Abstract
Stigmatic exudate, a secretion product recovered from the upper surface of Lilium longiflorum pistils, has been examined. Over 99% of the exudate is accounted for as water, carbohydrate, and protein. Exclusive of water, 95% is a high molecular weight, protein-containing polysaccharide composed of galactose, arabinose, rhamnose, glucuronic acid, and galacturonic acid.
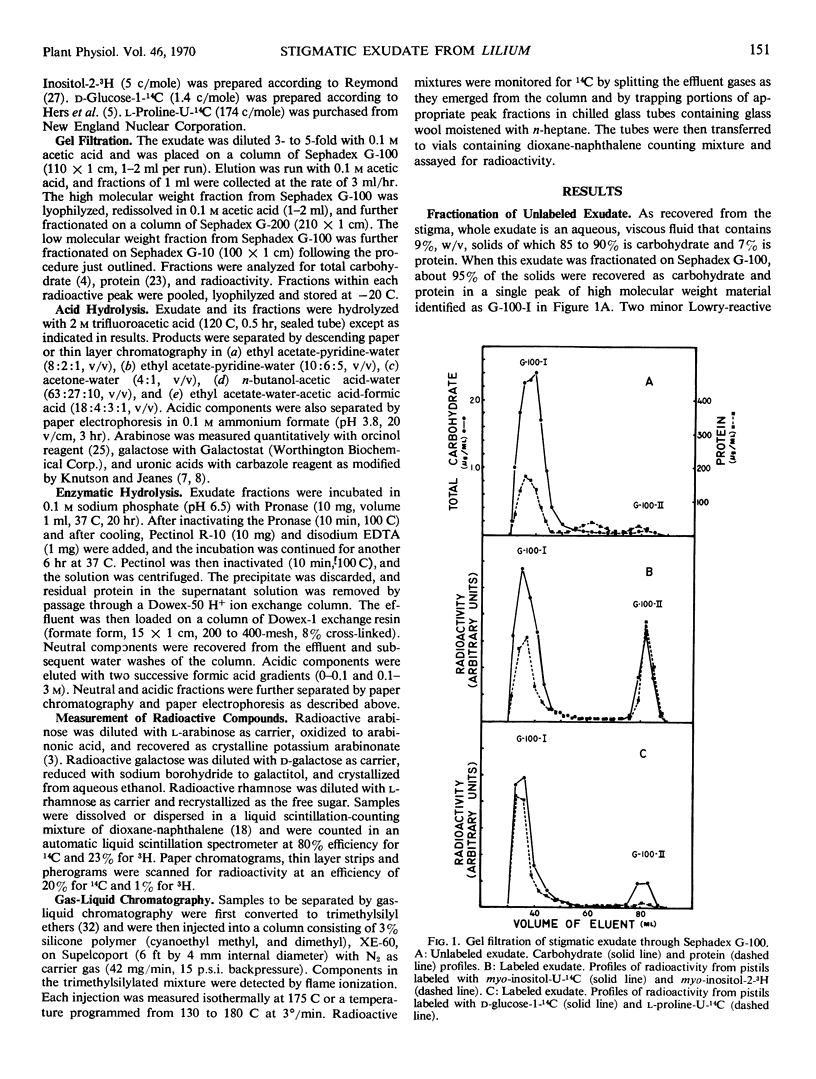
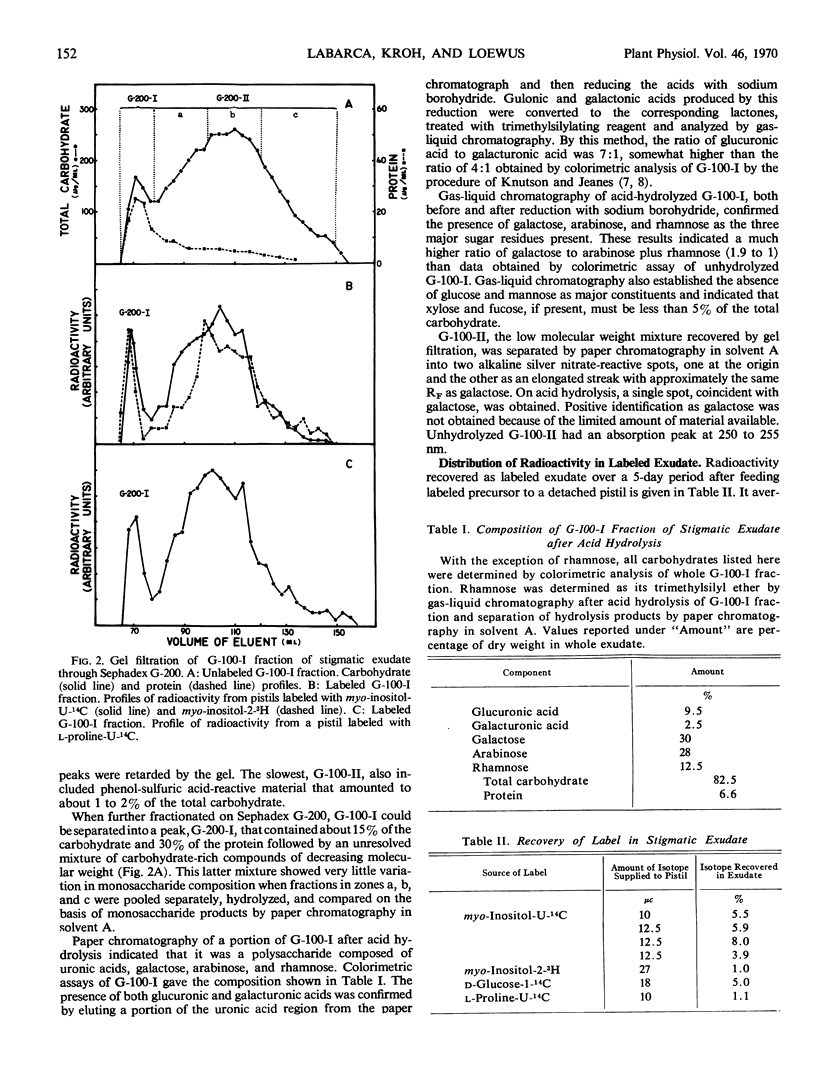
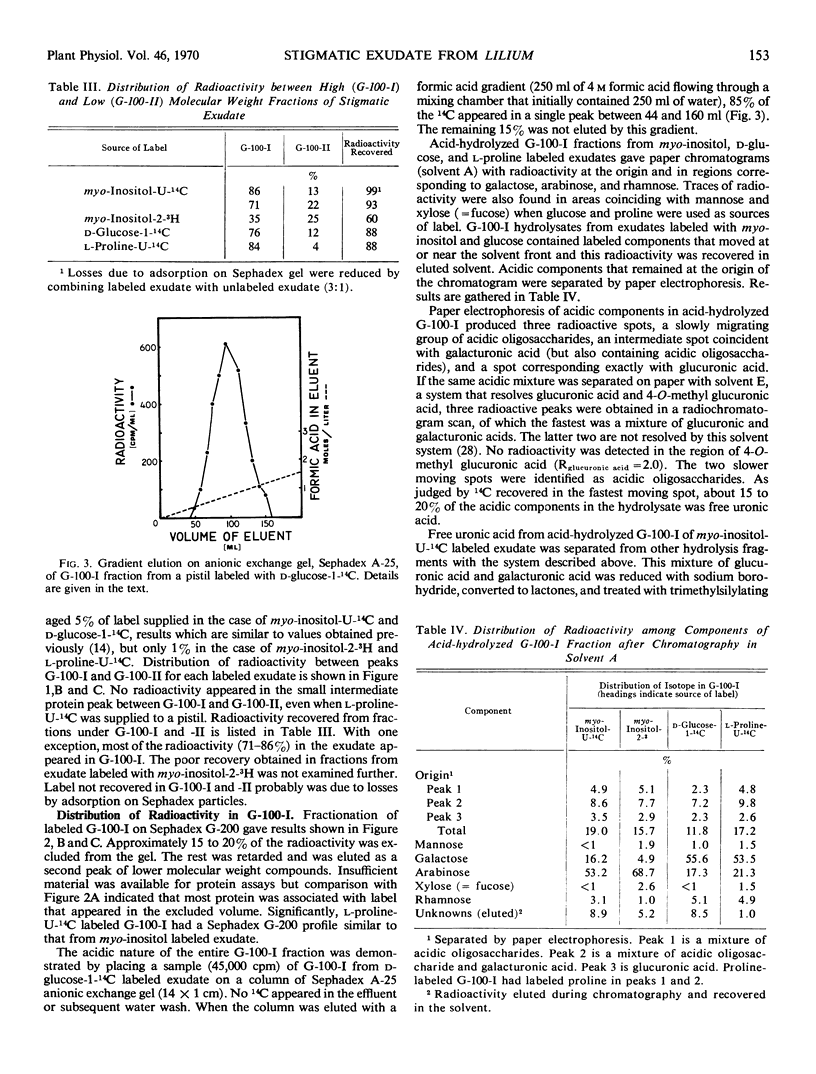
Detached pistils supplied with myo-inositol-U-14C, myo-inositol-2-3H, d-glucose-1-14C, or l-proline-U-14C produce labeled stigmatic exudate. When myo-inositol is supplied, the exudate is rich in labeled arabinose and uronic acids, but some label also recycles through the hexose phosphate pool of secreting cells, causing label to appear in galactose and rhamnose residues. When glucose is provided, galactose is the major constituent labeled but all of the other carbohydrate constituents are also labeled. Proline produces a pattern very similar to that obtained with glucose.
Stigmatic exudate also contains a small amount of low molecular weight carbohydrate. If myo-inositol is used to label exudate, free labeled myo-inositol cannot be detected in the low molecular weight fraction until it has been subjected to acid hydrolysis. Similarly, if d-glucose is the source of label, free labeled glucose is found in the low molecular weight fraction only after acid hydrolysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspinall G. O. The exudate gums and their structural relationship to other groups of plant polysaccharides. Pure Appl Chem. 1967;14(1):43–55. doi: 10.1351/pac196714010043. [DOI] [PubMed] [Google Scholar]
- Knutson C. A., Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968 Sep;24(3):470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- Knutson C. A., Jeanes A. Determination of the composition of uronic acid mixtures. Anal Biochem. 1968 Sep;24(3):482–490. doi: 10.1016/0003-2697(68)90155-3. [DOI] [PubMed] [Google Scholar]
- Kroh M., Loewus F. Biosynthesis of pectic substance in germinating pollen: labeling with myoinositol-2-14C. Science. 1968 Jun 21;160(3834):1352–1354. doi: 10.1126/science.160.3834.1352. [DOI] [PubMed] [Google Scholar]
- Kroh M., Miki-Hirosige H., Rosen W., Loewus F. Incorporation of label into pollen tube walls from myoinositol-labeled Lilium longiflorum pistils. Plant Physiol. 1970 Jan;45(1):92–94. doi: 10.1104/pp.45.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroh M., Miki-Hirosige H., Rosen W., Loewus F. Inositol metabolism in plants. VII. Distribution and utilization of label from myoinositol-U 14C and -2-3H by detached flowers and pistils of Lilium longiflorum. Plant Physiol. 1970 Jan;45(1):86–91. doi: 10.1104/pp.45.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWUS F. A. INOSITOL METABOLISM IN PLANTS. II. THE ABSOLUTE CONFIGURATION OF D-XYLOSE-5-T DERIVED METABOLICALLY FROM MYO-INOSITOL-2-T IN THE RIPENING STRAWBERRY. Arch Biochem Biophys. 1964 Jun;105:590–598. doi: 10.1016/0003-9861(64)90055-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- Loewus F. Metabolism of inositol in higher plants. Ann N Y Acad Sci. 1969 Oct 17;165(2):577–598. [PubMed] [Google Scholar]
- MYHILL D., JACKSON D. S. SEPARATION OF PROLINE AND HYDROXYPROLINE USING THIN-LAYER CHROMATOGRAPHY. Anal Biochem. 1963 Aug;6:193–198. doi: 10.1016/0003-2697(63)90110-6. [DOI] [PubMed] [Google Scholar]
- Roberts R. M., Shah R., Loewus F. Conversion of myo-inositol-2-14C to labeled 4-O-methyl-glucuronic acid in the cell wall of maize root tips. Arch Biochem Biophys. 1967 Mar;119(1):590–593. doi: 10.1016/0003-9861(67)90499-7. [DOI] [PubMed] [Google Scholar]



