Abstract
The early identification of children presenting ALKF1174L-mutated neuroblastoma, which are associated with resistance to the promising ALK inhibitor crizotinib and a marked poorer prognosis, has become a clinical priority. In comparing the radiology of the novel Th-ALKF1174L/Th-MYCN and the well-established Th-MYCN genetically-engineered murine models of neuroblastoma using MRI, we have identified a marked ALKF1174L-driven vascular phenotype. We demonstrate that quantitation of the transverse relaxation rate R2* (s−1) using intrinsic susceptibility-MRI under baseline conditions and during hyperoxia, can robustly discriminate this differential vascular phenotype, and identify MYCN-driven tumors harboring the ALKF1174L mutation with high specificity and selectivity. Intrinsic susceptibility-MRI could thus potentially provide a non-invasive and clinically-exploitable method to help identifying children with MYCN-driven neuroblastoma harboring the ALKF1174L mutation at the time of diagnosis.
Introduction
Neuroblastoma arises in the sympathetic nervous system during embryogenesis and is the most common extracranial solid tumor in children [1]. For the established subset of patients presenting with high-risk neuroblastoma, the current portfolio of therapeutic options has limited success, with five-year survival rates rarely exceeding 40%.
The poor clinical outcome and aggressive tumor phenotype of high-risk neuroblastoma strongly correlates with amplification of the proto-oncogene MYCN and enhanced tumor angiogenesis [2]. Recently, mutations in the anaplastic lymphoma kinase (ALK) tyrosine kinase gene have been identified in ∼8–10% of primary neuroblastoma, leading to constitutive activation of the ALK protein. The most common and potent ALK mutation, ALKF1174L, is associated preferentially with MYCN amplification, a markedly poorer prognosis, and confers resistance to the promising ALK inhibitor crizotinib [3]–.
With crizotinib in pediatric phase I clinical trials, and other ALK inhibitor studies in development, a current challenge is to rapidly identify upfront children with high-risk ALK mutated or amplified neuroblastoma who may benefit from or become resistant to ALK-targeted therapy.
As most pediatric cancers, including neuroblastoma, originate from only a few genetic anomalies during development, they are amenable to genetically engineered mouse (GEM) modeling approaches. GEM models of neuroblastoma, such as the Th-MYCN murine model, which develop spontaneous tumors mirroring the major pathophysiological, genetic and radiological features of high-risk MYCN-amplified childhood neuroblastoma, represent clinically-relevant tools for the study of neuroblastoma biology and response to novel therapeutics [6]–[8]. The development of GEM models co-expressing ALKF1174L and MYCN to the neural crest, such as the Th-ALKF1174L/Th-MYCN model, have recently been used to demonstrate how the ALKF1174L mutation potentiates the oncogenic activity of MYCN, and that ALKF1174L acquired resistance to crizotinib can be overcome through inhibition of key cellular modulators of n-myc [9]–[11].
In this study, we hypothesized that the presence of the ALKF1174L mutation results in a phenotypic difference in hemodynamic vasculature in tumors in the Th-ALKF1174L/Th-MYCN model, and which can be evaluated using intrinsic susceptibility magnetic resonance imaging (MRI). The aim of our study was to demonstrate that quantitation of the transverse relaxation rate, R2*, and changes in R2* induced by breathing 100% oxygen, ΔR2*oxygen-air, could discriminate between tumors arising in Th-ALKF1174L/Th-MYCN and Th-MYCN mice, and that intrinsic susceptibility MRI could thus potentially provide a non-invasive and clinically-translatable method to help identify children, presenting MYCN-amplified neuroblastoma harboring the ALKF1174L mutation.
Materials and Methods
Ethics statement
All procedures involving animals were approved by the Institute of Cancer Research Animal Ethics Committee and the UK Home Office and carried out according to the United Kingdom National Cancer Research Institute guidelines for the welfare of animals in cancer research [12].
Animal models
The generation of the Th-ALKF1174L/Th-MYCN mice has been recently described [11]. Th-ALKF1174L/Th-MYCN and Th-MYCN mice were identified by analyzing DNA from mice tails using real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR, Transnetyx Inc., Cordova, Tennessee), and mice with tumors were initially identified by palpation.
Magnetic Resonance Imaging
All the 1H MRI studies were performed on a 7T Bruker horizontal bore micro-imaging system (Bruker Instruments, Ettlingen, Germany) using a 3 cm birdcage coil. Anesthesia was induced by an intraperitoneal 0.1 ml injection of a combination of fentanyl citrate (0.315 mg/ml) plus fluanisone (10 mg/ml) (Hypnorm, Janssen Pharmaceutical, Oxford, UK) and midazolam (5 mg/ml) (Roche, Welwyn Garden City, UK) and water (1∶1∶2). A nose piece was positioned for oxygen delivery.
For all the mice, anatomical T2-weighted coronal and transverse images were acquired from twenty contiguous 1 mm-thick slices through the mouse abdomen, using a rapid acquisition with refocused echoes (RARE) sequence with 4 averages of 128 phase encoding steps over a 3×3 cm field of view, two echo times (TE) of 36 and 132 ms, a repetition time (TR) of 4.5 s and a RARE factor of 8. These images were used to determine tumor volumes, and for planning the intrinsic-susceptibility MRI measurements, which included optimization of the local field homogeneity. The baseline transverse relaxation rate R2*, sensitive to the concentration of paramagnetic species, principally deoxyhemoglobin, was quantified in tumors from Th-ALKF1174L/Th-MYCN (n = 23) and Th-MYCN (n = 21) mice, using a multiple gradient echo (MGE) sequence. MGE images were acquired from three 1 mm thick transverse slices through each tumor, using 8 averages of 128 phase encoding steps over a 3×3 cm field of view, and an acquisition time of 3 min 20 s. Images were acquired using 8 echoes spaced 3 ms apart, an initial echo time of 6 ms, a flip angle α = 45° and a repetition time of 200 ms. Subsequently, 100% oxygen (BOC Ltd, Guildford, UK) was delivered at a rate of 2 L/min to Th-ALKF1174L/Th-MYCN (n = 10) and Th-MYCN (n = 12) mice. After a 5 minutes equilibrium period, identical MGE images were acquired whilst the mouse continued to inhale oxygen.
All the MGE data were fitted voxelwise using in-house software (ImageView, working under IDL, ITT, Boulder, Colorado, USA) with a robust Bayesian approach that provided estimates of R2* and ΔR2*oxygen-air ( = R2*oxygen−R2*air) [13].
Histological Assessment
Formalin fixed paraffin embedded sections from Th-ALKF1174L/Th-MYCN and Th-MYCN mice were stained with haematoxylin and eosin, and visualized under light microscopy. The extent of functional vasculature in tumors from both models was assessed using the perfusion marker Hoechst 33342, and quantified as fluorescent area fractions (%), as previously described [8].
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, USA). The mean values for tumor volume, mean of median values for R2* and ΔR2*oxygen-air, and the mean fluorescent area fractions were used for statistical analysis. R2* and ΔR2*oxygen-air were assumed to be normally distributed, which was confirmed using the D'Agostino-Pearson omnibus K2 normality test [14]. Any significant difference in tumor volume, R2*, ΔR2*oxygen-air and the fluorescent area fractions between Th-ALKF1174L/Th-MYCN and Th-MYCN mice were identified using Student's 2-tailed unpaired t-test, with a 5% level of significance.
Results
ALKF1174Lmutation induces a differential radiological presentation of neuroblastoma
Anatomical T2-weighted RARE MR images revealed solid masses within the retroperitoneum in peri-renal and para-spinal abdominal regions of both the Th-ALKF1174L/Th-MYCN and Th-MYCN mice, typical of the clinical distribution and radiological presentation of human neuroblastoma (Figure 1A) [15], [16]. All the Th-ALKF1174L/Th-MYCN (n = 23) and Th-MYCN (n = 21) mice examined presented with abdominal tumors with wide range of volumes (525–3400 mm3 for tumors in Th-ALKF1174L/Th-MYCN mice and 335–2450 mm3 for tumors in Th-MYCN mice). Tumors in the Th-MYCN mice appeared heterogeneous, with 94% of tumors presenting with areas of hypointense signal, consistent with previous observations [8]. Tumors in the Th-ALKF1174L/Th-MYCN mice were generally hyperintense and significantly more homogeneous, with only 48% of the tumors having hypointense regions (p = 0.006, χ2-test). On T2*-weighted images (Figure 1B and C, and Figure 2A), tumors in the Th-MYCN mice were generally hypointense at longer echo times, whereas the signal from tumors in the Th-ALKF1174L/Th-MYCN remained hyperintense, providing a stark positive contrast with the surrounding organs for 74% of the Th-ALKF1174L/Th-MYCN mice (compared to 14% for the Th-MYCN mice, p<0.001, χ2-test).
Figure 1. Radiological comparison of the Th-ALKF1174L/Th-MYCN and Th-MYCN mice with abdominal neuroblastoma.
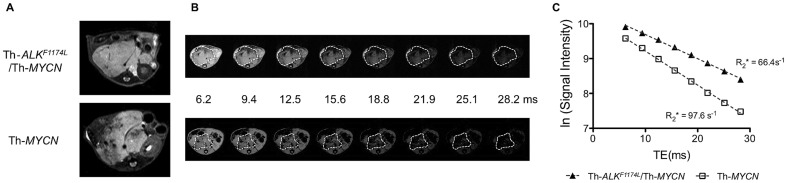
A) Anatomical transverse T2-weighted MR images acquired with a rapid acquisition with refocused echoes (RARE) sequence and B) anatomical transverse T2*-weighted MR images acquired at increasing gradient echo times as indicated, from representative presenting with abdominal neuroblastoma. C) Note the rapidly decaying tumor signal intensity in the Th-MYCN mouse, compared to the more sustained tumor signal observed in the Th-ALKF1174L/Th-MYCN mouse.
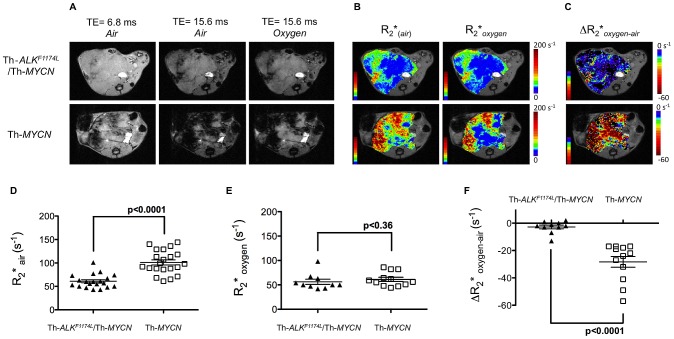
Figure 2. Identification of tumors harboring the ALKF1174L mutation in MYCN-driven transgenic mice with intrinsic susceptibility MRI.
A) Anatomical transverse T2*-weighted MR images acquired at TE = 6.8 and 15.6 ms from representative Th-ALKF1174L/Th-MYCN and Th-MYCN mice with abdominal neuroblastoma during initial air breathing, and at TE = 15.6 ms after 5 minutes of continuous inhalation of 100% oxygen. B) Corresponding parametric tumor transverse relaxation rate R2* maps calculated during initial air breathing and after 3 minutes of continuous inhalation of 100% oxygen. C) Resulting parametric tumor ΔR2*oxygen-air (R2*oxygen−R2*air) maps. D) Tumor R2* during initial air breathing, E) tumor R2* after 5 minutes of breathing 100% oxygen, and F) tumor ΔR2*oxygen-air (R2*oxygen−R2*air) were determined from Th-ALKF1174L/Th-MYCN and Th-MYCN mice with abdominal neuroblastoma. Individual data points represent the mean of the median values determined from all three imaging slices for each animal, as well as the mean ±1 s.e.m, p, Student's 2-tailed unpaired t-test with a 5% level of significance.
ALKF1174Lmutation results in a slower tumor transverse relaxation rate, R2 *, in neuroblastoma
Parametric maps revealed a heterogeneous distribution of R2* in tumors in both models (Figure 2B). Quantitation of the transverse relaxation R2* revealed significantly slower rates in tumors in the Th-ALKF1174L/Th-MYCN cohort, compared to the faster R2* rate determined in tumors in the Th-MYCN mice (Figure 2D). Baseline R2* detected tumors harboring the ALKF1174L mutation with a sensitivity of 90% (95% CI: 66.9–98.2) and a specificity of 81% (95% CI: 57.4–93.7).
ALKF1174Lmutation is associated with a stark differential BOLD MRI response to hyperoxic challenge in neuroblastoma
Continuous inhalation of 100% oxygen resulted in tumors in the Th-MYCN mice demonstrating a very strong, heterogeneous increase in T2*-weighted image signal intensity (blood oxygen level dependent (BOLD) effect) (Figure 2A). In contrast, tumors from Th-ALKF1174L/Th-MYCN mice showed a negligible BOLD effect on T2* weighted image intensity with hyperoxia. As with baseline R2*, parametric maps revealed a heterogeneous distribution of ΔR2*oxygen-air in tumors in both models (Figure 2B and C). Tumor regions showing a relatively fast baseline R2* remained so during hyperoxia, whereas regions of relatively slower baseline R2* typically showed a more marked reduction in R2* with 100% O2. The significant difference in R2* between the two models was lost with 100% oxygen challenge (Figure 2E). As a consequence, tumors in Th-ALKF1174L/Th-MYCN mice demonstrated a significantly lower absolute value of ΔR2*oxygen-air than the tumors in Th-MYCN mice (Figure 2F). Hyperoxia-induced ΔR2*oxygen-air detected tumors harboring the ALKF1174L mutation with a sensitivity of 90% (95% CI: 54.1–99.5) and a specificity of 94.1% (95% CI: 69.2–99.7).
No correlation between tumor R2* and ΔR2*oxygen-air, or between either R2* or ΔR2*oxygen-air and tumor volume, was determined across the cohorts, indicating that the differences in intrinsic susceptibility MRI between tumors in the Th-ALKF1174L/Th-MYCN and Th-MYCN mice were independent of tumor size.
ALKF1174Lmutation is associated with reduced functional vasculature in neuroblastoma
Gross examination of the tumors in situ, prior to excision, revealed an intense dark red coloration across the whole tumor in the Th-MYCN mice, in contrast to the pale appearance of tumors in the Th-ALKF1174L/Th-MYCN GEM model (Figure 3A). Histological examination with H&E staining revealed the presence of large hemorrhagic regions filled with stacked erythrocytes in 100% of the tumors from Th-MYCN mice (n = 10), but only in 10% the Th-ALKF1174L/Th-MYCN model (n = 10, p<0.0001, χ2-test) (Figure 3B and C). Fluorescence microscopy of Hoechst 33342 uptake revealed homogeneously and well-vascularized tumors in both models (Figure 3D). However, quantitation of the fluorescent area fractions revealed that tumors in the Th-MYCN mice had significantly higher uptake, consistent with the presence of more perfused, functional vasculature (Figure 3E).
Figure 3. Pathological comparison of tumors from Th-ALKF1174L/Th-MYCN and Th-MYCN mice with abdominal neuroblastoma.
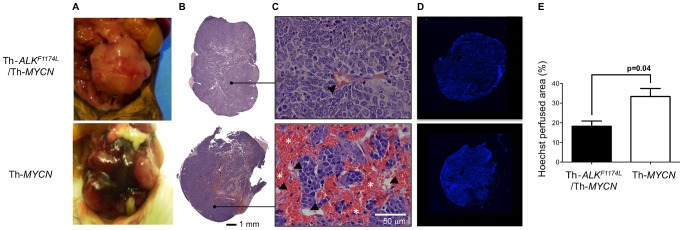
A) Gross pathology, B) composite images, and C) high magnification (x200) images from hematoxylin and eosin stained sections. Note the presence of large hemorrhagic regions filled with aggregated erythrocytes (*, blood lakes) extravasated from blood vessels (arrowed) in the tumor from the Th-MYCN mouse. D) Composite fluorescence images of uptake of the perfusion marker Hoechst 33342 into tumors from Th-ALKF1174L/Th-MYCN and Th-MYCN mice with abdominal neuroblastoma. E) Quantitation of Hoechst 33342 uptake revealed significantly lower functionally perfused vasculature in tumors of Th-ALKF1174L/Th-MYCN mice (n = 5) compared with tumors in Th-MYCN mice (n = 5). Data are mean ±1 s.e.m, p, Student's 2-tailed unpaired t-test with a 5% level of significance.
Discussion
In the current study we demonstrate that baseline R2* and hyperoxia-induced ΔR2* can discriminate a differential hemodynamic tumor vascular phenotype between tumors arising in Th-ALKF1174L/Th-MYCN and Th-MYCN models of high-risk, MYCN over-expressing neuroblastoma. With a sensitivity of 90% and a specificity of 81% for baseline R2*, and a sensitivity of 90% and a specificity of 94% for 100% oxygen-induced ΔR2*, intrinsic susceptibility MRI provides a robust method to discriminate and identify Th-MYCN transgenic mice harboring the ALKF1174L mutation.
The rapid and completely noninvasive quantitation of tumor R2* is suitable for the scanning of young children. Furthermore, such pediatric imaging sessions are usually performed under general anesthesia, providing an opportunity to transiently perturb the oxygen content inhaled by the patient, and enabling quantitation of tumor R2* under more hyperoxic conditions (from 21 to 30–40% O2). Upon successful translation, intrinsic susceptibility MRI could provide a rapid method to identify children with ALK-driven tumors, enabling the stratification of children with this ultra high-risk neuroblastoma at the time of diagnosis.
The baseline transverse relaxation rate R2* is sensitive to the concentration of paramagnetic deoxyhemoglobin in the vascular compartment in tissue (BOLD effect). Compared to most normal tissues, tumors exhibit relatively fast native R2* values, a consequence of the high concentration of deoxygenated erythrocytes within the vascular compartment associated with immature, irregular and unstable microcirculation [17]. The fast baseline tumor R2* measured in the Th-MYCN model is consistent with the aggregation of deoxygenated erythrocytes, described as blood lakes [18], and which are characteristic of childhood neuroblastoma [19]. The significantly slower baseline R2* and differential intrinsic susceptibility MRI presentation of tumors in the Th-ALKF1174L/Th-MYCN mice is consistent with the absence of such blood lakes.
Inhalation of high oxygen content gases results in the rapid re-oxygenation of hemoglobin, with paramagnetic deoxyhemoglobin within perfused tumor vessels being replaced by diamagnetic oxyhemoglobin, and a reduction in R2* [20]. Hyperoxia resulted in an overall significant reduction in R2* of tumors in the Th-MYCN mice, whereas the response in tumors in the Th-ALKF1174L/Th-MYCN mice was negligible, suggesting a clear difference in hemodynamic functional vasculature between the two GEM models. This was corroborated by the significant overall difference in Hoechst 33342 uptake. Interestingly, a spatially different hyperoxia ΔR2* response was apparent in tumors in the Th-MYCN mice. Tumor regions exhibiting relatively fast baseline R2* showed a less pronounced response with 100% O2 breathing, also consistent with the presence of blood lakes which are typically disconnected from the perfused vascular network [21]. In contrast, tumor regions with relatively slower baseline R2* showed a marked response to hyperoxia, indicative of functional vasculature.
Intriguingly, tumors from the Th-ALKF1174L/Th-MYCN mice showed no clear ΔR2* response to hyperoxia, despite Hoechst 33342 uptake indicating the presence of perfused blood vessels in both GEM models. This suggests a difference in vascular architecture that precludes erythrocyte delivery, but not plasma perfusion, in the tumor vessels in the Th-ALKF1174L/Th-MYCN mice, or in oxygen consumption by the tumor cells [17], [22]. The extensive hemorrhage and blood lakes present in the Th-MYCN model are indicative of vessel wall instability due to rapid endothelial cell proliferation and defective pericyte coverage [23]. This implies that the tumor vasculature in the Th-ALKF1174L/Th-MYCN mice may be more mature.
Given its relationship to blood oxygen saturation and partial pressure of oxygen in and around blood vessels, quantitation of baseline tumor R2* is also being investigated as an imaging biomarker of hypoxia [24]. For example, slow baseline R2* has been shown to correlate with increased hypoxia, determined by pimonidazole staining, in chemically-induced rat mammary tumors [25]. Conversely, fast baseline R2* is associated with hypoxia in prostate cancer [26]. In this context, we have recently shown that neuroblastoma from Th-MYCN mice exhibiting fast baseline R2*, induced by a hemorrhagic phenotype, are relatively oxic, as revealed by negligible pimonidazole staining [8]. R2* is first of all a marker of impaired hemodynamic function and as such shares a causal relationship to hypoxia. However this relationship is ambivalent as several differential hemodynamic phenotypes can in fact lead to hypoxia, while having an opposite effect on R2* values. In this study the suggested tumor vascular phenotype in the Th-ALKF1174L/Th-MYCN model, which hinders the delivery of erythrocytes and causes a significantly slower R2*, may result in increased tumour hypoxia in the Th-ALKF1174L/Th-MYCN model compared with the Th-MYCN model.
Recent reports have implicated a role of the ALKF1174L mutation in tumor angiogenesis in neuroblastoma. Selective targeted inhibition of ALK resulted in a significant reduction in vascular density in xenografts derived from MYCN non-amplified SH-SY5Y cells, which harbor the ALKF1174L mutation, accompanied with a decrease in vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) [27]. The expression of MMP-9 by stromal cells has previously been shown to regulate the vascular architecture in a murine orthotopic MYCN-amplified neuroblastoma xenograft model by promoting pericyte recruitment [28]. Collectively, these studies suggest that ALK and the ALKF1174L mutation contribute to tumor vasculogenesis and pericyte recruitment via the regulation of VEGF and MMP-9, leading to a dense vascular network with smaller, more stable vessels. The differential intrinsic susceptibility MRI vascular phenotype observed in tumors in the Th-MYCN and Th-ALKF1174L/Th-MYCN models reported herein demonstrates an important role for ALKF1174L in angiogenesis in MYCN-overexpressing neuroblastoma in vivo, strongly suggesting a role in vasculogenesis.
This study has some limitations. The Th-ALKF1174L mutation leads to the constitutive activation of the ALK protein in neuroblastoma [29]–[32], and the vascular phenotype associated with amplified or wild-type (WT) ALK expression is currently unknown. Amplification of ALK has also been shown to lead to activation of the ALK protein with, however, a 17-fold reduced kinase activity compared to ALKF1174L mutants [29]. In the absence of a Th-ALKwt GEM model, it is difficult to conclude if intrinsic susceptibility MRI would be solely able to identify ALKF1174L-mutated MYCN amplified neuroblastoma, or more generally ALK-driven MYCN amplified neuroblastoma.
There is a clear need to more deeply interrogate the role of the ALKF1174L mutation on vascular morphogenesis and architecture, which may be a major determinant of impaired drug delivery and a contributing factor to the poor prognosis of children with ALKF1174L-mutated MYCN-amplified neuroblastoma. The altered vascular phenotype may also impact on the response to anti-vascular therapies, including cediranib, currently being considered in clinical trials for the treatment of high-risk MYCN-amplified neuroblastoma [33]. Retrospective analysis of historical pathological samples for ALK mutations should provide sufficient statistical power to shed a light on the vascular phenotype of neuroblastoma associated with ALK amplification, and each of the rare ALK mutations, including the lethal ALKF1174L mutation.
Coupled with our recent identification of quantitation of R2* as a biomarker of treatment response to cediranib in the Th-MYCN GEM model [8], the present study reinforces R2* as a biomarker of vasculogenesis and its response to therapy in these clinically relevant GEM models. Furthermore, it provides a strong rationale for the evaluation of intrinsic susceptibility MRI for assessing any anti-angiogenic effects resulting from successful targeted inhibition of ALK signalling in ALKF1174L mutated neuroblastoma, which could ultimately used for the assessment of second generation ALK inhibitors [34], [35]. To conclude, this study has provided a strong rationale for the immediate incorporation of intrinsic susceptibility MRI into forthcoming imaging-embedded clinical trials of next generation ALK inhibitors in ALK-mutated and -amplified neuroblastoma.
Acknowledgments
Alex Oliver, consultant anaesthetist, (Royal Marsden Hospital, UK) for fruitful discussions regarding the potential use of hyperoxia during clinical pediatric imaging sessions.
Funding Statement
The authors acknowledge support received for The Institute of Cancer Research CR-UK (http://www.cancerresearchuk.org/) and EPSRC (http://www.epsrc.ac.uk) Cancer Imaging Centre, in association with the MRC (http://www.epsrc.ac.uk) and Department of Health (England) (https://www.gov.uk/government/organisations/department-of-health) grant C1060/A10334, and NHS funding to the NIHR Biomedical Research Centre. This work was supported by Cancer Research-UK project grants C16412/A6269, The Wellcome Trust (grant #091763Z/10/Z) (http://www.wellcome.ac.uk), and the Medical Research Council (grant #G1000391). Pre-clinical development of the TH-ALKF1174L/TH-MYCN model was funded by grants from The Neuroblastoma Society (http://www.nsoc.co.uk), the SPARKS charity (http://www.sparks.org.uk), Medical Research Council NC3R (grant #G1000121/94513)(http://www.nc3rs.org.uk/), and the National Institutes of Health (R01 CA148688) (http://www.nih.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362: 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meitar D, Crawford SE, Rademaker AW, Cohn SL (1996) Tumor angiogenesis correlates with metastatic disease, N-myc amplification, and poor outcome in human neuroblastoma. J Clin Oncol 14: 405–414. [DOI] [PubMed] [Google Scholar]
- 3. De Brouwer S, De Preter K, Kumps C, Zabrocki P, Porcu M, et al. (2010) Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res 16: 4353–4362. [DOI] [PubMed] [Google Scholar]
- 4. Sasaki T, Rodig SJ, Chirieac LR, Janne PA (2010) The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer 46: 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bresler SC, Wood AC, Haglund EA, Courtright J, Belcastro LT, et al. (2011) Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Science translational medicine 3: 108ra114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chesler L, Weiss WA (2011) Genetically engineered murine models—contribution to our understanding of the genetics, molecular pathology and therapeutic targeting of neuroblastoma. Semin Cancer Biol 21: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM (1997) Targeted expression of MYCN causes neuroblastoma in transgenic mice. Embo J 16: 2985–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jamin Y, Tucker ER, Poon E, Popov S, Vaughan L, et al. (2013) Evaluation of Clinically Translatable MR Imaging Biomarkers of Therapeutic Response in the Th-MYCN Transgenic Mouse Model of Neuroblastoma. Radiology 266: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu S, Lee JS, Guo F, Shin J, Perez-Atayde AR, et al. (2012) Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell 21: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heukamp LC, Thor T, Schramm A, De Preter K, Kumps C, et al. (2012) Targeted expression of mutated ALK induces neuroblastoma in transgenic mice. Science translational medicine 4: 141ra191. [DOI] [PubMed] [Google Scholar]
- 11. Berry T, Luther W, Bhatnagar N, Jamin Y, Poon E, et al. (2012) The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell 22: 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, et al. (2010) Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102: 1555–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walker-Samuel S, Orton M, McPhail LD, Boult JK, Box G, et al. (2010) Bayesian estimation of changes in transverse relaxation rates. Magn Reson Med 64: 914–921. [DOI] [PubMed] [Google Scholar]
- 14.D'agostino RB (1986) Tests for normal distribution. In: R. B. D'agostino and M. A. Stepenes, editors. Goodness-of-fit techniques. New York, NY: Macel Decker. pp. 367–413.
- 15. Goo HW (2010) Whole-body MRI of neuroblastoma. Eur J Radiol 75: 306–314. [DOI] [PubMed] [Google Scholar]
- 16. Brisse HJ, McCarville MB, Granata C, Krug KB, Wootton-Gorges SL, et al. (2011) Guidelines for imaging and staging of neuroblastic tumors: consensus report from the International Neuroblastoma Risk Group Project. Radiology 261: 243–257. [DOI] [PubMed] [Google Scholar]
- 17. Robinson SP, Rijken PF, Howe FA, McSheehy PM, van der Sanden BP, et al. (2003) Tumor vascular architecture and function evaluated by non-invasive susceptibility MRI methods and immunohistochemistry. J Magn Reson Imaging 17: 445–454. [DOI] [PubMed] [Google Scholar]
- 18. McDonald DM, Choyke PL (2003) Imaging of angiogenesis: from microscope to clinic. Nat Med 9: 713–725. [DOI] [PubMed] [Google Scholar]
- 19. Comstock JM, Willmore-Payne C, Holden JA, Coffin CM (2009) Composite pheochromocytoma: a clinicopathologic and molecular comparison with ordinary pheochromocytoma and neuroblastoma. American journal of clinical pathology 132: 69–73. [DOI] [PubMed] [Google Scholar]
- 20.Robinson SP (2006) Blood oxygenation level dependent (BOLD) imaging of tumours. In: A. R. Padhani and P. L. Choyke, editors. New Techniques in Oncologic Imaging. Boca Raton: Taylor & Francis. pp. 257–272.
- 21. Baluk P, Hashizume H, McDonald DM (2005) Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev 15: 102–111. [DOI] [PubMed] [Google Scholar]
- 22. Christen T, Lemasson B, Pannetier N, Farion R, Remy C, et al. (2012) Is T2* enough to assess oxygenation? Quantitative blood oxygen level-dependent analysis in brain tumor. Radiology 262: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, et al. (2002) Analysis of mural cell recruitment to tumor vessels. Circulation 105: 112–117. [DOI] [PubMed] [Google Scholar]
- 24. Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, et al. (2006) Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 82: 699–757. [DOI] [PubMed] [Google Scholar]
- 25. McPhail LD, Robinson SP (2010) Intrinsic susceptibility MR imaging of chemically induced rat mammary tumors: relationship to histologic assessment of hypoxia and fibrosis. Radiology 254: 110–118. [DOI] [PubMed] [Google Scholar]
- 26. Hoskin PJ, Carnell DM, Taylor NJ, Smith RE, Stirling JJ, et al. (2007) Hypoxia in prostate cancer: Correlation of BOLD-MRI with pimonidazole immunohistochemistry: initial observations. Int J Radiat Oncol Biol Phys 68: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 27. Di Paolo D, Ambrogio C, Pastorino F, Brignole C, Martinengo C, et al. (2011) Selective therapeutic targeting of the anaplastic lymphoma kinase with liposomal siRNA induces apoptosis and inhibits angiogenesis in neuroblastoma. Molecular therapy: the journal of the American Society of Gene Therapy 19: 2201–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chantrain CF, Shimada H, Jodele S, Groshen S, Ye W, et al. (2004) Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res 64: 1675–1686. [DOI] [PubMed] [Google Scholar]
- 29. Chen Y, Takita J, Choi YL, Kato M, Ohira M, et al. (2008) Oncogenic mutations of ALK kinase in neuroblastoma. Nature 455: 971–974. [DOI] [PubMed] [Google Scholar]
- 30. George RE, Sanda T, Hanna M, Frohling S, Luther W 2nd, et al. (2008) Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 455: 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, et al. (2008) Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455: 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Janoueix-Lerosey I, Lequin D, Brugieres L, Ribeiro A, de Pontual L, et al. (2008) Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 455: 967–970. [DOI] [PubMed] [Google Scholar]
- 33. Fox E, Aplenc R, Bagatell R, Chuk MK, Dombi E, et al. (2010) A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol 28: 5174–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carpenter EL, Haglund EA, Mace EM, Deng D, Martinez D, et al. (2012) Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene 31: 4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carpenter EL, Mosse YP (2012) Targeting ALK in neuroblastoma—preclinical and clinical advancements. Nat Rev Clin Oncol 9: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]