In a systematic review and meta-analysis, Eric Strunz and colleagues examine whether improvements in water, sanitation, and hygiene (WASH) practices are associated with reduced risk of infections with soil-transmitted helminths.
Please see later in the article for the Editors' Summary
Abstract
Background
Preventive chemotherapy represents a powerful but short-term control strategy for soil-transmitted helminthiasis. Since humans are often re-infected rapidly, long-term solutions require improvements in water, sanitation, and hygiene (WASH). The purpose of this study was to quantitatively summarize the relationship between WASH access or practices and soil-transmitted helminth (STH) infection.
Methods and Findings
We conducted a systematic review and meta-analysis to examine the associations of improved WASH on infection with STH (Ascaris lumbricoides, Trichuris trichiura, hookworm [Ancylostoma duodenale and Necator americanus], and Strongyloides stercoralis). PubMed, Embase, Web of Science, and LILACS were searched from inception to October 28, 2013 with no language restrictions. Studies were eligible for inclusion if they provided an estimate for the effect of WASH access or practices on STH infection. We assessed the quality of published studies with the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach. A total of 94 studies met our eligibility criteria; five were randomized controlled trials, whilst most others were cross-sectional studies. We used random-effects meta-analyses and analyzed only adjusted estimates to help account for heterogeneity and potential confounding respectively.
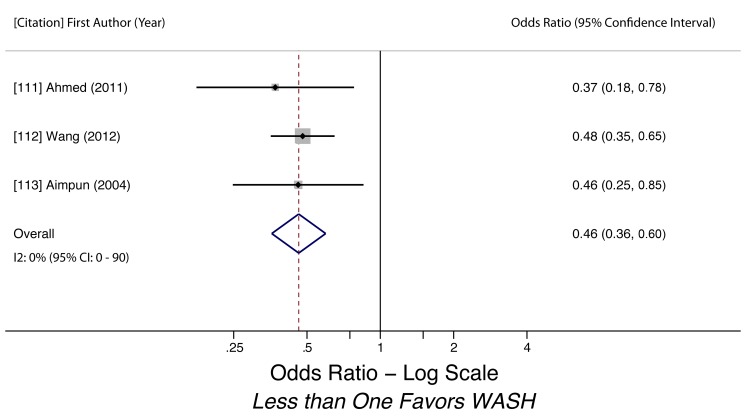
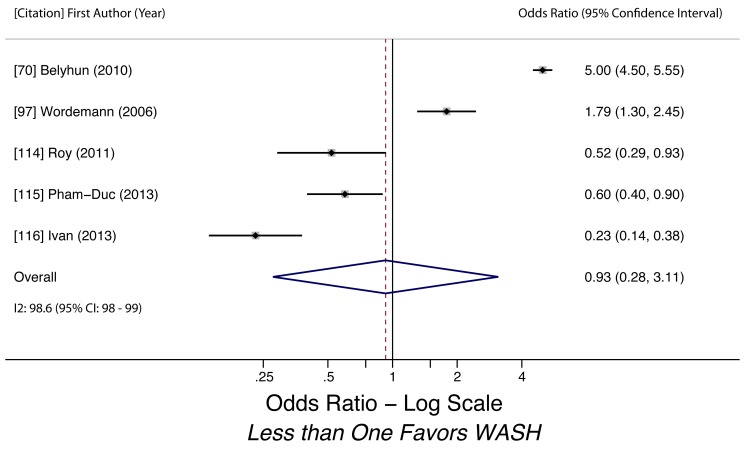
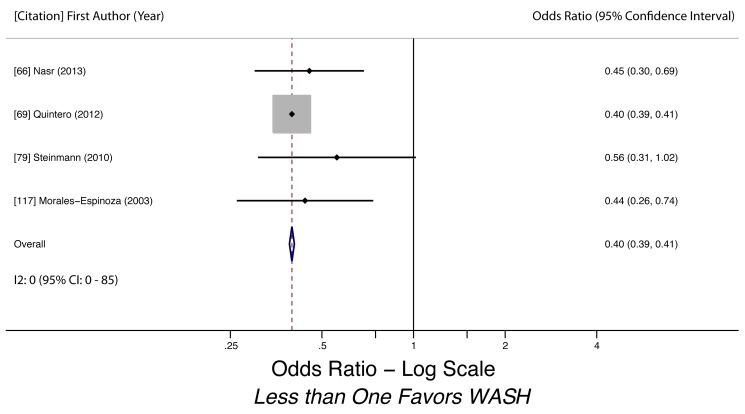
Use of treated water was associated with lower odds of STH infection (odds ratio [OR] 0.46, 95% CI 0.36–0.60). Piped water access was associated with lower odds of A. lumbricoides (OR 0.40, 95% CI 0.39–0.41) and T. trichiura infection (OR 0.57, 95% CI 0.45–0.72), but not any STH infection (OR 0.93, 95% CI 0.28–3.11). Access to sanitation was associated with decreased likelihood of infection with any STH (OR 0.66, 95% CI 0.57–0.76), T. trichiura (OR 0.61, 95% CI 0.50–0.74), and A. lumbricoides (OR 0.62, 95% CI 0.44–0.88), but not with hookworm infection (OR 0.80, 95% CI 0.61–1.06). Wearing shoes was associated with reduced odds of hookworm infection (OR 0.29, 95% CI 0.18–0.47) and infection with any STH (OR 0.30, 95% CI 0.11–0.83). Handwashing, both before eating (OR 0.38, 95% CI 0.26–0.55) and after defecating (OR 0.45, 95% CI 0.35–0.58), was associated with lower odds of A. lumbricoides infection. Soap use or availability was significantly associated with lower infection with any STH (OR 0.53, 95% CI 0.29–0.98), as was handwashing after defecation (OR 0.47, 95% CI 0.24–0.90).
Observational evidence constituted the majority of included literature, which limits any attempt to make causal inferences. Due to underlying heterogeneity across observational studies, the meta-analysis results reflect an average of many potentially distinct effects, not an average of one specific exposure-outcome relationship.
Conclusions
WASH access and practices are generally associated with reduced odds of STH infection. Pooled estimates from all meta-analyses, except for two, indicated at least a 33% reduction in odds of infection associated with individual WASH practices or access. Although most WASH interventions for STH have focused on sanitation, access to water and hygiene also appear to significantly reduce odds of infection. Overall quality of evidence was low due to the preponderance of observational studies, though recent randomized controlled trials have further underscored the benefit of handwashing interventions. Limited use of the Joint Monitoring Program's standardized water and sanitation definitions in the literature restricted efforts to generalize across studies. While further research is warranted to determine the magnitude of benefit from WASH interventions for STH control, these results call for multi-sectoral, integrated intervention packages that are tailored to social-ecological contexts.
Please see later in the article for the Editors' Summary
Editors' Summary
Background
Worldwide, more than a billion people are infected with soil-transmitted helminths (STHs), parasitic worms that live in the human intestine (gut). These intestinal worms, including roundworm, hookworm, and whipworm, mainly occur in tropical and subtropical regions and are most common in developing countries, where personal hygiene is poor, there is insufficient access to clean water, and sanitation (disposal of human feces and urine) is inadequate or absent. STHs colonize the human intestine and their eggs are shed in feces and enter the soil. Humans ingest the eggs, either by touching contaminated ground or eating unwashed fruit and vegetables grown in such soil. Hookworm may enter the body by burrowing through the skin, most commonly when bare-footed individuals walk on infected soil. Repeated infection with STHs leads to a heavy parasite infestation of the gut, causing chronic diarrhea, intestinal bleeding, and abdominal pain. In addition the parasites compete with their human host for nutrients, leading to malnutrition, anemia, and, in heavily infected children, stunting of physical growth and slowing of mental development.
Why Was This Study Done?
While STH infections can be treated in the short-term with deworming medication, rapid re-infection is common, therefore a more comprehensive program of improved water, sanitation, and hygiene (WASH) is needed. WASH strategies include improvements in water access (e.g., water quality, water quantity, and distance to water), sanitation access (e.g., access to improved latrines, latrine maintenance, and fecal sludge management), and hygiene practices (e.g., handwashing before eating and/or after defecation, water treatment, soap use, wearing shoes, and water storage practices). WASH strategies have been shown to be effective for reducing rates of diarrhea and other neglected tropical diseases, such as trachoma; however, there is limited evidence linking specific WASH access or practices to STH infection rates. In this systematic review and meta-analysis, the researchers investigate whether WASH access or practices lower the risk of STH infections. A systematic review uses predefined criteria to identify all the research on a given topic; a meta-analysis is a statistical method that combines the results of several studies.
What Did the Researchers Do and Find?
The researchers identified 94 studies that included measurements of the relationship between WASH access and practices with one or more types of STHs. Meta-analyses of the data from 35 of these studies indicated that overall people with access to WASH strategies or practices were about half as likely to be infected with any STH. Specifically, a lower odds of infection with any STH was observed for those people who use treated water (odd ratio [OR] of 0.46), have access to sanitation (OR of 0.66), wear shoes (OR of 0.30), and use soap or have soap availability (OR of 0.53) compared to those without access to these practices or strategies. In addition, infection with roundworm was less than half as likely in those who practiced handwashing both before eating and after defecating than those who did not practice handwashing (OR of 0.38 and 0.45, respectively).
What Do These Findings Mean?
The studies included in this systematic review and meta-analysis have several shortcomings. For example, most were cross-sectional surveys—studies that examined the effect of WASH strategies on STH infections in a population at a single time point. Given this study design, people with access to WASH strategies may have shared other characteristics that were actually responsible for the observed reductions in the risk of STH infections. Consequently, the overall quality of the included studies was low and there was some evidence for publication bias (studies showing a positive association are more likely to be published than those that do not). Nevertheless, these findings confirm that WASH access and practices provide an effective control measure for STH. Controlling STHs in developing countries would have a huge positive impact on the physical and mental health of the population, especially children, therefore there should be more emphasis on expanding access to WASH as part of development guidelines and targets, in addition to short-term preventative chemotherapy currently used.
Additional Information
Please access these websites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.1001620.
The US Centers for Disease Control and Prevention also provides detailed information on roundworm, whipworm, and hookworm infections
The World Health Organization provides information on soil-transmitted helminths, including a description of the current control strategy
Children Without Worms (CWW) partners with Johnson & Johnson, GlaxoSmithKline, the World Health Organization, national ministries of health and education, non-governmental organizations, and others to promote treatment and prevention of soil-transmitted helminthiasis. CWW advocates a four-pronged, comprehensive control strategy—Water, Sanitation, Hygiene Education, and Deworming (WASHED) to break the cycle of reinfection
The Global Network for Neglected Tropical Diseases, an advocacy initiative dedicated to raising the awareness, political will, and funding necessary to control and eliminate the most common neglected tropical diseases, provides information on infections with roundworm (ascariasis), whipworm (trichuriasis), and hookworm
WASH for the Neglected Tropical Diseases is a repository of information on WASH and the neglected tropical diseases (NTDs) such as soil-transmitted helminthiasis, and features a resource titled “WASH and the NTDs: A Manual for WASH Implementers.”
Two international programs promoting water sanitation are the World Health Organization Water Sanitation and Health program and the World Health Organization/United Nations Childrens Fund Joint Monitoring Programme for Water Supply and Sanitation
Introduction
More than a billion people are infected with soil-transmitted helminths (STHs) and many more live in high risk areas [1]. The global burden of STH infection is estimated at between 5 and 39 million disability-adjusted life years, largely attributable to anemia, stunting, and reduced cognitive development [2]–[4]. Humans are infected after ingesting eggs (A. lumbricoides and T. trichiura) or through penetration of the skin by infective larvae in the soil (hookworm [A. duodenale and N. americanus] and S. stercoralis) [1]. Current control strategies have focused on preventive chemotherapy through mass drug administration (MDA), in which at-risk populations are treated once or twice per year with benzimidazoles, primarily albendazole (usually given as a single oral dose of 400 mg) or mebendazole (500 mg) [5]. While preventive chemotherapy can greatly reduce morbidity from helminth infection, reinfection typically occurs rapidly after treatment [6].
Long-term STH control and eventual elimination require improvements to water, sanitation, and hygiene (WASH) access and practices [7]. The history of STH in the United States of America, South Korea, and Japan—where WASH improvements acted in concert with deworming to eliminate STH as a public health problem—supports the need for an integrated control paradigm [8]–[10]. WASH interventions are diverse, potentially including improvements in water access (e.g., water quality, water quantity, and distance to water), sanitation access (e.g., access to improved latrines, latrine maintenance, and fecal sludge management), and hygiene practices (e.g., handwashing before eating and/or after defecation, water treatment, soap use, wearing shoes, and water storage practices) [11]–[20]. Interventions often include multiple components, e.g., building ventilated-improved pit latrines while also providing hygiene education. Work in the WASH sector is often motivated by the view that access to clean water and adequate sanitation is a human right, but health outcomes are also broadly considered, with diarrheal disease burden representing a common measure of impact [21]–[23].
The successful integration of WASH into a disease control program has already been demonstrated for trachoma, which—like STH—is also considered a neglected tropical disease (NTD). The World Health Organization (WHO) endorses the “SAFE” strategy for trachoma control: surgery to correct advanced stages of trachoma, antibiotics to treat active infection, facial cleanliness to reduce disease transmission, and environmental change (including increased access to water and improved sanitation) [24]. The SAFE strategy explicitly calls for the implementation of improved access to, and use of, water, sanitation, and hygiene through improvements in delivery and/or specific interventions.
Such a fully integrated strategy—including guidelines and targets—does not yet exist for STH control, in part because evidence examining the relationship between WASH and STH is limited. A seminal review by Esrey and colleagues found few investigations that evaluated the association between WASH and STH infection [25]. A recent systematic review and meta-analysis by Ziegelbauer and colleagues found that individuals who have access to and use of sanitation facilities were at lower odds of infection with STH compared to individuals without sanitation [26]. Additional empirical evidence that links WASH improvements to reductions in STH infection is scarce, and an improved evidence-base may lead to better coordination between the NTD and WASH sectors [27],[28].
To fill this gap, we conducted a systematic review and set of meta-analyses to examine evidence of association between STH infection and WASH. We expanded the study's focus to include up-to-date meta-analyses for water and hygiene components, in addition to sanitation. We only used adjusted effect estimates in meta-analyses to help account for potential confounding and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews. Our use of the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach for quality assessment also provides a comprehensive accounting of the limitations of available evidence. We hypothesized that improvements in WASH would be associated with reductions in odds of STH infection. Thus, the purpose of this study was to quantitatively summarize the relationship between WASH access or practices on STH infection, while also synthesizing available data that did not qualify for meta-analysis.
Methods
Search Strategy, Inclusion Criteria, and Data Extraction
Our review adheres to the PRISMA and Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines (see Texts S1 and S2) [29]–[31]. The methods protocol is available in Text S3. A study investigator (ECS) and two research assistants (Rachel Stelmach [RS] and Claire Still [CS]) systematically searched PubMed, Embase, Web of Science, and LILACS for relevant articles from inception to October 28, 2013. We also indexed relevant studies from the bibliography of reviews by Ziegelbauer and colleagues [26] and Asaolu and Ofoezie [32]. Abstracts without published articles were considered eligible for inclusion. Additionally, we requested available unpublished research from the US Centers for Disease Control and Prevention, The Carter Center, The Task Force for Global Health, the WHO regional offices, and the authors' personal collections.
The native search engines within PubMed, Embase, Web of Knowledge, and LILACS were used to search each respective database using Boolean operators. The search included two clusters of terms: one for STH (i.e., helminth, soil-transmitted helminth, geohelminth, ascaris, lumbricoides, trichuris, trichiura, hookworm, ancylostoma, duodenale, necator, americanus, strongyloid*, stercoralis) and one for WASH (i.e., sanitation, sanitary engineering, water supply, waste management, environment*, excre*, faec*, fecal, feces, hand washing, handwashing, hygiene, latrine*, toilet*, water, soap). Results had to contain at least one term from both clusters. “Extensive search” was enabled when searching with Embase. Because Embase only allowed for exporting up to 5,000 records, results were stratified by date in order to screen and export all results in smaller segments. All search records were exported to bibliographic files and imported into Endnote X5 (Thomson Reuters), which was used to manage and screen search results. Titles, and when available, abstracts were scanned by an investigator (ECS) and also independently by research assistants (RS and CS) to determine possible relevance. Final selection was based on the full text of all potentially applicable articles. Ambiguous articles were examined by a senior reviewer (MCF).
Publications in all languages were considered. Studies in English, Spanish, Portuguese, and French were screened by investigators directly. Chinese-language articles were reviewed by a study collaborator (Shuyuan Huang [SH]) who assessed eligibility and extracted relevant data for the research team. Relevant data from all eligible studies was abstracted by a reviewer (ECS) and independently by assistants (RS and CS). Extracted data included study design, setting, year, population characteristics, WASH components measured, diagnostic approach, STH species, and relevant effect measures. Odds ratios (ORs) served as the primary effect measure in the reviewed literature. We collected both crude and adjusted estimates if available. Excel 2007 (Microsoft) was used to input and manage data using a long format to accommodate multiple effect estimates per study.
An article was eligible for inclusion if it presented a measure of effect between WASH and STH (e.g., an OR). For studies that pooled multiple intestinal parasites (e.g., Giardia intestinalis and STH) into one outcome measure, we contacted authors to request disaggregated data. We did not exclude studies based on methodology or population characteristics. Studies that evaluated multiple WASH components were included, as long as the components could be assessed separately from deworming medications and other non-WASH interventions.
There are few standard definitions for WASH access and practices, and it is difficult to measure WASH behaviors objectively [33]. We were unable to consistently connect water and sanitation variables reported in retrieved studies to the WHO and UNICEF Joint Monitoring Program's water and sanitation ladder definitions [34],[35]. For this review, “treated water” is defined as the use of any chemical or physical treatment of water to change its potability, whether conducted at the source or at the point of use. Two specific forms of treatment included boiling and filtering water at home. “Piped water” describes access to, or use of, water collected from a piped infrastructure, regardless of where the water is accessed (public/private) or how well maintained the infrastructure may be. “Sanitation access” was our primary sanitation exposure, defined as access to, or use of, any latrine. We did not exclude studies that lacked information about latrine quality, so access to sanitation could refer to anything from simple pit latrines to flush toilets. For hygiene, “washing after defecation” refers to the availability of handwashing resources (e.g., a wash basin) near sanitation facilities or reported handwashing behavior after defecation. “Soap use or availability” could refer to washing with water alone or no washing as the comparison group. Further, these definitions do not incorporate any criteria for compliance or consistency, since such details were rare in retrieved literature.
Statistical Methods
We conducted meta-analyses for groups of effect estimates that related similar WASH access or practices (e.g., latrine availability and/or use became “sanitation access”) to a common outcome. Potential outcomes included infection with a specific STH (i.e., A. lumbricoides, T. trichiura, hookworm, and S. stercoralis) or any STH generally. Note that “any STH” reflected infection with an individual species or co-infection with multiple species when authors reported aggregated STH infection results. Meta-analyses were performed for groups of independent effect estimates that numbered three or greater and shared a similar exposure and infection outcome. A study that measured several WASH components could contribute to multiple meta-analyses, but could only supply one effect estimate for any single meta-analysis.
We employed random-effects models to account for the expected heterogeneity between studies [36]. Only adjusted estimates were utilized to limit the impact of confounding on pooled effect measures [37]. When necessary, we inverted estimates to reflect the effect of WASH, rather than the absence of WASH. This inversion was necessary in order to ensure enough study estimates were available for meta-analysis, but could have resulted in additional heterogeneity. For example, the inverse of “no sanitation access” may be similar to, but distinct from, “sanitation access” when assessed by questionnaire due to bias associated with socially desirable responses. Further, the presence of WASH access or practices may not necessarily be the same as the inverse effect of their absence, especially if important confounders or effect modifiers remain unexplored. Estimates of effect not included in meta-analyses were summarized in the text. The meta-analysis package MAIS for Stata version 12 (StataCorp) was used to perform the random-effects meta-analyses with the DerSimonian and Laird method [38]. The natural log of reported ORs was the dependent variable. CIs use the 95% level unless otherwise noted.
Bias Assessment and Evidence Quality
We used the GRADE framework to assess potential sources of bias within studies and determine overall strength of evidence for each meta-analysis [39]. The GRADE approach is used to contextualize or justify intervention recommendations with four levels of evidence quality, ranging from very low to high. These levels correspond to how likely it would be for further research to alter conclusions drawn from the current evidence. “High quality” suggests that it is very unlikely for conclusions about effect estimates to change, whereas “very low quality” suggests that any estimate of effect is highly uncertain [40]. We formed our key bias categories from the literature, GRADE recommendations [41], and two instruments highlighted by the Cochrane Collaboration [42]: the Downs and Black tool [43] and the Newcastle-Ottawa scale [44]. We focused on five potential sources of bias in our assessment of individual studies: (i) diagnostic approach for assessing STH infection; (ii) exposure assessment; (iii) confounding assessment; (iv) response rate; and (v) selective reporting. Each study received one of three rankings for each source of bias: low risk, unclear risk, or high risk. Detailed criteria for these categories are available in Table 1. Bias was assessed independently by ECS and one of the two research assistants (RS and CS), compared, and reviewed by a senior assessor (DGA or MCF) if necessary.
Table 1. Criteria for study bias assessment.
| Criteria | Description |
| Infection diagnostics | Is a diagnostic assay clearly mentioned? Is there any form of quality control in the diagnostic process (e.g., a senior technician doing spot-checks)? |
| Exposure assessment | Was exposure assessment (e.g., access to clean water, washing hands) ascertained via a self-reported survey response (unreliable) or observed directly by investigators (more reliable)? Is there any attempt to gauge proper use of water, hygiene, or some form of “quality control” for the exposures? |
| Confounding assessment | Are only crude estimates computed? Has matching and/or multiple logistic regression been undertaken to control for important potential confounders? |
| Response rate | Is the response rate (or loss-to-follow-up) similar for infected versus non-infected individuals? |
| Selective reporting | Is there evidence of selective reporting within an article (e.g., outlining certain variables of interest in the methods but not providing any data on them in the results)? |
We assessed the overall quality of evidence for each meta-analysis after considering seven key characteristics. Each meta-analysis could receive a quality grade of very low, low, moderate, or high [45]. Meta-analyses of observational studies were classified as “low” by default, but could be downgraded (because of imprecision, indirectness, inconsistency, publication bias, and potential confounding) or upgraded (because of magnitude of effect, dose-response relationship, and potential confounding) on the basis of the overall strength of the evidence.
Inconsistency (i.e., heterogeneity) was assessed with Moran's I 2 and Cochran's Q-test [46]. I 2 provides an estimate of the proportion of variability in a meta-analysis that is explained by differences between the included studies instead of sampling error [47]. If a study exhibited an I 2 value over 50%, there was potential cause for concern, and the Q-test was also checked for a p-value less than 0.10. Values for I 2 over 70% or Q-test p-values lower than 0.05 resulted in the automatic downgrading of a body of evidence.
Publication bias was assessed through a visual inspection of funnel plots, though Egger's test also informed our interpretation [48]. Evidence quality was downgraded due to “imprecision” if the pooled effect estimate's 95% CI overlapped with the null (i.e., statistical significance at the 0.05 level). Although we provide CIs for pooled point estimates, imprecision remains a valuable criterion since not all consumers of reviews understand the importance of CIs and statistical uncertainty.
Evidence quality was upgraded owing to large magnitude of effect if the meta-analysis yielded a pooled OR less than 0.33 or greater than 3.0 [41]. Traditionally, risk ratios (RRs) are considered to show a large magnitude if they are less than 0.5 or greater than 2.0. However, ORs overstate the effect size compared to RRs, especially when initial risk (i.e., the prevalence of the outcome of interest) is high [49]. Because STH infection is relatively common, a more conservative threshold was needed for ORs in order to qualify as a large magnitude of effect.
Evidence quality could also be upgraded or downgraded on the basis of any unaccounted sources of potential confounding that would likely have a predictable direction on the effect estimate. For example, hygiene behaviors are typically over-reported in surveys, which could reduce the measured strength of effect for hygiene practices since the exposure group includes those who did not practice hygiene [50]–[52].
Due to the breadth of the review, indirectness was not a common concern, but would be more important for future reviews that focus on specific populations, settings, or interventions. Dose-response relationships were assessed by examining studies where exposures were discretized into ranked categories, e.g., analyzing “always washes hands” versus both “sometimes” and “never.” A dose-response relationship was considered possible if the point estimates improved between the ordinal categories, especially if relevant CIs did not overlap. Additional details about the meta-analysis GRADE criteria are available in Table 2.
Table 2. Criteria for meta-analysis GRADE assessment.
| Criteria | Description |
| Imprecision | Caused the evidence quality to be downgraded if the pooled effect estimate's 95% CI overlapped with the null (i.e., one for odds ratios). In this context, imprecision is synonymous with a pooled estimate being statistically non-significant at the 0.05 level. Imprecision is used to downgrade evidence quality because some consumers of reviews (e.g., policymakers and practitioners) often do not fully understand statistical uncertainty. |
| Indirectness | Did not cause any evidence quality to be downgraded. Our review had a broad scope that aimed to collect a wide array of evidence exploring different populations and contexts. Traditionally, indirectness refers to issues that may limit the generalizability of evidence's reported results to the review's specified research question. This could be caused by differences in study population, study design, co-interventions, etc. |
| Inconsistency (i.e., heterogeneity) | Assessed with Moran's I 2 and Cochran's Q-test [46]. If a study exhibited an I 2 value over 50%, there was potential cause for concern, and the Q-test was also checked for a p-value less than 0.10. Values for I 2 over 70% or Q-test p-values lower than 0.05 resulted in the downgrading of a body of evidence. |
| Publication bias | Assessed through a visual inspection of funnel plots, though Egger's test also informed our interpretation [48]. Detecting publication bias is difficult when dealing with dichotomous outcomes, especially when there is significant between-study heterogeneity. In such circumstances, the popular Egger's test is usually inappropriate, with the potential to result in many false positives. For this reason, qualitative funnel plot analysis served as our primary assessment tool, though we also computed Egger's statistics to inform our judgment. Tests described by Rücker et al. [135] and Peters et al. [136] were also considered, but not performed. |
| A large magnitude of effect (also called “effect size”) | Could upgrade overall evidence quality if pooled odds ratios were less than 0.33 or greater than 3.0 [41]. The standard criteria for risk ratios and hazard ratios is that effect estimates be less than 0.5 or greater than 2.0. However, since odds ratios will show a greater magnitude than risk ratios, especially when an outcome is common, a more conservative cut-off value is needed. No firm rules have been established in the literature, so we increased the relevant effect size magnitude for odds ratios by 50%. |
| Evidence of a dose-response relationship | Can upgrade evidence quality. Dose-response relationships were assessed by examining studies where exposures were discretized into ranked categories, e.g., analyzing “always washes hands” versus both “sometimes” and “never.” A dose-response relationship was considered possible if the point estimates improved between the ordinal categories, especially if relevant confidence intervals did not overlap. |
| Potential confounding | Can upgrade a body of evidence if there are plausible factors that may be artificially weakening the observed pooled measurement. In the case of hygiene, individuals are known to overreport handwashing behaviors, which would systematically lower any apparent benefits. Potential downgrades are also possible, however, especially if established confounding variables are not taken into account by an analysis. |
Results
Retrieved Studies
The search yielded a total of 47,589 articles from PubMed (n = 21,718), Embase (n = 18,188), Web of Knowledge (n = 7,502), and LILACS (n = 181), with 42,882 unique records. Our PRISMA flow diagram is available in Figure 1. After reviewing titles and abstracts, we examined 397 articles more intensively: 264 were excluded for lacking a relevant effect measure, 30 were excluded for aggregating non-STH infections in the outcome, and 11 were excluded for being review or editorial articles (see Tables 3–5 for included studies and S1 for excluded ones). We contacted 11 authors to obtain additional data [53]–[60], but only three authors responded [61]–[63]. A total of 94 studies ultimately met our inclusion criteria, yielding over 450 estimates of effect. Retrieved data included findings from one unpublished investigation [64] and one publication with information about two related studies [65].
Figure 1. PRISMA flow diagram.
Table 3. List of included studies with authors A–F.
| Author [cite ID], Year - Country | Title of Article | Setting and Population | Sample Size | Diagnosis Method | Exposure Assessment and Study Method | Main WASH Components | Adjustment or Controlled Variables |
| Ahmed [111], 2011 - Malaysiaa | The burden of moderate-to-heavy soil-transmitted helminth infections among rural malaysian aborigines: an urgent need for an integrated control programme | Satak, Raub district, Pahang-Sekolah Kebangsaan Satak school; Aboriginal schoolchildren, 6–13 years old | 254 | Kato-Katz and Harada Mori | Questionnaire, cross-sectional | Toilet, water source, playing in soil | Source of drinking water, toilet in house, domestic animals in the house, age, playing barefoot in soil |
| Aimpun [113], 2004 - Belizea | Survey for intestinal parasites in Belize, Central America | 5 villages in the Toledo district; all ages, Ketchi and Mopan ethnic groups | 533 | Formalin-ethyl-acetate concentration technique | Questionnaire, cross-sectional | Handwashing, shoes, water, latrine | Race, occupation, years of education, population density, presence of trash pit near home, drinking water source, water treatment, and ownership of electrical appliances |
| Alemu [137], 2011 - Ethiopia | Soil transmitted helminths and schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia | Elementary school children from Zarima town in NW Ethiopia | 319 | Kato-Katz | Questionnaire, observation, cross-sectional | Handwashing, shoe wearing, presence of latrine, latrine usage, water source | No adjusted WASH effect estimates identified |
| Al-Mekhlafi [138], 2007 - Malaysia | An unceasing problem: soil-transmitted helminthiases in rural malaysian communities | 18 villages around Pos Betau School, Kuala Lipis; Primary schoolchildren (7–12) of Pos Betau School, Kuala Lipis, Pahang, Malaysia. | 277 | Kato-Katz and Harada Mori | Questionnaire, cross-sectional | Latrine availability, water access | No adjusted WASH effect estimates identified |
| Al-Mekhlafi [139], 2008 - Malaysia | Pattern and predictors of soil-transmitted helminth reinfection among aboriginal schoolchildren in rural Peninsular Malaysia | Pos Betau, Kuala Lipis, Pahang; Orang Asli (aborigine) primary schoolchildren, age 7–12 | 120 | Modified cellophane thick smear and Harada Mori | Questionnaire, longitudinal | Toilet, water source | No adjusted WASH effect estimates identified |
| Alvarado [85], 2006 - Colombia | Social determinants, feeding practices and nutritional consequences of intestinal parasitism in children 7–18 months old in Guapi, Cauca | Guapi, Cauca; children 7–18 months old | 136 | Direct examination and concentrate Ritchie-Frick modified | Questionnaire, cross-sectional | Latrine type, floor type | No adjusted WASH effect estimates identified |
| Amahmid [140], 2005 - Morocco | Assessment of the health hazards associated with wastewater reuse: transmission of geohelminthic infections (Marrakech, Morocco) | Children (2–14 years) near Marrakech, Morocco | 610 | Formol-ether concentration | Questionnaire, observation, cross-sectional | Source of water | No adjusted WASH effect estimates identified |
| Asaolu [123], 2002 - Nigeriaa | Effect of water supply and sanitation on the prevalence and intensity of Ascaris lumbricoides among pre-school-age children in Ajebandele and Ifewara, Osun State, Nigeria. | Ajebandele and Ifewara, two peri-urban communities near Ile-Ife, Osun State, Nigeria; children aged 0 to 108 months from mix of different ethnic groups | 516 | Kato-Katz (modified) | Questionnaire, cross-sectional | Latrine type, water source | Final, full model not given. Used stepwise selection in multiple regression. Initial model included: village, water source, latrine type, mothers' age and education, fathers' age and education, and gender/age of the child |
| Awasthi [141], 2008 - India | Prevalence and risk factors associated with worm infestation in pre-school children (6–23 months) in selected blocks of Uttar Pradesh and Jharkhand, India | Preschool children (6–23 months) from Uttar Pradesh and Jharkhand, India | 909 | Formol-ether concentration | Questionnaire, cross-sectional | Drinking water source, toilets in home, washing hands after defecation | No adjusted WASH effect estimates identified |
| Balen [131], 2011 - Chinaa | Risk factors for helminth infections in a rural and a peri-urban setting of the Dongting Lake area, People's Republic of China | Wuyi and Laogang, two administrative villages in the Dongting Lake region of Hunan province; all ages from Wuyi, a rural village | 1,298 | Kato-Katz | Questionnaire, cross-sectional | Handwashing, water source | Village, occupation, socio-economic status, soil contact, animal ownership, washing hands w/soap before eating/after defecating |
| Barreto [142], 2010 - Brazil | Impact of a citywide sanitation program in Northeast Brazil on intestinal parasites infection in young children | Children (0–36 months) from Salvador, Brazil | 1,920 | Kato-Katz | Questionnaire, observation, cross-sectional | Regularity of water supply, hygiene behavior, indoor toilet, household excreta disposal | Different variables depending on model, but could include: drainage type, regularity of water supply, absence of rubbish dumps, paved road/sidewalk, hygiene behavior, indoor toilet, open sewage nearby, household excreta disposal, coverage with program sewerage connections |
| Basualdo [143], 2007 - Argentina | Intestinal parasitoses and environmental factors in a rural population of Argentina, 2002–2003 | Children (<15 years) and adults (≥15 years) from Buenos Aires, Argentina | 504 | Telemann | Survey, cross-sectional | Type of floors, water supply, public/private faucet, excrement disposal | Final multivariable model unclear |
| Belo [144], 2005 - São Tomé and Príncipe | Prevalence, behavioural and social factors associated with Schistosoma intercalatum and geohelminth infections in Sao Tome and Principe | Three primary schools in S. Marya, Guadalupe and Kilombo; schoolchildren | 130 | Kato-Katz and Teleman-Lima | Questionnaire, cross-sectional | Excreta location | No adjusted WASH effect estimates identified |
| Belyhun [70], 2010 - Ethiopiaa | Prevalence and risk factors for soil-transmitted helminth infection in mothers and their infants in Butajira, Ethiopia: a population based study | Butajira; infants | 908 | Formol-ether concentration method | Questionnaire, cross-sectional | Soap use, water source | Place of residence, age, domestic animals living together |
| Bieri [76], 2013 - China | Health-Education Package to Prevent Worm Infections in Chinese Schoolchildren | Rural Linxiang City District, Hunan province; children 9–10 years old | 1,718 | Kato-Katz with 10% quality control | Experimental, longitudinal | Handwashing | Clustering, school grade level, sex |
| Carneiro [145], 2002 - Brazil | The risk of Ascaris lumbricoides infection in children as an environmental health indicator to guide preventive activities in Caparao' and Alto Caparao', Brazil | Rural municipalities of Caparao and Alto Caparao, in Minas Gerais, Brazil; Children under 14 years of age | 760 | Kato-Katz | Questionnaire, cross-sectional | Sanitation index, hygiene index, water in washbasin | Crowding, water in washbasin, sanitation index, hygiene index, age, socioeconomic index |
| Chongsuvivatwong [65], 1996 - Thailanda | Predictors for the risk of hookworm infection: experience from endemic villages in southern Thailand | One village; All age groups (over 6 years old) | 245 | Kato-Katz | Questionnaire, observations, cross-sectional | Shoes, latrine availability | Education, income level, location in village, number of houses w/in 20 m, latrine, wearing shoes outside |
| Chongsuvivatwong [65], 1996 - Thailanda | Predictors for the risk of hookworm infection: experience from endemic villages in southern Thailand | Three villages; All age groups (over 6 years old) | 456 | Kato-Katz | Questionnaire, observations, cross-sectional | Shoes, latrine availability | Education, income level, location in village, number of houses w/in 20 m, latrine, wearing shoes outside |
| Corrales [124], 2006 - El Salvadora | Association between intestinal parasitic infections and type of sanitation system in rural El Salvador | Eight rural and semi-urban communities in the states of La Libertad and La Paz, El Salvador; Heads of households | 127 | Evergreen Scientific Fecal Parasite Concentrator kit | Questionnaire, cross-sectional | Latrine type | Household clustering, age, anthelmintic meds in past 3 months, having dirt floor, owning pigs |
| Cundill [67], 2011 - Brazil | Rates and intensity of re-infection with human helminths after treatment and the influence of individual, household, and environmental factors in a Brazilian community | Americaninhas, Minas Gerais State; Individuals aged over 5 years | 642 | Kato-Katz and formalin ether | Questionnaire, longitudinal | Water source, latrine | Parental education level, electricity access |
| Dumba [86], 2013 - Uganda | Design and implementation of participatory hygiene and sanitation transformation (PHAST) as a strategy to control soil-transmitted helminth infections in Luweero, Uganda | Children in 19 villages around Luweero, Uganda | 558 | Kato-Katz | Assignment, questionnaire, experimental | PHAST intervention (participatory hygiene/sanitation transformation) | Multivariable modeling used for one part of study, included maintenance condition of household, level of education |
| Ellis [146], 2007 - China | Familial aggregation of human susceptibility to co- and multiple helminth infections in a population from the Poyang Lake region, China | Five villages in Poyang Lake region, Jiangxi Province; Individuals aged over 5 years | 3,682 | Kato-Katz (duplicate) | Questionnaire, cross-sectional | Water contact | No adjusted WASH effect estimates identified |
| Ensink [147], 2005 - Pakistan | High risk of hookworm infection among wastewater farmers in Pakistan | Males involved in farming with wastewater or regular water or in textile work and their children (2–12 years) in Faisalabad, Pakistan | 1,704 | Formolin-ether concentration | Questionnaire, observation, cross-sectional | Type of water supply, toilet, wearing shoes | Toilet, house construction, type of water supply |
| Farook [148], 2002 - India | Intestinal Helminthic Infestations among Tribal Populations of Kottoor and Achankovil Areas in Kerala (India) | Kottoor and Acbankovil; All age groups | 258 | Formol-ether sedimentation technique | Questionnaire, cross-sectional | Proper handwashing | No adjusted WASH effect estimates identified |
| Ferreira [149], 2000 - Brazil | Secular trends in child intestinal parasitic diseases in S. Paulo city, Brazil (1984–1996) | Sao Paolo households; children (0–5 years old) in Sao Paulo | 1,044 | Sedimentation techniques, unstained and Lugol-stained | Questionnaire, Longitudinal | Improved sanitation | Age, year of survey, and maternal education (or, alternatively, per capita income), housing conditions, access to health services |
| Fonseca [119], 2010 - Brazila | Prevalence and factors associated with geohelminth infections in children living in municipalities with low HDI in North and Northeast Brazil | Ten Brazilian municipalities with low human development indices (HDI); Children | 2,523 | Kato-Katz and Sedimentation | Questionnaire, cross-sectional | Improved water | Maternal education, family income, presence of garbage near home, household crowding, urban/rural, gender (varied depending on worm outcome) |
| Freeman [27], 2013 - Kenya | The impact of a school-based hygiene, water quality, and sanitation intervention on soil-transmitted helminth reinfection: a cluster-randomized trial | 40 government primary schools in Nyanza Province; school-age children, 7–13 years old | 3,120 | Kato-Katz w/quality control | Experimental, longitudinal | Integrated WASH intervention | Clustering, baseline infection |
Studies contributed to a meta-analysis.
Table 5. List of included studies with authors N–Z.
| Author [cite ID], Year - Country | Title of Article | Setting and Population | Sample Size | Diagnosis Method | Exposure Assessment and Study Method | Main WASH Components | Adjustment or Controlled Variables |
| Narain [84], 2000 - Indiaa | Prevalence of Trichuris trichiura in relation to socio-economic and behavioral determinants of exposure to infection in rural Assam | Dibrugarh district in upper Assam; adults and children aged <15 years | 580 | Formol-ether concentration technique | Questionnaire, cross-sectional | Floor material, improved latrine, improved water | Age, open defecation, type of flooring, family size, number of children in household |
| Nasr [66], 2013 - Malaysiaa | Towards an effective control programme of soil-transmitted helminth infections among Orang Asli in rural Malaysia. Part 1: Prevalence and associated key factors | 13 villages in Lipis district, Pahang; Orang Asli children aged ≤15 years | 484 | Formalin-ether sedimentation, Kato Katz, and Harada Mori | Questionnaire, cross-sectional | Handwashing, water, sanitation | Age, family size, other WASH practices |
| Nguyen [165], 2006 - Vietnam | Intestinal helminth infections among reproductive age women in Vietnam: prevalence, co-infection and risk factors | 53 provinces; reproductive-age women | 5,127 | Kato-Katz | Questionnaire, cross-sectional | Latrine, manure fertilizer use | Adjusted for infection with A. lumbricoides, T. trichiura, and interaction term between them. |
| Nishiura [81], 2002 - Pakistan | Ascaris lumbricoides among children in rural communities in the Northern Area, Pakistan: prevalence, intensity, and associated socio-cultural and behavioral risk factors | Five rural villages in the northern area of Pakistan; school children | 492 | Kato-Katz | Questionnaire, cross-sectional | Washing hands, latrine, eating soil, soap | Age, sex, living with child under age of 5, other WASH practices |
| Norhayati [166], 1999 - Malaysia | Some risk factors of Ascaris and Trichuris infection in Malaysian aborigine (Orang Asli) children | Children ages 1–13 | 205 | Kato-Katz and Harada Mori | Questionnaire, cross-sectional | Usage of well-water, usage of toilets | No adjusted WASH effect estimates identified |
| Nwaneri [83], 2012 - Nigeria | Intestinal helminthiasis in children with chronic neurological disorders in Benin City, Nigeria: intensity and behavioral risk factors | Benin City child neurology clinic; Children with chronic neurological disorders | 155 | Kato-Katz | Questionnaire, case-control with matching on age/sex | Hygiene practices | Age, sex |
| Olsen [167], 2001 - Kenya | A study of risk factors for intestinal helminth infections using epidemiological and anthropological approaches | Villages in Kisumu District, Nyanza Province, Kenya; All inhabitants over the age of 4 years | 333 | Kato-Katz (duplicate) | Questionnaire, cross-sectional | Latrine, soap | Adjusted for crowding in households, children under five years of age, soap use, latrine presence. |
| Ortiz Valencia [168], 2005 - Brazil | Spatial ascariasis risk estimation using socioeconomic variables. | Children ages 1–9 | 1,550 | Unclear | Interview, cross-sectional | Water filtration | No adjusted WASH effect estimates identified |
| Parajuli [129], 2009 - Nepala | Behavioral and Nutritional Factors and Geohelminth Infection Among Two Ethnic Groups in the Terai Region, Nepal | Parsauni village in the Sakhawaparsauni Village Development Committee (VDC) of Parsa district, Nepal; Mushar and Tharu (ethnic groups) inhabitants, aged 20–60 years | 95 | Direct wetmount Lugol's iodine thin-smear method | Questionnaire, cross-sectional | Soap, walking barefoot | Adjusts for age, ethnicity, gender, height. |
| Pham-Duc [115], 2013 - Vietnama | Ascaris lumbricoides and Trichuris trichiura infections associated with wastewater and human excreta use in agriculture in Vietnam | Nhat Tan and Hoang Tay communes in Kim Bang district, Hanam province; Individuals over 1 year old | 1,425 | Kato-Katz thick smear and formalin-ether concentration techniques | Questionnaire, cross-sectional | Water, sanitation, handwashing | Age, sex, and season. |
| Phiri [134], 2000 - Malawia | Urban/rural differences in prevalence and risk factors for intestinal helminth infection in southern Malawi | Two sites in the Blantyre area of Malawi: Ndirande a densely populated, poor, urban township in Blantyre city; and Namitambo, a poor rural community in Chiradzulu district; children between the age of 3–14 years | 273 | Stoll's egg count technique | Questionnaire, cross-sectional | Sewage, walking barefoot | Age, sex, mother's education, school attendance, sewage around house |
| Quintero [69], 2012 - Venezuela | Household social determinants of ascariasis and trichuriasis in North Central Venezuela | 55 municipalities of the North Central Venezuela states Aragua, Carabobo, Miranda, Vargas and Capital District; Children and adults (3 months–60 years old) | 3,388; ∼4.7 million with weights | Kato-Katz | Questionnaire, cross-sectional | Improved water, soil floor, sewage disposal | Rural/urban, house vulnerability, waste disposal practices |
| Riess [169], 2013 - Tanzania | Hookworm Infection and Environmental Factors in Mbeya Region, Tanzania: A Cross-Sectional, Population-Based Study | Participants from nine different sites in Mbeya region, south-western Tanzania | 6,375 | Kato-Katz | Questionnaire | Latrine coverage, latrine type | Age, previous anthelmintic treatment, clustering |
| Rísquez [170], 2010 - Venezuela | Condiciones higiénico-sanitarias como factores de riesgo para las parasitosis intestinales en una comunidad rural venezolana | Students in the Panaquire-Miranda school district | 69 | Formol-ether concentration | Questionnaire | Defecation practices | No adjusted WASH effect estimates identified |
| Roy [114], 2011 - Bangladesha | Patterns and risk factors for helminthiasis in rural children aged under 2 in Bangladesh | 10 villages in Rural Mirzapur; Rural children under 2 years old | 252 | Formalin-ether sedimentation technique | Questionnaire, longitudinal | Improved water, excreta disposal | Adjusted by age, sex, breastfeeding, seasonality, and disposal site of child feces |
| Saathoff [171], 2002 - South Africa | Geophagy and its association with geohelminth infection in rural schoolchildren from northern KwaZulu-Natal, South Africa | Pupils in third grade (average age of 10.7 years) | 1,161 | Kato-Katz | Interview, cross-sectional | Geophagy | Family |
| Schmidlin [126], 2013 - Côte d'Ivoirea | Effects of hygiene and defecation behavior on helminths and intestinal protozoa infections in Taabo, Côte d'Ivoire | People in villages/hamlets in south-central that were small populations and similar pop. structure | 1,894 | Kato-Katz | Questionnaire, interview, cross-sectional | Sanitation behavior, hygiene behavior | Socioeconomic status, age group, and sex |
| Scolari [172], 2000 - Brazil | Prevalence and distribution of soil-transmitted helminth (STH) infections in urban and indigenous schoolchildren in Ortigueira, State of Parana, Brasil: implications for control | School children ages 5–15 | 236 | Kato-Katz | Questionnaires (verified by local field assistant), cross-sectional | Toilet ownership, location of toilet, safe water access | No adjusted WASH effect estimates identified |
| Sherkhonov [173], 2013 - Tajikistan | National intestinal helminth survey among schoolchildren in Tajikistan: Prevalences, risk factors and perceptions | Schools from across country; school children, 7–11 years old | 1,642 | Kato-Katz | Questionnaire, cross-sectional | Water, sanitation, handwashing | Clustering, other final covariates unclear |
| Soares Magalhaes [174], 2011 - Ghana, Mali, and Burkina Faso | Geographical analysis of the role of water supply and sanitation in the risk of helminth infections of children in West Africa | West African children | 18,812 | Kato-Katz | Questionnaire (health survey), cross-sectional | Water source, toilet, floor material | No adjusted WASH effect estimates identified |
| Steenhard [175], 2009 - Guinea-Bissau | Concurrent infections and socioeconomic determinants of geohelminth infection: a community study of schoolchildren in periurban Guinea-Bissau | Poor semirural area (Bandim II and Belem, near Bissau); school children aged 4–12 | 706 | McMaster technique, formol-ether technique | Questionnaire, cross-sectional | Improved water, improved sanitation | No adjusted WASH effect estimates identified |
| Steinmann [79], 2010 - Kyrgyzstana | Rapid appraisal of human intestinal helminth infections among schoolchildren in Osh oblast, Kyrgyzstan | Osh oblast; school children (grades 2 or 3, age: 6–15 years) | 1,262 | Kato-Katz | Questionnaire, cross-sectional | Washing vegetables, water source, toilet use | Age, sex, ethnic group, washing vegetables before eating, clustering |
| Stothard [120], 2008 - Zanzibara | Soiltransmitted helminthiasis among mothers and their preschool children on Unguja Island, Zanzibar with emphasis upon ascariasis | 10 Ungujan villages; mothers and their pre-SAC, 322 mothers, 359 children | 681 | Kato-Katz | Questionnaire, cross-sectional | Latrine access, wearing shoes, playing on ground | Clustering, having infected household member |
| Teixeira [176], 2004 - Brazil | Environmental factors related to intestinal helminth infections in subnormal settled areas, Juiz de Fora, MG | Children (1–5 years old) in the subnormal settlement areas in the municipality of Juiz de Fora, Mina Gerais. | 753 | Hoffmann-Pons-Janer method | Questionnaire | Water quality complaints, feces disposal | Family income, age of child |
| Trang [121], 2007 - Vietnama | Helminth infections among people using wastewater and human excreta in peri-urban agriculture and aquaculture in Hanoi, Vietnam | Yen So commune (population 10,500 at the time of study), a rural area located about 10 km south of central Hanoi; adults of 15–70 years of age engaged in agricultural activities and preschool children (less than 72 months of age) | 807 | Direct smear method | Questionnaire, cross-sectional | Water source, latrine | Age, sex, socioeconomic status, other WASH practices |
| Trang [177], 2006 - Vietnam | Low risk for helminth infection in wastewater-fed rice cultivation in Vietnam | All females and males from 15–94 years old from 2 communes using different irrigation for rice cultivation (wastewater and river water) | 1,139 | Direct smear method | Questionnaire, interview, cross-sectional | Latrine availability, latrine status, handwashing (soap), availability of drinking water | Clustering, age, gender, excreta agricultural use |
| Traub [118], 2004 - Indiaa | The prevalence, intensities and risk factors associated with geohelminth infection in tea-growing communities of Assam, India | Three tea-growing communities in Assam, India; tea-growing communities of rural Assam (no age restrictions) | 328 | Kato-Katz | Questionnaire, cross-sectional | Shoes, water source, latrine use | Socioeconomic status, age, household crowding, level of education, religion, use of footwear when outdoors, defecation practices, pig ownership, water source |
| Ugbomoiko [178], 2009 - Nigeria | Socio-environmental factors and ascariasis infection among school-aged children in Ilobu, Osun State, Nigeria | Small rural village of Ilobu in Irepodu Local Government Area of Osun State, Nigeria; children below 16 years of age | 440 | Kato-Katz | Questionnaire, cross-sectional | Water source, latrine, distance to waste disposal | Sex, age, which parent reside with child, number of playmates <6 or >5 years old, period of residency, and previous treatment status. |
| Walker [179], 2011 - Bangladesh | Individual Predisposition, Household Clustering and Risk Factors for Human Infection with Ascaris lumbricoides: New Epidemiological Insights | Dhaka; households | 2,929 | Ether sedimentation technique | Questionnaire, longitudinal | Shared latrines, shared water sources, floor material | Clustering, age, sex, household socioeconomic status, ethnicity, and household characteristics |
| Wang [112], 2012 - Chinaa | Soil-Transmitted Helminth Infections and Correlated Risk Factors in Preschool and School-Aged Children in Rural Southwest China | 141 impoverished rural areas of Guizhou and Sichuan Provinces in Southwest China; SAC and Pre-sac (3–5-year-old group and an 8–10-year-old group) | 1,707 | Kato-Katz | Questionnaire, cross-sectional | Washing hands, boiling water, latrine type, use of manure fertilizer | STH treatment history, individual characteristics, health and sanitation behaviors, and household characteristics |
| Wordemann [97], 2006 - Cubaa | Prevalence and risk factors of intestinal parasites in Cuban children | San Juan y Martinez and Fomento; Cuban schoolchildren aged 4–14 | 1,320 | Kato-Katz | Questionnaire, cross-sectional | Water source, latrine use | Age, sex, municipality, urban/rural background, and interaction between municipality and urban/rural background |
| Worrell [74], 2013 - Kenya | Water, Sanitation, and Hygiene-Related Risk Factors for Soil-Transmitted Helminth Infection in Urban School- and Pre-School-Aged Children in Kibera, Nairobi | Kibera; pre-school and school-aged children | 676 | Kato-Katz (three stools) | Questionnaire, observations, cross-sectional | Numerous | Age, presence of an infected sibling(s) in the household, household crowding, deworming in the last year, ability to meet water needs, treating water, and soap use |
| Xu [75], 2001 - China | On cleanliness of hands in diminution of Ascaris lumbricoides infection in children | Shaowu, Fujian Province; Children (pupils in preliminary school) | 654 | Kato-Katz | Experimental, longitudinal | Handwashing | No adjusted WASH effect estimates identified |
| Yajima [180], 2009 - Vietnam | High latrine coverage is not reducing the prevalence of soil-transmitted helminthiasis in Hoa Binh province, Vietnam | Residents of Tien Xuan commune, Hoa Binh province, Vietnam | 155 | Kato-Katz | Questionnaire, cross-sectional | Latrine at home | No adjusted WASH effect estimates identified |
| Yori [88], 2006 - Peru | Seroepidemiology of strongyloidiasis in the Peruvian Amazon | Residents of Santo Tomas, Peru | 908 | Direct smear, Baermann, simple sedimentation agar plate, serologic assays (ELISA) | Questionnaire, cross-sectional | Source and storage of drinking water, human waste disposal, wearing of shoes | Age |
| Young [82], 2007 - Tanzaniaa | Association of geophagia with Ascaris, Trichuris and hookworm transmission in Zanzibar, Tanzania | Pemba Island, Zanzibar; pregnant women | 970 | Kato-Katz | Questionnaire, cross-sectional | Geophagy, improved sanitation | Geophagia during current pregnancy, age, urban/rural, number of durable goods, pit toilet in HH, formal education |
Studies contributed to a meta-analysis.
HAZ, height for age Z score; SES, socioeconomic status.
Table 4. List of included studies with authors G–M.
| Author [cite ID], Year - Country | Title of Article | Setting and Population | Sample Size | Diagnosis Method | Exposure Assessment and Study Method | Main WASH Components | Adjustment or Controlled Variables |
| Geissler [150], 1998 - Kenya | Geophagy as a risk factor for geohelminth infections: A longitudinal study of Kenyan primary schoolchildren | Children (standards 5–6) | 200 | Kato-Katz | Questionnaire, verified, prospective cohort | Geophagy, having toilet at home | No adjusted WASH effect estimates identified |
| Glickman [151], 1999 - Guinea | Nematode intestinal parasites of children in rural Guinea, Africa: Prevalence and relationship to geophagia | Children (1–18 years) from rural Guinea, Africa | 286 | Direct smear and centrifugal flotation with sugar solution | Questionnaire, cross-sectional | Source of drinking water, sanitary facilities, geophagia | Age, sex |
| Gunawardena [130], 2004 - Sri Lankaa | Socio-economic and behavioural factors affecting the prevalence of Ascaris infection in a low-country tea plantation in Sri Lanka | Maliboda estate plantation (low country, <275 m above sea level); Tea plant workers, 2–50 years (median = 13 years) | 176 | Kato-Katz | Questionnaire, cross-sectional | Washing hands, boiling water | Full, final model not provided. Used step-wise variable selection in regression. The following variables were entered into the initial model: age, gender, living quarters, educational status and monthly income of each subject, availability of sanitary facilities, water supply source, use of boiled water, handwashing behavior, and cleanliness of each subject's house and immediate environment. |
| Gunawardena [152], 2005 - Sri Lanka | Effects of climatic, socio-economic and behavioural factors on the transmission of hookworm (Necator americanus) on two low-country plantations in Sri Lanka | The “low country” Maliboda and Ayr plantations; 2–74 years old | 477 | Kato-Katz | Questionnaire, observations, Longitudinal | Washing behavior, toilet | Occupation, level of education, toilet availability, usage, location, water source, use of footwear, playing with mud (if child), cleanliness of home environment |
| Gunawardena [153], 2011 - Sri Lanka | Soil-Transmitted Helminth Infections among Plantation Sector Schoolchildren in Sri Lanka: Prevalence after Ten Years of Preventive Chemotherapy | Nuwara Eliya, Badulla, Kegalle, Ratnapura, and Kandy. These five districts are centrally located in the southern half of Sri Lanka; School children (grade 4) | 1,890 | Kato-Katz | Questionnaire, cross-sectional | Better household sanitation, as reflected by a latrine score of 74 or more | Altitude, time since last school sanitary inspection, mother's education, latrine score, gender |
| Guo-Fei [154], 2011 - China | Analysis of influencing factors of Trichuris trichiura infection in demonstration plots of comprehensive control of parasitic diseases | Demonstration plots in multiple regions, including Anhui, Jiangxi, Hunan, Guangxi, Hainan, Sichuan, Guizhou, Yunnan; Unclear | Kato-Katz | Questionnaires, cross-sectional | Numerous | Agricultural activity, consumption of raw vegetables, previous anthelmintic treatment; could also have included sex, age, region, education level | |
| Gyorkos [155], 2011 - Peru | Exploring determinants of hookworm infection in Peruvian schoolchildren using a gender analysis | Primary schools in Belen, Peru; Grade 5 children | 927 | Kato-Katz | Questionnaire, cross-sectional | Shoes, improved water | Dirty fingernails, presence of potable water at home, wearing shoes |
| Gyorkos [77], 2013 - Peru | Impact of Health Education on Soil-Transmitted Helminth Infections in Schoolchildren of the Peruvian Amazon: A Cluster-Randomized Controlled Trial | Grade 5 schoolchildren in Peruvian Amazon | 1,089 | Kato-Katz | Assignment, questionnaire, experimental | Hygiene education intervention | Clustering, age, sex, SES status, presence of running water in the home, baseline values of outcome measures (e.g., baseline STH values, baseline knowledge values), time of year of baseline visit, length of follow-up |
| Habbari [156], 2001 - Morocco | Geohelminthic infections associated with raw wastewater reuse for agricultural purposes in Beni-Mellal, Morocco | Students (7–14) attending primary school in Beni Mallal, Morocco | 1,999 | Formaldehyde-ether | Questionnaire, cross-sectional | Source of water, toilet at home, hand-washing | No adjusted WASH effect estimates identified |
| Hall [72], 1994 - Bangladesh | Strongyloides stercoralis in an urban slum community in Bangladesh: factors independently associated with infection | Urban slum in Dhaka; older than 1 year | 880 | Ether sedimentation technique | Questionnaire, longitudinal | Sanitation, water source, soil | No adjusted WASH effect estimates identified |
| Halpenny [157], 2013 - Panama | Regional, Household and Individual Factors that Influence Soil Transmitted Helminth Reinfection Dynamics in Preschool Children from Rural Indigenous Panama | The comarca Ngabe-Bugle, a semi-autonomous political region; children from 0–48 months of age | 356 | FLOTAC and Kato-Katz | Questionnaire, longitudinal | Sanitation | Clustering, other covariates depended on worm outcome, but could include household density, child HAZ score, maternal education |
| Henry [158], 1988 - St. Lucia | Reinfection with Ascaris lumbricoides after chemotherapy: a comparative study in three villages with varying sanitation | Children (0–36 months) from St. Lucia | 219 | Formol-ether concentration | Questionnaire, observation, prospective cohort | Having piped water, having a water-sealed toilet | No adjusted WASH effect estimates identified |
| Hidayah [122], 1997 - Malaysiaa | Socio-environmental predictors of soil-transmitted helminthiasis in a rural community in Malaysia | Bachok; children | 363 | Formol-ether method | Questionnaire, cross-sectional | Hygiene, indiscriminate defecation | Age, location of household |
| Hohmann [80], 2001 - Lao PDRa | Relationship of intestinal parasites to the environment and to behavioral factors in children in the Bolikhamxay province of Lao PDR | Bolikhamxay province; children aged below 15 years | 709 | Kato-Katz | Questionnaire, cross-sectional | Washing hands | Mountainous region, age, material possessions, cleaning after defecation |
| Huat [159], 2012 - Malaysia | Prevalence and Risk Factors of Intestinal Helminth Infection Among Rural Malay Children | Beris Lalang, a rural Muslim community; children 7–9 years old | 79 | Saline wet mounting technique | Questionnaire, cross-sectional | Eating raw salad | BMI, mother's education level |
| Hughes [73], 2004 - Pacific Islandsa | Environmental influences on helminthiasis and nutritional status among Pacific schoolchildren | 27 primary schools in 13 Pacific Island countries; Primary school children, aged 5–12 years | 1,996 | Kato-Katz | Questionnaire, observations, cross-sectional | Water supply, soap available, sanitation facilities (many covariates) | All estimates age, sex, nutritional status and school/cluster. |
| Humphries [132], 2011 - Ghanaa | Epidemiology of Hookworm Infection in Kintampo North Municipality, Ghana: Patterns of Malaria Coinfection, Anemia, and Albendazole Treatment Failure | Four communities in Kintampo North Municipality: Jato-Akuraa (JA), Cheranda (C), Kawampe (KA), and Gulumpe (GU); study results include only those >15 years old (adults) | 126 | Kato-Katz | Questionnaire, cross-sectional | Latrine use, shoes | Age, gender, and community. |
| Ivan [116], 2013 - Rwandaa | Helminthic infections rates and malaria in HIV-infected pregnant women on anti-retroviral therapy in Rwanda | HIV-positive pregnant women | 980 | Kato-Katz | Questionnaire, cross-sectional | Water source, shoe wearing, washing hands after defecation | ART, employment, handwashing, CD4 count |
| Jiraanankul [133], 2011 - Thailanda | Incidence and Risk Factors of Hookworm Infection in a Rural Community of Central Thailand | Tungsor Hongsa community, Chachoengsao Province, 228 km east of Bangkok, Thailand; all ages | 585 | Kato-Katz, water-ethyl acetate sedimentation technique | Questionnaire, longitudinal | Latrine use, shoes, washing hands | Age, raising cats or buffalo |
| Khieu [87], 2013 - Cambodia | Diagnosis, Treatment and Risk Factors of Strongyloides stercoralis in Schoolchildren in Cambodia | Semi-rural villages in Kandal province; Primary school children | 458 | Kato-Katz, KAP culture, and Baermann technique | Questionnaire, cross-sectional | Sanitation, handwashing, shoes | No adjusted WASH effect estimates identified |
| Knopp [125], 2011 - Zanzibara | From morbidity control to transmission control: time to change tactics against helminths on Unguja Island, Zanzibar | Individuals on the island of Unguja | 2,858 | Kato-Katz, koga agar plate method (KAP), and Baermann technique (BM) | Questionnaire, interview, cross-sectional | Latrine at home, washing hands before eating, washing hands after defecation | Sex, age, and village |
| Kounnavong [68], 2011 - Lao PDR | Soil-transmitted helminth infections and risk factors in preschool children in southern rural Lao People's Democratic Republic | Three rural remote districts of Savannakhet Province in southern Lao PDR; Pre-school children aged 12–59 months | 570 | Kato-Katz | Questionnaire, cross-sectional | Latrine access, improved water access | No adjusted WASH effect estimates identified |
| Koura [160], 2011 - Benin | Prevalence and risk factors for soil-transmitted helminth infection in Beninese women during pregnancy | Pregnant women at two maternity wards | 300 | Kato-Katz | Questionnaire, cross-sectional | Wearing shoes | No adjusted WASH effect estimates identified |
| Lee [161], 2007 - Brunei | Hookworm infections in Singaporean soldiers after jungle training in Brunei Darussalam | Singaporean soldiers returning from jungle training in Brunei Darussalam | 113 | Fecal screens via microscopy | Questionnaire, interview, cross-sectional | Water supply source, crawling on ground/soil, shoe use | No adjusted WASH effect estimates identified |
| Luoba [162], 2005 - Kenya | Earth-eating and reinfection with intestinal helminths among pregnant and lactating women in western Kenya | Pregnant women in Nyanza Province | 824 | Kato-Katz | Interview, prospective cohort (longitudinal intervention) | Geophagy | No adjusted WASH effect estimates identified |
| Mahmud [127], 2013 - Ethiopiaa | Risk factors for intestinal parasitosis, anaemia, and malnutrition among school children in Ethiopia | 12 primary schools; School children aged 6–15 | 600 | Kato-Katz and direct saline wetmount, formalin ethyl concentration technique | Questionnaire, observations, cross-sectional | Latrine, hygiene, water source | Age and sex |
| Matthys [71], 2007 - Côte d'Ivoire | Risk factors for Schistosoma mansoni and hookworm in urban farming communities in western Côte d'Ivoire | Six agricultural zones in the town of Man, western Côte d'Ivoire; Households | 716 | Kato-Katz | Questionnaire, cross-sectional | Water source, latrine use | Clustering, sex, age, education level, socioeconomic status, household crowding |
| Mihrshahi [128], 2009 - Vietnama | The effectiveness of 4 monthly albendazole treatment in the reduction of soil-transmitted helminth infections in women of reproductive age in Viet Nam | Women of reproductive age in Yen Bai province | 366 | Kato-Katz | Questionnaire, cross-sectional | Sanitary latrine system, shoe use | Age, education status, work (inside/outside), number of children, meat consumption, shoe use, latrine type, socio-economic status, and handwashing |
| Moraes [163], 2004 - Brazil | Impact of drainage and sewerage on intestinal nematode infections in poor urban areas in Salvador, Brazil | Nine poor urban areas of the city of Salvador (pop. 2.44 million), capital of Bahia State, in Northeast Brazil; children aged between 5 and 14 years old | 1,893 | Kato-Katz | Questionnaire, cross-sectional | Sanitation | Child's sex, child's age, number of children aged 5–14 years in the household, crowding (number of people per room), years of schooling of the household head, monthly per capita income, religion, animals in the house, and the house floor material |
| Moraes [164], 2007 - Brazil | [Household solid waste bagging and collection and their health implications for children living in outlying urban settlements in Salvador, Bahia State, Brazil]. | Nine peri-urban settlements of the city of Salva-pain, Bahia, Brazil; Children 5–14 years old | 1,893 | Kato-Katz | Questionnaire, longitudinal | Solid waste collection | Age and sex of the child, number of household members, number of persons/room, monthly family income per capita, religion, presence of lavatory, floor of the home, and excreta disposal of sewage |
| Morales-Espinoza [117], 2003 - Mexicoa | Intestinal parasites in children, in highly deprived areas of the border region of Chiapas, Mexico | Chiapas, 32 communities; children under 15 years of age | 1,148 | Faust Method | Questionnaire, cross-sectional | Water source, latrine | Age, overcrowding, living conditions, and educational level |
Studies contributed to a meta-analysis.
Most included studies were published in English (n = 86), though articles in Portuguese (n = 4), Chinese (n = 2), and Spanish (n = 2) were also included. Studies researched populations in Asia (n = 42), Africa (n = 29), and the Americas (n = 23). Studies investigated access and practices relating to water (n = 56), sanitation (n = 79), and hygiene (n = 53) (Figure 2); the most commonly explored were access to sanitation (n = 63), access to water (n = 45), handwashing (n = 30), and wearing shoes (n = 27). Studies reported investigating infection with A. lumbricoides (n = 69), T. trichiura (n = 60), hookworm (n = 63), S. stercoralis (n = 12), and any STH collectively (n = 52). Tables 6 and 7 illustrate the number of articles in which both specific WASH components and helminth infections were investigated.
Figure 2. Retrieved articles by WASH group.
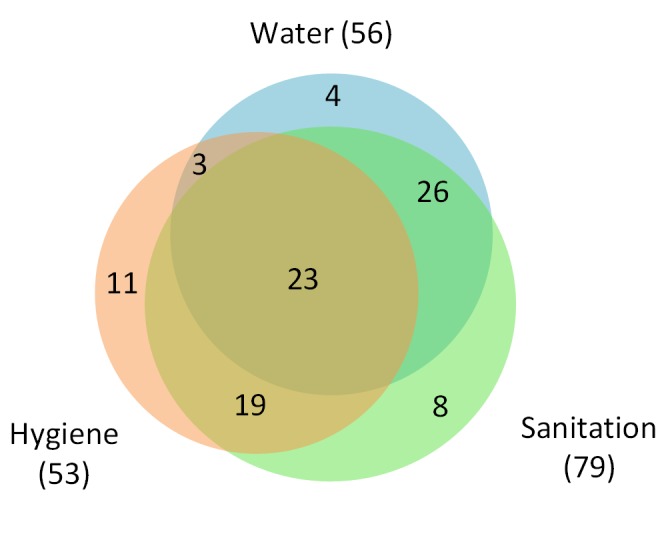
Table 6. Number of studies (n = 94) that investigated STH species and WASH domains.
| Studies | Water | Sanitation | Hygiene | Water and Sanitation | Water and Hygiene | Sanitation and Hygiene | Water, Sanitation, and Hygiene |
| Any STH (grouped) | 34 | 44 | 28 | 32 | 16 | 21 | 15 |
| A. lumbricoides | 43 | 59 | 37 | 38 | 20 | 30 | 18 |
| Hookworm | 34 | 53 | 37 | 28 | 17 | 30 | 14 |
| T. trichiura | 38 | 52 | 34 | 34 | 20 | 28 | 18 |
| S. stercoralis | 10 | 11 | 6 | 9 | 4 | 5 | 3 |
| Total (all studies) | 56 | 79 | 53 | 49 | 26 | 42 | 23 |
Each cell indicates the number of reviewed studies that investigated both an STH species (or any STH) and WASH domains. Higher numbers suggest that certain WASH-STH relationships are more commonly explored in the literature.
Table 7. Number of studies that investigated STH species and WASH access and practices.
| STH Species | Water | Sanitation | Hygiene | ||||||||||
| Water Access | Water Typesa | Treat Water | Sanit. Access | Latrine Typesa | Sharing Latrines | Latrine Maint. | Washing Hands | Soap | Washing Vegetables | Shoe Use | Geophagy | Hygiene Education | |
| Any STH | 30b | 5 | 9b | 34b | 8 | 3 | 2 | 17b | 7b | 2 | 13b | 4 | 4 |
| A. lumbricoides | 33b | 3 | 15 | 45b | 13 | 5 | 2 | 20b | 9 | 2 | 14 | 8 | 4 |
| Hookworm | 28 | 2 | 11 | 44b | 11 | 3 | 2 | 16 | 5 | 1 | 20b | 8 | 2 |
| T. trichiura | 31b | 3 | 12 | 41b | 12 | 3 | 2 | 18 | 7 | 2 | 12 | 7 | 3 |
| S. stercoralis | 8 | 1 | 5 | 11 | 2 | 1 | 0 | 5 | 2 | 0 | 3 | 1 | 0 |
Cells with high numbers but no meta-analysis (no footnote) indicate that effect measures were not reported (selective reporting), reported measures were not statistically adjusted, or that the WASH access and practice was too diverse to be effectively grouped in a meta-analysis (e.g., handwashing can be measured before eating or after defecating).
Water Types and Latrine Types refer to studies that measured multiple sanitation comparisons, not just “latrine versus no latrine.” For example, a study could examine water collected from rivers, wells, or piped connections.
Gray cells indicate that a meta-analysis was conducted for that WASH variable and STH outcome.
Of 94 studies, 89 were observational: 75 used a cross-sectional epidemiologic design, 13 were prospective, and the remaining was a case-control study. Most studies investigated multiple potential risk factors for STH infection. Exposure status for WASH access and practices was typically determined through self-report, although 15 studies also used some form of observation to validate self-reported information. All included studies reported the diagnostic method used to assess helminth infection, with the Kato-Katz technique most frequently mentioned (n = 63). To assess the independent effect of WASH components on STH infection, authors typically used multiple regression analysis (n = 68), though adjusted effect estimates were often not reported for WASH covariates if they were not statistically significant. Not all multivariable models were reported with a full list of included covariates either. Slightly more than one-third of the studies (n = 33) reported at least one non-significant adjusted effect estimate. Study bias assessment is presented in Table S2. Meta-analysis results are available in Table 8 and grades summarized in Table 9.
Table 8. Meta-analysis results.
| Meta-Analysis | Odds Ratio (95% CI) | Tau Squared | Q p-Value | I 2 (95% Uncertainty) | Egger's Test P | n Studies | GRADE |
| Piped water use (any STH) | 0.93 (0.28–3.11) | 1.86 | <0.01 | 98.6 (98–99) | <0.01 | 5 | Very low |
| Piped water use (A. lumbricoides) | 0.40 (0.39–0.41) | 0 | 0.62 | 0 (0–85) | 0.08 | 4 | Low |
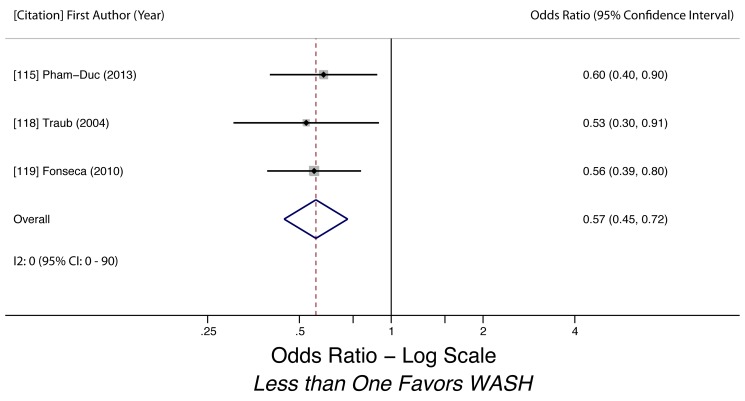
| Piped water use (T. trichiura) | 0.57 (0.45–0.72) | 0 | 0.93 | 0 (0–90) | 0.67 | 3 | Low |
| Treated water use (any STH) | 0.46 (0.36–0.60) | 0 | 0.82 | 0 (0–90) | 0.36 | 3 | Low |
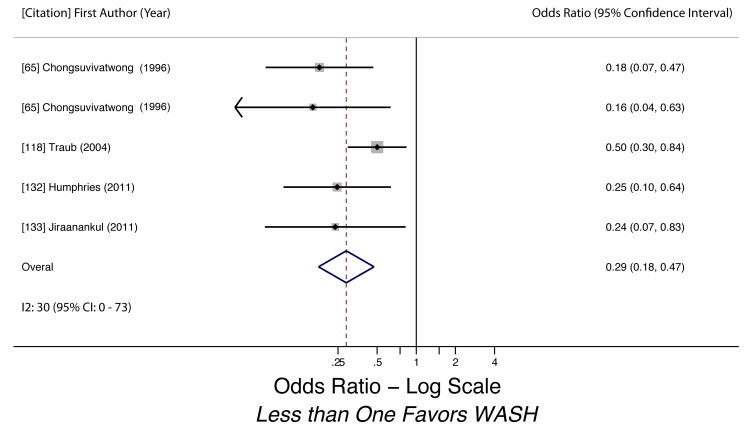
| Wearing shoes (hookworm) | 0.29 (0.18–0.47) | 0.09 | 0.09 | 30 (0–73) | 0.03 | 5 | Moderate |
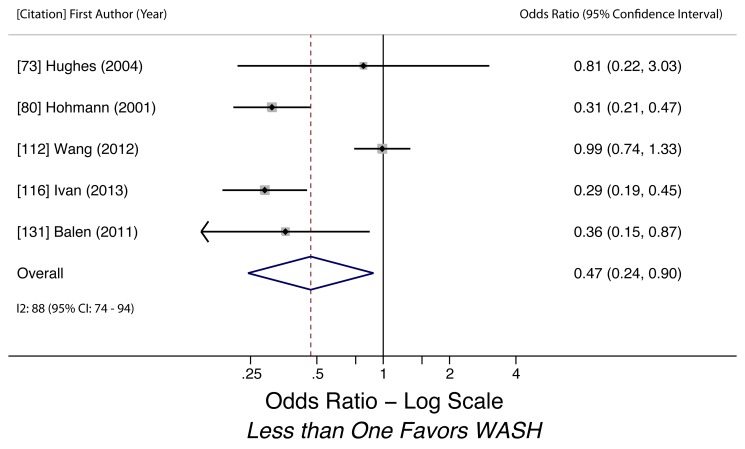
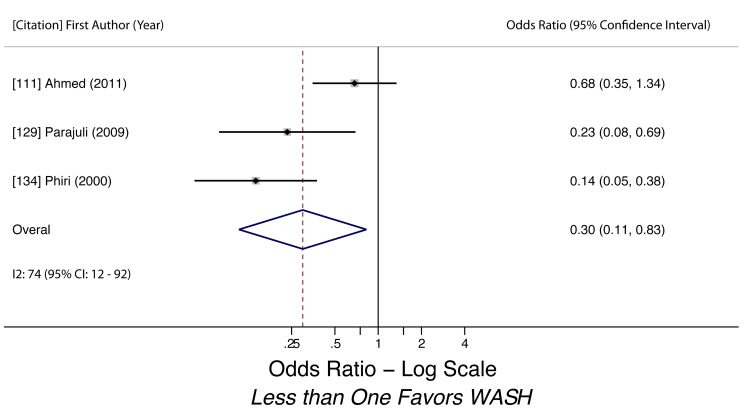
| Wearing Shoes (any STH) | 0.30 (0.11–0.83) | 0.60 | 0.02 | 74 (12–92) | 0.29 | 3 | Low |
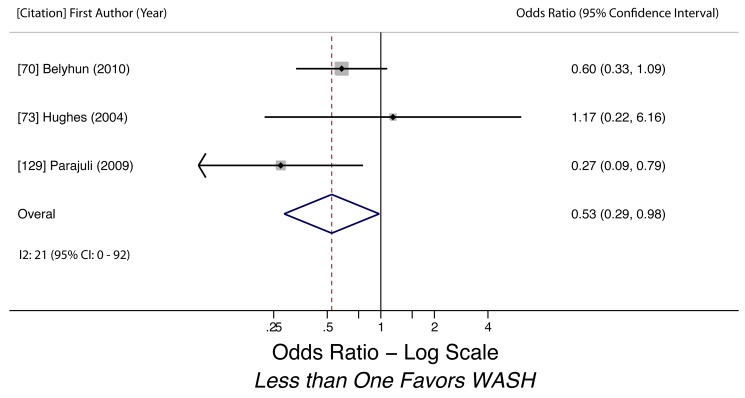
| Soap use/availability (any STH) | 0.53 (0.29–0.98) | 0.07 | 0.28 | 21 (0–92) | 0.98 | 3 | Low |
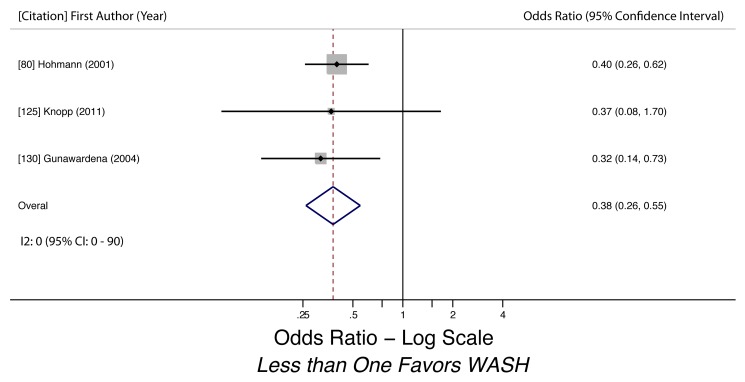
| Handwashing before eating (A. lumbricoides) | 0.38 (0.26–0.55) | 0 | 0.90 | 0 (0–90) | 0.59 | 3 | Low |
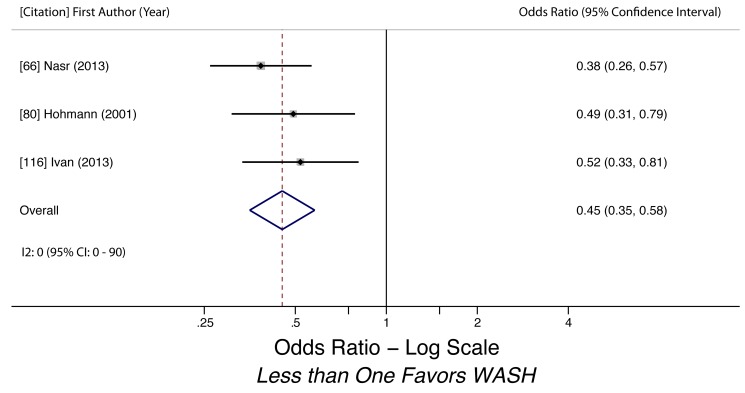
| Handwashing after defecation (A. lumbricoides) | 0.45 (0.35–0.58) | 0 | 0.55 | 0 (0–90) | 0.29 | 3 | Low |
| Handwashing after defecation (any STH) | 0.47 (0.24–0.90) | 0.44 | <0.01 | 88 (74–94) | 0.58 | 5 | Very low |
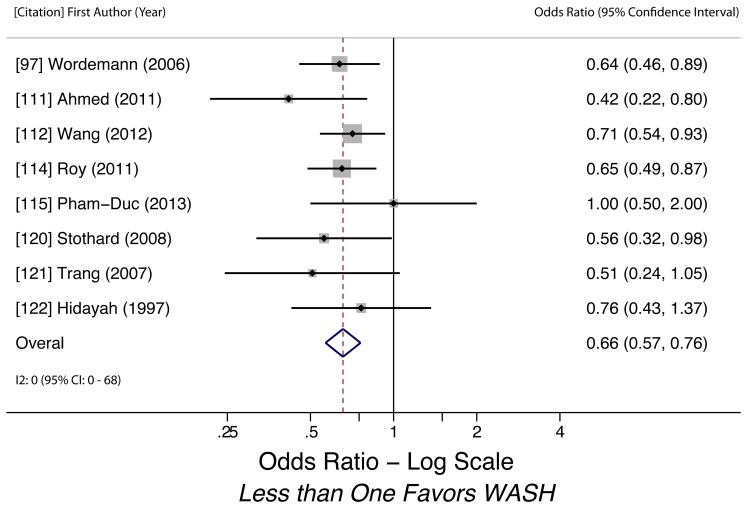
| Sanitation access (any STH) | 0.66 (0.57–0.76) | 0 | 0.70 | 0 (0–68) | 0.57 | 8 | Low |
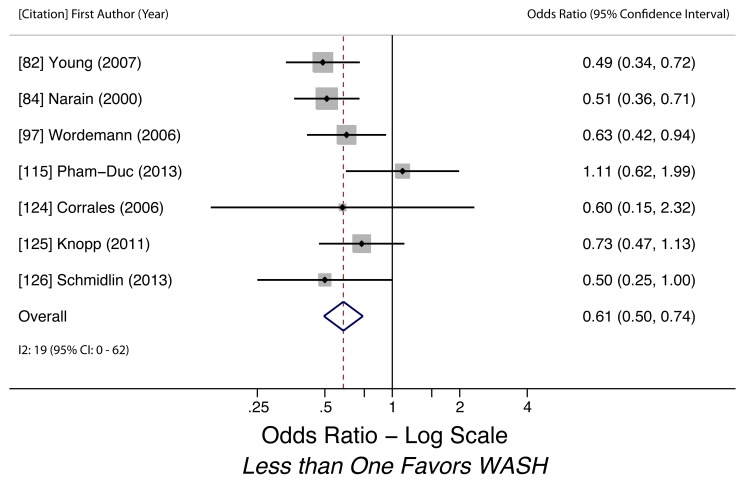
| Sanitation access (T. trichiura) | 0.61 (0.50–0.74) | 0.01 | 0.29 | 19 (0–62) | 0.49 | 7 | Low |
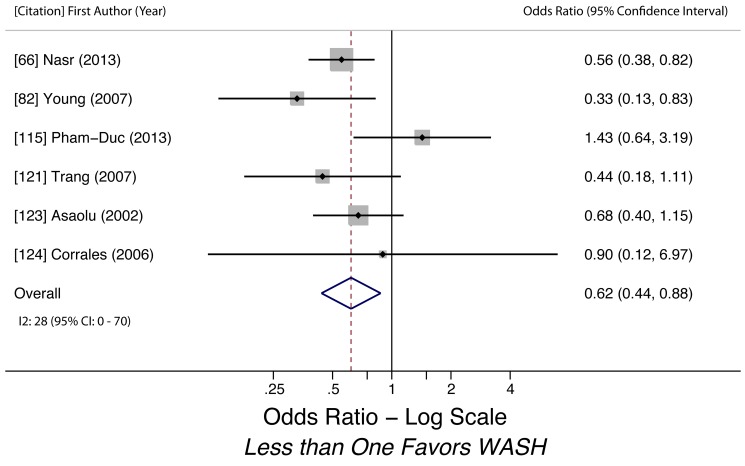
| Sanitation access (A. lumbricoides) | 0.62 (0.44–0.88) | 0.05 | 0.22 | 28 (0–70) | 0.83 | 6 | Low |
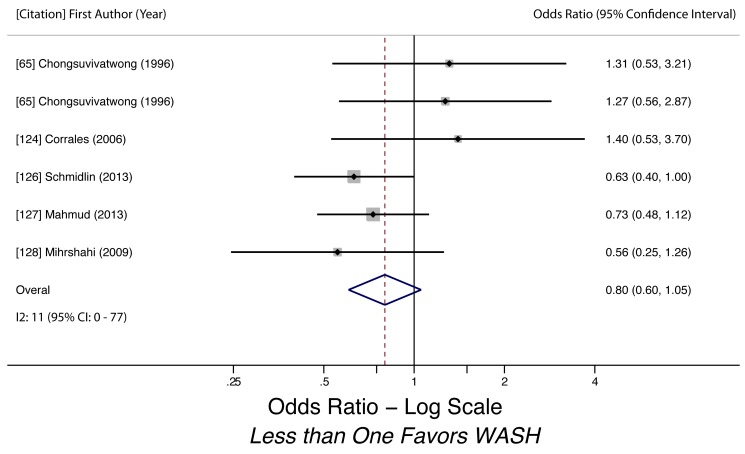
| Sanitation access (hookworm) | 0.80 (0.61–1.06) | 0.01 | 0.34 | 11 (0–77) | 0.13 | 6 | Very low |
Table 9. Meta-analysis grades.
| Meta-Analysis Group | Internal Bias | Inconsistency | Indirect | Imprecise | Publication Bias | Large Effect | Dose Response | Confounding Towards Null | Overall |
| Piped water access (any STH) | Moderate, used help of observations to assess exposure and used adjusted estimates | Yes, I 2 = 98.6% | Nothing serious | Yes, 95% CI includes null | Likely, but unclear due to strong heterogeneity | Nothing strong | Not found | Nothing strong | Very low, due to heterogeneity and wide confidence interval |
| Piped water access (A. lumbricoides) | Moderate, observational studies but all use adjusted estimates | I 2 = 0%, 95% CI (0%–85%) | Nothing serious | Nothing serious | Likely, but direction suggests slightly more protective effect | Nothing strong | Not found | Nothing strong | Low |
| Piped water access (T. trichiura) | Moderate, observational studies but all use adjusted estimates | I 2 = 0%, 95% CI (0%–90%) | Nothing serious | Nothing serious | Undetected | Nothing strong | Not found | Nothing strong | Low |
| Treated water use (any STH) | Moderate, observational studies but all use adjusted estimates | I 2 = 0%, 95% CI (0%–90%) | Nothing serious | Nothing serious | Undetected | Nothing strong | Not found | Nothing strong | Low |
| Wearing shoes (hookworm) | Moderate, observational studies but all use adjusted estimates | I 2 = 29.7%, 95% CI (0%–73%) | Nothing serious | Nothing serious | Likely | Strong effect evident (OR 0.29) | Not found | Yes, hygiene behaviors overreported | Moderate, due to strong effect size |
| Wearing shoes (any STH) | Moderate, observational studies but all use adjusted estimates | Yes, I 2 = 74% | Nothing serious | Nothing serious | Likely, but unclear due to strong heterogeneity | Strong effect evident (OR 0.30) | Not found | Yes, hygiene behaviors overreported | Low, upgraded from effect size, downgraded from heterogeneity |
| Soap use/availability (any STH) | Moderate, observational studies but all use adjusted estimates | I 2 = 20.8%, 95% CI (0%–92%) | Nothing serious | Nothing serious | Undetected | Nothing strong | Not found | Yes, hygiene behaviors overreported | Low |
| Handwashing before eating (A. lumbricoides) | Moderate, observational studies but all use adjusted estimates | I 2 = 0%, 95% CI (0%–90%) | Nothing serious | Nothing serious | Undetected | Nothing strong | Not found | Yes, hygiene behaviors overreported | Low |
| Handwashing after defecation (A. lumbricoides) | Moderate, observational studies but all use adjusted estimates | I 2 = 0%, 95% CI (0%–90%) | Nothing serious | Nothing serious | Undetected | Nothing strong | Not found | Yes, hygiene behaviors overreported | Low |
| Handwashing after defecation (any STH) | Moderate, observational studies but all use adjusted estimates | Yes - I 2 = 88% | Nothing serious | Yes, 95% CI includes null | Undetected | Nothing strong | Not found | Yes, hygiene behaviors overreported | Very low, due to high heterogeneity |
| Sanitation access (any STH) | Moderate, observational studies but all use adjusted estimates | I 2 = 0%, 95% CI (0%–68%) | Nothing serious | Nothing serious | Undetected | Nothing strong | N/A | Nothing strong | Low |
| Sanitation access (T. trichiura) | Moderate, observational studies but all use adjusted estimates | I 2 = 19%, 95% CI (0%–62%) | Nothing serious | Nothing serious | Undetected | Nothing strong | N/A | Nothing strong | Low |
| Sanitation access (A. lumbricoides) | Moderate, observational studies but all use adjusted estimates | I 2 = 28%, 95% CI (0%–70%) | Nothing serious | Nothing serious | Undetected | Nothing strong | N/A | Nothing strong | Low |
| Sanitation access (hookworm) | Moderately Low, used help of observations to assess exposure and used adjusted estimates | I 2 = 11%, 95% CI (0%–77%) | Nothing serious | Yes, 95% CI includes null | Likely, but direction suggests slightly more protective effect | Nothing strong | N/A | Nothing strong | Very low, due to confidence interval including null |
Water
Water-related access and practices were generally associated with lower odds of STH infection. We conducted meta-analyses to examine the association of piped water access and use of treated water on STH infection. Using treated water (filtered or boiled) was associated with lower likelihood of having any STH infection (k = 3, OR 0.46, 95% CI 0.36–0.60). The quality of evidence for the analysis was low, as all three studies were observational (Figure 3). Use of piped water was not associated with STH infection in general (k = 5, OR 0.93, 95% CI 0.28–3.11). The quality of evidence for the pooled estimate was very low due to high heterogeneity (I2 = 98.6%, 95% CI 98%–99%, Q p-value<0.01) among the studies (Figure 4). The heterogeneity could have stemmed from multiple factors, as the five studies shared few methodological characteristics. Use of piped water was associated with reduced likelihood of A. lumbricoides infection (k = 4, OR 0.40, 95% CI 0.39–0.41) and T. trichiura infection (k = 3, OR 0.57, 95% CI 0.45–0.72). Evidence quality for these two meta-analyses was low, based on four studies and three studies respectively (Figures 5 and 6). We did not find a sufficient number of studies to conduct a similar meta-analysis for hookworm infection, although Nasr and colleagues found a significantly lower adjusted odds of infection (OR 0.59, 95% CI 0.34–0.91) for Malaysian children with access to piped water [66]. Other researchers found no statistically significant associations between piped water access and hookworm infection [67],[68].
Figure 3. Meta-analysis of the association between use of treated water and infection with any STH [111]–[113].
Figure 4. Meta-analysis of the association between use of piped water use and any STH infection [70],[97],[114]–[116].
Figure 5. Meta-analysis of the association between use of piped water and A. lumbricoides infection [66],[69],[79],[117].
Figure 6. Meta-analysis of the association between use of piped water and T. trichiura infection [115],[118],[119].
Other water-related exposures for STH infection were reported in the literature, but not with sufficient frequency for meta-analyses. In one study examining storage of water, Quintero and colleagues found a significantly higher adjusted odds of T. trichiura infection for Venezuelan children and adults collecting water in “inappropriate” receptacles (OR 1.12, 95% CI 1.09–1.15) [69]. Limited evidence also was retrieved on the influence of water source location; Belyhun and colleagues [70] found a beneficial association of using an outside water pipe compared to an indoor tap for infection with any STH among Ethiopian infants (OR 0.21, 95% CI 0.09–0.51). Matthys and colleagues [71] found that having a private well significantly increased the odds of hookworm infection for farming households in western Côte d'Ivoire (OR 2.32, 95% CI 1.24–4.05). No evidence was found of an association between public or private water source and S. stercoralis infection [72]. Having “inadequate water supply” in schools was strongly associated with increased infection with any STH among school children living on Pacific islands (OR 4.93, 95% CI 2.24–10.88) [73].
Sanitation
Sanitation access (availability or use of latrines) was associated with lower likelihood of infection with any STH (k = 8, OR 0.66, 95% CI 0.57–0.76), T. trichiura (k = 7, OR 0.61, 95% CI 0.50–0.74), and A. lumbricoides (k = 6, OR 0.62, 95% CI 0.44–0.88) (Figures 7–9). The quality of evidence for these meta-analyses was low due to the observational nature of included studies. We did not find evidence that sanitation access was associated with hookworm infection (k = 6, OR 0.80, 95% CI 0.61–1.06), which had very low evidence quality due to imprecision (Figure 10).
Figure 7. Meta-analysis of the association between sanitation access and infection with any STH [97],[111],[112],[114],[115],[120]–[122].
Figure 9. Meta-analysis of the association between sanitation access and T. trichiura infection [82],[84],[97],[115],[124]–[126].
Figure 10. Meta-analysis of the association between sanitation access and hookworm infection [65],[124],[126]–[128]. Note: Chongsuvivatwong et al [65]. reported on two separate studies in their 1996 article.
Figure 8. Meta-analysis of the association between sanitation access and A. lumbricoides infection [66],[82],[115],[121],[123],[124].
We found limited evidence that use of shared or private sanitation facilities influenced odds of STH infection. Worrell and colleagues [74] found in Kenya that participants using toilets located outside of their household premises had significantly increased odds of infection with any STH. In contrast, another study found that sharing latrines with neighboring households, compared with private latrine use, was associated with significantly lower odds of hookworm infection [71]. Few details were provided to contextualize this finding.
Hygiene
Three randomized controlled trials, two carried out in China and one in the Peruvian Amazon, found strong benefits for interventions that focused on promoting hygiene in schools [75]–[77]. Xu and colleagues [75] assessed a randomized intervention that promoted handwashing with soap, both before eating and after defecation among 657 school children in three schools. All infected children were treated at baseline. At the 1-year follow-up, A. lumbricoides prevalence for children in the experimental group had declined by 35.7% (pre-intervention prevalence, 68.3%; post-intervention cumulative infection rate, 43.9%) compared with an increase in the control group of 78% (pre-intervention, 41.4%; post-intervention, 73.7%); this was a statistically significant difference (p<0.01). The study's primary limitation was that schools were the unit of randomization, with two primary schools becoming controls and the third receiving the intervention. With so few clusters, it is highly possible that confounding factors were not comparable between the control and experimental groups.
More recently, Bieri and colleagues [76] reported on a single-blind, unmatched, cluster-randomized intervention trial involving 1,718 children (aged 9–10) in 38 schools over the course of one school year. Schools were randomly assigned to a health-education package, which included an entertainment-education cartoon video, or to a control package, which only displayed a health-education poster. All participants were treated with albendazole at baseline. At follow-up at the end of the school year, knowledge about STH was significantly higher in the intervention group, and almost twice as many intervention children (63.3% versus 33.4%, p<0.01) reported washing their hands after defecating. The incidence of STH infection (predominantly T. trichiura and A. lumbricoides) was also significantly improved in the experimental schools: 50% lower in the intervention group than in the control group (4.1% versus 8.4%, p<0.01).
Gyorkos and colleagues [77] conducted an open-label, cluster-randomized controlled trial using a hygiene education intervention in Peruvian primary schools. Within paired groups, 18 schools (1,089 fifth grade student participants) were randomly allocated to receive albendazole and the hygiene intervention or albendazole alone. The health intervention included a helminth-oriented class for students, a health curriculum workshop for teachers, and educational print materials. Four months after the intervention, the experimental group showed a significant reduction in A. lumbricoides intensity compared to deworming alone (adjusted incidence rate ratio [IRR] 0.42, 95% CI: 0.21–0.85). T. trichiura and hookworm intensity did not show statistically significant improvements in the experimental group, nor did prevalence of any single STH species. Children in the intervention group showed significant improvements in STH knowledge and water treatment behaviors compared to the control, but not in most other hygiene practices (e.g., handwashing). The authors also noted that the prevalence of hookworm was low (about 5% compared to 30% for A. lumbricoides and 50% for T. trichiura) and that albendazole was less efficacious against T. trichiura than it was against A. lumbricoides.
Our meta-analyses of hygiene-related observational evidence provided estimates that are consistent with findings from these randomized controlled trials. Soap use or availability was significantly associated with lower odds of STH infection at the 5% level (k = 3, OR 0.53, 95% CI 0.29–0.98). The quality of the evidence was low, though the possibility of respondents' over-reporting hygiene behaviors could have underestimated the strength of the association (Figure 11). Handwashing, both before eating (k = 3, OR 0.38, 95% CI 0.26–0.55) and after defecating (k = 3, OR 0.45, 95% CI 0.35–0.58), was associated with lower odds of A. lumbricoides infection (Figures 12 and 13). Both analyses were of low quality due to the observational evidence available. Handwashing after defecation also was associated with reduced odds of any STH infection (k = 5, OR 0.47, 95% CI 0.24–0.90). This meta-analysis had very low evidence quality due to high heterogeneity among estimates from the five pooled studies (I 2 = 88%, 95% CI 74%–94%, Q p-value<0.01, Figure 14). All studies used Kato-Katz for diagnosis, but varied considerably in most other study characteristics, including population age, baseline prevalence, and geographic setting. Balen and colleagues reported limited evidence of a dose-response effect for handwashing; respondents who more frequently washed their hands with soap after defecation had lower odds of infection with any STH, but confidence intervals of the handwashing groups overlapped [78].
Figure 11. Meta-analysis of the association between soap use and infection with any STH [70],[73],[129].
Figure 12. Meta-analysis of the association between handwashing before eating and infection with A. lumbricoides [80],[125],[130].
Figure 13. Meta-analysis of the association between handwashing after defecation and infection with A. lumbricoides [66],[80],[116].
Figure 14. Meta-analysis of the association between handwashing after defecation and infection with any STH [73],[80],[112],[116],[131].
Washing vegetables was found to be associated with lower odds of STH infection in two studies. Steinmann and colleagues [79] found washing vegetables to be negatively associated with A. lumbricoides infection in school children (OR 0.69, 95% CI 0.50–0.95), while Hohmann and colleagues [80] found washing was associated with lower odds of T. trichiura (OR 0.50, 95% CI 0.31–0.79) and any STH infection (OR 0.71, 95% CI 0.51–0.99).
Our meta-analysis found evidence of a strong association between wearing shoes and lower odds of hookworm infection (k = 5, OR 0.29, 95% CI 0.18–0.47). The quality of the evidence was moderate, upgraded due to the magnitude of effect (Figure 15). Wearing shoes was also associated with lower odds of infection with any STH (k = 3, OR 0.30, 95% CI 0.11–0.83). The evidence quality for that analysis was low, downgraded by heterogeneity (I2 = 74%, 95% CI 12–92%, Q p-value = 0.02) (Figure 16) but upgraded by a strong effect magnitude. Heterogeneity could have been introduced by many different factors, as the studies shared few characteristics. Three studies found mostly non-significant associations between geophagy (i.e., consumption of soil) and STH infection [81]–[83]. In adjusted models, households with dirt floors in India and Venezuela were found to have higher odds of T. trichiura and A. lumbricoides infection than were houses with other more elaborate flooring material [69],[84]. Young children living with dirt floors in Colombia also showed higher odds of infection with any STH compared to those with tile or cement floors [85].
Figure 15. Meta-analysis of the association between wearing shoes and hookworm infection [65],[118],[132],[133]. Note: Chongsuvivatwong et al. [65] reported on two separate studies in their 1996 article.
Figure 16. Meta-analysis of the association between wearing shoes and infection with any STH [111],[129],[134].
Integrated Interventions
In a cluster-randomized controlled trial, Freeman and colleagues examined a comprehensive WASH intervention in Kenyan schools that included hygiene promotion, water treatment and storage, and installation of sanitation infrastructure [27]. The intervention reduced reinfection prevalence (OR 0.56, 95% CI 0.31–1.00) and egg count (IRR 0.34, 95% CI 0.15–0.75) of A. lumbricoides, but not of T. trichiura or hookworm. Effects of the intervention differed by sex, with girls in the intervention group showing a significantly reduced A. lumbricoides infection intensity compared to the control group; boys in the intervention group did not show any significant difference from controls. Shoe-wearing and geophagy also emerged as effect modifiers for hookworm and T. trichiura infection intensity, respectively.
Dumba and colleagues found no statistically significant benefit of a participatory hygiene and sanitation transformation (PHAST) intervention when compared with a control group that only received deworming [86]. PHAST uses training sessions to encourage communities to identify problems in their own environment, decide what aspects need to be improved, and then implement changes. Parents or guardians of participating children in 19 villages received three PHAST education sessions. Participants in both control and experimental villages received albendazole and showed significant reductions in helminth prevalence compared with baseline, but the prevalence in the experimental group did not decline more than that among the control children. This study grouped Hymenolepis nana and Enterobius vermicularis with STH in analysis, but only a handful of participants were infected by H. nana or E. vermicularis, whereas STH prevalence was very high (>80%).
Strongyloides stercoralis
We found 12 studies that investigated the relationship between WASH and S. stercoralis infection, but only located relevant effect estimates in five. Among school children in Cambodia, Khieu and colleagues found crude associations between infection and handwashing, shoe-wearing, and sanitation access [87]. Hall and colleagues found mixed results for a range of sanitation-related exposures, with some evidence that open defecation and use of community latrines were associated with higher odds of S. stercoralis infection in children [72]. In a multivariable model using data from a rural Peruvian community, Yori and colleagues found that wearing shoes never or occasionally (versus more frequently) was associated with higher odds of infection (OR 1.89, 95% CI 1.10–3.27) [88]. Knopp and colleagues did not find a significant association between S. stercoralis infection and home latrine ownership or handwashing after defecation [89].
Discussion
We conducted a systematic review and meta-analysis of the relationship between WASH access and practices and STH infection. Our analysis revealed that WASH access and practices are generally, but not universally, associated with lower odds of STH infection. Particularly strong associations emerged between wearing shoes and hookworm infection (OR 0.29, 95% CI 0.18–0.47), piped water use and A. lumbricoides infection (OR 0.40, 95% CI 0.39–0.41), and treated water use and infection by any STH (OR 0.46, 95% CI 0.36–0.60). Pooled estimates for all meta-analyses, except for two (i.e., piped water use for any STH and sanitation access for hookworm), indicated at least a 33% lower odds of STH infection associated with specific WASH behaviors or access (Table 8). All but two of the meta-analyses were statistically significant at the 5% level.
On the basis of the evidence available, this review primarily draws upon observational studies. Observational research typically has greater risks to internal validity than randomized controlled trials, but such research is also key to providing a broad evidence base. When conducted well, randomized controlled trials provide the strongest evidence of a causal relationship between an exposure (e.g., an intervention) and an outcome. In the WASH context, however, conducting RCTs can be ethically and financially challenging. Traditional randomized designs can be costly and require that a subset of the target population be allocated to the control group, receiving only a limited intervention. Observational studies can be conducted more quickly and affordably in a wide array of contexts, allowing for WASH access and practices to be investigated in different social-ecological systems. This diversity is critical, since the effectiveness of specific WASH interventions can vary widely across settings, and interventions will most likely provide the greatest impact after being tailored to local conditions. Looking forward, a stepped wedge design represents a powerful compromise between ethics, operational feasibility, and internal validity. With a stepped wedge approach, the rollout of an intervention is randomized so that all participants eventually receive the study benefits, but at different times. Because many WASH interventions require staggered implementation owing to limited financial and human resources, randomizing the order in which communities are visited is often feasible. Combined with longitudinal data analysis, this design allows for robust assessments that can integrate with many interventions without radically altering implementing organizations' plans.
This review highlights important gaps in the WASH and STH body of literature. For example, only a few of the studies that met our inclusion criteria investigated the impact of sharing latrines (n = 6) or latrine maintenance (n = 3) on STH infection. The effect of treating water (n = 7) and geophagy (n = 10) were also infrequently explored. S. stercoralis was by far the least commonly investigated STH infection, reflecting another important knowledge gap.
A total of 35 studies contributed data to the 14 meta-analyses. A lack of standardized WASH definitions across studies limited our ability to pool results via additional meta-analyses. More consistent use of the Joint Monitoring Program's water and sanitation ladder definitions would aid future review efforts. Additional meta-analyses could have been conducted if all reviewed studies had provided relevant adjusted estimates of association. For example, many studies investigated the relationship between “toilet sharing” on any STH infection and “water access” on hookworm infection, but a dearth of reported adjusted estimates stymied meta-analyses of these relationships (Table 7).
Few studies analyzed the relationship between fecal egg count, a proxy for intensity of infection, and WASH [27],[81],[90], even though intensity of infection represents a more relevant predictor for morbidity than prevalence alone [91]. A lack of measures on this relationship represents a considerable gap in the literature, though many studies did report broadly on intensity of infection. Zero-inflated modeling strategies have recently shown promise in analyzing fecal egg count datasets, which often contain excess zero counts due to some individuals not harboring infections [92]–[94]. Contemporary analysis of existing data represents a potentially cost-effective mechanism for yielding additional insights into this topic.
Our findings build upon past reviews by Asaolu and Ofoezie [32] and Ziegelbauer and colleagues [26], which both concluded that WASH represents a valuable strategy for STH control. Although Asaolu and Ofoezie did not conduct a meta-analysis, their comprehensive review found broad evidence of reductions in STH prevalence and intensity resulting from multiple types of WASH interventions. Asaolu and Ofoezie concluded that improvements in sanitation systems and hygiene practices were important tools to not only sustain preventive chemotherapy benefits, but also help protect the uninfected. Results from our meta-analyses support their conclusion using systematically aggregated quantitative data. Ziegelbauer and colleagues focused more specifically on latrine access and use, conducting a rigorous meta-analysis using primarily crude odds ratios. The results from our meta-analyses, which drew upon adjusted odds ratios, are consistent with their findings and lend additional support to the value of sanitation improvements for STH control. Our meta-analyses also broadened focus to include water and hygiene components, allowing for a quantitative summary of currently available evidence across the three core WASH domains.
Our analysis of the relationship between access to a piped water source and STH infection yielded significantly protective associations for A. lumbricoides and T. trichiura, but not for any STH infection generally. The meta-analysis of any STH yielded strong heterogeneity statistics, reflecting a spread in observed effects. While the inclusion of hookworm infections in the “any STH” analysis may seem like a possible source of the variability, we found no clear evidence to support this explanation. The only study that analyzed hookworm infection and piped water use with an adjusted model found a significantly protective association, so other sources of heterogeneity should be considered.
The presence of heterogeneity can be systematically investigated by statistics like Moran's I 2 and Cochran's Q, but these global tests do not themselves uncover specific causes of heterogeneity. Diversity among studies can originate from a plethora of sources: population, setting, diagnostic approach, study design, analytic method, definitions, and so on. Without additional subgroup analysis or meta-regression, which both require a large body of studies, it is difficult to investigate the myriad potential causes of heterogeneity. Without clarification, the presence of heterogeneity indicates that pooled results are averaging multiple related, but distinct effects. For example, access to piped water could have different levels of benefit depending on distance to the source [95],[96], water quality [70],[97], or other unknown factors—especially when studies use different diagnostic assays and are conducted in a variety of community settings.
Concerning sanitation, our meta-analyses of access to sanitation yielded considerably lower odds of infection with A. lumbricoides, T. trichiura, or any STH for those with latrine access. We did not find evidence of a statistically significant association between sanitation and hookworm, though the pooled estimate suggested reduced odds of infection. Our sanitation findings were comparable to those found by Ziegelbauer and colleagues, who asserted that improved sanitation access should be prioritized alongside preventive chemotherapy to achieve a sustainable reduction in helminthiasis burden. They found an overall pooled odds ratio of 0.51 (95% CI 0.44–0.61) for the effect of sanitation availability and use, while we found an odds ratio of 0.66 (95% CI 0.57–0.76). Species-specific results were similar as well, with the exception of hookworm. Differences in the magnitude of our findings may be attributed to the use of adjusted measures in our analysis, since Ziegelbauer and colleagues used unadjusted estimates. In addition, we did not include separate estimates for sanitation use and access. Taken together, these two reviews support the hypothesis that improved access to, and use of, sanitation prevents STH infection. Additional research could help explore the complementarity of sanitation promotion with MDA.
For hygiene, three randomized controlled trials provided strong evidence linking hygiene practices—especially handwashing with soap—to reductions in STH infection [75]–[77]. However, not all hygiene interventions may be effective in reducing STH infection [86]. Our meta-analyses of the effect of handwashing before eating and after defecation for A. lumbricoides infection, along with handwashing after defecation and soap use for any STH infection, also yielded significant results that suggest protective effects. Accurately assessing handwashing is challenging; self-reported and observed measures are often highly biased [33]. Many studies rely on self-report, but individuals have consistently been shown to over-report handwashing behaviors [98]. Heterogeneity was exhibited in the analysis of handwashing after defecation, suggesting that the benefits of handwashing may vary considerably depending on circumstances and definitions. Beyond handwashing, our analysis also showed that wearing shoes was associated with significantly lower odds of infection with hookworm and any STH.
These results may be of interest to several audiences. Researchers can take note of the gaps in the literature identified by this review and focus investigation on key outstanding questions (e.g., the impact of WASH on S. stercoralis infections). Policymakers should understand that, despite gaps in data, these findings provide a broad evidence base in support of WASH for STH control—especially from randomized trials for hygiene interventions. WASH practitioners will recognize that these findings provide further support for their efforts and, we hope, will consider partnering with STH researchers to evaluate future interventions.
Strengths and Limitations
Our review included only adjusted effect estimates in meta-analyses, which lends greater strength to our pooled results [37]. Many different variables were controlled across studies, which may contribute to heterogeneity. However, this variation in adjusted models may also serve as a small buffer against the inherent heterogeneity across observational studies. Different covariates will vary in importance for different populations and circumstances, so a broad review like ours may benefit from pooling estimates from models that were adapted by researchers to best fit their data and contexts. There are many factors that could confound the relationship between WASH access or practices and STH prevalence, including socioeconomic status, age, and gender. Consideration of only crude associations would likely overstate the magnitude of effect for WASH exposures or even misinterpret the true direction of effect [99]. Limiting our focus to adjusted measures of effect reduces the number of eligible studies, which may impact the generalizability of our results. This strategy also amplifies the impact of selective reporting, since many authors reported only statistically significant adjusted estimates.
Evidence quality was typically “low”—the default GRADE for observational research—meaning that our confidence in pooled effect estimates is limited, and that the true effect may be markedly different from the results reported here [40]. A much stronger case can be made for the benefit of hygiene because of the evidence provided by recent randomized controlled trials, but results from our meta-analyses suggest that the protective effect of hygiene practices on STH infection may be variable depending on context.
Publication bias also represents a concern. Five meta-analyses (piped water for any STH and A. lumbricoides, wearing shoes for hookworm and any STH, sanitation access for hookworm) showed evidence of publication bias in funnel plot assessments. However, two of those plots (piped water for A. lumbricoides and sanitation access for hookworm) showed that larger studies yielded more protective associations, suggesting that the results from those analyses may be underestimating the true relationship strength. This was unexpected—and possibly caused by the natural heterogeneity across observational studies—since larger studies are traditionally expected to show smaller magnitudes of effect. Heterogeneity creates great difficulty in assessing publication bias accurately with statistical tests, so it is impossible to know how pronounced publication bias may be throughout our meta-analyses [100].
Conclusion
A vibrant discussion continues in the literature about the role of MDA in measurably mitigating morbidity from STH infection at the population level [101]–[106]. MDA alone is unlikely to permanently interrupt STH transmission. Our review provides evidence that WASH is a valuable component for STH control strategies, but guidelines and targets for the integration of these approaches are needed. Increased attention towards WASH for STH also has great potential to catalyze synergies with integrated NTD control programs, while jointly elevating awareness of WASH and NTDs [5],[28],[107]. Additional high-quality research into the potential of integrated WASH interventions is merited, specifically on the complementarity of WASH and MDA. Recent and ongoing research continues to build an evidence-base that can guide policymaking and programmatic decisions [27],[28],[108]. Increased collaboration between the health and WASH sectors represents a key enterprise for the future of NTD control and elimination [109],[110].
Supporting Information
Funnel plot for treated water use and any STH infection.
(EPS)
Funnel plot for piped water use and any STH infection.
(EPS)
Funnel plot for piped water use and A. lumbricoies infection.
(EPS)
Funnel plot for piped water use and T. trichiura infection.
(EPS)
Funnel plot for sanitation access and any STH infection.
(EPS)
Funnel plot for sanitation access and A. lumbricoides infection.
(EPS)
Funnel plot for sanitation access and T. trichiura infection.
(EPS)
Funnel plot for sanitation access and hookworm infection.
(EPS)
Funnel plot for soap use and any STH infection.
(EPS)
Funnel plot for handwashing before eating and A. lumbricoides infection.
(EPS)
Funnel plot for handwashing after defecating and A. lumbricoides infection.
(EPS)
Funnel plot for handwashing after defecating and any STH infection.
(EPS)
Funnel plot for wearing shoes and hookworm infection.
(EPS)
Funnel plot for wearing shoes and any STH infection.
(EPS)
Excluded studies.
(DOC)
Study bias assesment.
(DOC)
PRISMA checklist.
(DOC)
MOOSE checklist.
(DOC)
Original methods protocol.
(DOC)
Acknowledgments
We thank Rachel Stelmach and Claire Still for their stellar work on data collection and extraction. We also thank Shuyuan Huang, Peiling Yap, and Yingsi Lai for their assistance translating Chinese-language articles.
Abbreviations
- GRADE
Grades of Recommendation, Assessment, Development and Evaluation
- MDA
mass drug administration
- NTD
neglected tropical disease
- OR
odds ratio
- PHAST
participatory hygiene and sanitation transformation
- RR
risk ratio
- STH
soil-transmitted helminth
- WASH
water, sanitation, and hygiene
- WHO
World Health Organization
Funding Statement
M.C.F. was funded in part by UK aid from the Department for International Development (DFID) as part of the SHARE Research Programme (www.SHAREResearch.org). However, the views expressed do not necessarily reflect the Department's official policies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, et al. (2006) Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 2. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, et al. (2013) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 3. Brooker S (2010) Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers – A review. Int J Parasitol 40: 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan MS (1997) The global burden of intestinal nematode infections — fifty years on. Parasitol Today 13: 438–443. [DOI] [PubMed] [Google Scholar]
- 5. Utzinger J, Raso G, Brooker S, De Savigny D, Tanner M, et al. (2009) Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology 136: 1859–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jia T-W, Melville S, Utzinger J, King CH, Zhou X-N (2012) Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartram J, Cairncross S (2010) Hygiene, sanitation, and water: forgotten foundations of health. PLoS Med 7: e1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO (2006) Preventive chemotherapy in human helminthiasis. Geneva: World Health Organization. 71 p. [Google Scholar]
- 9. Hong ST, Chai JY, Choi MH, Huh S, Rim HJ, et al. (2006) A successful experience of soil-transmitted helminth control in the Republic of Korea. Korean J Parasitol 44: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobayashi A, Hara T, Kajima J (2006) Historical aspects for the control of soil-transmitted helminthiases. Parasitol Int 55 Suppl: S289–291. [DOI] [PubMed] [Google Scholar]
- 11. Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, et al. (2005) Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis 5: 42–52. [DOI] [PubMed] [Google Scholar]
- 12. Clasen T, Roberts I, Rabie T, Schmidt W, Cairncross S (2006) Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev CD004794. [DOI] [PubMed] [Google Scholar]
- 13. Arnold B, Arana B, Mäusezahl D, Hubbard A, Colford JM Jr (2009) Evaluation of a pre-existing, 3-year household water treatment and handwashing intervention in rural Guatemala. Int J Epidemiol 38: 1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cairncross S, Hunt C, Boisson S, Bostoen K, Curtis V, et al. (2010) Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol 39 Suppl 1: i193–i205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clasen TF, Bostoen K, Schmidt W-P, Boisson S, Fung ICH, et al. (2010) Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database Syst Rev CD007180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graf J, Zebaze Togouet S, Kemka N, Niyitegeka D, Meierhofer R, et al. (2010) Health gains from solar water disinfection (SODIS): evaluation of a water quality intervention in Yaoundé, Cameroon. J Water Health 8: 779–796. [DOI] [PubMed] [Google Scholar]
- 17. Hunter PR, Ramírez Toro GI, Minnigh HA (2010) Impact on diarrhoeal illness of a community educational intervention to improve drinking water quality in rural communities in Puerto Rico. BMC Public Health 10: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freeman MC, Clasen T (2011) Assessing the impact of a school-based safe water intervention on household adoption of point-of-use water treatment practices in southern India. Am J Trop Med Hyg 84: 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greene LE, Freeman MC, Akoko D, Saboori S, Moe C, et al. (2012) Impact of a school-based hygiene promotion and sanitation intervention on pupil hand contamination in Western Kenya: a cluster randomized trial. Am J Trop Med Hyg 87: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gruber JS, Reygadas F, Arnold BF, Ray I, Nelson K, et al. (2013) A stepped wedge, cluster-randomized trial of a household UV-disinfection and safe storage drinking water intervention in rural Baja California Sur, Mexico. Am J Trop Med Hyg 89: 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The PLoS Medicine Editors (2009) Clean water should be recognized as a human right. PLoS Med 6: e1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pink R (2012) Child rights, right to water and sanitation, and human security. Health Hum Rights 14: E78–E87. [PubMed] [Google Scholar]
- 23. Luh J, Baum R, Bartram J (2013) Equity in water and sanitation: developing an index to measure progressive realization of the human right. Int J Hyg Environ Health 216: 662–671. [DOI] [PubMed] [Google Scholar]
- 24. Emerson P, Kollmann M, MacArthur C, Bush S, Haddad D (2012) SAFE strategy for blinding trachoma addresses sanitation, the other half of MDG7. Lancet 380: 27–28. [DOI] [PubMed] [Google Scholar]
- 25. Esrey SA, Potash JB, Roberts L, Shiff C (1991) Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull World Health Organ 69: 609–621. [PMC free article] [PubMed] [Google Scholar]
- 26. Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, et al. (2012) Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med 9: e1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freeman MC, Clasen T, Brooker S, Akoko D, Rheingans R (2013) The impact of a school-based hygiene, water quality and sanitation intervention on soil-transmitted helminth re-infection: a cluster-randomized trial. Am J Trop Med Hyg 89: 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Freeman MC, Ogden S, Jacobson J, Abbott D, Addiss DG, et al. (2013) Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: a rationale for inter-sectoral collaboration. PLoS Negl Trop Dis 7: e2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Hest R, Grant A, Abubakar I (2011) Quality assessment of capture-recapture studies in resource-limited countries. Trop Med Int Health 16: 1019–1041. [DOI] [PubMed] [Google Scholar]
- 30. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Asaolu SO, Ofoezie IE (2003) The role of health education and sanitation in the control of helminth infections. Acta Trop 86: 283–294. [DOI] [PubMed] [Google Scholar]
- 33. Ram PK, Halder AK, Granger SP, Jones T, Hall P, et al. (2010) Is structured observation a valid technique to measure handwashing behavior? Use of acceleration sensors embedded in soap to assess reactivity to structured observation. Am J Trop Med Hyg 83: 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO (2006) Core Questions on Drinking-Water and Sanitation for Household Surveys: World Health Organization. 24 p. [Google Scholar]
- 35.WHO, UNICEF (2012) Progress on sanitation and drinking-water 2012 update. Geneva; New York: World Health Organization; Unicef. [Google Scholar]
- 36. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 37.Reeves B, Deeks J, Higgins J, Wells G (2008) Chapter 13: Including non-randomized studies. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 1st edition. Chichester (UK); Hoboken (New Jersey): Wiley. pp. 672. [Google Scholar]
- 38. Kawai K, Saathoff E, Antelman G, Msamanga G, Fawzi WW (2009) Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg 80: 36–43. [PMC free article] [PubMed] [Google Scholar]
- 39. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A (2011) GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 64: 380–382. [DOI] [PubMed] [Google Scholar]
- 40. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, et al. (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 41. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, et al. (2011) GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 64: 407–415. [DOI] [PubMed] [Google Scholar]
- 42.Higgins JP, Green S (2008) Cochrane handbook for systematic reviews of interventions: Wiley Online Library. [Google Scholar]
- 43. Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wells G, Shea B, O'Connell D, Peterson J, Welch V, et al. (2008) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 45. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, et al. (2011) GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64: 383–394. [DOI] [PubMed] [Google Scholar]
- 46.Deeks J, Higgins J, Altman D (2008) Chapter 9: Analysing data and undertaking meta-analyses. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 1st edition. Chichester (UK); Hoboken (New Jersey): Wiley. pp. 672. [Google Scholar]
- 47. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 48. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Davies HTO, Crombie IK, Tavakoli M (1998) When can odds ratios mislead? BMJ 316: 989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Manun'Ebo M, Cousens S, Haggerty P, Kalengaie M, Ashworth A, et al. (1997) Measuring hygiene practices: a comparison of questionnaires with direct observations in rural Zaire. Trop Med Int Health 2: 1015–1021. [DOI] [PubMed] [Google Scholar]
- 51. Curtis V, Cousens S, Mertens T, Traore E, Kanki B, et al. (1993) Structured observations of hygiene behaviours in Burkina Faso: validity, variability, and utility. Bull World Health Organ 71: 23–32. [PMC free article] [PubMed] [Google Scholar]
- 52. Jenner EA, Fletcher BC, Watson P, Jones FA, Miller L, et al. (2006) Discrepancy between self-reported and observed hand hygiene behaviour in healthcare professionals. J Hosp Infect 63: 418–422. [DOI] [PubMed] [Google Scholar]
- 53. Alemu A, Atnafu A, Addis Z, Shiferaw Y, Teklu T, et al. (2012) Soil transmitted helminths and schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. Parasitol Int 61: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Curtale F, Pezzotti P, Sharbini AL, Al Maadat H, Ingrosso P, et al. (1998) Knowledge, perceptions and behaviour of mothers toward intestinal helminths in Upper Egypt: Implications for control. Health Policy Plan 13: 423–432. [DOI] [PubMed] [Google Scholar]
- 55. Quihui L, Valencia ME, Crompton DW, Phillips S, Hagan P, et al. (2006) Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. Vet Immunol Immunopathol 114: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nematian J, Nematian E, Gholamrezanezhad A, Asgari AA (2004) Prevalence of intestinal parasitic infections and their relation with socio-economic factors and hygienic habits in Tehran primary school students. Acta Trop 92: 179–186. [DOI] [PubMed] [Google Scholar]
- 57. Carneiro FF, Cifuentes E, Tellez-Rojo MM, Romieu I (2002) The risk of Ascaris lumbricoides infection in children as an environmental health indicator to guide preventive activities in Caparao and Alto Caparao, Brazil. Rev Saude Publica 36: 69–74. [PMC free article] [PubMed] [Google Scholar]
- 58. Alyousefi NA, Mahdy MAK, Mahmud R, Lim YAL (2011) Factors associated with high prevalence of intestinal protozoan infections among patients in Sana'a city, Yemen. PLoS ONE 6: e22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ruiz Lopes FM, Goncalves DD, Dos Reis CR, Bregano RM, Filho FA, et al. (2006) Occurrence of enteroparasitosis in schoolchildren of the municipal district of Jataizinho, State of Parana, Brazil. Acta Scientiarum - Health Sciences 28: 107–111. [Google Scholar]
- 60. Alaofe H, Zee J, Dossa R, O'Brien HT (2008) Intestinal parasitic infections in adolescent girls from two boarding schools in southern Benin. Trans R Soc Trop Med Hyg 102: 653–661. [DOI] [PubMed] [Google Scholar]
- 61. Chirdan OO, Akosu JT, Adah SO (2010) Intestinal parasites in children attending day care centers in Jos, Central Nigeria. Niger J Med 19: 219–222. [DOI] [PubMed] [Google Scholar]
- 62. Escobedo AA, Canete R, Nunez FA (2008) Prevalence, risk factors and clinical features associated with intestinal parasitic infections in children from San Juan y Martinez, Pinar del Rio, Cuba. West Indian Med J 57: 377–382. [PubMed] [Google Scholar]
- 63. Krause RJ, Koski KG, Scott ME (2012) Evidence that multisector food security intervention program in rural panama reduces hookworm infection in preschool children. Am J Trop Med Hyg 87: 341. [Google Scholar]
- 64. Worrell CM, Davis SM, Wiegand RE, Lopez G, Cosmas L, et al. (2012) Water- and sanitation-related risk factors for soil-transmitted helminth infection in urban school- and preschool-aged children in Kibera, Nairobi. Am J Trop Med Hyg 87: 380–381. [Google Scholar]
- 65. Chongsuvivatwong V, Pas-Ong S, McNeil D, Geater A, Duerawee M (1996) Predictors for the risk of hookworm infection: experience from endemic villages in southern Thailand. Trans R Soc Trop Med Hyg 90: 630–633. [DOI] [PubMed] [Google Scholar]
- 66. Nasr NA, Al-Mekhlafi HM, Ahmed A, Roslan MA, Bulgiba A (2013) Towards an effective control programme of soil-transmitted helminth infections among Orang Asli in rural Malaysia. Part 1: prevalence and associated key factors. Parasit Vectors 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cundill B, Alexander N, Bethony JM, Diemert D, Pullan RL, et al. (2011) Rates and intensity of re-infection with human helminths after treatment and the influence of individual, household, and environmental factors in a Brazilian community. Parasitology 138: 1406–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Quintero K, Duran C, Duri D, Medina F, Garcia J, et al. (2012) Household social determinants of ascariasis and trichuriasis in North Central Venezuela. International Health 4: 103–110. [DOI] [PubMed] [Google Scholar]
- 70. Belyhun Y, Medhin G, Amberbir A, Erko B, Hanlon C, et al. (2010) Prevalence and risk factors for soil-transmitted helminth infection in mothers and their infants in Butajira, Ethiopia: a population based study. BMC Public Health 10: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Matthys B, Tschannen AB, Tian-Bi NT, Comoe H, Diabate S, et al. (2007) Risk factors for Schistosoma mansoni and hookworm in urban farming communities in western Cote d'Ivoire. Trop Med Int Health 12: 709–723. [DOI] [PubMed] [Google Scholar]
- 72. Hall A, Conway DJ, Anwar KS, Rahman ML (1994) Strongyloides stercoralis in an urban slum community in Bangladesh: factors independently associated with infection. Trans R Soc Trop Med Hyg 88: 527–530. [DOI] [PubMed] [Google Scholar]
- 73. Hughes RG, Sharp DS, Hughes MC, Akau'ola S, Heinsbroek P, et al. (2004) Environmental influences on helminthiasis and nutritional status among Pacific schoolchildren. Int J Environ Health Res 14: 163–177. [DOI] [PubMed] [Google Scholar]
- 74.Worrell C, Davis S, Wiegand R, Lopez G, Odero K, et al.. (2013) Water, sanitation, and hygiene-related risk factors for soil-transmitted helminth infection in urban school- and pre-school-aged children in Kibera, Nairobi 2013; ASTMH 61st Annual Meeting; 11–15 November 2013, Atlanta. [DOI] [PMC free article] [PubMed]
- 75. Xu LQ, Xiao DH, Zhou CH, Zhang XQ, Lan SG, et al. (2001) [On cleanliness of hands in diminution of Ascaris lumbricoides infection in children]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese journal of parasitology & parasitic diseases] 19: 294–297. [PubMed] [Google Scholar]
- 76. Bieri FA, Gray DJ, Williams GM, Raso G, Li YS, et al. (2013) Health-education package to prevent worm infections in Chinese schoolchildren. N Engl J Med 368: 1603–1612. [DOI] [PubMed] [Google Scholar]
- 77. Gyorkos TW, Maheu-Giroux M, Blouin B, Casapia M (2013) Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: a cluster-randomized controlled trial. PLoS Negl Trop Dis 7: e2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Balen J, Raso G, Li YS, Zhao ZY, Yuan LP, et al. (2011) Risk factors for helminth infections in a rural and a peri-urban setting of the Dongting Lake area, People's Republic of China. Int J Parasitol 41: 1165–1173. [DOI] [PubMed] [Google Scholar]
- 79. Steinmann P, Usubalieva J, Imanalieva C, Minbaeva G, Stefiuk K, et al. (2010) Rapid appraisal of human intestinal helminth infections among schoolchildren in Osh oblast, Kyrgyzstan. Acta Trop 116: 178–184. [DOI] [PubMed] [Google Scholar]
- 80. Hohmann H, Panzer S, Phimpachan C, Southivong C, Schelp FP (2001) Relationship of intestinal parasites to the environment and to behavioral factors in children in the Bolikhamxay Province of Lao PDR. Southeast Asian J Trop Med Public Health 32: 4–13. [PubMed] [Google Scholar]
- 81. Nishiura H, Imai H, Nakao H, Tsukino H, Changazi MA, et al. (2002) Ascaris lumbricoides among children in rural communities in the Northern Area, Pakistan: prevalence, intensity, and associated socio-cultural and behavioral risk factors. Acta Trop 83: 223–231. [DOI] [PubMed] [Google Scholar]
- 82. Young SL, Dave G, Farag TH, Said MA, Khatib MR, et al. (2007) Geophagia is not associated with Trichuris or hookworm transmission in Zanzibar, Tanzania. Trans R Soc Trop Med Hyg 101: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nwaneri DU, Ibadin MO, Ofovwe GE, Sadoh AE (2012) Intestinal helminthiasis in children with chronic neurological disorders in Benin City, Nigeria: intensity and behavioral risk factors. World J Pediatr 10.1007/s12519-012-0394-9. [DOI] [PubMed] [Google Scholar]
- 84. Narain K, Rajguru SK, Mahanta J (2000) Prevalence of Trichuris trichiura in relation to socio-economic & behavioural determinants of exposure to infection in rural Assam. Indian J Med Res 112: 140–146. [PubMed] [Google Scholar]
- 85. Alvarado BE, Vasquez LR (2006) [Social determinants, feeding practices and nutritional consequences of intestinal parasitism in young children]. Biomedica 26: 82–94. [PubMed] [Google Scholar]
- 86. Dumba R, Kaddu JB, Wabwire-Mangen F (2013) Design and implementation of participatory hygiene and sanitation transformation (PHAST) as a strategy to control soil-transmitted helminth infections in Luweero, Uganda. Afr Health Sci 13: 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Khieu V, Schar F, Marti H, Sayasone S, Duong S, et al. (2013) Diagnosis, treatment and risk factors of Strongyloides stercoralis in schoolchildren in Cambodia. PLoS Negl Trop Dis 7: e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yori PP, Kosek M, Gilman RH, Cordova J, Bern C, et al. (2005) Seroepidemiology of strongyloidiasis in the Peruvian Amazon. Cad Saude Publica 21: 1778–1784.16410862 [Google Scholar]
- 89. Knopp S, Stothard JR, Rollinson D, Mohammed KA, Khamis IS, et al. (2013) From morbidity control to transmission control: time to change tactics against helminths on Unguja Island, Zanzibar. Acta Trop 128: 412–422. [DOI] [PubMed] [Google Scholar]
- 90. Gyorkos TW, Maheu-Giroux M, Blouin B, Casapia M (2013) Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: a cluster-randomized controlled trial. PLoS Negl Trop Dis 7: e2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hall A, Hewitt G, Tuffrey V, de Silva N (2008) A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr 4 Suppl 1: 118–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chipeta MG, Ngwira B, Kazembe LN (2013) Analysis of Schistosomiasis haematobium infection prevalence and intensity in Chikhwawa, Malawi: an application of a two part model. PLoS Negl Trop Dis 7: e2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nodtvedt A, Dohoo I, Sanchez J, Conboy G, DesCjteaux L, et al. (2002) The use of negative binomial modelling in a longitudinal study of gastrointestinal parasite burdens in Canadian dairy cows. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire 66: 249–257. [PMC free article] [PubMed] [Google Scholar]
- 94. Vounatsou P, Raso G, Tanner M, N'Goran E K, Utzinger J (2009) Bayesian geostatistical modelling for mapping schistosomiasis transmission. Parasitology 136: 1695–1705. [DOI] [PubMed] [Google Scholar]
- 95. Wang X, Hunter PR (2010) A systematic review and meta-analysis of the association between self-reported diarrheal disease and distance from home to water source. Am J Trop Med Hyg 83: 582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pickering AJ, Davis J (2012) Freshwater availability and water fetching distance affect child health in sub-saharan Africa. Environ Sci Technol 46: 2391–2397. [DOI] [PubMed] [Google Scholar]
- 97. Wordemann M, Polman K, Menocal Heredia LT, Diaz RJ, Madurga AM, et al. (2006) Prevalence and risk factors of intestinal parasites in Cuban children. Trop Med Int Health 11: 1813–1820. [DOI] [PubMed] [Google Scholar]
- 98.Johnston RB, Halder AK, Huda TMN, Akhter S, Huque MR, et al.. (2009) Monitoring impacts of WASH interventions: The case of SHEWAB; 18–22 May 2009; United Nations Conference Centre, Addis Ababa, Ethiopia. Loughborough University of Technology. pp. 352–359.
- 99. Dumba R, Kaddu JB, Wabwire Mangen F (2008) Intestinal helminths in Luweero district, Uganda. Afr Health Sci 8: 90–96. [PMC free article] [PubMed] [Google Scholar]
- 100. Harbord RM, Egger M, Sterne JA (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25: 3443–3457. [DOI] [PubMed] [Google Scholar]
- 101. Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P (2012) Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance. Cochrane Database Syst Rev 11: CD000371. [DOI] [PubMed] [Google Scholar]
- 102. Awasthi S, Peto R, Read S, Richards SM, Pande V, et al. (2013) Population deworming every 6 months with albendazole in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet 381: 1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Anderson RM, Truscott JE, Pullan RL, Brooker SJ, Hollingsworth TD (2013) How effective is school-based deworming for the community-wide control of soil-transmitted helminths? PLoS Negl Trop Dis 7: e2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Addiss DG (2013) Epidemiologic models, key logs, and realizing the promise of WHA 54.19. PLoS Negl Trop Dis 7: e2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Montresor A, Gabrielli AF, Engels D, Daumerie D, Savioli L (2013) Has the NTD community neglected evidence-based policy? PLOS NTDs 2013 expert commentary of the viewpoint by Nagpal S, Sinclair D, Garner P. PLoS Negl Trop Dis 7: e2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nagpal S, Sinclair D, Garner P (2013) Has the NTD community neglected evidence-based policy? PLoS Negl Trop Dis 7: e2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Spiegel JM, Dharamsi S, Wasan KM, Yassi A, Singer B, et al. (2010) Which new approaches to tackling neglected tropical diseases show promise? PLoS Med 7: e1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Clasen T, Boisson S, Routray P, Cumming O, Jenkins M, et al. (2012) The effect of improved rural sanitation on diarrhoea and helminth infection: design of a cluster-randomized trial in Orissa, India. Emer Themes Epidemiol 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nakagawa J, Ehrenberg JP, Nealon J, Furst T, Aratchige P, et al. (2013) Towards effective prevention and control of helminth neglected tropical diseases in the Western Pacific Region through multi-disease and multi-sectoral interventions. Acta Trop In press. [DOI] [PubMed] [Google Scholar]
- 110. Singer BH, de Castro MC (2007) Bridges to sustainable tropical health. Proc Natl Acad Sci U S A 104: 16038–16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ahmed A, Al-Mekhlafi HM, Choy SH, Ithoi I, Al-Adhroey AH, et al. (2011) The burden of moderate-to-heavy soil-transmitted helminth infections among rural malaysian aborigines: an urgent need for an integrated control programme. Parasit Vectors 4: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang X, Zhang L, Luo R, Wang G, Chen Y, et al. (2012) Soil-transmitted helminth infections and correlated risk factors in preschool and school-aged children in rural southwest China. PLoS ONE 7: e45939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Aimpun P, Hshieh P (2004) Survey for intestinal parasites in Belize, Central America. Southeast Asian J Trop Med Public Health 35: 506–511. [PubMed] [Google Scholar]
- 114. Roy E, Hasan KZ, Haque R, Fazlul Haque AKM, Siddique AK, et al. (2011) Patterns and risk factors for helminthiasis in rural children aged under 2 in Bangladesh. SAJCH South African Journal of Child Health 5: 78–84. [Google Scholar]
- 115. Pham-Duc P, Nguyen-Viet H, Hattendorf J, Zinsstag J, Phung-Dac C, et al. (2013) Ascaris lumbricoides and Trichuris trichiura infections associated with wastewater and human excreta use in agriculture in Vietnam. Parasitol Int 62: 172–180. [DOI] [PubMed] [Google Scholar]
- 116. Ivan E, Crowther NJ, Mutimura E, Osuwat LO, Janssen S, et al. (2013) Helminthic infections rates and malaria in HIV-infected pregnant women on anti-retroviral therapy in Rwanda. PLoS Negl Trop Dis 7: e2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Morales-Espinoza EM, Sanchez-Perez HJ, Garcia-Gil Mdel M, Vargas-Morales G, Mendez-Sanchez JD, et al. (2003) Intestinal parasites in children, in highly deprived areas in the border region of Chiapas, Mexico. Salud Publica Mex 45: 379–388. [DOI] [PubMed] [Google Scholar]
- 118. Traub RJ, Robertson ID, Irwin P, Mencke N, Andrew Thompson RC (2004) The prevalence, intensities and risk factors associated with geohelminth infection in tea-growing communities of Assam, India. Trop Med Int Health 9: 688–701. [DOI] [PubMed] [Google Scholar]
- 119. Fonseca EO, Teixeira MG, Barreto ML, Carmo EH, Costa Mda C (2010) [Prevalence and factors associated with geohelminth infections in children living in municipalities with low HDI in North and Northeast Brazil]. Cad Saude Publica 26: 143–152. [DOI] [PubMed] [Google Scholar]
- 120. Stothard JR, Imison E, French MD, Sousa-Figueiredo JC, Khamis IS, et al. (2008) Soil-transmitted helminthiasis among mothers and their pre-school children on Unguja Island, Zanzibar with emphasis upon ascariasis. Parasitology 135: 1447–1455. [DOI] [PubMed] [Google Scholar]
- 121. Do TT, Molbak K, Phung DC, Dalsgaard A (2007) Helminth infections among people using wastewater and human excreta in peri-urban agriculture and aquaculture in Hanoi, Vietnam. Trop Med Int Health 12 Suppl 2: 82–90. [DOI] [PubMed] [Google Scholar]
- 122. Hidayah NI, Teoh ST, Hillman E (1997) Socio-environmental predictors of soil-transmitted helminthiasis in a rural community in Malaysia. Southeast Asian J Trop Med Public Health 28: 811–815. [PubMed] [Google Scholar]
- 123. Asaolu SO, Ofoezie IE, Odumuyiwa PA, Sowemimo OA, Ogunniyi TA (2002) Effect of water supply and sanitation on the prevalence and intensity of Ascaris lumbricoides among pre-school-age children in Ajebandele and Ifewara, Osun State, Nigeria. Trans R Soc Trop Med Hyg 96: 600–604. [DOI] [PubMed] [Google Scholar]
- 124. Corrales LF, Izurieta R, Moe CL (2006) Association between intestinal parasitic infections and type of sanitation system in rural El Salvador. Trop Med Int Health 11: 1821–1831. [DOI] [PubMed] [Google Scholar]
- 125. Knopp S, Stothard JR, Rollinson D, Mohammed KA, Khamis IS, et al. (2011) From morbidity control to transmission control: time to change tactics against helminths on Unguja Island, Zanzibar. PLoS Genet 7: e1001384. [DOI] [PubMed] [Google Scholar]
- 126. Schmidlin T, Hurlimann E, Silue KD, Yapi RB, Houngbedji C, et al. (2013) Effects of hygiene and defecation behavior on helminths and intestinal protozoa infections in Taabo, Cote d'Ivoire. PLoS ONE 8: e65722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mahmud MA, Spigt M, Bezabih AM, Pavon IL, Dinant GJ, et al. (2013) Risk factors for intestinal parasitosis, anaemia, and malnutrition among school children in Ethiopia. Pathog Glob Health 107: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mihrshahi S, Casey GJ, Montresor A, Phuc TQ, Thach DTC, et al. (2009) The effectiveness of 4 monthly albendazole treatment in the reduction of soil-transmitted helminth infections in women of reproductive age in Viet Nam. Int J Parasitol 39: 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Parajuli RP, Umezaki M, Watanabe C (2009) Behavioral and nutritional factors and geohelminth infection among two ethnic groups in the Terai region, Nepal. Am J Hum Biol 21: 98–104. [DOI] [PubMed] [Google Scholar]
- 130. Gunawardena GS, Karunaweera ND, Ismail MM (2004) Socio-economic and behavioural factors affecting the prevalence of Ascaris infection in a low-country tea plantation in Sri Lanka. Ann Trop Med Parasitol 98: 615–621. [DOI] [PubMed] [Google Scholar]
- 131. Balen J, Raso G, Li YS, Zhao ZY, Yuan LP, et al. (2011) Risk factors for helminth infections in a rural and a peri-urban setting of the Dongting Lake area, People's Republic of China. Int J Parasitol 41: 1165–1173. [DOI] [PubMed] [Google Scholar]
- 132. Humphries D, Mosites E, Otchere J, Twum WA, Woo L, et al. (2011) Epidemiology of hookworm infection in Kintampo North Municipality, Ghana: patterns of malaria coinfection, anemia, and albendazole treatment failure. Am J Trop Med Hyg 84: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, et al. (2011) Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am J Trop Med Hyg 84: 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Phiri K, Whitty CJ, Graham SM, Ssembatya-Lule G (2000) Urban/rural differences in prevalence and risk factors for intestinal helminth infection in southern Malawi. Folia Parasitol (Praha) 47: 111–117.10945735 [Google Scholar]
- 135. Rücker G, Schwarzer G, Carpenter J (2008) Arcsine test for publication bias in meta-analyses with binary outcomes. Stat Med 27: 746–763. [DOI] [PubMed] [Google Scholar]
- 136. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2006) Comparison of two methods to detect publication bias in meta-analysis. JAMA 295: 676–680. [DOI] [PubMed] [Google Scholar]
- 137. Alemu A, Atnafu A, Addis Z, Shiferaw Y, Teklu T, et al. (2011) Soil transmitted helminths and schistosoma mansoni infections among school children in zarima town, northwest Ethiopia. BMC Infect Dis 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Al-Mekhlafi MS, Atiya AS, Lim YA, Mahdy AK, Ariffin WA, et al. (2007) An unceasing problem: soil-transmitted helminthiases in rural Malaysian communities. Southeast Asian J Trop Med Public Health 38: 998–1007. [PubMed] [Google Scholar]
- 139. Al-Mekhlafi HM, Surin J, Atiya AS, Ariffin WA, Mohammed Mahdy AK, et al. (2008) Pattern and predictors of soil-transmitted helminth reinfection among aboriginal schoolchildren in rural Peninsular Malaysia. Acta Trop 107: 200–204. [DOI] [PubMed] [Google Scholar]
- 140. Amahmid O, Bouhoum K (2005) Assessment of the health hazards associated with wastewater reuse: transmission of geohelminthic infections (Marrakech, Morocco). Ann Agric Environ Med 12: 35–38. [DOI] [PubMed] [Google Scholar]
- 141. Awasthi S, Verma T, Kotecha P, Venkatesh V, Joshi V, et al. (2008) Prevalence and risk factors associated with worm infestation in pre-school children (6–23 months) in selected blocks of Uttar Pradesh and Jharkhand, India. Indian J Med Sci 62: 484–491. [PubMed] [Google Scholar]
- 142. Barreto ML, Genser B, Strina A, Teixeira MG, Assis AMO, et al. (2010) Impact of a citywide sanitation program in Northeast Brazil on intestinal parasites infection in young children. Environ Health Perspect 118: 1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Basualdo JA, Cordoba MA, de Luca MM, Ciarmela ML, Pezzani BC, et al. (2007) Intestinal parasitoses and environmental factors in a rural population of Argentina, 2002–2003. Bull Soc Pathol Exot 100: 174–175. [DOI] [PubMed] [Google Scholar]
- 144. Belo S, Rompao H, Goncalves L, Gracio MA (2005) Prevalence, behavioural and social factors associated with Schistosoma intercalatum and geohelminth infections in Sao Tome and Principe. Parassitologia 47: 227–231. [PubMed] [Google Scholar]
- 145. Carneiro FF, Cifuentes E, Tellez-Rojo MM, Romieu I (2002) The risk of Ascaris lumbricoides infection in children as an environmental health indicator to guide preventive activities in Caparao and Alto Caparao, Brazil. Bull World Health Organ 80: 40–46. [PMC free article] [PubMed] [Google Scholar]
- 146. Ellis MK, Raso G, Li YS, Rong Z, Chen HG, et al. (2007) Familial aggregation of human susceptibility to co- and multiple helminth infections in a population from the Poyang Lake region, China. Int J Parasitol 37: 1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ensink JH, van der Hoek W, Mukhtar M, Tahir Z, Amerasinghe FP (2005) High risk of hookworm infection among wastewater farmers in Pakistan. Proc Natl Acad Sci U S A 102: 12449–12454. [DOI] [PubMed] [Google Scholar]
- 148. Farook MU, Sudharmini S, Remadevi S, Vijayakumar K (2002) Intestinal helminthic infestations among tribal populations of Kottoor and Achankovil areas in Kerala (India). J Commun Dis 34: 171–178. [PubMed] [Google Scholar]
- 149. Ferreira MU, Ferreira CD, Monteiro CA (2000) Secular trends in child intestinal parasitic diseases in S. Paulo city, Brazil (1984–1996). Rev Saude Publica 34: 73–82. [PubMed] [Google Scholar]
- 150. Geissler PW, Mwaniki D, Thiong'o F, Friis H (1998) Geophagy as a risk factor for geohelminth infections: A longitudinal study of Kenyan primary schoolchildren. Trans R Soc Trop Med Hyg 92: 7–11. [DOI] [PubMed] [Google Scholar]
- 151. Glickman LT, Camara AO, Glickman NW, McCabe GP (1999) Nematode intestinal parasites of children in rural Guinea, Africa: prevalence and relationship to geophagia. Int J Epidemiol 28: 169–174. [DOI] [PubMed] [Google Scholar]
- 152. Gunawardena GS, Karunaweera ND, Ismail MM (2005) Effects of climatic, socio-economic and behavioural factors on the transmission of hookworm (Necator americanus) on two low-country plantations in Sri Lanka. Ann Trop Med Parasitol 99: 601–609. [DOI] [PubMed] [Google Scholar]
- 153. Gunawardena K, Kumarendran B, Ebenezer R, Gunasingha MS, Pathmeswaran A, et al. (2011) Soil-transmitted helminth infections among plantation sector schoolchildren in Sri Lanka: prevalence after ten years of preventive chemotherapy. PLoS Negl Trop Dis 5: e1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Guo-Fei W, Ying-Dan C, Chang-Hai Z, Ting-Jun Z (2011) [Analysis of influencing factors of Trichuris trichiura infection in demonstration plots of comprehensive control of parasitic diseases]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi [Chinese journal of schistosomiasis control] 23: 495–500. [PubMed] [Google Scholar]
- 155. Gyorkos TW, Maheu-Giroux M, Blouin B, Casapia M (2011) Exploring determinants of hookworm infection in peruvian schoolchildren using a gender analysis. Am J Epidemiol 173: S224. [Google Scholar]
- 156. Habbari K, Tifnouti A, Bitton G, Mandil A (2001) Geohelminthic infections associated with raw wastewater reuse for agricultural purposes in Beni-Mellal, Morocco. J Parasitol 87: 169–172. [DOI] [PubMed] [Google Scholar]
- 157. Halpenny CM, Paller C, Koski KG, Valdes VE, Scott ME (2013) Regional, household and individual factors that influence soil transmitted helminth reinfection dynamics in preschool children from rural indigenous Panama. PLoS Negl Trop Dis 7: e2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Henry FJ (1988) Reinfection with Ascaris lumbricoides after chemotherapy: a comparative study in three villages with varying sanitation. Ann Parasitol Hum Comp 63: 448–454. [DOI] [PubMed] [Google Scholar]
- 159. Huat LB, Mitra AK, Noor Jamil NI, Dam PC, Jan Mohamed HJ, et al. (2012) Prevalence and risk factors of intestinal helminth infection among rural Malay children. Journal of Global Infectious Diseases 4: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Koura GK, Briand V, Massougbodji A, Cot M, Garcia A (2011) Prevalence and risk factors for soil-transmitted helminth infection in beninese women during pregnancy. Am J Epidemiol 173: S225. [Google Scholar]
- 161. Lee VJ, Ong A, Lee NG, Lee WT, Fong KL, et al. (2007) Hookworm infections in Singaporean soldiers after jungle training in Brunei Darussalam. Vet Parasitol 150: 128–138 Epub 2007 Oct 2024. [DOI] [PubMed] [Google Scholar]
- 162. Luoba AI, Geissler PW, Estambale B, Ouma JH, Alusala D, et al. (2005) Earth-eating and reinfection with intestinal helminths among pregnant and lactating women in western Kenya. Trop Med Int Health 10: 220–227. [DOI] [PubMed] [Google Scholar]
- 163. Moraes LR, Cancio JA, Cairncross S (2004) Impact of drainage and sewerage on intestinal nematode infections in poor urban areas in Salvador, Brazil. Trans R Soc Trop Med Hyg 98: 197–204. [DOI] [PubMed] [Google Scholar]
- 164. Moraes LRS (2007) Household solid waste bagging and collection and their health implications for children living in outlying urban settlements in Salvador, Bahia State, Brazil. Cad Saude Publica 23: S643–S649. [DOI] [PubMed] [Google Scholar]
- 165. Nguyen PH, Nguyen KC, Nguyen TD, Le MB, Bern C, et al. (2006) Intestinal helminth infections among reproductive age women in Vietnam: prevalence, co-infection and risk factors. Southeast Asian J Trop Med Public Health 37: 865–874. [PubMed] [Google Scholar]
- 166. Norhayati M, Oothuman P, Fatmah MS (1999) Some risk factors of Ascaris and Trichuris infection in Malaysian aborigine (Orang Asli) children. Med J Malaysia 54: 96–101. [PubMed] [Google Scholar]
- 167. Olsen A, Samuelsen H, Onyango-Ouma W (2001) A study of risk factors for intestinal helminth infections using epidemiological and anthropological approaches. J Biosoc Sci 33: 569–584. [DOI] [PubMed] [Google Scholar]
- 168. Ortiz Valencia LI, Drumond Fortes BDPM, De Andrade Medronho R (2005) Spatial Ascariasis risk estimation using socioeconomic variables. Int J Environ Health Res 15: 411–424. [DOI] [PubMed] [Google Scholar]
- 169. Riess H, Clowes P, Kroidl I, Kowuor DO, Nsojo A, et al. (2013) Hookworm infection and environmental factors in mbeya region, Tanzania: a cross-sectional, population-based study. PLoS Negl Trop Dis 7: e2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Rísquez PA, Márquez TMD, Quintero PGdC, Ramírez DJP, Requena JG, et al. (2010) Condiciones higiénico-sanitarias como factores de riesgo para las parasitosis intestinales en una comunidad rural venezolana. Rev Fac Med (Caracas) 33: 151–158. [Google Scholar]
- 171. Saathoff E, Olsen A, Kvalsvig JD, Geissler WP (2002) Geophagy and its association with geohelminth infection in rural schoolchildren from northern KwaZulu-Natal, South Africa. Trans R Soc Trop Med Hyg 96: 485–490. [DOI] [PubMed] [Google Scholar]
- 172. Scolari C, Torti C, Beltrame A, Matteelli A, Castelli F, et al. (2000) Prevalence and distribution of soil-transmitted helminth (STH) infections in urban and indigenous schoolchildren in Ortigueira, State of Parana, Brasil: implications for control. Rev Inst Med Trop Sao Paulo 42: 115–117. [DOI] [PubMed] [Google Scholar]
- 173. Sherkhonov T, Yap P, Mammadov S, Sayfuddin K, Martinez P, et al. (2013) National intestinal helminth survey among schoolchildren in Tajikistan: prevalences, risk factors and perceptions. Acta Trop 126: 93–98. [DOI] [PubMed] [Google Scholar]
- 174. Soares Magalhaes RJ, Barnett AG, Clements ACA (2011) Geographical analysis of the role of water supply and sanitation in the risk of helminth infections of children in West Africa. Proc Natl Acad Sci U S A 108: 20084–20089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Steenhard NR, Ornbjerg N, Molbak K (2009) Concurrent infections and socioeconomic determinants of geohelminth infection: a community study of schoolchildren in periurban Guinea-Bissau. Trans R Soc Trop Med Hyg 103: 839–845. [DOI] [PubMed] [Google Scholar]
- 176. Teixeira JC, Heller L (2004) Fatores ambientais associados às helmintoses intestinais em áreas de assentamento subnormal, Juiz de Fora, MG. Eng sanit ambient 9: 301–305. [Google Scholar]
- 177. Trang DT, van der Hoek W, Cam PD, Vinh KT, Hoa NV, et al. (2006) Low risk for helminth infection in wastewater-fed rice cultivation in Vietnam. East Mediterr Health J 12: 137–143. [DOI] [PubMed] [Google Scholar]
- 178. Ugbomoiko US, Dalumo V, Ofoezie IE, Obiezue RNN (2009) Socio-environmental factors and ascariasis infection among school-aged children in Ilobu, Osun State, Nigeria. Trans R Soc Trop Med Hyg 103: 223–228. [DOI] [PubMed] [Google Scholar]
- 179. Walker M, Hall A, Basanez MG (2011) Individual predisposition, household clustering and risk factors for human infection with Ascaris lumbricoides: new epidemiological insights. PLoS Negl Trop Dis 5: e1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Yajima A, Jouquet P, Do TD, Dang TC, Tran CD, et al. (2009) High latrine coverage is not reducing the prevalence of soil-transmitted helminthiasis in Hoa Binh province, Vietnam. Parasitol Res 104: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot for treated water use and any STH infection.
(EPS)
Funnel plot for piped water use and any STH infection.
(EPS)
Funnel plot for piped water use and A. lumbricoies infection.
(EPS)
Funnel plot for piped water use and T. trichiura infection.
(EPS)
Funnel plot for sanitation access and any STH infection.
(EPS)
Funnel plot for sanitation access and A. lumbricoides infection.
(EPS)
Funnel plot for sanitation access and T. trichiura infection.
(EPS)
Funnel plot for sanitation access and hookworm infection.
(EPS)
Funnel plot for soap use and any STH infection.
(EPS)
Funnel plot for handwashing before eating and A. lumbricoides infection.
(EPS)
Funnel plot for handwashing after defecating and A. lumbricoides infection.
(EPS)
Funnel plot for handwashing after defecating and any STH infection.
(EPS)
Funnel plot for wearing shoes and hookworm infection.
(EPS)
Funnel plot for wearing shoes and any STH infection.
(EPS)
Excluded studies.
(DOC)
Study bias assesment.
(DOC)
PRISMA checklist.
(DOC)
MOOSE checklist.
(DOC)
Original methods protocol.
(DOC)