Abstract
The chemokine receptor CCR4 is expressed by Th2 and Tregs and directs their migration along gradients of the chemokines CCL17 and CCL22. Both chemokines and receptor are upregulated in allergic disease, making CCR4 a therapeutic target for the treatment of allergy. We set out to assess the mechanisms underlying a previous report that CCL22 is a dominant ligand of CCR4, which may have implications for its therapeutic targeting. Human T-cells expressing endogenous CCR4 and transfectants engineered to express CCR4 were assessed for receptor function using assays of calcium release, chemotaxis, receptor endocytosis and ligand binding. Despite the two ligands having equal potency in calcium flux and chemotaxis assays, CCL22 showed dominance in both receptor endocytosis assays and heterologous competitive binding assays. Using two different CCR4-specific antibodies, we showed that CCR4 exists in at least two distinct conformations, which are differentially activated by ligand. A major population is activated by both CCL17 and CCL22, whilst a minor population is activated only by CCL22. Mutation of a single C-terminal residue K310 within a putative CCR4 antagonist binding site, ablated activation of CCR4 by CCL17 but not by CCL22, despite having no effect on the binding of either ligand. We conclude that CCL17 and CCL22 are conformationally selective ligands of CCR4 and interact with the receptor by substantially different mechanisms. This suggests that the selective blockade of CCR4 in allergy may be feasible where one CCR4 ligand dominates, allowing the inhibition of Th2 signalling via one ligand whilst sparing Treg recruitment via another.
INTRODUCTION
Chemokines constitute a family of approximately 50 low molecular weight proteins that regulate the recruitment of leukocytes into inflammatory sites and also maintain a homeostatic lymphoid environment (1). Chemokines exert their effects through the activation of G protein coupled receptors on the leukocyte cell surface, and can be grouped into four subfamilies according to the number and positioning of their amino-terminal cysteine residues (2). CC Chemokine Receptor 4 (CCR4) is the sole receptor identified to date for the chemokines CCL22/Macrophage Derived Chemokine (MDC) and CCL17/Thymus and Activation-Regulated Chemokine (TARC), and was first shown to be highly expressed in the thymus and by peripheral blood mononuclear cells (3-6). Both CCL22 and CCL17 are also expressed in the thymus; one role of the receptor may be to regulate the intrathymic movement of CCR4+CD4+CD8+ thymocytes during the process of T lymphocyte education and differentiation (7, 8). Subsequent studies have identified CCR4 as being preferentially expressed by Th2 cells (9), regulatory T cells (10), and mast cells (11) suggestive of a role in allergic disease. High levels of CCR4 expression on specific subpopulations of T cells, including skin-homing cutaneous lymphocyte antigen (CLA)+ T cells (12), implicates the receptor in the pathology of atopic dermatitis (AD) (13, 14). In vivo studies suggest that CCR4 is expressed by the majority of murine Th2 lymphocytes and facilitates CCL17- and CCL22-mediated chemotaxis (15). Whilst deletion of CCR4 has no effect on either Th2 lymphocyte differentiation in vitro or on a Th2-dependent model of allergic airway inflammation (16), the CCR4/CCL17/CCL22 axes have been shown to play a pivotal role in the late phase of allergic airways inflammation, in studies employing treatment with blocking antibodies specific for the murine orthologues of CCL22 and CCL17 (17-19). Moreover, in clinical studies of allergen-challenged atopic asthmatics and rhinitics the majority of T lymphocytes present in bronchial biopsies were found to be CCR4 positive (20, 21). Consequently, CCR4 arouses much interest as a potential therapeutic target for the treatment of allergic disease (22). However, one potential caveat of targeting CCR4 is its expression on T regulatory cells (Tregs) (10). Blockade of CCR4 function on these cells might be envisaged to worsen rather than dampen allergic inflammation since Tregs have the capacity to suppress Th2-mediated inflammation in vivo (23).
It has previously been reported that of the two CCR4 agonists, CCL22 shows a degree of dominance over CCL17 with respect to CCR4 internalisation and desensitization (5, 24), suggestive of a different mode of interaction of either ligand with the receptor. Similarly, recent studies of prototypic CCR4 antagonists have uncovered two classes of compounds, one which likely binds to a transmembrane binding site and another which interacts with the intracellular C-terminus of CCR4 (25). Here we show that the chemokines CCL17 and CCL22 bind to distinct molecular conformations of CCR4, providing opportunities for the selective antagonism of the receptor.
EXPERIMENTAL PROCEDURES
Materials
Reagents were purchased from Sigma Aldrich and Invitrogen unless otherwise stated. Recombinant human CCL17 and CCL22 were purchased from Peprotech Ltd. (London, UK). Radiolabelled 125I-CCL17 and 125I-CCL22 were purchased from Perkin Elmer (Cambridge, MA). 96 well ChemoTx® chemotaxis plates (101-5, pore size 5 μm) were purchased from Neuroprobe (Gaithersburg MD, USA). The anti-CCR4 antibodies 1G1 and 10E4 have been previously described (15, 26) and were generated by Millennium Pharmaceuticals (Cambridge, MA). The anti-haemagglutinin (HA) anti-HA.11 antibody was from Covance (Berkeley, CA, USA). A PE-conjugated form of 1G1 and its isotype control were purchased from BD Biosciences (Oxford, UK). An APC-conjugated rat anti-mouse IgG2a (clone m2-15F8) was purchased from eBioscience (Hatfield, UK).
Generation of CCR4 K310N mutant
HA-tagged CCR4 cDNA contained within the pcDNA3 plasmid was used as a template for mutagenesis. Point mutants were generated using the QuikChange™ Site-Directed Mutagenesis Kit (Stratagene, Stockport, UK). Mutation was confirmed by DNA sequencing (MWG Biotech, Ebersberg, Germany).
Cell culture and transfection
The murine pre-B lymphoma L1.2 cell line and the human T cell lines Hut78 and CEM-4 were cultured in suspension in RPMI+glutaMAX (Invitrogen, Paisley UK) containing 10% heat-inactivated fetal calf serum, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, and non-essential amino acids at 37°C, 5% CO2. The cells were maintained at a density below 1×106/ml prior to transfection. L1.2 cells were transiently transfected with 1 μg plasmid DNA and 50 μl tRNA per 1×106 cells by electroporation as previously described (27) and cultured overnight with 10 mM sodium butyrate. For the generation of highly polarized human Th2 cells, peripheral blood was taken from healthy volunteers following informed consent and according to an approved protocol. Naïve CD4+ cells were isolated and polarized following 4 weeks in culture using previously described methods (28).
Flow cytometry
16 hours following transfection, 5×105 cells were washed in fluorescence-activated cells sorting (FACS) buffer (0.25% BSA, 0.01% NaN3 in PBS) and incubated with 10 μg/ml 1G1/10E4 antibody (or the appropriate isotype control (26)) in 100 μl FACS buffer for 20 minutes. The cells were washed and then incubated in the same manner with 50 μg/ml FITC-conjugated goat anti-mouse secondary antibody. After this, the cells were washed again, resuspended in 500 μl FACS buffer and analysed on a FACS Calibur as described previously (29). Dead cells were excluded by forward/side-scatter gating and TOPRO-3 exclusion. To assess the number of 10E4 and 1G1 antibody binding sites on Hut78 cells, the Cellquant Calibrator system was used according to the manufacturer’s instructions (Biocytex, Marseille, France). To assess whether 10E4 and 1G1 staining was additive or competitive, staining of Hut78 cells was performed using a directly conjugated 1G1-PE mAb with or without unconjugated 10E4 or their respective isotype controls (10 μg/ml). 10E4 staining was revealed by use of an APC-conjugated rat anti-mouse IgG2a.
Chemotaxis assay
After confirmation of receptor expression by flow cytometry, the chemotactic ability of the cells was measured using ChemoTx™ plates (Neuroprobe, Gathersburg MD, USA), as described previously (27). Briefly, 0.1% BSA in RPMI was used to compose several chemokine concentrations, which were pipetted into previously blocked wells of the plate. The membrane was placed on top, and droplets containing 2×105 cells added on top of the membrane to each well. After 5 hours incubation at 37°C and 5% CO2, the membrane was removed and migration assessed by either cell counting with a haemocytometer or by staining with the live cell dye CellTiter Glo® (Promega, Southampton, UK) and reading luminescence counts. Haemocytometer counts were expressed as a percentage of total cells migrated. CellTiter Glo luminescence counts were converted to chemotactic index (counts/buffer control counts). In inhibition studies, prior to their use, cells were preincubated for 15 minutes at room temperature with 10μg/ml of 10E4, 1G1 or the respective isotype controls either alone or in combination.
Internalisation assay
5×105 cells were incubated in simple RPMI in the presence or absence of 100 nM CCL17/CCL22, for 30 minutes at 37°C. The samples were then washed and stained for flow cytometry as described above. The fluorescence of the chemokine-treated cells was expressed as a percentage of untreated cell fluorescence. Staining of cells incubated with either ligand on ice confirmed that loss of cell surface expression was due to receptor internalization and not simply by steric hindrance of antibody binding.
Intracellular calcium flux
Chronically activated human Th2 lymphocytes were prepared as previously described (30) and were labelled with the dye Fluo-3 (Molecular Probes, Eugene, OR) at a final concentration of 4 μM. Following washing, cells were plated at 300,000 cells per well and stimulated with chemokine at varying concentrations. Ca2+ mobilization was then measured on a 96-well FLIPR System (Molecular Devices, Sunnyvale, CA) as previously described (31).
Radiolabelled chemokine binding assay
Receptor expression was first confirmed by flow cytometry, after which cells were incubated with a fixed concentration of radiolabelled chemokine and a varying concentration of unlabelled chemokine, as previously described (27). Briefly, serial dilutions of chemokine were added to a 96 well plate along with 0.1 nM 125I chemokine, the highest 100nM concentration of unlabelled chemokine representing a 1000-fold excess of cold ligand. 1 million cells were added to each well. After 90 minutes incubation, a 0.5 M NaCl salt wash was applied and cell-associated chemokine separated from free chemokine by centrifugation through silicone oil. Cell pellets were counted for 10 minutes on a gamma counter, and the results presented as the percentage of total binding in the absence of competitor following subtraction of non-specific binding. Despite the use of the salt wash, 125I-CCL22 notably gave higher levels of non-specific binding (NSB) than 125I-CCL17, especially when L1.2 cells were used as a source of CCR4 (over 40% NSB). To assess the number of ligand binding sites/cell on CEM-4 cells, data was analysed in GraphPad Prism using an appropriate binding model and taking into account the relative specific activity of the radiolabelled chemokine (2000 Ci/mmol).
Homology modelling of CCR4
A simple homology model of the human CCR4 receptor was constructed by comparative modelling of the full length CCR4 sequence to the crystal structures of Bovine Rhodopsin (PDB Code 1F88), squid opsin (PDB Code: 2Z73), and CXCR4 (magenta PDB Code: 3OE6). The model was constructed and coarsely refined using the automated modelling process in MOE2011.10 (Chemical Computing Group Inc., Montreal, Canada). Structures were aligned on the Cα atoms of Helix VII and Helix VIII.
Data and statistical analyses
Data are presented as the mean ± SEM of at least three independent experiments. Data were analysed and graphed using Prism software (GraphPad, San Diego, CA, USA).
RESULTS
The CCR4 ligands CCL17 and CCL22 have equal potency and maximal effect in chemotaxis but drive the endocytosis of different CCR4 conformations
The human cell line Hut78 is a T cell lymphoma that endogenously expresses the chemokine receptor CCR4, and therefore serves as a useful model system to examine ligand-receptor interactions. Hut78 cells (Fig. 1A) migrated in a dose-dependent manner to soluble CCL17 and CCL22, exhibiting the typical bell-shaped response with both chemokines demonstrating similar potency and maximal effect. Similar data was observed with the cell line CEM-4 (data not shown). We and others have previously described a panel of CCR4-specific antibodies raised against the human receptor, notably the antibodies 1G1 (15) and 10E4 (26). Using identical concentrations of both antibodies in flow cytometry (10μg/ml) we obtained quite different profiles of CCR4 expression on Hut-78 cells (Fig.1B, 1C), with much more intensive staining observed with the 10E4 mAb than with the 1G1 mAb. Using a commercially available kit (Cellquant Calibrator) the number of binding sites per cell for 1G1 and 10E4 on Hut78 cells were estimated at 590 ± 356 and 10,749 ± 417 respectively. Likewise, more intensive staining of CEM-4 cells, CCR4-L1.2-transfecants and Th2 lymphocytes was observed when the 10E4 mAb was used compared with 1G1 (Figure 2).
Figure 1. Responses of Hut-78 T cells to CCR4 ligands.
(A) Hut78 cell migration to increasing concentrations of CCL17 and CCL22. CCR4 expression on Hut78 cells using 1G1 (B) and 10E4 (C) mAbs; shaded histograms are isotype controls. (D) CCR4 endocytosis on Hut78 cells following chemokine treatment measured using CCR4 mAbs. n=3, *p<0.05, **p<0.01 and ***p<0.001.
Figure 2. Comparison of staining of CEM-4 cells, CCR4 L1.2 transfectants and primary Th2 lymphocytes with the 10E4 and 1G1 antibodies.
Data are representative of at least three separate experiments.
We then employed both mAbs in endocytosis assays using an optimal dose of either CCL17 or CCL22 to induce receptor internalisation (24). The ability of each ligand to drive CCR4 endocytosis was assessed by determining the loss of cell surface CCR4. In keeping with previous observations obtained with the 1G1 antibody (24), CCL22 but not CCL17 was able to drive significant CCR4 endocytosis (Fig. 1.D). However, using the 10E4 antibody, both ligands were apparently able to induce a significant reduction in cell-surface CCR4 expression. Collectively, this suggests the existence of distinct conformations of CCR4 which differ in their ability to respond to CCL17 stimulation.
The CCR4 ligands CCL17 and CCL22 have equal potency and maximal effect in calcium flux release but display differential abilities to desensitize CCR4 populations
We subsequently investigated the ability of CCL17 and CCL22 to induce calcium mobilisation in Th2 lymphocytes, in particular, assessing the ability of each chemokine to desensitize responses to a subsequent stimulus of the same or alternate ligand (homologous and heterologous desensitization). Initial treatment of Th2 cells with a 50 nM concentration of CCL17, resulted in intracellular calcium release, and rendered the cells unresponsive to a second identical CCL17 stimulus (Fig. 3A). Similar results were observed when two consecutive doses of 50 nM CCL22 were employed (Fig. 3B). However, when heterologous desensitization was examined in the same fashion, CCL22 rendered cells unresponsive to subsequent CCL17 treatment (Fig. 3C), but the converse was not observed, with CCL17 having little if any impact on subsequent CCL22 responses (Fig. 3D). Dose response analysis of calcium release in human Th2 cells in response to increasing concentrations of chemokine found that CCL17 and CCL22 had equal potency and maximal effects (Fig. 3E), as was the case with their activity in chemotaxis assays (Fig. 1A).
Figure 3. Responses of human Th2 cells to CCR4 ligands.
Homologous (A & B)/heterologous (C & D) desensitization of calcium responses following CCL17/CCL22 treatment. Arrows indicate addition of chemokine. Data are representative of three independent experiments. (E) Calcium release of Th2 cells after CCL17/CCL22 treatment. EC50 values = 1.6 nM and 2.5 nM respectively. n=3.
CCL17 and CCL22 are conformationally selective ligands of CCR4
Radioligand binding assays were then carried out to investigate further the interaction of CCR4 with CCL17 and CCL22. Unlabelled CCL17 and CCL22 dose-dependently displaced 125I-CCL17 on both CEM-4 and CCR4-L1.2 transfectants cells in an identical fashion with a 1000-fold excess of either unlabelled chemokine (100 nM) reducing 125I-CCL17 binding to similar levels (Fig. 4A-B, respectively). When 125I-CCL22 was used as a radioligand, higher levels of non-specific binding were observed than with 125I-CCL17, especially when CCR4-L1.2 transfectants were used. Using both CEM-4 cells and CCR4-L1.2 transfectants, the displacement of 125I-CCL22 from both cell types by unlabelled CCL22 was much more effective than its counterpart (almost three fold difference in IC50 values, Table 1) with a significant proportion of 125I-CCL22 resistant to displacement by a 1000-fold excess of CCL17 (Fig. 4C-D). This suggests that CCL22 and CCL17 are conformationally selective ligands of CCR4, a phenomenon that has previously been reported by ourselves and others for ligands of the chemokine receptor CXCR3 (32, 33). Non-linear regression analysis of the binding data for homologous competition of either radioligand from CEM-4 cells suggested that there were more binding sites for CCL22 (16,163 ± 11,522) than for CCL17 (13,997 ± 3,974).
Figure 4. CCL22 and CCL17 are conformationally selective ligands of CCR4.
Displacement of 125I-CCL17 (A & B) and 125I-CCL22 (C & D) from CEM-4 cells (A & C) or CCR4-L1.2s (B & D) by increasing concentrations of unlabelled chemokine. n=3.
Table 1. Ligand binding properties of CCR4 expressing cells in heterologous competition binding assays.
Mean IC50 values are from at least three experiments.
| Hot Tracer ligand | ||||
|---|---|---|---|---|
| 125I-CCL17 | 125I-CCL22 | |||
| Cold Competitor |
IC50 CCL17 (nM) |
IC50 CCL22 (nM) |
IC50 CCL17 (nM) |
IC50 CCL22 (nM) |
| CEM-4 | 2.5 ± 0.8 | 2.1 ± 0.8 | 3.8 ± 0.8 | 3.5 ± 0.7 |
| L1.2 CCR4 | 3.1 ± 0.8 | 3.2 ± 0.8 | 4.1 ± 0.7 | 1.5 ± 0.8 |
The 10E4 antibody recognizes a population of CCR4 that can bind CCL17
We have previously mapped the 10E4-specific epitope of CCR4 to the receptor N-terminus, a key ligand-binding domain (26). We subsequently used this antibody to assess the role of the receptor. In keeping with earlier data from that study, pre-treatment of CEM-4 cells with 10E4 - but not an isotype control - reduced 125I-CCL17 binding to the levels observed with a 1000-fold excess of unlabelled CCL17 or CCL22 (Fig. 5A). In contrast, although 10E4 pre-treatment significantly lowered the level of 125I-CCL22 to those obtained with a 1000-fold excess of unlabelled CCL17, a significantly greater displacement of 125I-CCL22 was observed when a 1000-fold excess of CCL22 was employed (Fig. 5B). Together with the earlier heterologous competition assays, this suggests that the majority of CCR4 is in a 10E4-sensitive conformation that can bind both ligands, whilst a 10E4-insensitive remnant can bind CCL22.
Figure 5. 10E4 blockade of CCL17 and CCL22 binding.
Binding of 125I-CCL17 (A) and 125I-CCL22 (B) to CCR4 transfectants in the presence of unlabelled chemokine, an isotype control (IgG2a) mAb or the CCR4-specific mAb 10E4. (B) and (C) - Migration of CCR4 transfectants to CCL17 (C) or CCL22 (D) following pre-treatment with either 10E4, 1G1, or both antibodies combined (closed symbols and dashed lines). Isotype treated cells are shown as open symbols and solid lines. Competitive staining of Hut78 cells with the 10E4 and 1G1 antibodies (E). n=3,*p<0.05, **p<0.01 and ***p<0.001.
To assess whether this 10E4-insensitive remnant was functional, we assessed chemotaxis responses of transfectants to both ligands following 10E4 pre-treatment. As a comparator, the antibody 1G1 was also employed which has previously been shown to partially block CCL22 and CCL17 chemotactic responses in Th2 lymphocytes (15).
Whilst maximal chemotactic responses to CCL17 were significantly inhibited by 10E4, notably at the optimal concentration of 1 nM (Fig 5C), responses to CCL22 were left largely intact (Fig 5D), with a rightward shift in the potency of CCL22 observed. The 1G1 mAb had no significant inhibitory activity for either CCL17 or CCL22 responses and combinations of 10E4 and 1G1 had no additional inhibitory activity to that seen with 10E4 alone. We therefore conclude that the 10E4-insensitive remnant of CCR4 is able to facilitate migration in response to CCL22. To address whether or not 10E4 and 1G1 are competitive or additive in CCR4 staining we incubated Hut78 cells with a PE-conjugated version of 1G1 together with unlabelled 10E4 or an isotype control. 10E4 staining with assessed with an APC-conjugated anti-IgG2a mAb (Fig. 5E). 10E4 was observed to significantly inhibit 1G1 binding, whilst the inclusion of the isotype control had no effect. In contrast, 1G1 did not interfere with recognition of CCR4 by the 10E4 mAb. We therefore conclude that 1G1 recognizes a fraction of the 10E4 population, which likely explains its inability to effectively block CCR4 responses.
An amino acid within the CCR4 C-terminus is key for activation of CCR4 by CCL17 but not by CCL22
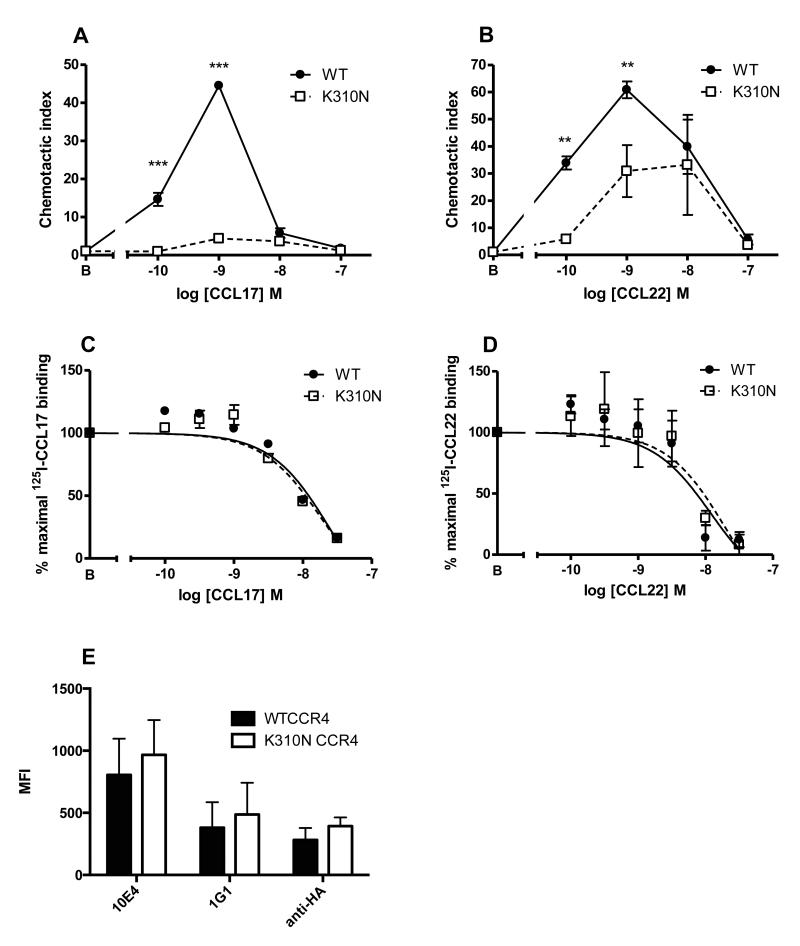
Of late, a conserved C-terminal region known as Helix VIII found within class A GPCRs has come under scrutiny, with several studies alluding to its significance in receptor signalling and ligand activity, notably that of the Herpes virus encoded chemokine receptor ORF74 (34). Interestingly, the C-terminal region of CCR4 has been reported to be the principal site of action of a novel class of CCR4 small molecule antagonist, suggesting the importance of this region in CCR4 activation (25). To examine this region we created a point mutation within Helix VIII of CCR4 at lysine 310 (K310N) to test the hypothesis that Helix VIII was involved in the discrimination of CCL17 and CCL22 in the two observed CCR4 conformations. We chose this particular residue since a lysine in an analogous position is highly conserved within class A GPCRs and our intention was to disrupt any likely ionic interactions of K310 either within the GPCR or with other intracellular proteins such as a G protein. Following transfection of cells, the K310N construct was found to be singularly unresponsive to CCL17 in chemotaxis assays (Fig. 6A). In contrast, the K310N mutant displayed a robust chemotactic response to CCL22, albeit at a reduced potency and with a reduced maximal effect compared to WT CCR4 (Fig. 6B). In light of this disparity in chemotaxis, transfectants were then assayed for their ability to bind both chemokines. In contrast to the chemotaxis data, no significant differences were observed in the IC50 values for either ligand (Fig. 6C-D). Comparisons of staining of cells expressing WT CCR4 or the K310N mutant by flow cytometry showed a trend towards increased expression of the mutant regardless of the primary antibody used for detection (Fig. 6E). Thus K310 of Helix VIII is required for effective activation of CCR4 by CCL17 but plays no role in either receptor expression or the binding of either ligand.
Figure 6. A role for K310N in CCR4 activation by CCL17.
Migration of WT CCR4-L1.2 or K310N CCR4-L1.2 to increasing concentrations of CCL17 (A) or CCL22 (B). Homologous displacement binding curves for 125I-CCL17 (C) and 125I-CCL22 (D). Staining of CCR4-L1.2 or K310N CCR4-L1.2 transfectants using 10E4, 1G1 or anti-HA mAbs (E). n=5, *p<0.05, **p<0.01 and ***p<0.001.
DISCUSSION
The role of CCR4 and its ligands CCL17 and CCL22 in allergic disease is well established, with the receptor a key target for antagonist development. Promisingly, CCR4 blockade by a monoclonal antibody in a human PBMC-reconstituted SCID mouse model, was found to abolish several key features of allergic airways inflammation, notably bronchial hyperreactivity, airway eosinophilia, goblet cell hyperplasia and IgE synthesis (35). Blockade of CCR4 in vitro, with a prototypic small molecule antagonist has also been shown to be successful in inhibiting the actin polymerization responses of human Th2 cells, a population increased in numbers in nasal biopsies of rhinitis patients following allergen challenge (36). However, one potential caveat of CCR4-specific therapy is the potential for also blocking Treg cell recruitment, with the majority of human Tregs expressing CCR4 (37, 38). Thus, approaches which spare Treg recruitment but inhibit Th2 recruitment are likely to be the most effective. The data we present here suggests that CCL17 and CCL22 interact with distinct conformations of CCR4 on leukocytes, allowing for the blockade of signalling via one conformation but not the other.
Figure 7 shows a simplistic model of CCR4 activation based upon the data we present here. Distinct populations of CCR4 with differing conformations are shown; a major species denoted R1 and one or more minor species denoted collectively as R2. The R1 conformation is activated by both CCL17 and CCL22, and is recognized by the antibody 10E4. The smaller population of receptors in the R2 conformation can only be activated by CCL22. R1 and R2 are not interconvertable conformations of the receptor but are fixed states, since neither CCL17 nor the mAb 10E4 were able to completely displace 125I-CCL22 from cells (Fig. 5B) nor was 10E4 able to completely inhibit CCL22 mediated migration (Fig. 5D). Mutation of Helix VIII within the CCR4 C-terminus (K310N mutant) ablated CCL17 but not CCL22 responses in chemotaxis assays. This was at the level of CCR4 activation rather than receptor expression or ligand engagement, since the expression levels and the affinities of CCL17 and CCL22 for the K310N receptor were not significantly different from WT CCR4. Notably, the profile of the CCL22 chemotactic responses of the K310 mutant were reminiscent of those obtained in cells pre-treated with the 10E4 antibody, with reduced potency (Fig. 5D) suggesting that the R1 but not the R2 state relies upon K310 for activation by chemokine. Molecular modelling suggests that K310 of Helix VIII likely forms a salt bridge with an aspartic acid residue (D76) in the cytoplasmic region of Helix II. (Fig. 8) and possibly influences interactions with intracellular G proteins.
Figure 7. A two-state model for CCR4 activation.
Cartoon of CCR4 showing two receptor conformations (R1 and R2) which are differentially bound and activated by CCL17 and CCL22. R1 is the major species as assessed by ligand binding assays and staining with the 10E4 antibody (and to a lesser extent by the 1G1 antibody in grey). The importance of residue K310 in CCR4 activation by CCL17 is highlighted in the R1 conformation, although this residue has no effect upon the 10E4 sensitive-conformation of the R1 state.
Figure 8. Putative salt bridge in CCR4 predicted by homology modelling.
Crystal structures of bovine rhodopsin (blue N-terminus through red C-terminus), squid opsin (gold) and CXCR4 (magenta), and a CCR4 homology model (green). The cutout shows the residues corresponding to lysine 310 of CCR4, which is thought to form a salt bridge with a highly conserved aspartate residue in TMII.
Our model also adequately explains the apparent appearance of CCL22 as a dominant ligand by other groups, either in terms of receptor endocytosis (24) or in heterologous desensitization assays (39). In receptor endocytosis assays, CCL22 can engage with and induce the internalisation of both R1 and R2 conformations. CCL22 appears dominant to CCL17 when the 1G1 antibody is employed, as in these assays, the 1G1 antibody recognises only a fraction of the R1 conformation to which CCL17 binds specifically. When the 10E4 antibody is employed, then the entire R1 population is stained and both CCL17 and CCL22 are observed to induce CCR4 internalization with a similar degree of efficacy (Fig 1D). Likewise, in assays of heterologous desensitization observed by calcium flux, CCL22 can interact with both the R1 and R2 conformations and can therefore desensitize all subsequent responses to CCL22 or CCL17. In contrast, CCL17 binds only to the R1 conformation and presumably sufficient levels of the R2 state remain to mediate the observed CCL22 calcium flux response.
It is evident that the apparent dominance of one ligand over another can be dependent upon the experimental readout. A recent study by Bonner and colleagues showed that CCL17 could potently induce the synthesis of αCGRP by CCR4+ bronchial epithelial cells with implications for the pathogenesis of asthma (40). Notably, CCL17 induced αCGRP transcription with around a 20,000-fold higher efficacy than an equivalent concentration of CCL22. Such biased signalling at a chemokine receptor may hint at why higher organisms have often developed two or more ligands to activate the same chemokine receptor. Originally termed ‘functional redundancy’, the notion was that this duplication of ligands and receptors helped to maintain a minimal level of leukocyte trafficking should a single component of the system be subverted by microbes (41). In light of subsequent study it is apparent that each chemokine may play a specific role in a context outside of leukocyte migration, such as cellular differentiation.
So how do our observations affect the status quo in terms of the current development of small molecule CCR4 antagonists (42-45)? If our model is correct, then blockade of both the R1 and R2 conformations is desirable for complete CCR4 blockade, for example by a small molecule which locks CCR4 into an alternative conformation to which neither CCL22 nor CCL17 can bind or activate the receptor. However, as mentioned previously, a potential caveat of treatment with such a drug is that recruitment of immunosuppressive CCR4+ Tregs, to the site of allergic inflammation is also likely to be hampered. It is notable that of a range of adult biomarkers, CCL17 had the highest odds ratio for the likelihood of having atopic dermatitis (AD) (46), consistent with in vitro studies showing that corneal and dermal fibroblasts are important sources of CCL17 but not CCL22 following stimulation with IL-4 and IL-13 (47). In a study of AD patients with significant improvement of symptoms following subcutaneous immunotherapy with a house dust mite allergoid, it was observed that serum IL-10 levels were increased significantly after 6 months of treatment (48). This correlated with significantly reduced serum levels of CCL17 but not CCL22, suggesting that CCL22 is primarily responsible for Treg recruitment. This concurs with data from the original publication in which CCR4 was described as a key receptor for Treg migration (10). Using a supernatant from activated DCs as a source of chemoattractants, Iellem and colleagues showed that antibody blockade of CCL17 had negligible effects upon Treg recruitment whereas blockade of CCL22 reduced Treg migration by around 50%. A previous in vitro study showed that some classes of prototypic small molecule CCR4 antagonist were less effective at inhibiting CCR4 signalling in Tregs compared with other CD4+ T cell subsets, presumably due to greater levels of CCR4 expression on the Tregs (49). Further fine-tuning of such molecules to block CCL17 but spare CCL22 signaling, may provide a beneficial therapeutic option for the treatment of allergic diseases such as AD.
Acknowledgments
Grant Support: The work was supported by BBSRC/GSK Case Studentships awarded to JMV and PP and a Wellcome Trust Project grant awarded to JEP (068226/Z/02/Z). DJC and MJ-W were funded and supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London.
Abbreviations
- AD
atopic dermatitis
- GPCR
G protein-coupled receptor
- PTX
pertussis toxin
References
- 1.Charo IF, Ransohoff RM. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 2.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Power CA, Meyer A, Nemeth K, Bacon KB, Hoogewerf AJ, Proudfoot AEI, Wells TNC. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J Biol Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- 4.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 5.Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 1998;273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 6.Andrew DP, Chang M-S, McNinch J, Wathen ST, Rihanek M, Tseng J, Spellberg JP, Elias CG. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998;161:5027–5038. [PubMed] [Google Scholar]
- 7.Chantry D, Romagnani P, Raport CJ, Wood CL, Epp A, Romagnani S, Gray PW. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3(+), CD4(+), CD8(low) thymocytes. Blood. 1999;94:1890–1898. [PubMed] [Google Scholar]
- 8.Annunziato F, Romagnani P, Cosmi L, Beltrame C, Steiner BH, Lazzeri E, Raport CJ, Galli G, Manetti R, Mavilia C, Vanini V, Chantry D, Maggi E, Romagnani S. Macrophage-derived chemokine and EBI1-ligand chemokine attract human thymocytes in different stage of development and are produced by distinct subsets of medullary epithelial cells: possible implications for negative selection. J. Immunol. 2000;165:238–246. doi: 10.4049/jimmunol.165.1.238. [DOI] [PubMed] [Google Scholar]
- 9.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juremalm M, Olsson N, Nilsson G. Selective CCL5/RANTES-induced mast cell migration through interactions with chemokine receptors CCR1 and CCR4. Biochem. Biophys. Res. Commun. 2002;297:480–485. doi: 10.1016/s0006-291x(02)02244-1. [DOI] [PubMed] [Google Scholar]
- 12.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 13.Kakinuma T, Nakamura K, Wakugawa M, Mitsui H, Tada Y, Saeki H, Torii H, Asahina A, Onai N, Matsushima K, Tamaki K. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J. Allergy Clin. Immunol. 2001;107:535–541. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 14.Vestergaard C, Bang K, Gesser B, Yoneyama H, Matsushima K, Larsen CG. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. The Journal of investigative dermatology. 2000;115:640–646. doi: 10.1046/j.1523-1747.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- 15.Andrew DP, Ruffing N, Kim CH, Miao W, Heath H, Li Y, Murphy K, Campbell JJ, Butcher EC, Wu L. C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J. Immunol. 2001;166:103–111. doi: 10.4049/jimmunol.166.1.103. [DOI] [PubMed] [Google Scholar]
- 16.Chvatchko Y, Hoogewerf AJ, Meyer A, Alouani S, Juillard P, Buser R, Conquet F, Proudfoot AE, Wells TN, Power CA. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J. Exp. Med. 2000;191:1755–1764. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, Powers CA, Proudfoot AE, Coyle AJ, Gearing D, Gutiérrez-Ramos JC. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- 18.Kawasaki S, Takizawa H, Yoneyama H, Nakayama T, Fujisawa R, Izumizaki M, Imai T, Yoshie O, Homma I, Yamamoto K, Matsushima K. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J. Immunol. 2001;166:2055–2062. doi: 10.4049/jimmunol.166.3.2055. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez-A C, Coyle AJ, Gutiérrez-Ramos JC. CC chemokine receptor (CCR) 3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J. Exp. Med. 2000;191:265–274. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, Arrigoni G, Fabbri LM, Sinigaglia F. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J. Clin. Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Till S, Jopling L, Wachholz P, Robson R, Qin S, Andrew D, Wu L, van Neerven J, Williams T, Durham S, Sabroe I. T cell phenotypes of the normal nasal mucosa: induction of Th2 cytokines and CCR3 expression by IL-4. J. Immunol. 2001;166:2303–2310. doi: 10.4049/jimmunol.166.4.2303. [DOI] [PubMed] [Google Scholar]
- 22.Pease JE. Targeting chemokine receptors in allergic disease. Biochem. J. 2011;434:11–24. doi: 10.1042/BJ20101132. [DOI] [PubMed] [Google Scholar]
- 23.Saito K, Torii M, Ma N, Tsuchiya T, Wang L, Hori T, Nagakubo D, Nitta N, Kanegasaki S, Hieshima K, Yoshie O, Gabazza EC, Katayama N, Shiku H, Kuribayashi K, Kato T. Differential regulatory function of resting and preactivated allergen-specific CD4+ CD25+ regulatory T cells in Th2-type airway inflammation. J. Immunol. 2008;181:6889–6897. doi: 10.4049/jimmunol.181.10.6889. [DOI] [PubMed] [Google Scholar]
- 24.Mariani M, Lang R, Binda E, Panina-Bordignon P, D’ambrosio D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur. J. Immunol. 2004;34:231–240. doi: 10.1002/eji.200324429. [DOI] [PubMed] [Google Scholar]
- 25.Andrews G, Jones C, Wreggett KA. An Intracellular Allosteric Site for a Specific Class of Antagonists of the CC Chemokine G Protein-Coupled Receptors CCR4 and CCR5. Mol. Pharmacol. 2007;73:855–867. doi: 10.1124/mol.107.039321. [DOI] [PubMed] [Google Scholar]
- 26.Jopling LA, Sabroe I, Andrew DP, Mitchell TJ, Li Y, Hodge MR, Williams TJ, Pease JE. The identification, characterization, and distribution of guinea pig CCR4 and epitope mapping of a blocking antibody. J. Biol. Chem. 2002;277:6864–6873. doi: 10.1074/jbc.M109974200. [DOI] [PubMed] [Google Scholar]
- 27.Vaidehi N, Pease JE, Horuk R. Modeling small molecule-compound binding to G-protein-coupled receptors. Meth. Enzymol. 2009;460:263–288. doi: 10.1016/S0076-6879(09)05213-6. [DOI] [PubMed] [Google Scholar]
- 28.Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines. J. Immunol. 2002;169:2498–2506. doi: 10.4049/jimmunol.169.5.2498. [DOI] [PubMed] [Google Scholar]
- 29.de Mendonça FL, Fonseca P. C. A. da, Phillips RM, Saldanha JW, Williams TJ, Pease JE. Site-directed mutagenesis of CC chemokine receptor 1 reveals the mechanism of action of UCB 35625, a small molecule chemokine receptor antagonist. J. Biol. Chem. 2005;280:4808–4816. doi: 10.1074/jbc.M412267200. [DOI] [PubMed] [Google Scholar]
- 30.Zabel BA, Agace WW, Campbell JJ, Heath HM, Parent D, Roberts AI, Ebert EC, Kassam N, Qin S, Zovko M, LaRosa GJ, Yang LL, Soler D, Butcher EC, Ponath PD, Parker CM, Andrew DP. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 1999;190:1241–1256. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilbanks A, Zondlo SC, Murphy K, Mak S, Soler D, Langdon P, Andrew DP, Wu L, Briskin M. Expression cloning of the STRL33/BONZO/TYMSTR ligand reveals elements of CC, CXC, and CX3C chemokines. J. Immunol. 2001;166:5145–5154. doi: 10.4049/jimmunol.166.8.5145. [DOI] [PubMed] [Google Scholar]
- 32.Cox MA, Jenh CH, Gonsiorek W, Fine J, Narula SK, Zavodny PJ, Hipkin RW. Human interferon-inducible 10-kDa protein and human interferon-inducible T cell alpha chemoattractant are allotopic ligands for human CXCR3: differential binding to receptor states. Mol. Pharmacol. 2001;59:707–715. doi: 10.1124/mol.59.4.707. [DOI] [PubMed] [Google Scholar]
- 33.Nedjai B, Li H, Stroke IL, Wise EL, Webb ML, Merritt JR, Henderson I, Klon AE, Cole AG, Horuk R, Vaidehi N, Pease JE. Small-molecule chemokine mimetics suggest a molecular basis for the observation that CXCL10 and CXCL11 are allosteric ligands of CXCR3. Br J Pharmacol. 2012;166:912–923. doi: 10.1111/j.1476-5381.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verzijl D, Pardo L, Van Dijk M, Gruijthuijsen YK, Jongejan A, Timmerman H, Nicholas J, Schwarz M, Murphy PM, Leurs R, Smit MJ. Helix 8 of the Viral Chemokine Receptor ORF74 Directs Chemokine Binding. Journal of Biological Chemistry. 2006;281:35327–35335. doi: 10.1074/jbc.M606877200. [DOI] [PubMed] [Google Scholar]
- 35.Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64:995–1002. doi: 10.1111/j.1398-9995.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 36.Banfield G, Watanabe H, Scadding G, Jacobson MR, Till SJ, Hall DA, Robinson DS, Lloyd CM, Nouri-Aria KT, Durham SR. CC Chemokine Receptor 4 (CCR4) in human allergen-induced late nasal responses. Allergy. 2010 doi: 10.1111/j.1398-9995.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, Badoual C, Gey A, Ravel P, Marcheteau E, Batteux F, Sandoval F, Adotevi O, Chiu C, Garcia S, Tanchot C, Lone YC, Ferreira LC, Nelson BH, Hanahan D, Fridman WH, Johannes L, Tartour E. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood. 2011;118:4853–4862. doi: 10.1182/blood-2011-01-329656. [DOI] [PubMed] [Google Scholar]
- 38.Hirahara K, Liu L, Clark RA, Yamanaka K-I, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J. Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 39.Struyf S, Proost P, Sozzani S, Mantovani A, Wuyts A, De Clercq E, Schols D, Van Damme J. Enhanced anti-HIV-1 activity and altered chemotactic potency of NH2-terminally processed macrophage-derived chemokine (MDC) imply an additional MDC receptor. J. Immunol. 1998;161:2672–2675. [PubMed] [Google Scholar]
- 40.Bonner K, Pease JE, Corrigan CJ, Clark P, Kay AB. CCL17/thymus and activation-regulated chemokine induces calcitonin gene-related peptide in human airway epithelial cells through CCR4. J. Allergy Clin. Immunol. 2013;132:942–950.e3. doi: 10.1016/j.jaci.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- 42.Allen S, Newhouse B, Anderson AS, Fauber B, Allen A, Chantry D, Eberhardt C, Odingo J, Burgess LE. Discovery and SAR of trisubstituted thiazolidinones as CCR4 antagonists. Bioorg. Med. Chem. Lett. 2004;14:1619–1624. doi: 10.1016/j.bmcl.2004.01.072. [DOI] [PubMed] [Google Scholar]
- 43.Burdi DF, Chi S, Mattia K, Harrington C, Shi Z, Chen S, Jacutin-Porte S, Bennett R, Carson K, Yin W, Kansra V, Gonzalo J-A, Coyle A, Jaffee B, Ocain T, Hodge M, LaRosa G, Harriman G. Small molecule antagonists of the CC chemokine receptor 4 (CCR4) Bioorg. Med. Chem. Lett. 2007;17:3141–3145. doi: 10.1016/j.bmcl.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Purandare AV, Gao A, Wan H, Somerville J, Burke C, Seachord C, Vaccaro W, Wityak J, Poss MA. Identification of chemokine receptor CCR4 antagonist. Bioorg. Med. Chem. Lett. 2005;15:2669–2672. doi: 10.1016/j.bmcl.2005.02.084. [DOI] [PubMed] [Google Scholar]
- 45.Procopiou PA, Ford AJ, Graves RH, Hall DA, Hodgson ST, Lacroix YML, Needham D, Slack RJ. Lead optimisation of the N1 substituent of a novel series of indazole arylsulfonamides as CCR4 antagonists and identification of a candidate for clinical investigation. Bioorg. Med. Chem. Lett. 2012;22:2730–2733. doi: 10.1016/j.bmcl.2012.02.104. [DOI] [PubMed] [Google Scholar]
- 46.Kou K, Aihara M, Matsunaga T, Chen H, Taguri M, Morita S, Fujita H, Yamaguchi Y, Kambara T, Ikezawa Z. Association of serum interleukin-18 and other biomarkers with disease severity in adults with atopic dermatitis. Archives of dermatological research. 2012;304:305–312. doi: 10.1007/s00403-011-1198-9. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda K, Fujitsu Y, Seki K, Kumagai N, Nishida T. Differential expression of thymus- and activation-regulated chemokine (CCL17) and macrophage-derived chemokine (CCL22) by human fibroblasts from cornea, skin, and lung. Journal of Allergy and Clinical Immunology. 2003;111:520–526. doi: 10.1067/mai.2003.59. [DOI] [PubMed] [Google Scholar]
- 48.Bussmann C, Maintz L, Hart J, Allam JP, Vrtala S, Chen KW, Bieber T, Thomas WR, Valenta R, Zuberbier T, Sager A, Novak N. Clinical improvement and immunological changes in atopic dermatitis patients undergoing subcutaneous immunotherapy with a house dust mite allergoid: a pilot study. Clin. Exp. Allergy. 2007;37:1277–1285. doi: 10.1111/j.1365-2222.2007.02783.x. [DOI] [PubMed] [Google Scholar]
- 49.Hall DA. Insurmountable CCR4 antagonists are less effective at inhibiting CCR4-agonist induced actin polymerisation in regulatory T cells than other T cell phenotypes. Proceedings of the British Pharmacological Society. 2010:137. [Google Scholar]