioral plasticity (i.e., plasticity in prey defenses countered by plasticity in predator offenses and vice versa) and consistent individual behavioral variation (i.e., behavioral types) within both predator and prey populations. We assessed the behavior of a predator species (northern pike) and a prey species (three-spined stickleback) during one-on-one encounters. We also measured additional behavioral and morphological traits in each species. Using structural equation modeling, we found that reciprocal behavioral plasticity as well as predator and prey behavioral types influenced how individuals behaved during an interaction. Thus, the progression and ultimate outcome of predator-prey interactions depend on both the dynamic behavioral feedback occurring during the encounter and the underlying behavioral type of each participant. We also examined whether predator behavioral type is underlain by differences in metabolism and organ size. We provide some of the first evidence that behavioral type is related to resting metabolic rate and size of a sensory organ (the eyes). Understanding the extent to which reciprocal behavioral plasticity and intraspecific behavioral variation influence the outcome of species interactions could provide insight into the maintenance of behavioral variation as well as community dynamics.
Keywords: antipredator behavior, metabolic rate, northern pike, pace-of-life syndrome, structural equation modeling, three-spined stickleback
Introduction
Species interactions can cause plastic changes in the behavior, morphology, and/or physiology of interacting individuals that have both ecological and evolutionary consequences (Agrawal 2001; Miner et al. 2005; Fordyce 2006; Berg and Ellers 2010). For example, exposure to predators often triggers inducible defenses in prey (e.g., Kishida et al. 2010). These inducible defenses, be they morphological or behavioral, can have strong effects on the entire community (Werner and Peacor 2003; Schmitz et al. 2004; Miner et al. 2005; Preisser et al. 2005; Fordyce 2006). While predator-prey interactions and resulting phenotypic plasticity are often studied from the prey perspective (Lima 2002), prey defenses induced by predator exposure are often matched by plastic changes in predator offenses (Sih 1984; Kopp and Tollrian 2003; Kishida et al. 2010). This reciprocal phenotypic plasticity can result in a “back-and-forth response” on an ecological timescale, whereby phenotypic changes in species A change the environment experienced by species B, resulting in a phenotypic change in species B, and vice versa (Agrawal 2001; Alonzo 2002). With reversible behavioral plasticity, this back-and-forth can occur during a single interaction between individuals (i.e., reciprocal behavioral plasticity). An increasing number of models suggest that reciprocal phenotypic plasticity can alter the outcome of species interactions, with potential consequences for population dynamics and community stability (Mitchell 2009; Mougi and Kishida 2009; Cortez 2011; Flaxman et al. 2011; Mougi 2012).
There is also growing evidence that intraspecific trait variation among individuals within populations can substantially affect species interactions (Bolnick et al. 2003, 2011; Schreiber et al. 2011; Griffen et al. 2012; Pruitt et al. 2012a, 2012b; Sih et al. 2012; Wolf and Weissing 2012). For example, within a population, prey often differ consistently from one another in their tendency to take risks in the presence and absence of predators (Sih et al. 2003; Smith and Blumstein 2010; Griffen et al. 2012), which could affect their likelihood of being attacked. Similarly, within a population, individual predators often vary consistently in their foraging behavior and diet (i.e., specialization vs. generalization; Bolnick et al. 2003; Estes et al. 2003; Smith and Blumstein 2010; Nyqvist et al. 2012). The coexistence of a range of consistent behavioral types within prey (or predator) populations (Sih et al. 2004a, 2004c) creates a situation where individual prey (or predators) are not equivalent to one another in terms of predation risk (or threat). Furthermore, the combination of predator behavioral types and prey behavioral types can dramatically impact predator-prey interactions (Sih et al. 2012; Wolf and Weissing 2012). For example, Pruitt et al. (2012b) found that active sea star predators (Pisaster ochraceus) disproportionally consumed sedentary snail prey, and inactive sea star predators tended to capture more active snail prey (Chlorostoma funebralis). While this work suggests that species interactions can maintain behavioral variation, a number of outstanding questions remain. For example, what behavioral mechanisms underlie the different outcomes of predator behavioral type–prey behavioral type interactions? Can behavioral types influence the outcome of predator-prey interactions when there is reciprocal behavioral plasticity between predators and prey? Finally, what are the proximate causes of variation in behavioral types?
Variation among individuals in relatively unchanging traits such as metabolic rate or organ size has been suggested as a proximate cause generating differences among individuals in similarly stable behavioral traits (Biro and Stamps 2010; Reale et al. 2010; Wolf and McNamara 2012). Consistent individual differences in metabolic rate, for example, might promote consistent individual differences in behaviors that provide net energy such as foraging behavior (Biro and Stamps 2010; Houston 2010). Moreover, if a “big engine” is required to support a fast, active life-style, then we might expect positive correlations between energetically demanding behaviors, metabolic rate, and energetically expensive organs (Careau et al. 2008; Biro and Stamps 2010; Reale et al. 2010). Correlations between behaviors and these relatively nonplastic traits might help explain the maintenance of behavioral variation via life-history trade-offs (Biro and Stamps 2010). For example, active fast-growing individuals might have high fitness when environmental conditions are favorable and have low fitness when they are poor. These fitness trade-offs might maintain variation in metabolic rate and traits associated with it (Burton et al. 2011; formalized as the “pace-of-life syndrome” hypothesis [Reale et al. 2010]).
There is support for this pace-of-life-syndrome hypothesis in the literature, with studies reporting significant correlations at the intraspecific level between resting metabolic rate and growth (reviewed in Burton et al. 2011), between resting metabolic rate and the size of metabolically demanding organs such as the intestines, liver, kidneys, and heart (reviewed in Biro and Stamps 2010; Burton et al. 2011), and between resting metabolic rate and behavioral type (Huntingford et al. 2010; Barreto and Volpato 2011; Careau et al. 2011; reviewed in Careau et al. 2008; Biro and Stamps 2010; see also Careau et al. 2010). However, a number of studies have also reported either no relationship between resting metabolic rate and behavioral type (Farwell and McLaughlin 2009; Lantova et al. 2011; Timonin et al. 2011; Le Galliard et al. 2013) or context-dependent links between resting metabolic rate and behavioral type (Killen et al. 2011, 2012; Reid et al. 2012). Moreover, while there is growing interest in whether metabolism might underlie behavioral type, it is quite possible that behavioral type is underlain by other traits that contribute to an animal’s physiological architecture (e.g., organ sizes) and possibly constrain metabolism. In addition, variation among individuals in their sensory system or abilities might generate variation in perception and decision making as well as variation in performance traits such as foraging accuracy and reaction time (see also Ronald et al. 2012). Indeed, measuring these architecture traits might be preferable to measuring metabolism because estimates of metabolic rates are influenced by how individuals respond to being measured (e.g., freezing vs. hyperactivity; discussed in Careau et al. 2008). Regardless of the physiological and sensory traits measured, exploring their relationship with behavior will provide insight into whether these relatively nonplastic traits might impose a constraint on certain behaviors.
Therefore, we address three questions: (1) Does reciprocal behavioral plasticity between predators and prey affect the outcome of encounters? (2) Are the behaviors of predators and prey during an encounter influenced by their own respective behavioral types? (3) Is variation in predator behavior associated with differences in metabolic rate and organ size? We used northern pike (Esox lucius) as the predator species and three-spined stickleback (Gasterosteus aculeatus) as the prey species—two species that interact in nature. We used structural equation modeling (SEM) to examine how the outcome of predator-prey interactions is influenced by the reciprocal behavioral plasticity that occurs during an encounter as well as the intraspecific behavioral variation present in both predators and prey. This statistical approach considers multivariate relationships and is thus particularly well suited for examining the behavioral back-and-forth aspect of predator-prey interactions (i.e., reciprocal behavioral plasticity) as well as the direct and indirect influences of correlated traits (Grace 2006, 2008). We used correlation analyses to examine whether variation in predator behavior is related to underlying differences in stable states such as metabolic rate and organ size.
Methods
Predators
The northern pike (Esox lucius) we used as predators were hatchery reared (Spirit Lake Fish Hatchery, Spirit Lake, IA) and transported to the University of Illinois by car 5 weeks prior to the experiment. These pike were accustomed to eating only live prey. They were housed singly in 83.3-L tanks (107×33×24 cm) on a separate water flow system and were visually separated from the stickleback in the lab. They were maintained at 18°C on a photoperiod that matched seasonal changes. We used 12 juvenile pike that ranged in size from 18.2 to 22.3 cm in length. Water was cleaned in all tanks via a recirculating flow-through system with particulate, biological, and UV filters (Aquaneering, San Diego, CA). Approximately 10% of the water volume in the tanks was replaced each day. Pike tanks had a gravel bottom, two artificial plants, two pieces of PVC pipe on the bottom, and two PVC stand-pipes on the back wall for prey refuge (PVC pipes = 2 cm diameter).
Prey
The three-spined stickleback (Gasterosteus aculeatus) we used as prey were lab-reared juvenile F1 descendants of a wild population (Putah Creek, CA). Eggs were artificially fertilized. Offspring were housed in full-sibling groups of 10 juveniles per 9-L rearing tank. We fed juveniles a slurry of frozen adult Artemia, mysis shrimp, bloodworms, and Cyclop-eeze decapods (Argent, Redmond, WA) once a day. All tanks had a recirculating flow-through system and were maintained at 18°C on a photoperiod that matched seasonal changes.
These juveniles were part of a larger study on maternal effects whose field-collected mothers had received either a predator exposure or control treatment while producing eggs. Details of the maternal treatment protocol and the results in terms of the maternal effect on stickleback behavior and survival are discussed elsewhere (McGhee et al. 2012). Stickleback maternal treatment and family were randomized with respect to both pike identity and day of testing, thus each pike preyed on stickleback of both maternal treatments. Here we focus on offspring from the control treatment whose mothers were not exposed to a predator (N = 9 control mothers; number of offspring per maternal family = 8.6 ± 0.9, mean ± SE). All pike interacted with 12–14 stickleback over the course of the maternal effects experiment (McGhee et al. 2012). Because stickleback maternal treatment was random with respect to pike identity, individual pike interacted with a variable number of stickleback offspring from the maternal control treatment (N = 3–8 interactions). These offspring were behaviorally tested when they were approximately 6 months old and 3 cm in length (30.7 ± 0.4 mm).
Quantifying Individual Variation in Prey Behavior in the Absence of a Predator (“Prey-Alone Assay”)
The day before behavioral testing, a randomly selected stickleback was netted from a rearing tank, measured for standard length, and isolated in an observation tank (37.8 L) overnight. Rearing tanks were tested in a random order, and no more than one fish from a single rearing tank was tested per day. The observation tank had a gravel bottom, opaque plastic covering its sides, and a single plastic plant on the right side of the tank. The tank front was covered with opaque plastic until the behavioral assays were run to minimize disturbance.
The next day between 9:30 and 11:30 a.m., behavioral assays were performed on the stickleback. Individuals were tested singly. Freezing, where individuals stop moving and hold still, is an important antipredator behavior. In staged predator-prey interactions, stickleback who spent more than 75% of their time frozen survived nearly 3 times longer than those who spent less than 25% of their time frozen (McGhee et al. 2012). First, to examine how stickleback reacted to a novel object, we added a glass flask filled with water to the left side of the tank (opposite the plant) and for 3 min recorded the time the stickleback spent frozen (holding still for 12 s). Second, to examine how individuals responded to a shoal of unfamiliar conspecifics, we added four conspecific juveniles to the water-filled flask and for 3 min recorded the time the stickleback spent frozen. Finally, we measured freezing behavior of each individual stickleback in the presence of a live pike (described below). Tanks were videotaped from behind a blind. Videotapes were later scored using JWatcher (Blumstein and Daniel 2007).
Quantifying Predator and Prey Behavior during an Interaction (“Predator-Prey-Interaction Assay”)
Two hours after completing the prey-alone assay, the individual stickleback was gently herded into a cup of water and then immediately released into a randomly chosen individual pike tank where the predator-prey-interaction assays were conducted. The rationale for the predator-prey-interaction assay was to quantify differences in individual behavior of both predators and prey during a direct encounter.
To reduce the chances that the pike would capture the stickleback immediately on release, we used two identical feeding cups: a decoy cup containing only water and a cup containing water and one stickleback. The cups were on opposite sides of the tank, and the one containing the stickleback was positioned so that it was furthest from the pike. The cups were partially submerged in the water, and their contents were simultaneously poured out gently into the tank. The cups were removed and data recording began immediately. Data were recorded live using JWatcher, and trials lasted until the stickleback was captured or a maximum of 10 min. Pike were tested once per day in a random order. On days when pike were not used in the experiment, they were fed in an identical way (two cups, one stickleback or one goldfish). Neither prey nor predators were fed prior to a trial on the day of testing (i.e., they were starved for 24 h).
During a trial, we simultaneously recorded the following stickleback and pike behaviors. For the stickleback, we recorded (1) the time spent frozen (holding still for >2 s) and (2) the time spent oriented toward the pike. Since stickleback survived for variable amounts of time, stickleback freezing and orienting behavior were converted to proportions (time spent frozen/oriented divided by capture time). For the pike, we recorded (1) latency to orient toward the stickleback for the first time and (2) latency to attack the stickleback for the first time. We also recorded the capture time (i.e., the time until the pike successfully caught the stickleback). Capture time was often longer than latency to attack because pike were not always successful in capturing the stickleback on the first attempt. Capture time does not include the handling time required for the pike to manipulate and swallow the stickleback. Note that capture time reflects a combination of prey vulnerability and predator foraging performance.
Measuring Predator Metabolic Rate and Organ Size
During the pike-stickleback interactions, we noticed that some pike appeared to be able to chase and attack the stickleback better than others, and others took longer to recover after an attack. Although the literature has emphasized the relationship between resting, rather than active, metabolic rate and behavioral types (Careau et al. 2008; Biro and Stamps 2010; Reale et al. 2010; but see Martins et al. 2011), we suspected that an important axis of variation for the pike might be differences in their ability to recover after exertion. Therefore, after completing all of the predator-prey-interaction assays, we measured pike breathing rates at rest and after exertion as proxies for resting and active metabolic rates, respectively, to test the hypothesis that an individual’s resting metabolic rate and/or elevated metabolic rate after exertion (active metabolic rate) influence/s predatory behavior. We recorded the number of open-close motions of the operculum for 30 s to measure opercular beat rate (OBR) or ventilation rate per minute. We use these ventilation rates as estimates for metabolic rates. Other studies on many fish species have found a tight positive relationship between ventilation rate and metabolic rate as measured in a respirometer (van Rooij and Videler 1996; Grantner and Taborsky 1998; Dalla Valle et al. 2003; Millidine et al. 2008; Frisk et al. 2012). To obtain an estimate of pike resting metabolic rate, we measured the resting OBR of each pike by watching the pike undisturbed in its home tank between 9:00 and 9:30 a.m. each day for five consecutive days (N = 5 measures per pike). Resting OBR measures were taken approximately 2 weeks after the predator-prey-interaction assays were completed. Approximately 1 month after measuring resting OBR, we measured active OBR after exertion (i.e., active metabolic rate after exertion) at 10:00 a.m. each day for three consecutive days (N = 3 measures per pike). Specifically, we measured the OBR of each pike 5 min after chasing it vigorously throughout its home tank with a hand net for 15 s. All OBR measurements were taken after at least 24 h of starvation, and the order in which we observed the pike each day was randomized by drawing numbers out of a cup.
Pike were euthanized with an overdose of MS222, measured for standard length and body mass, and dissected. We dissected out organs that we could reliably measure and that we hypothesized might play a role in predatory behavior. We quantified liver and brain masses because both are energetically costly organs; but while the liver is important for energy (glycogen) storage, the brain is important in learning and processing information. Since pike are visual predators, we also quantified the mass of both whole eyeballs. Organ masses were measured both wet and after being dried in a drying oven at 60°C for 5 days. The organ wet and dry masses were strongly correlated (r range = 0.83–0.98). We use the wet masses here in order to have body mass on the same scale (i.e., we did not dry the entire bodies). Sex was determined by visual inspection of the gonads (3 males, 9 females). We did not detect an effect of sex on behavior, metabolic rate, body size, and organ mass; therefore, sex was not considered further.
Data Analysis: Structural Equation Modeling with Predator and Prey Traits
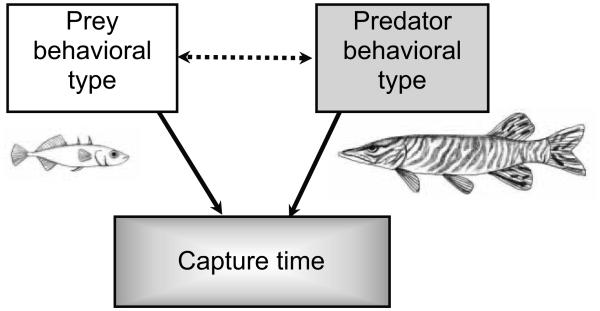
SEM is used to quantify the direct and indirect effects of factors while holding other factors constant (Grace 2006, 2008). It also has the additional benefit of being able to statistically compare alternative models. We tested two general hypotheses, (1) that there is reciprocal behavioral plasticity between predators and prey and (2) that behavioral types of both predators and prey contribute to the outcome of a predator-prey interaction (fig. 1). From these general hypotheses we constructed our a priori predator-prey model, in which we included behavioral data on both the predator and the prey. We included the behaviors of each stickleback alone (from the prey-alone assay) and with the pike (from the predator-prey-interaction assay; N = 77 stickleback). We also included the behaviors exhibited by the pike during each interaction with an individual stickleback (N = 77 interactions).
Figure 1.
The general hypotheses underlying the a priori structural equation model. Solid arrows indicate direct effects, and the dashed double-headed arrow indicates where reciprocal behavioral plasticity might occur. The white box indicates prey traits, and the gray box indicates predator traits. Line drawings by K. E. McGhee.
Since the 12 individual pike were reused (N = 3–8 interactions per pike), each pike occurs more than once in these data. While we recognize that the repeated use of the same individual pike could be viewed as pseudoreplication, there are several reasons why the relevant unit of replication in this study is at the level of interaction rather than the level of pike. First, the goal of this study was to assess whether knowledge about each player’s behavioral tendencies is enough to understand the behaviors that each will show in an interaction with another. Thus, examining average pike behavior across different prey individuals does not allow us to explore what happens during single interactions. Second, each interaction involves a novel partner/prey individual, making it a unique interaction regardless of the pike involved. Finally, this design is a conservative approach to estimating reciprocal behavioral plasticity: if pike identity is the major driver of predator-prey interactions, then we should see no evidence for reciprocal behavioral plasticity.
We compared the fit of our original a priori predator-prey model to the fit of two a priori alternative models. First, to examine whether including reciprocal behavioral plasticity (i.e., behavioral feedback) between predator and prey improved the fit of the model, we compared our original a priori predator-prey model (with feedback) to an alternative a priori model without feedback. We defined reciprocal behavioral plasticity, or feedback, as the prey adjusting their behavior to the predator and vice versa (fig. 1, dashed double-headed arrow). In our a priori model, we designated four paths as potential feedback paths. In two of these feedback paths, the predator’s behavior can affect the prey’s behavior. Specifically, the pike’s first orientation toward the prey can affect stickleback behavior (freezing or orienting). In the other two feedback paths, the prey’s behavior can affect the predator’s behavior. Specifically, the stickleback’s freezing behavior and/or orienting behavior can affect the timing of the pike’s first attack. Second, to examine whether there were family-level differences in stickleback behavior, we compared our original a priori predator-prey model (without stickleback mother) to an alternative a priori model including stickleback mother identity as an observed variable. We subtracted the χ2 value of our original model from that of the “no feedback” model (and likewise from the “stickleback mother included” model) and determined whether this difference was statistically significant at the difference in degrees of freedom between the two models (Grace 2006).
We used AMOS (Arbuckle 2006) to create our hypothesized a priori model described above and assessed its adequacy (confirmatory analyses sensu Grace 2006, 2008). If the a priori model adequately fit the data, we were not able to reject it based on the χ2 value (P > .05; Grace 2008), and the comparative fit index (CFI; an index appropriate for small sample sizes) was “close to” 0.95 (Iacobucci 2010). We did not remove any nonsignificant relationships from the hypothesized a priori model. For variables in which we expected that errors might be correlated (i.e., covariance between residuals) and not related in an obvious directional fashion, we included double-headed arrows between the errors (Grace 2006, 2008). Transformation of variables was determined based on the assessment of normality in AMOS to ensure that the data were approximately multivariate normal. Latency behavioral variables (e.g., time to first orient, time to first attack, capture time) were natural log transformed. The proportion of time an individual stickleback spent doing a particular behavior (e.g., proportion of time spent frozen with a novel object or shoal out of the 3-min trial or proportion of time spent frozen or oriented at the pike before capture during the predation assay) was arcsine square root transformed.
It is important to note that the pike became more experienced with the predation assay as the experiment progressed. To examine whether experience with the predation assay affected our results, we reran our a priori predator-prey model with pike behavioral data accounting for the day of the experiment (i.e., residuals of a regression of pike orienting/attacking/capturing behavior on test day) instead of the raw behavioral data. Our results (not shown) are essentially identical, suggesting that experience with the assay does not substantially alter the behavioral patterns.
Data Analysis: Repeatability Estimates of Predator and Prey Traits
We examined whether prey showed behavioral types in terms of their freezing behavior across different stimuli. Each stickleback had repeated measures of their freezing behavior—once with a novel object stimulus, once with the unfamiliar shoal stimulus, and once with a live pike. If the proportion of time spent frozen is significantly repeatable across stimuli, we can conclude that individuals vary consistently in the tendency to freeze across different situations, suggesting consistent behavioral types. We calculated repeatabilities of freezing behavior from the mean squares of general linear models using the protocol in Lessells and Boag (1987). Specifically, we used general linear models to examine whether stickleback identity (random effect) accounted for a significant amount of the variation in freezing behavior across the three stimuli. We accounted for mean level differences across stimuli by including “stimulus” as a fixed effect. Unfortunately, we could not examine whether stickleback had consistent behavioral types within the predator-prey-interaction assay because they encountered the predator only once. However, our repeatability analysis, as well as the SEM described below, allows us to examine whether the amount of freezing behavior an individual stickleback exhibits when alone, whether with a novel object or a shoal, is related to its freezing behavior with a live predator.
We also examined variation in predators. First, we tested if predators showed behavioral types, that is, consistent individual differences in their latency to orient and attack prey for the first time. Second, we tested for consistent individual differences among predators in metabolism as measured by their resting ventilation rate and active ventilation rate after exertion. Each pike had repeated measures of its behavior during the predator-prey-interaction assay with a stickleback (N = 3–8 interactions per pike) as well as repeated measures of resting ventilation rate (N = 5 measures per pike) and active ventilation rate (N = 3 measures per pike). As above, we used general linear models to examine whether pike identity accounted for a significant amount of the pike behavioral variation in the predator-prey-interaction assay (latency to first orient, latency to first attack, capture time) and of the variation in resting and active ventilation rates. As in the SEM, the proportion of time the individual stickleback spent frozen (with a novel object, with a shoal, and with the pike) were arcsine square root transformed prior to analysis. Similarly, all latency behavioral variables for the individual pike (e.g., time to first orient, time to first attack, capture time) were natural log transformed. For all repeatability estimates, we calculated standard errors according to Becker (1992).
Data Analysis: Correlations among Predator Traits
We examined whether an individual pike’s average behavior across all its predator-prey interaction assays was related to its metabolic rate (i.e., average resting ventilation rate and average active ventilation rate after exertion), body size, and organ size (i.e., liver, brain, and eye masses). Due to the limited number of pike (N = 12), and since pike orienting and attack behaviors are strongly correlated (Pear son’s product-moment correlation coefficient [rP] = 0.84, P = .006), we restricted our analyses to the average latency with which a pike first attacks the stickleback. We controlled for the effect of body size on organ size and ventilation rate by dividing organ mass and ventilation rates by body mass. We used Pearson’s correlations to examine whether attack behavior was related to (1) body mass, (2) organ sizes (divided by body mass), and (3) resting and active ventilation rates (divided by body mass). In these analyses, latency to first attack prey as well as liver mass divided by body mass were natural log transformed. These analyses only include the average values for each of the 12 individual pike, thus each pike occurs only once in these data.
Analyses were conducted using SAS software, version 9.3 (SAS Institute, Cary, NC). All data are deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.7h7tk (McGhee et al. 2013).
Ethical Note
Allowing predators to interact freely with their prey was essential to quantifying normal predatory and antipredator behavior. However, we provided numerous refuges in the pike’s tanks in order to give stickleback an opportunity to hide or escape from the pike. In planning the maternal effects study (McGhee et al. 2012), we used power analyses based on results from a previous study (Giesing et al. 2011) to minimize the number of subjects used (Animal Behavior Society/Association for the Study of Animal Behaviour [ABS/ASAB] guidelines 2004–2007). This experiment was approved by the Animal Care and Use Committee of University of Illinois (protocol 09204).
Results
Does Reciprocal Behavioral Plasticity between Predators and Prey Affect the Outcome of Encounters?
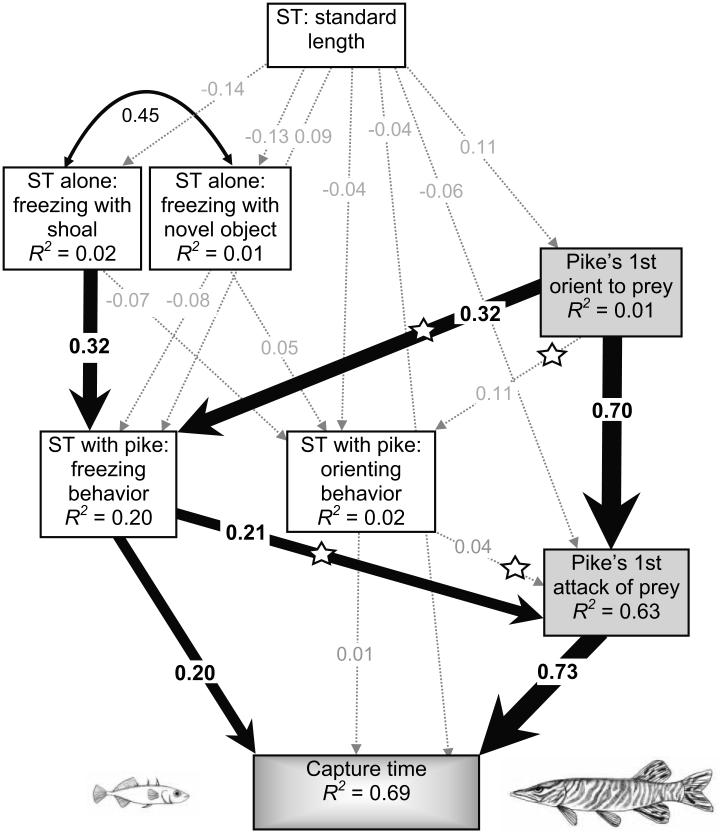
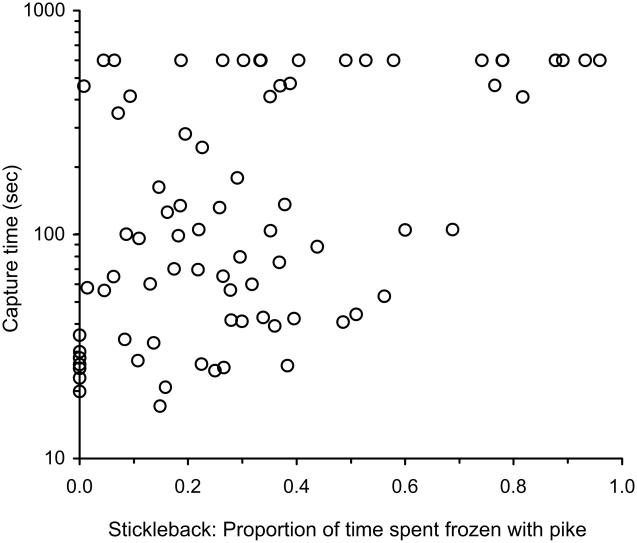
How a pike behaved toward a stickleback influenced the stickleback’s behavior and vice versa (fig. 2, paths marked with stars). For example, the sooner a pike first oriented toward the stickleback, the longer the stickleback froze in response. At the same time, the behavior of the stickleback influenced the behavior of the pike: the longer a stickleback froze, the longer the pike’s first attack was delayed and the longer the stickleback survived (fig. 3). However, freezing behavior’s positive influence on stickleback survival (delaying capture time) was due to its direct effect on capture time (standardized direct effect = 0.20) as well as its indirect effect on capture time via its effect on the pike’s latency to attack (standardized indirect effect = 0.16; fig. 2; see table A1 for all direct and indirect effects; see fig. A1 for unstandardized coefficients; tables A1, A2 and fig. A1 available online). Thus, there is evidence that reciprocal plasticity occurred during the predator-prey interaction; the time when the predator first noticed the prey affected the prey’s antipredator behavior, which in turn affected the predator’s first attempted attack.
Figure 2.
Path diagram for the structural equation model with standardized regression weights for stickleback (ST) behavior (white boxes) and pike behavior (gray boxes). Statistically significant paths are indicated by solid arrows, and the strength of these relationships is indicated by the width of the arrows. Nonsignificant paths are indicated by gray dashed arrows. Straight arrows reflect causal paths; curved arrows designate correlations. The R2 values in each box indicate the amount of variation in that variable that is explained by the input arrows. Stars indicate the paths allowing behavioral feedback between predator and prey; N = 77 interactions. The standardized coefficients in these path diagrams are measured in standard deviation units.
Figure 3.
Proportion of time stickleback spent frozen with pike relative to time to capture. The greater the proportion of time prey spent frozen during an encounter with a live predator, the longer they survived.
The importance of reciprocal plasticity on the outcome of a predator-prey encounter was statistically confirmed by comparing models with and without feedback between pike behavior (orienting and attacking) and stickleback antipredator behaviors (freezing and orienting). Our original a priori predator-prey model that included feedback fit our data adequately and was not rejected (original model with feedback: χ2 = 5.57, df = 8, P =.695, Akaike Information Criterion [AIC] = 61.57, CFI = 1.0; Iacobucci 2010). Removing the four feedback paths between pike behavior and stickleback antipredator behavior resulted in a model that did not adequately fit the data (no feedback model: χ2 = 23.52, df = 12, P = .024, AIC = 71.52, CFI = 0.937). Indeed, removing the four feedback paths between the predator and prey (no-feedback model) resulted in a significant reduction in model fit compared to the original a priori predator-prey model with feedback (χ2 difference = 17.95, df = 4, P = .001). In addition, less behavioral variance was explained in the no-feedback model compared to the original a priori predator-prey model with feedback (stickleback freezing behavior: R2 = 0.09 vs. 0.20; orienting behavior: R2 = 0.01 vs. 0.02; pike attack behavior: R2 = 0.59 vs. 0.63; capture time: R2 = 0.65 vs. 0.69).
Are the Behaviors of Predators and Prey during an Encounter Influenced by Their Respective Behavioral Types?
Although reciprocal behavioral plasticity occurred when individual pike and stickleback encountered one another, how each player behaved during the interaction was also influenced by its behavioral type. Stickleback showed significantly repeatable differences in their freezing behavior across three different stimuli, suggesting behavioral types (table 1). These consistent behavioral differences emerged despite changes in the mean level of freezing behavior that individuals exhibited across the three stimuli (F2, 152 = 94.87, P< .0001; table 1). Our a priori model revealed, however, that while stickleback freezing behavior in the predator-prey interaction was positively related to freezing behavior when encountering a shoal (standardized regression weight = 0.32, P = .005), it was not strongly related to freezing behavior when encountering a novel object (standardized regression weight = −0.08, P = .506; fig. 2). The role of prey behavioral type does not seem to be due to family effects and relatedness among individuals; including stickleback mother identity as an observed variable connecting all of the stickleback traits did not result in an improved model (χ2 = 9.38, df = 11, P = .587, AIC = 77.383, CFI = 1.0), explained no additional variation in capture time (R2 = 0.69 vs. 0.69), and was not a significant improvement from the simpler original a priori predator-prey model without mother (χ2 difference = 3.81, df = 3, P = .282). Additionally, behavioral type was not related to variation in body size; stickleback standard length was unrelated to stickleback behavior alone or in the presence of the predator or in the predator’s behavior during the interaction (fig. 2; see table A2 for prey trait means).
Table 1.
Repeatability estimates for stickleback freezing behavior and pike predatory behaviors and metabolism (from ventilation rates)
| Mean ± SE | Repeatability ± SEa | F value | P value | |
|---|---|---|---|---|
| Stickleback: | ||||
| Proportion of time spent frozen:b | ||||
| With novel object | .84 ± .03 | .29 ± .07 | 2.22 | <.0001 |
| With shoal | .61 ± .03 | |||
| With live pike | .32 ± .03 | |||
| Pike: | ||||
| Predatory behaviors:c | ||||
| Time to first orient to prey (s) | 92 ± 25 | .18 ± .12 | 2.38 | .015 |
| Time to first attack of prey (s) | 171 ± 36 | .23 ± .12 | 2.89 | .004 |
| Time to capture prey (s) | 254 ± 46 | .21 ± .12 | 2.69 | .006 |
| Metabolism: | ||||
| Resting ventilation rate (bpm)d | 28 ± 2 | .60 ± .13 | 8.42 | <.0001 |
| Active ventilation rate (bpm)e | 67 ± 4 | .54 ± .16 | 4.48 | .001 |
Note: All repeatability estimates were statistically significant after accounting for multiple tests using sequential Dunn-Sidak. bpm = beats per minute.
Standard errors were calculated according to Becker (1992).
Number of observations per stickleback was 3 (no = 3); df = 76, 152. The fixed effect of context (flask, shoal, and pike) accounting for mean level differences was statistically significant (F = 94.87, P < .001).
Number of observations per pike ranged from 3 to 8 (no = 6.4); df = 11, 61.
Number of observations per pike was 5 (no = 5); df = 11, 61.
Number of observations per pike was 3 (no = 3); df = 11,61.
We also found evidence for pike behavioral types. Individual differences among pike in their predatory behavior toward their prey (orienting and attacking) were consistent across multiple predator-prey interactions and significantly repeatable, leading to repeatable capture times (table 1; see table A2 for predator trait means). Thus, some individual pike consistently oriented to and attacked their prey faster than others, despite encountering variable (and randomly assigned) prey and despite behavioral feedback between predators and prey.
Is Variation in Predator Behavior Associated with Differences in Metabolic Rate and Organ Size?
There was substantial variation among individual pike in both their resting ventilation rates (range = 20–36 beats per min [bpm]) and their active ventilation rates after exertion (range = 48–85 bpm), which we are using as proxies for resting and active metabolic rates, respectively. Average resting and active ventilation rates were positively associated (rP = 0.59, P = .045). Differences in ventilation rate among pike were strongly and significantly repeatable (table 1). Pike were also highly variable in their body mass and in the mass of their organs (see table A2).
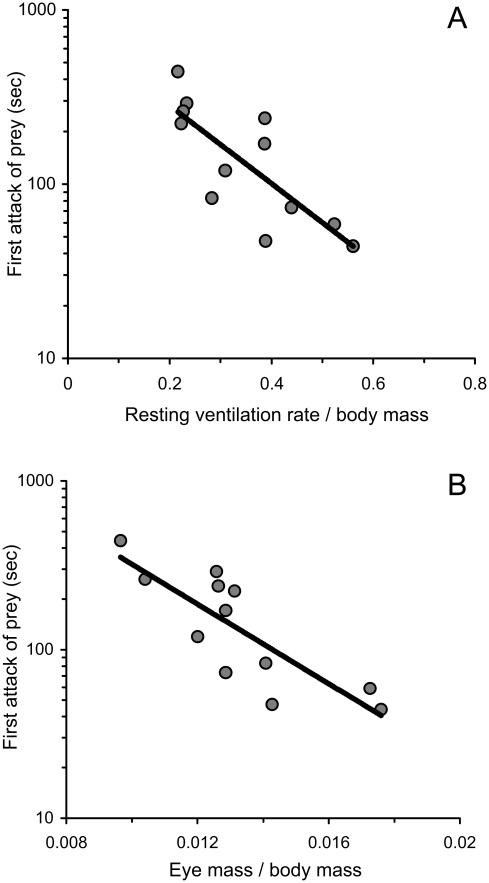
Furthermore, we found that the average speed at which a pike launched its first attack on the prey was related to variation among pike in body mass, organ mass, and metabolism (table 2). Larger pike tended to be slower predators and took longer to attack prey, although this was not strictly significant after accounting for multiple testing. In addition, individuals with larger eyes for their body size attacked their prey faster than individuals with smaller eyes for their body size (fig. 4B). There was also a link between resting ventilation rate and predatory behavior: pike with relatively high resting metabolic rates quickly attacked prey, even after controlling for the effect of body size (fig. 4A). However, this pattern was not significant after accounting for multiple testing. Liver mass, brain mass, and active ventilation rates were not significantly related to behavioral type. Finally, while we acknowledge that these results are based on a relatively small sample of individual pike (N = 12), our results do suggest that behavioral differences among predators are associated with individual differences in relative organ size and resting metabolic rate, even after controlling for body size.
Table 2.
Pearson’s correlations between the average latency for pike to first attack the stickleback and their body mass, organ masses, and average metabolic rates
| Time to first attack of prey (s) |
||
|---|---|---|
| rP | P value | |
| Body mass (g) | .70 | .012 |
| Organ sizes, accounting for body size:a | ||
| Eye mass | − .76 | .004 |
| Liver massb | .10 | .758 |
| Brain mass | −.40 | .202 |
| Metabolism, accounting for body size:a | ||
| Resting ventilation rate | −.66 | .019 |
| Active ventilation rate | −.46 | .136 |
Note: Correlation coefficients in bold are statistically significant after accounting for multiple tests using sequential Dunn-Sidak.
Divided by body mass (g).
Natural log transformed.
Figure 4.
Average predatory behavior during the predation assay was negatively correlated with resting metabolic rate (A) and eye mass (B), after accounting for body mass.
Discussion
Our results indicate that the progression and eventual outcome of one-on-one encounters between predators and prey were affected by reciprocal behavioral plasticity during the encounter, as well as the underlying behavioral types of both predator and prey. During an interaction, an individual predator’s behavior was influenced by its prey’s antipredator behavior. At the same time, an individual prey’s antipredator behavior was influenced by its predator’s behavior. Although we envision this reciprocal behavioral plasticity as a back-and-forth behavioral sequence between predator and prey, future studies should aim to directly test this by examining the temporal behavioral dynamics of predator and prey in real time. In addition to the evidence for reciprocal behavioral plasticity, our results also indicate that there was consistent intraspecific behavioral variation in both predators and prey. Prey showed distinct behavioral types, with freezing behavior carrying over across different stimuli. Importantly, a stickleback’s tendency to freeze with conspecifics was correlated with the tendency to freeze with a live predator. Similarly, there were consistent behavioral differences among individual pike, with some pike consistently orienting and attacking their prey faster than others. Thus, capture time, the eventual outcome of the predator-prey interaction, was influenced by both the dynamic behavioral plasticity occurring during the encounter as well as the behavioral type of each participant, emphasizing that the presence of behavioral types does not imply that plasticity is absent (Neff and Sherman 2004; Sih et al. 2004b).
This study also provides insights into how behavioral variation among individuals might be related to differences among individuals in underlying morphological and physiological traits (Careau et al. 2008; Biro and Stamps 2010). We found some of the strongest links to date between organ size, metabolic rate, and behavioral type. We detected evidence that pike with higher resting metabolic rates, as measured by ventilation rate, launched their first attack more quickly, consistent with the idea that the “idling cost” of the metabolic engine is related to predatory ability (reviewed in Careau et al. 2008; Biro and Stamps 2010). While the literature has emphasized the importance of energetically costly organs, such as the liver, for behavioral type (Biro and Stamps 2010; Reale et al. 2010; but see Wolf and McNamara 2012), we offer some of the first evidence at the intraspecific level that differences in sensory organs might also be important drivers of behavioral variation (see Ronald et al. 2012). One of the strongest, yet unexpected, patterns was that individual pike with relatively large eyes were “fast” predators and quicker to launch their initial attack, a finding that is potentially consistent with interspecific studies of avian predators (Brooke et al. 1999; Garamszegi et al. 2002). Pike are visual predators that prey on a number of fish species, including other pike (reviewed in Craig 2008). Thus, larger eyes in this species may aid in prey detection as well as in the detection of cannibalistic pike predators.
An important ecological implication of these results is that if pike are constrained in their behavioral flexibility because of their sensory system or metabolism, they might adjust other associated behaviors, such as the habitat they occupy or their prey selection, to maximize foraging success. For example, particular pike with large eyes might actively seek out certain environments for foraging (e.g., open water vs. near shore) and target particular prey (e.g., fish vs. invertebrates). Similarly, pike with higher metabolic rates might be able to engage in high levels of energetically costly predatory behavior such as actively pursuing prey rather than using a sit-and-wait strategy. This might impact which individuals within a prey population predators target, as well as the species of prey individual predators pursue. Thus, variation in metabolism and sensory system might also serve as a proximate cause for within-population diet diversification and habitat use. Consistent with this hypothesis, northern pike exhibit consistent individual differences in habitat use within natural lakes (Kobler et al. 2009) and individual specialization in both short-term diet and long-term trophic position (Beaudoin et al. 1999).
Growing evidence for consistent differences among individuals within a population indicates that it is unrealistic to assume that traits are uniform within species (e.g., individual predators of the same species exert an equal level of threat; Lima 2002; Bolnick et al. 2003; Sih et al. 2004a, 2004c). Furthermore, behavioral variation among individuals of each interacting species can influence how species interactions proceed (Schreiber et al. 2011; Sih et al. 2012). Indeed, incorporating the behavioral type of both partners in an interaction can reveal predictable predation patterns that might not have been detected otherwise (e.g., Pruitt et al. 2012b). Our results suggest, however, that it is also important to examine the behaviors that occur during an interaction. We found that not only does behavioral variation among individual predators directly affect predator-prey interactions but that predators also indirectly affect predator-prey interactions through alterations to prey behavior. In our study, individual predators and prey were paired randomly with respect to their behavioral type, metabolic rate, or organ size. Thus, it remains unknown whether particular predator behavioral types are more successful at capturing particular prey behavioral types and whether the pattern and intensity of reciprocal plasticity between predators and prey depends on their respective behavioral types.
Our results provide compelling support for the idea that behavioral variation in both predators and prey might be maintained via environment- and frequency-dependent selection arising from species interactions. First, the reciprocal behavioral plasticity that occurs between predators and prey during interactions might change the selective biotic environment that individuals experience (Agrawal 2001; Miner et al. 2005; Fordyce 2006). In other words, the plastic response of an individual can affect the fitness surface of an interacting partner. Second, the selective environment experienced by particular predator (prey) behavioral types might depend on the frequency of similar behavioral types in the population as well as the frequency of particular prey (predator) behavioral types in the population (Sih et al. 2012). Thus, reciprocal plasticity has the potential to alter the frequency-dependent selective benefits of particular predator and prey behavioral types in a population leading to evolutionary consequences. In addition, our results provide compelling support for the hypothesis that the consistent behavioral variation we see among predator individuals is underlain by their morphological, physiological, and sensory system architecture. The behavioral link to relatively nonplastic traits might be particularly important for the maintenance of variation in behavioral type. For example, Wolf and McNamara (2012) have shown that when a “stable trait” that underlies behavior (i.e., physiological architecture) is incorporated into frequency-dependent models, both behavioral consistency and strong behavioral correlations emerge.
Recent theory suggests that phenotypic plasticity and rapidly induced defenses tend to stabilize community dynamics and dampen the population oscillations in predator-prey systems (Mougi and Kishida 2009; Cortez 2011). Communities tend to be further stabilized when one incorporates multiple interaction types (e.g., combinations of antagonistic and mutualistic interactions) and increasing complexity (Mougi and Kondoh 2012). Our results suggest that it is worth incorporating an additional level of complexity to reflect the consistent variation among individuals in their behavior within interactions (see also Fordyce 2006; Berg and Ellers 2010; Schreiber et al. 2011; Sih et al. 2012) and worth examining more closely the relationships between behavioral variation and differences among individuals in relatively nonplastic traits such as sensory organs. Species interactions are influenced by reciprocal behavioral plasticity as well as intraspecific behavioral variation, and the extent to which these factors determine the outcome of species interactions could have far-reaching consequences on community dynamics and diversity.
Acknowledgments
We thank the Spirit Lake Fish Hatchery (Iowa) for providing the pike, the Jake Wolf Fish Hatchery (Illinois) for holding the pike, and B. Mommer and M. Schrader for help transporting the pike. We thank D. Roche for help collecting behavioral data on both stickleback and pike, J. Hamilton, S. Khoo, V. Sefton, and J. Wang for watching stickleback videos, and E. Suhr for help with fish care. We thank J. Bruskotter and B. Inouye for looking over the SEM. We thank the Bell Lab at the University of Illinois, J. Bronstein, B. Cole, and two reviewers for insightful comments that greatly improved the manuscript. K.E.M. was supported by a National Institutes of Health/National Institute of Child Health and Human Development (NIH/ NICHD) fellowship (T32 HD007333) and a National Science Foundation grant to A.M.B. and K.E.M. (IOS-1121980). L.M.P. was supported by University of Illinois start-up funds to A.M.B.
Literature Cited
- Agrawal AA. Ecology: phenotypic plasticity in the interactions and evolution of species. Science. 2001;294:321–326. doi: 10.1126/science.1060701. [DOI] [PubMed] [Google Scholar]
- Alonzo SH. State-dependent habitat selection games between predators and prey: the importance of behavioural interactions and expected lifetime reproductive success. Evolutionary Ecology Research. 2002;4:759–778. [Google Scholar]
- Arbuckle JL. Amos. Version 7.0. SPSS; Chicago: 2006. [Google Scholar]
- Barreto RE, Volpato GL. Ventilation rates indicate stress-coping styles in Nile tilapia. Journal of Biosciences. 2011;36:851–855. doi: 10.1007/s12038-011-9111-4. [DOI] [PubMed] [Google Scholar]
- Beaudoin CP, Tonn WM, Prepas EE, Wassenaar LI. Individual specialization and trophic adaptability of northern pike (Esox lucius): an isotope and dietary analysis. Oecologia (Berlin) 1999;120:386–396. doi: 10.1007/s004420050871. [DOI] [PubMed] [Google Scholar]
- Becker WA. Academic Enterprises; Pullman, WA: 1992. Manual of quantitative genetics. [Google Scholar]
- Berg MP, Ellers J. Trait plasticity in species interactions: a driving force of community dynamics. Evolutionary Ecology. 2010;24:617–629. [Google Scholar]
- Biro PA, Stamps JA. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends in Ecology and Evolution. 2010;25:653–659. doi: 10.1016/j.tree.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC. Sinauer; Sunderland, MA: 2007. Quantifying behavior the JWatcher way. [Google Scholar]
- Bolnick DI, Amarasekare P, Araujo MS, Burger R, Levine JM, Novak M, Rudolf VHW, Schreiber SJ, Urban MC, Vasseur DA. Why intraspecific trait variation matters in community ecology. Trends in Ecology and Evolution. 2011;26:183–192. doi: 10.1016/j.tree.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. The ecology of individuals: incidence and implications of individual specialization. American Naturalist. 2003;161:1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- Brooke MD, Hanley S, Laughlin SB. The scaling of eye size with body mass in birds. Proceedings of the Royal Society B: Biological Sciences. 1999;266:405–412. [Google Scholar]
- Burton T, Killen SS, Armstrong JD, Metcalfe NB. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proceedings of the Royal Society B: Biological Sciences. 2011;278:3465–3473. doi: 10.1098/rspb.2011.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careau V, Reale D, Humphries MM, Thomas DW. The pace of life under artificial selection: personality, energy expenditure, and longevity are correlated in domestic dogs. American Naturalist. 2010;175:753–758. doi: 10.1086/652435. [DOI] [PubMed] [Google Scholar]
- Careau V, Thomas D, Humphries MM, Reale D. Energy metabolism and animal personality. Oikos. 2008;117:641–653. [Google Scholar]
- Careau V, Thomas D, Pelletier F, Turki L, Landry F, Garant D, Reale D. Genetic correlation between resting metabolic rate and exploratory behaviour in deer mice (Peromyscus maniculatus) Journal of Evolutionary Biology. 2011;24:2153–2163. doi: 10.1111/j.1420-9101.2011.02344.x. [DOI] [PubMed] [Google Scholar]
- Cortez MH. Comparing the qualitatively different effects rapidly evolving and rapidly induced defences have on predator-prey interactions. Ecology Letters. 2011;14:202–209. doi: 10.1111/j.1461-0248.2010.01572.x. [DOI] [PubMed] [Google Scholar]
- F Craig, J. A short review of pike ecology. Hydrobiologia. 2008;601:5–16. [Google Scholar]
- Dalla Valle AZ, Rivas-Diaz R, Claireaux G. Opercular differential pressure as a predictor of metabolic oxygen demand in the starry flounder. Journal of Fish Biology. 2003;63:1578–1588. [Google Scholar]
- Estes JA, Riedman ML, Staedler MM, Tinker MT, Lyon BE. Individual variation in prey selection by sea otters: patterns, causes and implications. Journal of Animal Ecology. 2003;72:144–155. [Google Scholar]
- Farwell M, McLaughlin RL. Alternative foraging tactics and risk taking in brook charr (Salvelinus fontinalis) Behavioral Ecology. 2009;20:913–921. [Google Scholar]
- Flaxman SM, Lou YA, Meyer FG. Evolutionary ecology of movement by predators and prey. Theoretical Ecology. 2011;4:255–267. [Google Scholar]
- Fordyce JA. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. Journal of Experimental Biology. 2006;209:2377–2383. doi: 10.1242/jeb.02271. [DOI] [PubMed] [Google Scholar]
- Frisk M, Skov PV, Steffensen JF. Thermal optimum for pikeperch (Sander lucioperca) and the use of ventilation frequency as a predictor of metabolic rate. Aquaculture. 2012;324:151–157. [Google Scholar]
- Garamszegi LZ, Møller AP, Erritzoe J. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proceedings of the Royal Society B: Biological Sciences. 2002;269:961–967. doi: 10.1098/rspb.2002.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesing ER, Suski CD, Warner RE, Bell AM. Female sticklebacks transfer information via eggs: effects of maternal experience with predators on offspring. Proceedings of the Royal Society B: Biological Sciences. 2011;278:1753–1759. doi: 10.1098/rspb.2010.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace JB. Cambridge University Press; New York: 2006. Structural equation modeling and natural systems. [Google Scholar]
- Grace JB. Structural equation modeling for observational studies. Journal of Wildlife Management. 2008;72:14–22. [Google Scholar]
- Grantner A, Taborsky M. The metabolic rates associated with resting, and with the performance of agonistic, submissive and digging behaviours in the cichlid fish Neolamprologus pulcher (Pisces: Cichlidae) Journal of Comparative Physiology B. 1998;168:427–433. [Google Scholar]
- Griffen BD, Toscano BJ, Gatto J. The role of individual behavior type in mediating indirect interactions. Ecology. 2012;93:1935–1943. doi: 10.1890/11-2153.1. [DOI] [PubMed] [Google Scholar]
- Houston AI. Evolutionary models of metabolism, behaviour and personality. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:3969–3975. doi: 10.1098/rstb.2010.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntingford FA, Andrew G, Mackenzie S, Morera D, Coyle SM, Pilarczyk M, Kadri S. Coping strategies in a strongly schooling fish, the common carp Cyprinus carpio. Journal of Fish Biology. 2010;76:1576–1591. doi: 10.1111/j.1095-8649.2010.02582.x. [DOI] [PubMed] [Google Scholar]
- Iacobucci D. Structural equations modeling: fit indices, sample size, and advanced topics. Journal of Consumer Psychology. 2010;20:90–98. [Google Scholar]
- Killen SS, Marras S, McKenzie DJ. Fuel, fasting, fear: routine metabolic rate and food deprivation exert synergistic effects on risk-taking in individual juvenile European sea bass. Journal of Animal Ecology. 2011;80:1024–1033. doi: 10.1111/j.1365-2656.2011.01844.x. [DOI] [PubMed] [Google Scholar]
- Killen SS, Marras S, Ryan MR, Domenici P, McKenzie DJ. A relationship between metabolic rate and risk-taking behaviour is revealed during hypoxia in juvenile European sea bass. Functional Ecology. 2012;26:134–143. [Google Scholar]
- Kishida O, Trussell GC, Mougi A, Nishimura K. Evolutionary ecology of inducible morphological plasticity in predator-prey interaction: toward the practical links with population ecology. Population Ecology. 2010;52:37–46. [Google Scholar]
- Kobler A, Klefoth T, Mehner T, Arlinghaus R. Coexistence of behavioural types in an aquatic top predator: a response to resource limitation? Oecologia (Berlin) 2009;161:837–847. doi: 10.1007/s00442-009-1415-9. [DOI] [PubMed] [Google Scholar]
- Kopp M, Tollrian R. Reciprocal phenotypic plasticity in a predator-prey system: inducible offences against inducible defences? Ecology Letters. 2003;6:742–748. [Google Scholar]
- Lantova P, Zub K, Koskela E, Sichova K, Borowski Z. Is there a linkage between metabolism and personality in small mammals? the root vole (Microtus oeconomus) example. Physiology and Behavior. 2011;104:378–383. doi: 10.1016/j.physbeh.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Le Galliard JF, Paquet M, Cisel M, Montes-Poloni L. Personality and the pace-of-life syndrome: variation and selection on exploration, metabolism and locomotor performances. Functional Ecology. 2013;27:136–144. [Google Scholar]
- Lessells CM, Boag PT. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- Lima SL. Putting predators back into behavioral predator-prey interactions. Trends in Ecology and Evolution. 2002;17:70–75. [Google Scholar]
- Martins CIM, Castanheira MF, Engrola S, Costas B, Conceicao LEC. Individual differences in metabolism predict coping styles in fish. Applied Animal Behaviour Science. 2011;130:135–143. [Google Scholar]
- McGhee KE, Pintor LM, Bell AM. Data from: Reciprocal behavioral plasticity and behavioral types during predator-prey interactions. American Naturalist, Dryad Digital Repository. 2013 doi: 10.1086/673526. http://dx.doi.org/10.5061/dryad.7h7tk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee KE, Pintor LM, Suhr EL, Bell AM. Maternal exposure to predation risk decreases offspring antipredator behaviour and survival in threespined stickleback. Functional Ecology. 2012;26:932–940. doi: 10.1111/j.1365-2435.2012.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millidine KJ, Metcalfe NB, Armstrong JD. The use of ventilation frequency as an accurate indicator of metabolic rate in juvenile Atlantic salmon (Salmo salar) Canadian Journal of Fisheries and Aquatic Sciences. 2008;65:2081–2087. [Google Scholar]
- Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. Ecological consequences of phenotypic plasticity. Trends in Ecology and Evolution. 2005;20:685–692. doi: 10.1016/j.tree.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Mitchell WA. Multi-behavioral strategies in a predator-prey game: an evolutionary algorithm analysis. Oikos. 2009;118:1073–1083. [Google Scholar]
- Mougi A. Unusual predator-prey dynamics under reciprocal phenotypic plasticity. Journal of Theoretical Biology. 2012;305:96–102. doi: 10.1016/j.jtbi.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Mougi A, Kishida O. Reciprocal phenotypic plasticity can lead to stable predator-prey interaction. Journal of Animal Ecology. 2009;78:1172–1181. doi: 10.1111/j.1365-2656.2009.01600.x. [DOI] [PubMed] [Google Scholar]
- Mougi A, Kondoh M. Diversity of interaction types and ecological community stability. Science. 2012;337:349–351. doi: 10.1126/science.1220529. [DOI] [PubMed] [Google Scholar]
- Neff BD, Sherman PW. Behavioral syndromes versus Darwinian algorithms. Trends in Ecology and Evolution. 2004;19:621–622. [Google Scholar]
- Nyqvist MJ, Gozlan RE, Cucherousset J, Britton JR. Behavioural syndrome in a solitary predator is independent of body size and growth rate. PLoS ONE. 2012;7:e31619. doi: 10.1371/journal.pone.0031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisser EL, Bolnick DI, Benard MF. Scared to death? the effects of intimidation and consumption in predator-prey interactions. Ecology. 2005;86:501–509. [Google Scholar]
- Pruitt JN, Cote J, Ferrari MCO. Behavioural trait variants in a habitat-forming species dictate the nature of its interactions with and among heterospecifics. Functional Ecology. 2012a;26:29–36. [Google Scholar]
- Pruitt JN, Stachowicz JJ, Sih A. Behavioral types of predator and prey jointly determine prey survival: potential implications for the maintenance of within-species behavioral variation. American Naturalist. 2012b;179:217–227. doi: 10.1086/663680. [DOI] [PubMed] [Google Scholar]
- Reale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO. Personality and the emergence of the pace-of-life syndrome concept at the population level. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:4051–4063. doi: 10.1098/rstb.2010.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid D, Armstrong JD, Metcalfe NB. The performance advantage of a high resting metabolic rate in juvenile salmon is habitat dependent. Journal of Animal Ecology. 2012;81:868–875. doi: 10.1111/j.1365-2656.2012.01969.x. [DOI] [PubMed] [Google Scholar]
- Ronald KL, Fernandez-Juricic E, Lucas JR. Taking the sensory approach: how individual differences in sensory perception can influence mate choice. Animal Behaviour. 2012;84:1283–1294. [Google Scholar]
- Schmitz OJ, Křivan V, Ovadia O. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecology Letters. 2004;7:153–163. [Google Scholar]
- Schreiber SJ, Burger R, Bolnick DI. The community effects of phenotypic and genetic variation within a predator population. Ecology. 2011;92:1582–1593. doi: 10.1890/10-2071.1. [DOI] [PubMed] [Google Scholar]
- Sih A. The behavioral-response race between predator and prey. American Naturalist. 1984;123:143–150. [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology and Evolution. 2004a;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sih A. Reply to Neff and Sherman: behavioral syndromes versus Darwinian algorithms. Trends in Ecology and Evolution. 2004b;19:622–623. [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE. Behavioral syndromes: an integrative overview. Quarterly Review of Biology. 2004c;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- Sih A, Cote J, Evans M, Fogarty S, Pruitt J. Ecological implications of behavioural syndromes. Ecology Letters. 2012;15:278–289. doi: 10.1111/j.1461-0248.2011.01731.x. [DOI] [PubMed] [Google Scholar]
- Sih A, Kats LB, Maurer EF. Behavioural correlations across situations and the evolution of antipredator behaviour in a sunfish-salamander system. Animal Behaviour. 2003;65:29–44. [Google Scholar]
- Smith BR, Blumstein DT. Behavioral types as predictors of survival in Trinidadian guppies (Poecilia reticulata) Behavioral Ecology. 2010;21:919–926. [Google Scholar]
- Timonin ME, Carriere CJ, Dudych AD, Latimer JGW, Unruh ST, Willis CKR. Individual differences in the behavioural responses of meadow voles to an unfamiliar environment are not correlated with variation in resting metabolic rate. Journal of Zoology. 2011;284:198–205. [Google Scholar]
- van Rooij JM, Videler JJ. Estimating oxygen uptake rate from ventilation frequency in the reef fish Sparisoma viride. Marine Ecology Progress Series. 1996;132:31–41. [Google Scholar]
- Werner EE, Peacor SD. A review of trait-mediated indirect interactions in ecological communities. Ecology. 2003;84:1083–1100. [Google Scholar]
- Wolf M, McNamara JM. On the evolution of personalities via frequency-dependent selection. American Naturalist. 2012;179:679–692. doi: 10.1086/665656. [DOI] [PubMed] [Google Scholar]
- Wolf M, Weissing FJ. Animal personalities: consequences for ecology and evolution. Trends in Ecology and Evolution. 2012;27:452–461. doi: 10.1016/j.tree.2012.05.001. [DOI] [PubMed] [Google Scholar]