Abstract
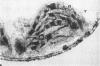
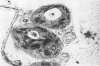
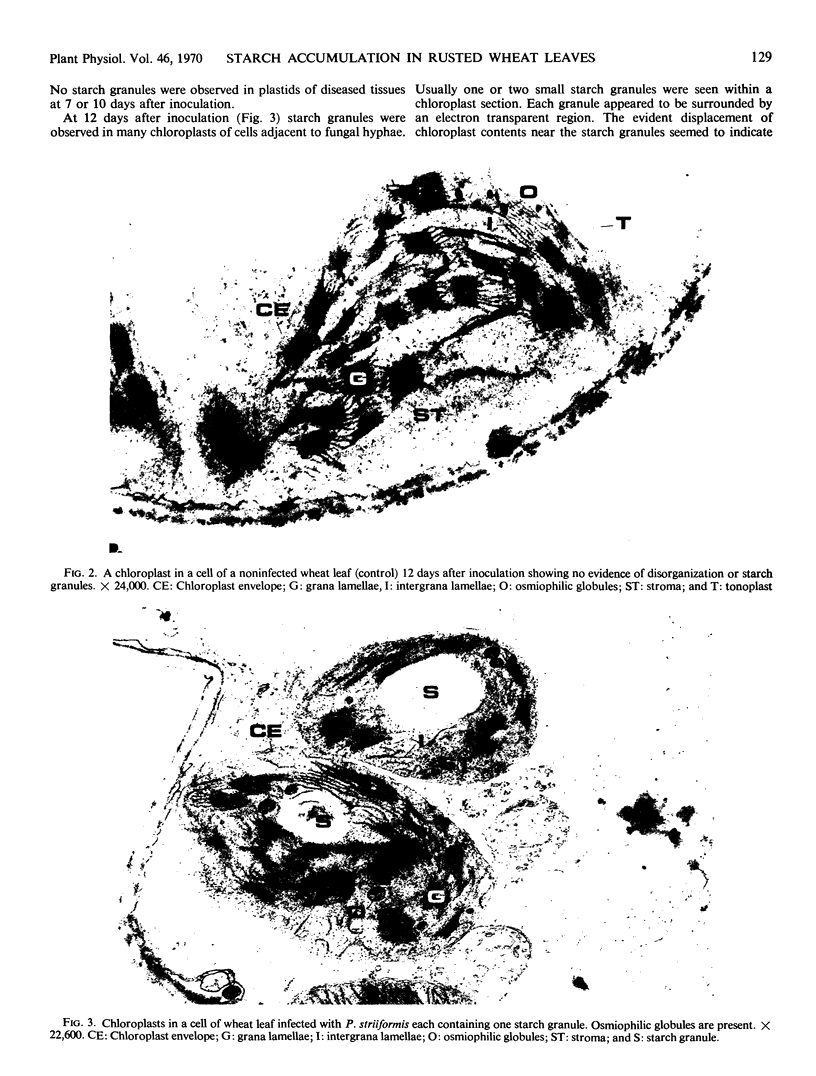
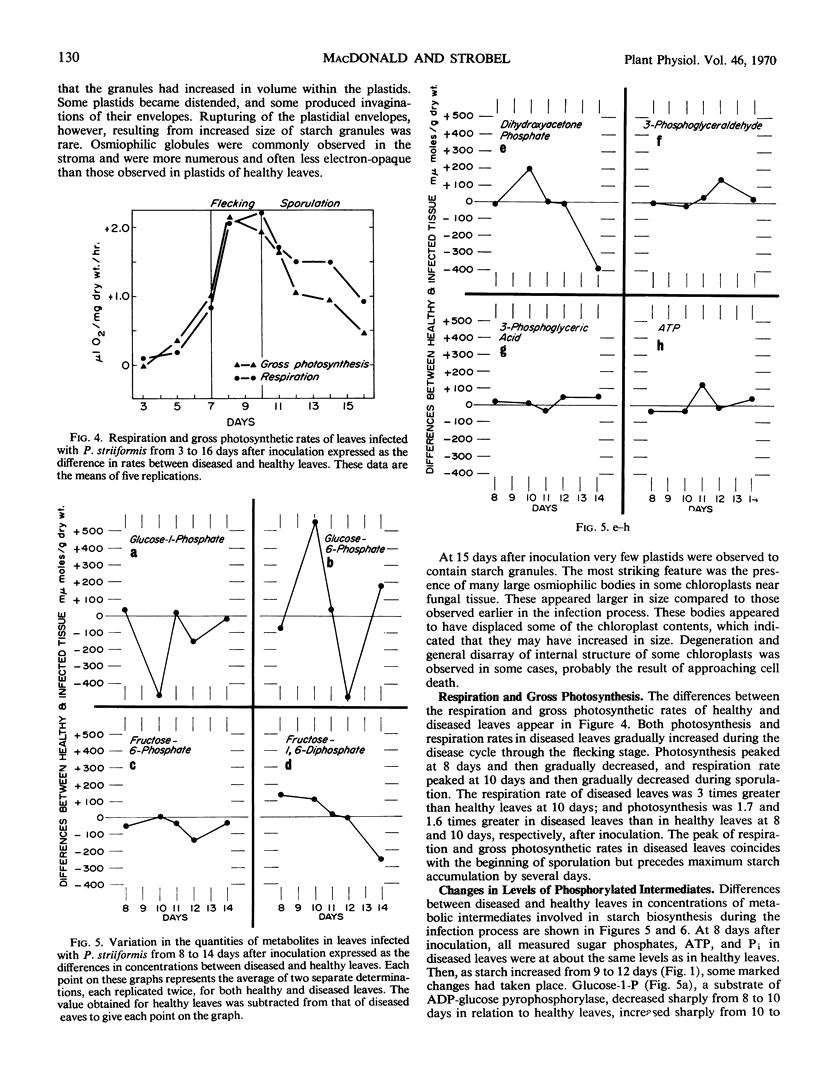
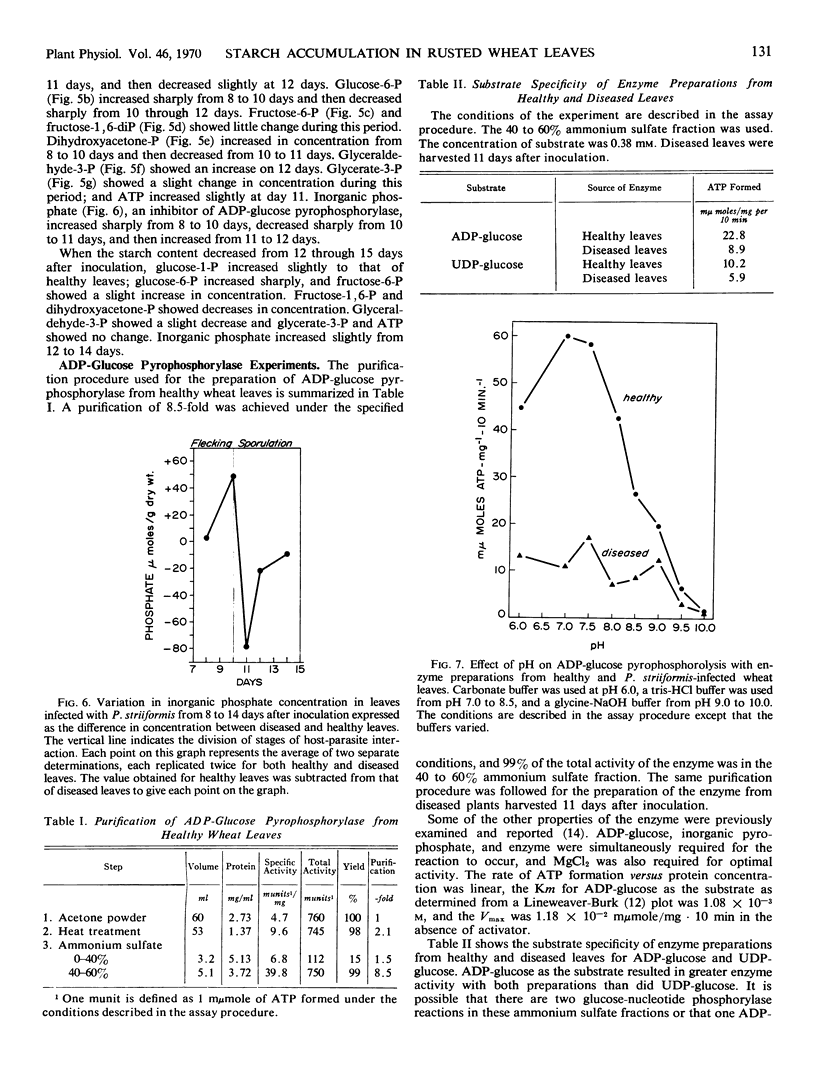
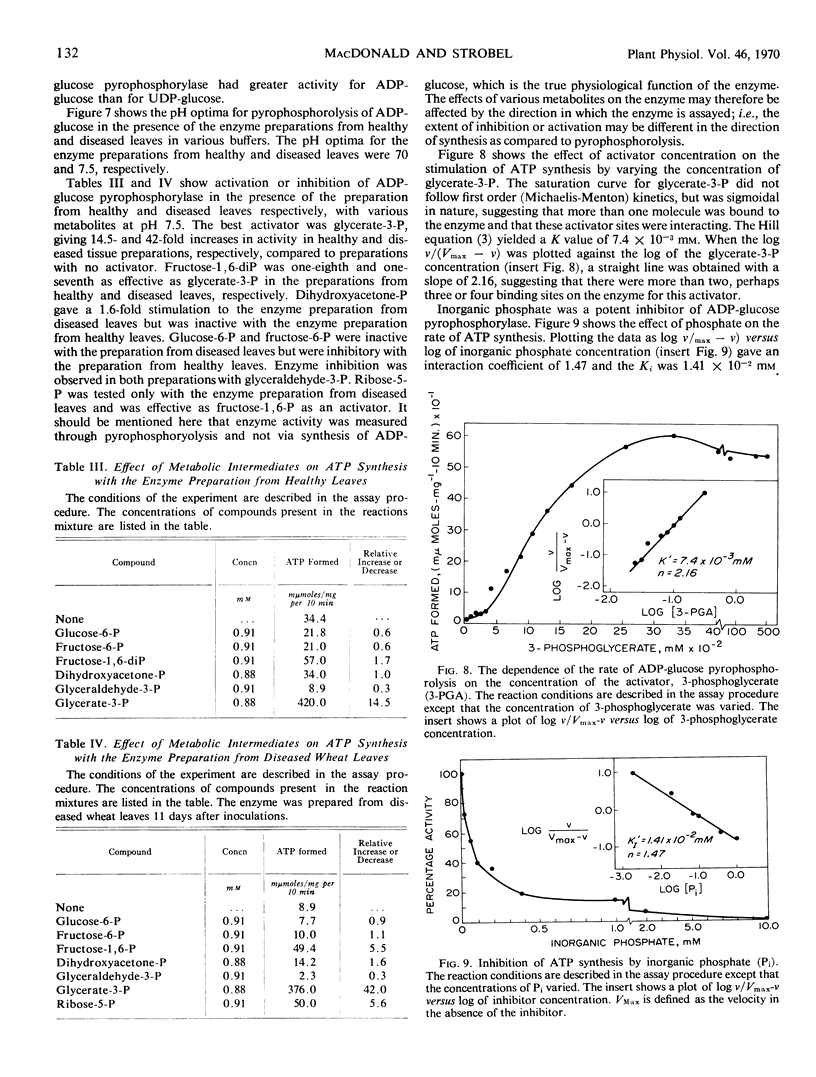
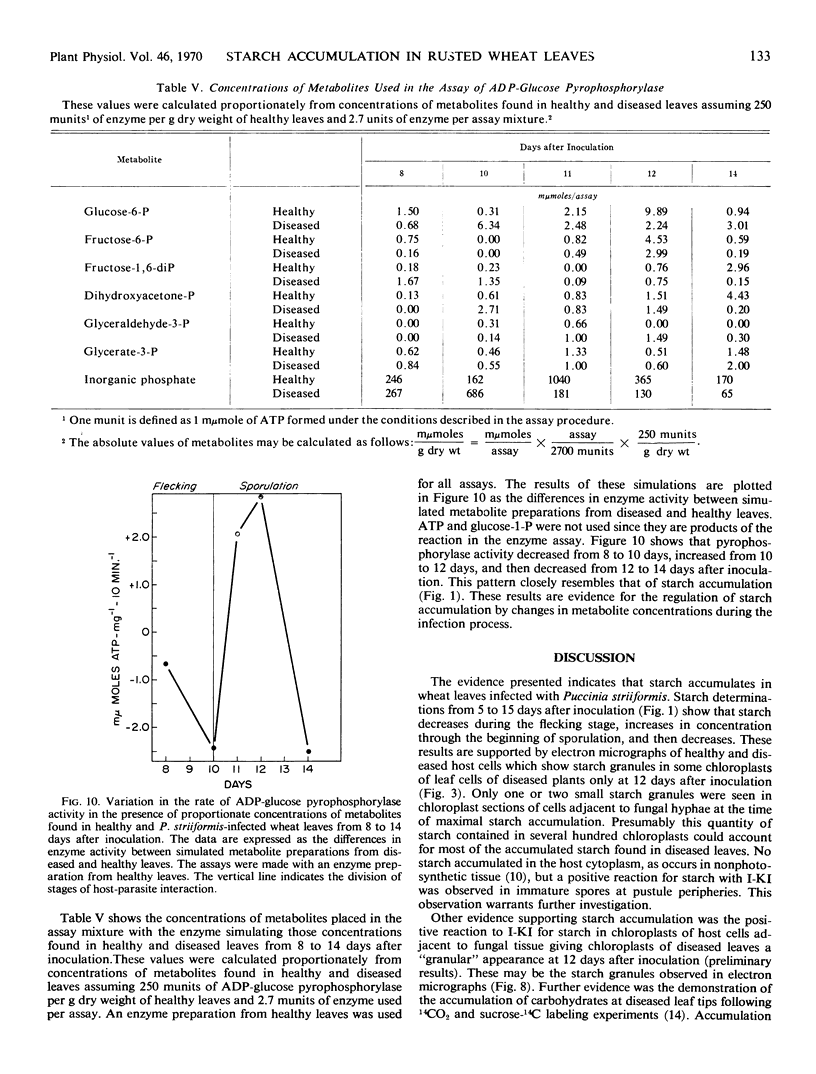
The variation in starch content in healthy and Puccinia striiformsi-infected wheat leaves was measured from 5 to 15 days after inoculation. The starch content of diseased leaves relative to healthy leaves decreased from 5 to 9 days, increased from 9 to 12 days to twice that of healthy leaves, and decreased from 12 to 15 days after inoculation. Electron micrographs of plant tissues indicated that the starch accumulated in the chloroplasts of host cells adjacent to fungal hyphae. Variations in sugar phosphates, ATP, and inorganic phosphate were measured during the infection process. ADP-glucose pyrophosphorylase was extracted and partially purified from healthy and diseased leaves. When proportionate concentrations of sugar phosphates and inorganic phosphate found in healthy and diseased leaves during the infection process were placed in the assay mixture, ADP-glucose pyrophosphorylase activity was similar to the pattern of starch accumulation and was almost the inverse of the variation observed in inorganic phosphate in diseased leaves during the infection process. A mechanism to explain the accumulation of starch is presented and discussed. This mechanism is based on the regulation of ADP-glucose pyrophosphorylase by changes in effector molecule concentrations during the infection process. Reasons for these changes are presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carroll T. W., Kosuge T. Changes in structure of chloroplasts accompanying necrosis of tobacco leaves systemically infected with tobacco virus. Phytopathology. 1969 Jul;59(7):953–962. [PubMed] [Google Scholar]
- FRYDMAN R. B. STARCH SYNTHETASE OF POTATOES AND WAXY MAIZE. Arch Biochem Biophys. 1963 Aug;102:242–248. doi: 10.1016/0003-9861(63)90177-2. [DOI] [PubMed] [Google Scholar]
- GHOSH H. P., PREISS J. THE BIOSYNTHESIS OF STARCH IN SPINACH CHLOROPLASTS. J Biol Chem. 1965 Feb;240:960–962. [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Biosynthesis of starch in spinach chloroplasts. Biochemistry. 1965 Jul;4(7):1354–1361. doi: 10.1021/bi00883a020. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Level of photosynthetic intermediates in isolated spinach chloroplasts. Plant Physiol. 1969 Mar;44(3):396–402. doi: 10.1104/pp.44.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Greenberg E. Biosynthesis of starch in Chlorella pyrenoidosa. I. Purification and properties of the adenosine diphosphoglucose: alpha-1, 4-glucan, alpha-4-glucosyl transferase from Chlorella. Arch Biochem Biophys. 1967 Mar 20;118(3):702–708. doi: 10.1016/0003-9861(67)90407-9. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal G. G., Greenberg E., Hardie J., Cameron E. C., Preiss J. Regulation of starch biosynthesis in plant leaves: activation and inhibition of ADPglucose pyrophosphorylase. Plant Physiol. 1968 Mar;43(3):417–427. doi: 10.1104/pp.43.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal G. G., Preiss J. Biosynthesis of starch in Chlorella pyrenoidosa. II. Regulation of ATP: alpha-D-glucose 1-phosphate adenyl transferase (ADP-glucose pyrophosphorylase) by inorganic phosphate and 3-phosphoglycerate. Arch Biochem Biophys. 1967 Mar;119(1):454–469. doi: 10.1016/0003-9861(67)90477-8. [DOI] [PubMed] [Google Scholar]