Abstract
Introduction
Depression during pregnancy can have serious consequences for families. Indications of fetal aneuploidy can induce maternal stress, a risk factor for depression. Few studies have assessed symptoms of depression in pregnant women soon after they receive results indicating increased risk for fetal aneuploidy.
Purpose
We compared symptoms of depression in women who had increased risks for fetal aneuploidy with two other groups of pregnant women at similar gestational ages: Controls, and women taking antidepressant medication (MEDS).
Methods
81 women attending the BC Medical Genetics Programme (MG) regarding positive maternal serum screens or ultrasound soft marker findings completed the Edinburgh Postnatal Depression Scale (EPDS). Control (n=41) and MEDS (n=41) groups were recruited from the community or the BC Reproductive Mental Health program. A threshold score of 12 on the EPDS was used to calculate percentages of women likely to be depressed. Mean EPDS scores were compared using ANOVA, followed by post-hoc tests.
Results
In the Control, MG, and MEDS groups, 2.4%, 35%, and 52.4% of women, respectively, scored above 12. Mean EPDS score was significantly higher in the MG group than in the Control group (p<.0001).
Conclusions
These results suggest a place for depression screening in prenatal genetic counselling.
Keywords: DEPRESSION, FETAL ANEUPLOIDY, GENETIC COUNSELLING, MATERNAL SERUM SCREEN, MOOD, PREGNANCY, PRENATAL, ULTRASOUND SOFT MARKER
Introduction
Depression is a debilitating illness characterized by persistent anhedonia, generalized sadness, and somatic changes (1), with a point prevalence of 10–15% (2–4) among women of childbearing age. While the perinatal period is often thought of as a joyful time, depression occurs both during and after pregnancy; approximately half of women with postpartum depression (PPD) experience symptom onset during pregnancy (5,6). Indeed, longitudinal studies of perinatal depression symptoms show a peak during pregnancy’s third trimester, when 12.8–17% of women screen positive on a depression-screening tool (6,7). Women with a history of psychiatric illnesses have an increased risk for PPD (8–11), and women with a history of these illnesses may be more vulnerable to prenatal depression because ~1/2 of women with PPD experience symptom onset during pregnancy.
Depression symptoms in the perinatal period are associated with risks to mothers and children, including: intrauterine growth retardation, premature delivery, impaired mother-infant attachment, impaired cognitive and emotional development, and lower social competency scores in school-age children (12–17). Considering this, and the fact that there are efficacious treatments for perinatal depression (pharmacologic interventions and/or psychotherapy can ameliorate symptoms (18,19), it has been suggested that depression screening should be standard care in obstetric clinics (20), and that all health professionals should offer pregnant women information about PPD, assessments for depression, and referrals to mental health services (21).
Depression and Medical Genetics
Depression symptoms have not been formally assessed in pregnant women attending Medical Genetics clinics to discuss positive maternal serum screens or ultrasound soft markers, both of which indicate an increased risk for fetal chromosome abnormality. However, several qualitative studies shed some light on the issue. Two examined women’s reactions to positive maternal serum screens; one found reports of depression, fear, anxiety, and changes to sleeping patterns (22), the other found that 92% of women reported experiencing anxiety after receiving positive results for Down syndrome (23). In another study, 100% of women receiving an ultrasound diagnosis of choroid plexus cysts reported a negative emotional reaction, and 68% reported that this continued for several weeks (24).
Women receiving a result increasing risk for fetal aneuploidy could be facing an imminent diagnosis. Symptoms of anxiety are, to an extent, expected in women who understand this information. Indeed, the process of appraising an “uncertain threat” almost inevitably generates anxiety prior to the mobilization of coping strategies (25). Symptoms of depression, however, can interfere with an individual’s ability to process information (26), make decisions (27), and to cope with health-related stress (28).
Little is known about current clinical practice regarding depression screening in prenatal genetic counselling. One study found that 28.57% of pregnant women referred for genetic counselling for advanced maternal age scored above cut-off on the Beck Depression Inventory-II (BDI-II) (29), but that genetic counsellors could not readily identify depression symptoms (30). The authors of that study recommended the integration of a formal depression-screening tool into prenatal genetic counselling.
Study Purpose
This was an exploratory study that used the Edinburgh Postnatal Depression Scale (EPDS) 1) to quantitatively assess depression symptoms amongst pregnant women attending the BC Medical Genetics Programme for positive maternal serum screens indicating an increased risk for fetal aneuploidy or ultrasound soft markers, and 2) to compare these EPDS scores to those of two other groups of women at similar gestational ages: controls, and a group taking antidepressants (MEDS). The following hypotheses were tested: 1) mean EPDS scores would differ significantly across the groups, and 2) women with a personal history of a psychiatric illness would be more likely to have EPDS scores ≥12 (an established cut-off).
Materials and Methods
Participants and Procedures
Medical Genetics Group (MG)
Pregnant women (age ≥19) who were fluent in English, and who attended Medical Genetics between January 10 and March 2, 2007, were considered for participation in the MG group. Women were eligible if they were attending an appointment for a prenatal ultrasound soft marker (including, but not limited to, increased nuchal thickening, echogenic focus, choroid plexus cysts, pyelectasis, and echogenic bowel) or a maternal serum screen that was positive for Down syndrome, open spina bifida, or trisomy 18 (results that increase risk for fetal aneuploidy). Women were included even if they later received a diagnosis of fetal aneuploidy. Women were excluded if the screen was positive for Smith-Lemli-Opitz syndrome, given that this result is predominantly associated with increased risk for fetal demise, anatomic abnormalities, and single gene disorders, rather than fetal aneuploidy (31). Women were also excluded if complicating factors arose, such as structural abnormalities on prenatal ultrasound or prenatal diagnosis for a genetic condition. Additionally, women were not invited to participate if genetic counsellors judged their distress level to be high and, thus, did not feel comfortable extending the invitation to participate in research.
At the conclusion of genetic counselling appointments, genetic counsellors (at their discretion) invited eligible patients to participate. If interested, participants were handed a study package which included: cover letter, demographics questionnaire, EPDS, and a pamphlet about PPD, available at: http://www.heretohelp.bc.ca/publications/factsheets/postpartum. Questionnaires were returned anonymously, and so it was not possible to obtain any follow-up information, such as subsequent diagnoses, rule out of fetal aneuploidy, or pregnancy outcome. The Institutional Review Boards (IRBs) of the University of British Columbia and BC Women’s Hospital approved this study.
Comparison Groups
Women in the MEDS (n=41) and Control (n=41) groups were recruited early in their second trimester of pregnancy (26 weeks gestation) as part of a previous (IRB-approved) prospective, longitudinal study of infant development and behavior following prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants and maternal mood disorders (32). Women were fluent in English, and self-referred from the community or the BC Reproductive Mental Health Program between 2002 and 2005. At enrollment, demographic data were collected, and women were assessed using the MINI-International Neuropsychiatric Interview (a 15-minute structured diagnostic interview) (33), clinician-rated Hamilton Anxiety Rating Scale (HAM-A) and Hamilton Depression Rating Scale (HAM-D) (34,35), and the EPDS (19). MEDS women had a previous psychiatric diagnosis (Axis I, unipolar mood disorder) and were taking SSRI medications from the time of recruitment through to delivery, with the exception of two mothers who were started on medication during the second trimester and continued through to delivery. Medication side effects and adherence were monitored on a weekly basis. Women were included in the Control group if the Major Depressive Episode module of the MINI was negative, and they were not taking any psychiatric medications. One participant was re-categorized from MG to MEDS because she reported current use of paroxetine, resulting in final group sizes: MEDS=42 and MG=80.
Measures
The EPDS is a 10-item self-report questionnaire that assesses cognitive and affective depression symptoms (36). This scale is unique amongst depression screening instruments because it was designed specifically for perinatal use, and so does not assess somatic features of depression, which are confounded by physiological changes of pregnancy. It is also the only depression scale that has been validated for use pre- and post-natally (36,37), including in the context of high-risk pregnancies(38). Each EPDS item has four possible responses (scored from 0–3, giving a total EPDS score of 0–30), and participants are asked to select the response that reflects their feelings of the previous seven days.
Demographic questionnaires included items regarding age, marital and employment status, ethnicity, education history, and current pregnancy. Three questions in the demographic questionnaire assessed participants’ psychiatric histories: 1) “Have you ever received a diagnosis of a psychiatric condition (e.g. depression, schizophrenia, schizoaffective disorder, bipolar disorder, postpartum psychosis)?” (Tick boxes to indicate ‘Yes’ or ‘No’), 2) “If Yes, please specify:” (space to elaborate), and 3) “If Yes, are you currently receiving treatment for a psychiatric condition?” (Tick boxes to indicate ‘Yes’ or ‘No’).
Analysis
First, percentages of women with total EPDS scores ≥ 12 were calculated for each group, and compared descriptively. Second, the groups’ mean EPDS scores were compared using a one-way ANOVA, followed by post-hoc analysis using Tukey’s tests (α < .05). Finally, data from all three groups were stratified into two new groups according to whether total EPDS score was above or below the threshold of 12. Chi square analysis was used to compare the number of women in each group with a psychiatric history.
A threshold score of 12 was chosen because validation studies of the EPDS demonstrated that this score is indicative of major depression with a sensitivity of 88%, specificity of 92.5%, and positive predictive value of 56.8% (36). The EPDS developers recommended that women with scores above this threshold be referred for a clinical evaluation. Additionally, the threshold of 12 is generally accepted as indicative of major depression, and is employed in the majority of literature using the EPDS to assess symptoms of perinatal depression.
Results
Response Rate
In the MG group, genetic counsellors invited 130 women to participate (appointment indications: serum screen positive = 71, serum screen positive and soft marker = 11, soft marker = 48), and 81 (62.3%) returned a complete EPDS. Of these 81 women, 66 (81.5%) kept the PPD pamphlet. Several women who declined to participate requested the PPD pamphlet. Response rate for the MEDS and Control groups is not applicable due to recruitment method (self-referral).
Demographics
In the total group (N=163), the majority of women were Caucasian (66.3%), married or living with a partner (96.9%), and had completed some college or university (88.3%) (Table 1). Mean age was 32.1 years, and mean gestational age was 23.3 weeks. Gestational age was significantly lower for MG as compared to Control and MEDS (F(2,159)=127.6, p<.0001). There were no other significant differences between groups with respect to demographic variables.
Table 1.
Summary of Demographic Information
| Medical Genetics Group (n=81)
|
Total Sample (N=163)
|
|||
|---|---|---|---|---|
| Number | % | Number | % | |
| Marital Statusa | ||||
| Single | 2 | 2.5 | 3 | 1.8 |
| Married/Living with | 79 | 97.5 | 158 | 96.9 |
| Widowed | 0 | 0 | 0 | 0 |
| Separated/Divorced | 2 | 2.5 | 4 | 2.5 |
| Other | 0 | 0 | 0 | 0 |
| Ethnicity | ||||
| Caucasian | 45 | 55.6 | 108 | 66.3 |
| African American | 1 | 1.2 | 2 | 1.2 |
| Asian | 27 | 33.3 | 40 | 24.5 |
| Native American | 1 | 1.2 | 2 | 1.2 |
| Mixed | 4 | 4.9 | 4 | 2.5 |
| Other | 3 | 3.7 | 7 | 4.3 |
| Education | ||||
| Some high school | 0 | 0 | 0 | 0 |
| Finished high school | 12 | 15 | 19 | 11.7 |
| Attended college/university | 68 | 85 | 143 | 88.3 |
Note. Percentages are based on non-missing data. For ethnicity, “other” includes Latin American/Hispanic.
For marital status, it was possible to report more than one response.
Psychiatric History
Percentages of women reporting personal psychiatric histories were significantly different for Control (26.8%), MG (11.3%), and MEDS groups (97.6%) (χ2(2)=90.4, p<.0001). Previous diagnoses included depression, PPD, and anxiety disorders. It was somewhat unexpected that more Controls than MG women reported a psychiatric history. There may have been a selection bias in the Control group, whereby women with a psychiatric history were more likely to volunteer for a study investigating SSRIs and pregnancy.
Scores Above EPDS Threshold
Numbers of women who scored above EPDS threshold were as follows: MG group (n=28): 35%, Control group (n=1): 2.4%, and MEDS group (n=22): 52.4%. Amongst MG women who scored above threshold, 23/28 (82.1%) did not report a psychiatric history.
Mean EPDS Scores
EPDS scores for the Control group were not normally distributed. A square root transformation normalized data of the Control, MG, and MEDS groups. Mean EPDS scores for the three groups (Figure 1) were significantly different (F(2,160)=26.19, p<.0001). Post-hoc analysis using Tukey’s tests revealed significantly more symptoms of depression reported by MG and MEDS, as compared to Controls (p<.0001), and no significant difference between MG and MEDS mean EPDS scores.
Figure 1.
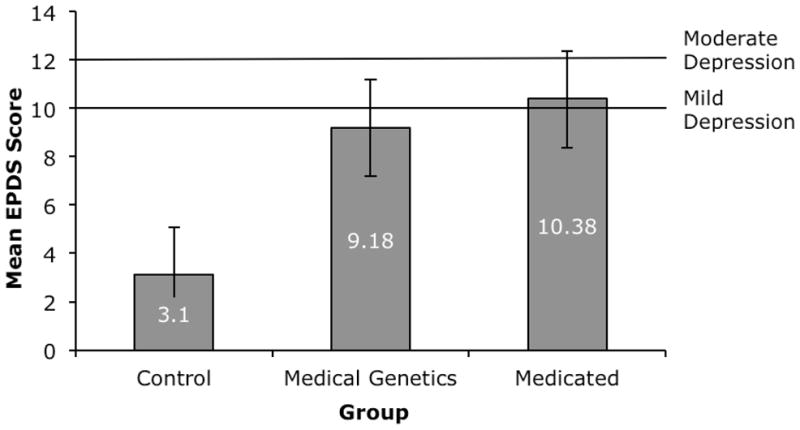
Mean score on the EPDS (+SE) for the Control group (n=41), the MG group (n=80), and the MEDS group (n=42), with lines illustrating cut-off scores for mild depression (10) and moderate depression (12).
Psychiatric History as a Predictor of Depression
In the total sample (N=163), women scoring above EPDS threshold were more likely than women scoring below threshold to have a history of psychiatric illness (χ2(1)=5.83, p<.05).
Discussion
In this study, 35% of pregnant MG women scored above the EPDS threshold that is generally accepted as being indicative of major depression. This substantially exceeds the proportion of pregnant women in the general population who scored above the same EPDS cutoff (17%) (6). Of particular note, over 80% of MG women did not report a psychiatric history.
It is important to highlight that this was an exploratory study that did not attempt to separate out the contribution of anxiety symptoms from the EPDS scores in the MG group, investigate whether symptoms were transitory or persistent, or elucidate the mechanisms underlying symptoms. Further, at the time of collecting EPDS data, women were facing uncertain pregnancy outcomes, and it is possible that depression symptoms resolved, particularly when the fetus was not affected by aneuploidy.
Nevertheless, the high frequency (35%) of women experiencing depression symptoms worthy of referral for psychiatric evaluation following prenatal results indicating an increased risk for fetal aneuploidy is of concern, particularly for prenatal genetic counselling practice. The detrimental impact of depression symptoms on ability to process information and make decisions is well established (26–28), and these abilities are crucial in the face of such a prenatal result. Moreover, it is vital for genetic counsellors to recognize patient inability in the areas of information processing and decision making as potential challenges in the process of genetic counselling, the definition of which includes “counseling to promote informed choices” (39).
Indeed, according to the Practice-Based Competencies of the American Board of Genetic Counseling (ABGC), genetic counsellors should be trained to “recognize psychopathology” (40), but no studies have yet been published that provide insight into content of genetic counselling training programs in this regard. Further, aside from the one study that showed that prenatal genetic counsellors failed to recognize depression symptoms in 70% of women scoring above threshold on the BDI-II (30), there is little research addressing whether genetic counsellors can successfully identify psychopathology. Based on the limited data that are currently available, it appears that current training practices are insufficient to equip genetic counsellors with the skills needed to recognize depression symptoms, and that perhaps there is a need for systematic use of depression screening tools in the prenatal setting.
Screening for personal history of psychiatric illness could be considered as such a tool, given the association with depression symptoms during the perinatal period (8–11), and that women with such histories were more likely to have EPDS scores above threshold in this study. However, we found that 82.1% of MG women with EPDS scores above threshold did not report a personal history of a psychiatric disorder, so history as a screening tool may not be adequate. To address this problem, a tool like the EPDS could be employed. The EPDS is quick and easy to administer, has been validated for use prenatally, is acceptable to depressed and non-depressed women (41), is not confounded by somatic changes related to pregnancy, and has greater sensitivity than using personal psychiatric history alone to screen for depression symptoms.
With a view to establishing best practice guidelines, there is a need to consider appropriate measures that should be taken in follow-up to the prenatal identification of women experiencing depression symptoms. It is worth looking towards the current definition of genetic counseling, and then seeking to build upon established skills and practices. Genetic counseling involves “helping people understand and adapt to the medical, psychological and familial implications of genetic contributions to disease” (39). In the context of depression symptoms during pregnancy, this might include providing information about PPD and connecting patients with appropriate community resources. Women in this study were very receptive to information about PPD, but studies have yet to characterize the potential benefits to pregnant women of receiving such information. Reproductive Mental Health Programs and psychiatrists interested in perinatal issues are ideal referrals for clinical confirmation or rule out of depression, and discussion of treatment options as appropriate. Mental health counsellors can provide long-term support for women experiencing symptoms of depression during pregnancy, whether or not a diagnosis of depression is made.
Limitations
Importantly, the 35% of MG women experiencing depression symptoms worthy of referral for psychiatric evaluation is likely an underestimate for two main reasons. First, several eligible women were excluded because their distress levels were judged to be high, and genetic counsellors did not feel comfortable extending the invitation to participate in research. Second, the EPDS threshold score of 12 was conservative. EPDS threshold scores of 9–10 have been associated with mild depression (diagnosed by psychiatric evaluation), and have been recommended for use in routine prenatal care (36). Additionally, the difference in mean EPDS score between Control and MG groups may have been more dramatic had there not been a significant difference in gestational age between them (Control: 26 weeks, MG: 20.5 weeks), given that studies have shown that depression peaks during the third trimester (42).
Also, the EPDS is a screening tool, not a diagnostic tool, and so is inherently associated with a false positive rate. To avoid unnecessary anxiety for patients, and work for genetic counsellors and mental health professionals, it will be important to assess the use of different EPDS threshold scores in the prenatal genetic counselling setting before integrating the EPDS into clinical practice.
Another limitation was the use of antidepressant medications by the MEDS group. Various SSRIs were used over different durations prior to enrollment, which complicates the interpretation of the difference in mean EPDS scores between MG and MEDS groups.
Finally, depression symptoms reported by MG women may have been only transiently associated with the prenatal result indicating an increased risk for fetal aneuploidy. Whether or not such results represent risk factors for later development of PPD is a question worthy of future investigation, especially given that receiving such news could be considered a “stressful life event”, and that stressful life events predispose women to depression (43,44). More generally, given that women are at greater risk for depression than are men, and that pregnancy increases risk for depression, prenatal results increasing risk for fetal aneuploidy could be conceptualized as a stressor in a stress-diathesis model of depression, and investigated as such.
Conclusion
Given that perinatal depression can have severe consequences for mothers and infants, and that treatment options can ameliorate symptoms, there is a strong argument for early identification and intervention. While prenatal screening programs for fetal aneuploidy have clear benefits, the health care system perhaps has an obligation to manage negative emotional consequences experienced by some women who participate in these programs. It is appropriate for genetic counsellors to adopt depression screening, given that they are expected to “recognize psychopathology”. We propose that all women presenting for genetic counselling due to a prenatal test result indicating an increased risk for fetal aneuploidy (maternal serum screen result or ultrasound soft marker finding) should be screened for symptoms of depression, using a tool like the EPDS, and that research should be conducted to determine the optimal EPDS cut-off score to use in this context for referral to a mental health provider.
Acknowledgments
C. H. was a student of the University of British Columbia (UBC) MSc in Genetic Counselling program when this research was conducted. Special thanks to the participants, the genetic counsellors (for their vital role in data collection), Ursula Brain, and Drs. Jan Friedman and Geoff Smith. T. F. O. is the R. Howard Webster Professor in Child Development (UBC, Faculty of Graduate Studies) and was supported by a Human Early Learning Partnership Senior Career Award, March of Dimes Foundation (USA) (#12-FY01-30), Canadian Institutes of Health Research (CIHR) #MOP 54490 and 57837. S. M. has participated in speakers’ bureaus for GlaxoSmithKline Inc., Lundbeck, Wyeth, AstraZeneca, and Eli Lilly and Company; has served as a consultant for GlaxoSmithKline Inc., AstraZeneca, and Wyeth; and has conducted research for AstraZeneca, GlaxoSmithKline Inc., Lundbeck, March of Dimes, The BC Medical Research Foundation, the Vancouver Foundation, and CIHR. W. G. H. and J. A. were supported by CIHR NET54013, the Michael Smith Foundation for Health Research, and BC Mental Health and Addictions Services.
References
- 1.Association, A.P. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.O’Hara MW, et al. Controlled prospective study of postpartum mood disorders: comparison of childbearing and nonchildbearing women. J Abnorm Psychol. 1990;99(1):3–15. doi: 10.1037//0021-843x.99.1.3. [DOI] [PubMed] [Google Scholar]
- 3.O’Hara MW, Swain AM. Rates and risk of postpartum depression- a meta-analysis. International Review of Psychiatry. 1996;8(1):37–54. [Google Scholar]
- 4.Oberlander TF, et al. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63(8):898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 5.Gotlib IH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269–74. doi: 10.1037//0022-006x.57.2.269. [DOI] [PubMed] [Google Scholar]
- 6.Josefsson A, et al. Prevalence of depressive symptoms in late pregnancy and postpartum. Acta Obstet Gynecol Scand. 2001;80(3):251–5. doi: 10.1034/j.1600-0412.2001.080003251.x. [DOI] [PubMed] [Google Scholar]
- 7.Ross LE, et al. Measurement issues in postpartum depression part 1: anxiety as a feature of postpartum depression. Arch Womens Ment Health. 2003;6(1):51–7. doi: 10.1007/s00737-002-0155-1. [DOI] [PubMed] [Google Scholar]
- 8.Howard L. Postnatal depression. Clinical Evidence. 2004;12:2000–15. [PubMed] [Google Scholar]
- 9.Bosanac P, Buist A, Burrows G. Motherhood and schizophrenic illnesses: a review of the literature. Australian and New Zealand Journal of Psychiatry. 2003;37(1):24–30. doi: 10.1046/j.1440-1614.2003.01104.x. [DOI] [PubMed] [Google Scholar]
- 10.Jones I, Craddock N. Familiality and the puerperal trigger in bipolar disorder: results of a family study. American Journal of Psychiatry. 2001;158:913–17. doi: 10.1176/appi.ajp.158.6.913. [DOI] [PubMed] [Google Scholar]
- 11.Marks MN, Wieck A, Checkley SA, Kumar R. Contribution of psychological and social factors ro psychotic and non-psychotic relapse after childbirth in women with previous histories of affective disorder. Journal of Affective Disorders. 1992;24(4):253–63. doi: 10.1016/0165-0327(92)90110-r. [DOI] [PubMed] [Google Scholar]
- 12.Steer RA, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093–9. doi: 10.1016/0895-4356(92)90149-h. [DOI] [PubMed] [Google Scholar]
- 13.Misri S, Duke M. Depression during pregnancy and postpartum. Journal of the Society of Obstetrics & Gynecology of Canada. 1995;17:657–63. [Google Scholar]
- 14.Rahman A, Iqbal Z, Bunn J, Lovel H, Harrington R. Impact of maternal depression on infant nutritional status and illness. Archives of General Psychiatry. 2004;61:946–52. doi: 10.1001/archpsyc.61.9.946. [DOI] [PubMed] [Google Scholar]
- 15.Teti DM, Gelfland DM, Messinger DS, Isabella R. Maternal depression and the quality of early attachment: an examination of infants, preschoolers and their mothers. Developmental Psychology. 1995;31:364–76. [Google Scholar]
- 16.Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12(1):12–20. doi: 10.1016/s0883-9417(98)80004-6. [DOI] [PubMed] [Google Scholar]
- 17.Luoma I, Tamminen T, Kaukonen P, Laippala P, Puura K, Salmelin R, Almqvist F. Longitudinal study of maternal depressive symptoms and child well-being. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(12):1367–74. doi: 10.1097/00004583-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Misri S, et al. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry. 2004;65(9):1236–41. doi: 10.4088/jcp.v65n0913. [DOI] [PubMed] [Google Scholar]
- 19.Spinelli MG, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry. 2003;160(3):555–62. doi: 10.1176/appi.ajp.160.3.555. [DOI] [PubMed] [Google Scholar]
- 20.Thoppil J, Riutcel TL, Nalesnik SW. Early intervention for perinatal depression. American Journal of Obstetrics and Gynaecology. 2005;192:1446–48. doi: 10.1016/j.ajog.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 21.Program, B.R.C. Reproductive Mental Health Guideline 3: Identification and assessment of reproductive mental illness during the preconception and perinatal periods. 2003 [cited; Available from: http://www.bcwomens.ca/Services/HealthServices/ReproductiveMentalHealth/BestPractices.htm.
- 22.Santalahti P, et al. Women’s experiences of prenatal serum screening. Birth. 1996;23(2):101–7. doi: 10.1111/j.1523-536x.1996.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 23.Weinans MJ, et al. How women deal with the results of serum screening for Down syndrome in the second trimester of pregnancy. Prenat Diagn. 2000;20(9):705–8. doi: 10.1002/1097-0223(200009)20:9<705::aid-pd904>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 24.Cristofalo EA, et al. Women’s response to fetal choroid plexus cysts detected by prenatal ultrasound. J Perinatol. 2006;26(4):215–23. doi: 10.1038/sj.jp.7211489. [DOI] [PubMed] [Google Scholar]
- 25.Lazarus RS. From psychological stress to the emotions: a history of changing outlooks. Annu Rev Psychol. 1993;44:1–21. doi: 10.1146/annurev.ps.44.020193.000245. [DOI] [PubMed] [Google Scholar]
- 26.Hammar A, Lund A, Hugdahl K. Selective impairment in effortful information processing in major depression. J Int Neuropsychol Soc. 2003;9(6):954–9. doi: 10.1017/S1355617703960152. [DOI] [PubMed] [Google Scholar]
- 27.Murphy FC, et al. Decision-making cognition in mania and depression. Psychol Med. 2001;31(4):679–93. doi: 10.1017/s0033291701003804. [DOI] [PubMed] [Google Scholar]
- 28.Heckman TG, et al. Depressive symptomatology, daily stressors, and ways of coping among middle-age and older adults living with HIV disease. J Ment Health Aging. 1999;5:311–22. [Google Scholar]
- 29.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 30.Jurek M, Edwards J, Lovell C, Singletary C, Vincent V, Wolpert C. Evidence that depression screening is useful in prenatal genetic counselling. Journal of Genetic Counselling. 2001;10:494–95. [Google Scholar]
- 31.Craig WY, et al. Major fetal abnormalities associated with positive screening tests for Smith-Lemli-Opitz syndrome (SLOS) Prenat Diagn. 2007;27(5):409–14. doi: 10.1002/pd.1699. [DOI] [PubMed] [Google Scholar]
- 32.Oberlander TF, et al. Infant serotonin transporter (SLC6A4) promoter genotype is associated with adverse neonatal outcomes after prenatal exposure to serotonin reuptake inhibitor medications. Mol Psychiatry. 2008;13(1):65–73. doi: 10.1038/sj.mp.4002007. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 34.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 36.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10 item Edinburgh postnatal depression scale. British Journal of Psychiatry. 1987;150:782–86. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 37.Murray D, Cox J. Screening for depression during pregnancy with the Edinburgh Postnatal Depression Scale (EPDS) Journal of Reproductive and Infant Psychology. 1990;8:99–107. [Google Scholar]
- 38.Adouard F, Glangeaud-Freudenthal NM, Golse B. Validation of the Edinburgh postnatal depression scale (EPDS) in a sample of women with high-risk pregnancies in France. Arch Womens Ment Health. 2005;8(2):89–95. doi: 10.1007/s00737-005-0077-9. [DOI] [PubMed] [Google Scholar]
- 39.Resta R, Bowles Biesecker B, Bennett RL, Blum S, Estabrooks Hahn S, Strecker MN, Williams JL. A new definition of genetic counseling: National Society of Genetic Counselors’ task force report. Journal of Genetic Counseling. 2006;15(2):77–83. doi: 10.1007/s10897-005-9014-3. [DOI] [PubMed] [Google Scholar]
- 40.ABGC. Practice-Based Competencies. [cited Apr. 24, 2008]; Available from: http://abgc.iamonline.com/english/view.asp?x=1529&all=true.
- 41.Gemmill AW, et al. A survey of the clinical acceptability of screening for postnatal depression in depressed and non-depressed women. BMC Public Health. 2006;6:211. doi: 10.1186/1471-2458-6-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans J, et al. Cohort study of depressed mood during pregnancy and after childbirth. Bmj. 2001;323(7307):257–60. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156(6):837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 44.Wurtman RJ. Genes, stress, and depression. Metabolism. 2005;54(5 Suppl 1):16–9. doi: 10.1016/j.metabol.2005.01.007. [DOI] [PubMed] [Google Scholar]


