Abstract
Elusive for more than half a century, corticotropin-releasing factor (CRF) was finally isolated and characterized in 1981 from ovine hypothalami and shortly thereafter, from rat brain. Thirty years later, much has been learned about the function and localization of CRF and related family members (Urocortins 1, 2 and 3) and their 2 receptors, CRF receptor type 1 (CRFR1) and CRF receptor type 2 (CRFR2). Here, we report the stepwise development of peptide CRF agonists and antagonists, which led to the development of the CRFR1 agonist Stressin1; the long-acting antagonists Astressin2-B which is specific for CRFR2; and Astressin B, which binds to both CRFR1 and CRFR2. This analog has potential for the treatment of CRF-dependent diseases in the periphery, such as irritable bowel syndrome.
Keywords: oCRF, h/rCRF, α–hel CRF(9–41), astressin, astressin B, [DPhe12] CRF, astressin-2B, CRF antagonists, CRFR1, CRFR2, CRF, stress, stressin1
A. Introduction
On September 18, 1981, W. Vale, C. Rivier, J. Spiess and J. Rivier reported in Science, the characterization of the 41 residue ovine hypothalamic peptide that stimulates the secretion of adrenocorticotropin hormone (ACTH) and beta-endorphin (Vale et al., 1981). Each member of the team contributed according to his/her own experimental background and expertise. W. Vale headed the overall projects that dealt with the isolation and characterization of ovine hypothalamic (Spiess et al., 1981; Vale et al., 1981) and rat brain (Rivier et al., 1983b) corticotropin-releasing factors (CRF), and provided the critical in vitro cultured rat anterior pituitary cells (RAP) assay (Vale et al., 1972a) in collaboration with C. Rivier, who developed a radio-immuno assay specific for rat ACTH (Rivier et al., 1973a). It allowed the detection and identification of the active chromatographic zones (Rivier et al., 1983b; Vale et al., 1981) and was used in the in vivo bioassay that demonstrated the ability of the new CRF to release ACTH in rats (Rivier et al., 1973a). J. Rivier developed the reversed phase high performance liquid chromatography (RP-HPLC) systems (column supports, solvent systems, flow rates and temperature) that provided the best separations, reproducibility and recoveries for a peptide the size of CRF (Hoeger et al., 1987; Miller and Rivier, 1996; Rivier and McClintock, 1989; Rivier et al., 1982c), while J. Spiess developed the micro-sequencing that provided the putative sequences of ovine and rat CRF (Spiess et al., 1983; Vale et al., 1981). J. Rivier then further used improved protocols of solid phase peptide synthesis, along with preparative RP-HPLC, to obtain the replicate of ovine and rat CRFs and their methionine oxide derivatives (Rivier et al., 1983b; Vale et al., 1981), which confirmed the sequencing results described by Spiess et al. (1983). Each step presented many technical challenges that are well described in the cited literature, and will not be discussed here. Independently, and concomitantly, the structure of sauvagine isolated from the skin of the frog Phyllomedusa Sauvagei (Montecucchi and Henschen, 1981) and urotensin I from two teleost fish species (Cyprinus Carpio and Catostomus Commersoni) (Lederis et al., 1982) were reported.
Immediately upon availability of these sequences, a multi-pronged approach was developed for the extensive characterization of these peptides, including (but not limited to) their distribution in the brain, as well as biological, molecular, chemical, immunological, structural and functional characteristics. Analyses were initiated that used rational, systematic as well as intuitive analoging, a process whereby analogs were designed to fulfill needs that were not satisfied by CRF family members. For example, CRF analogs with higher affinity for their receptor(s) and higher resistance to biodegradation than CRF family members were more potent in vitro and longer acting in vivo than CRF itself. Some other substitutions resulted in improved chemical stability (for example, substitution of a methionine by norleucine or norvaline).
In the absence of competitive peptide antagonists, we developed specific CRF neutralizing antibodies that helped define, in a first phase, CRF’s physiological role (Rivier et al., 1982b). The intravenous administration of a rabbit antiserum to ovine CRF (oCRF) markedly reduced the oCRF-induced rise of plasma ACTH in intact non-stressed adult male rats, while blocking more than 75 percent of the ACTH release observed in rats exposed to ether stress. Furthermore, immunoneutralisation of oCRF significantly lowered ACTH levels in adrenalectomized animals. These results provided evidence that endogenous CRF played an important physiological role in regulating ACTH secretion under a variety of basal and stimulated conditions. Antibodies, however, are not without shortcomings associated with their high molecular weight, species specificity, antigenicity and poor ability to be fully distributed in the brain. In view of the above, designing potent competitive antagonists of CRF became our top priority, so that we could assess the physiologic and patho-physiologic significance of endogenous CRF in experimental animals and, ultimately, in human.
B. Lessons learned from structure-activity relationship (SAR) studies of thyrotropin releasing hormone (TRH), gonadotropin releasing hormone (GnRH) and somatotropin release inhibiting factor (SRIF somatostatin) agonists
1. Agonists
In the late 70’s, studies were initiated to establish the SARs of the then known hypothalamic peptides, namely TRH, GnRH, and somatostatin. To this effect, peptides were synthesized by solid phase method (Märki et al., 1981). Purification of the crude synthetic peptides generated after treatment with HF and cleavage from the methyl-benzhydryl-amine (MBHA) and Merrifield resins, respectively, were achieved by preparative RP-HPLC, using three different solvent systems (Gulyas et al., 1995). Peptides were then characterized by analytical HPLC under various conditions (other than that used for purification), amino acid analysis, capillary zone electrophoresis (CZE), mass spectrometry (MS) and circular dichroism in some cases. The RAP assay (Vale et al., 1972a) was used to determine the potency of our analogs relative to a given concentration of oCRF. The same cells were also used to test analogs for antagonism (Vale et al., 1972b) while in vivo experiments were performed in rats (Rivier et al., 1982a; Rivier et al., 1982b).
The first priority in peptide analog design was to identify the shortest bioactive sequence by deleting amino acids (AAs) one or two at a time from the N-, C- (Rivier et al., 1974; Rivier et al., 1973b) or N- and C-termini (Vale et al., 1976), respectively. In the case of GnRH, deletion of the Gly10 residue and replacement by an ethylamide resulted in a 3-fold increase in potency (Fujino et al., 1973). However, further deletions resulted in significant, if not total loss of potency. Deletion of two AAs simultaneously from the N- and C-termini of Des-Ala-Gly-somatostatin, led to the discovery of the mini-somatostatins in general (Vale et al., 1976), and octapeptide DTrp8 SRIF (ODT-8) (des-Ala1-Gly2, Lys4, Asn5, Thr12, Ser13-[DTrp8]-SRIF in particular (Rivier et al., 1975). The second priority was to replace AAs by their optical isomers (Rivier et al., 1993) (or DAla in lieu of Gly) in order to identify the presence of putative turns in GnRH (Monahan et al., 1973) and SRIF (Rivier et al., 1975). Ala scans such as that of SRIF were also commonly used to gain an appreciation of the contribution of each native AA side chain to biological activity and potency (Rivier et al., 1975). Finally, additional substitutions by unnatural amino acids offered the possibility to constrain tertiary structures (Rizo et al., 1996), modulate solubility, [ImBzl-DHis6, Pro9-NHEt]-GnRH (Karten and Rivier, 1986), and increase potency of TRH analogs, [3-MeHis2]TRH (Rivier et al., 1971), among others.
2. Antagonists
Generation of an antagonist of small (5–15 residues) and middle size (25–45 residues) peptides has generally resulted from some specific AA substitutions (often unnatural or of the D-configuration) (Rivier et al., 1983a), or deletion. Gonadotropin-releasing hormone (GnRH) (Vale et al., 1972b) and human parathyroid hormone (PTH) (Rosenblatt et al., 1977; Rosenblatt et al., 1978) exemplify the former and latter, respectively. In the case of GnRH, systematic deletions of one AA at a time, and of His2 in particular, allowed us to identify His2 as a critical amino acid for receptor activation, but less so for binding affinity. This provided the first lead for the development of GnRH antagonists. Substituting His2 by a D-phenylalanine further improved antagonist potency significantly (Karten and Rivier, 1986). We therefore hypothesized that multiple substitutions might have additive effects such as, in the case of GnRH, increased duration of action, chemical/biological stability in solution (Karten and Rivier, 1986), and reduced toxicity (Karten et al., 1990). These findings were epithomized by the discovery of Fe200486 (Degarelix/Firmagon) (Broqua et al., 2002; Jiang et al., 2001), which blocks gonadotropin secretion upon monthly sc administration, lowers testosterone to castrate levels, and significantly reduces prostate specific antibodies (PSA) within a few days in men with prostate cancer. In the case of PTH, an antagonist resulted from the deletion of six amino acids at the NH2 terminus of the fully active fragment, yielding the antagonist PTH(7–34)-NH2 (Horiuchi et al., 1983) and its long-acting derivative [DTrp12, Tyr34]bPTH(7–34) (Goldman et al., 1988).
C. CRF
1. Receptors
As briefly discussed in the Introduction, in 1982 our laboratory reported the isolation and structure identification of the hypothalamic peptide CRF. This was followed by the report, in 1993, of the primary structure of the CRF receptors type 1 [CRFR1 (Chen et al., 1993)] and in 1995, that of the CRF receptors type 2 [CRFR2 (Perrin et al., 1995)]. With the discovery of these two receptors, the design of non peptide ligands became possible. Two CRF/Ucn receptors, encoded by different genes and existing in multiple forms as splice variants, have currently been cloned: CRFR1 (Chang et al., 1993; Chen et al., 1993; Vita et al., 1993) and CRFR2 (Kishimoto et al., 1995; Kostich et al., 1998; Lovenberg et al., 1995; Perrin et al., 1995; Stenzel et al., 1995). CRFR1 is the predominant receptor type in the pituitary (Chalmers et al., 1995; Potter et al., 1994) and is also widely distributed throughout the central nervous system. The second CRF receptor, called type 2β (CRFR2β), is expressed not only in the rat brain, but also in peripheral tissues such as the heart, gastrointestinal tract, and epididymis (Perrin et al., 1995). Several reviews have appeared that summarize present knowledge about their distribution and respective roles (Bale and Vale, 2004; Broadbear, 2006; Dautzenberg and Hauger, 2002; Farrokhi et al., 2007; Hauger et al., 2006; Hauger et al., 2009; Krohg et al., 2008; Liapakis et al., 2011; Slominski et al., 2006; Smith et al., 1998; Van Pett et al., 2000; Zorrilla and Koob, 2010).
We will now describe, in chronological order, the development of long-acting agonists and antagonists to CRFR1 and CRFR2 and their potential clinical relevance. Specifically, we retrace the rationale behind the discovery of the main peptide CRF agonists and antagonists that became the workhorses for studies of tissue distribution, pharmacology and physiology of CRF and their receptors.
2. Agonists
CRF release during acute stress is essential for the survival of the organism [for ref., see for example (Gold et al., 1987; Habib et al., 2001; Pecoraro et al., 2006; Sapolsky, 2000)]. However, it is now also well documented that CRF released as a result of exposure to chronic stressors can influence emotions (such as fear and anxiety), and exert negative effects on the homeostasis of most physiological functions including the cardiovascular, gastrointestinal, immune and reproductive systems, the gastrointestinal tract, and the skin. Indeed, these findings have provided fascinating and challenging evidence for the links between emotions and disease (Sternberg, 1997). This review highlights thirty years of CRF peptide SAR studies carried out mostly in our laboratories (yielding ca. 1500 analogs, ca. 20 papers on SAR, and at least 15 different compounds for sale by commercial suppliers of reagents). In addition, Spiess and collaborators contributed two papers on CRF SAR, that dealt with the properties of the CRF receptor (Spiess et al., 1998), and the development of CRFR2β-selective anti-sauvagine-30 (Rühmann et al., 1998). Beyermann et al. achieved signaling selectivity of ligands for CRFR1 by modifying the agonist’s signaling domain (Beyermann et al., 2007). Also, using a single-point slight alteration set as a tool for structure-activity relationships, studies of oCRF yielded unique information on the role of each amino acid demonstrating that most analogs, with the exception of the inactive Thr to Ser7, Arg to Lys16, Glu to Asp17 and Gln to Asn34, remained relatively potent (Beyermann et al., 1996). Mazur et al. developed sauvagine analogs selective for CRFR2 while introducing substitutions at positions 35 and 39 (Mazur et al., 2005). Finally, Yamada et al. improved the potencies of the C-terminus 12 AAs of astressin (Yamada et al., 2004) while substituting a number of residues to yield [DAla31,Glu32,Cha38,Asp39]-Ac-Ast(19–30) with a Ki of 3.1 nM as compared to 2.0 nM for astressin.
a) Alpha-helical CRF and α-helical CRF(9–41) (Rivier et al., 1984)
The design of α-helical CRF with increased potency over that of oCRF, sauvagine (Svg) and urotensin I (U1), allowed us to hypothesize that a fragment could be found that would retain high binding affinity for the CRF receptors without causing their activation. Based on Chou and Fassman’s data (Chou and Fasman, 1978; Chou and Fasman, 1987), Montecucchi and Gozzini (Montecucchi and Gozzini, 1982) proposed that sauvagine and CRF assumed a similar pattern of α-helixes and β-turns. Independently, we presented spectroscopic and physicochemical evidence for such a secondary structure for oCRF, rCRF, sauvagine, and U1 (Lau et al., 1983; Pallai et al., 1983). Specifically, we hypothesized that such a feature might be essential to receptor recognition and binding. Using Chou and Fassman predictive and statistical analysis (Chou and Fasman, 1978), we designed and synthesized a 41-residue analog with optimized α-helix formation by introduction of the amino acid with the highest Pα value at positions where the aligned, naturally occurring members of the CRF family had nonidentical residues [see Rivier et al. (Rivier et al., 1984) for additional substitutions] (Table 1). When tested in the RAP cells assay, this analog (named α-helical CRF) was 2–3 times more potent than any of the then known members of the CRF family found to be equally potent in releasing ACTH in vitro (Vale et al., 1981) (Table 2). Knowing that amidation of the COOH-terminal of CRF was critical for potency, we then investigated the effects of systematic deletion of the NH2-terminal amino acids of α-helical CRF on its biopotency in vitro. Most of the intrinsic activity was conserved even after deletion of residues 1 to 6. Deletion of the next three amino acids, however, led to α-helical CRF(9–41) that showed limited partial agonism with low potency when tested in vitro, and inhibited CRF-mediated release of ACTH when tested for antagonism in rats (see Figure 1) (Rivier et al., 1984).
Table 1.
Sequences of CRF family members and analogs
| oCRF | S QEPPISLDLT | FHLLREVLEM | TKADQLAQQA | HSNRKLLDIA-NH2 |
| h/rCRF | S EEPPISLDLT | FHLLREVLEM | ARAEQLAQQA | HSNRKLMEII-NH2 |
| hUcn1 | DNPSLSIDLT | FHLLRTLLEL | ARTQSQRERA | EQNRIIFDSV-NH2 |
| hUcn2 | IVLSLDVP | IGLLQILLEQ | ARARAAREQA | TTNARILARV-NH2 |
| hUcn3 | FTLSLDVP | TNIMNLLFNI | AKAKNLRAQA | AANAHLMAQI-NH2 |
| Sauvagine | UGPPISIDLS | LELLRKMIEI | EKQEKEKQQA | ANNRLLLDTI-NH2 |
| Stressin1 | Ac-PPISLDLT | fHLLREVLEZ | ARAEQLAQQE | HSKRKLZEII-NH2 |
| Alpha-helical CRF | S QEPPISLDLT | FHLLREMLEM | AKAEQEAEQA | ALNRLLLEEA-NH2 |
| Alpha-helical CRF(9–41) | DLT | FHLLREMLEM | AKAEQEAEQA | ALNRLLLEEA-NH2 |
| [DPhe12,Nle21,38]-hCRF(12–41) | fHLLREVLEZ | ARAEQLAQQA | HSNRKLZEII-NH2 | |
| [DPhe12,Nle21,38,Leu(Me)37]-hCRF(12–41) | fHLLREVLEZ | ARAEQLAQQA | HSNRKXZEII-NH2 | |
| Astressin | fHLLREVLEZ | ARAEQLAQEA | HKNRKLZEII-NH2 | |
| Astressin B | Ac-DLT | fHLLREVLEZ | ARAEQXAQEA | HKNRKLZEXI-NH2 |
| Astressin2-B | Ac-DLS | fHXLRKZIEI | EKQEKEKQQA | ENNKLLLDXI-NH2 |
| Anti-Sauvagine-30 | fHLLRKMIEI | EKQEKEKQQA | ANNRLLLDTI-NH2 |
Note: Z = Nle; X = Leu(Me); Ac = acetyl; U = pGlu.
hCRF differs from oCRF in that residues Gln2, Thr22, Lys23, Asp25, Leu38, Asp39, Ala41 in oCRF are substituted by the corresponding Amino Acids Glu2, Ala22, Arg23, Glu25, Met38, Glu39 and Ile41 in hCRF.
Stressin1 differs from hCRF in that residues 1 to 3 in hCRF are deleted and replaced by an acetyl group. In addition, Phe12, Met21, Ala31, Asn34, Met38 in hCRF are substituted by the corresponding DPhe12, Nle21, Glu31, Arg34 and Nle38 in stressin1. Substitution of Met by Nle precludes oxydation of Met to Met (O) therefore adding to overall chemical stability. In addition, a lactam bridge between Glu31 and Lys34 has been formed to lock in a specific turn favorable for CRFR1 selectivity.
Overall rationale in the design of α-helical-CRF was to optimize α-helicity resulting from the substitution of amino acids in oCRF by α-helical inducing residues found in other members of the CRF family (sauvagine, urotensins, hCRF) with greater P values.
α-helical CRF(9–41) is a C-terminal 30 amino acid fragment of α-helical CRF. Other fragments of active linear human sequences include [DPhe12,Nle21,38]-hCRF(12–41) and [DPhe12,Nle21,38,Leu(Me)37]-hCRF(12–41) with known substitutions such as DPhe12,Nle21,Nle38 and CαMeLeu37 that increased potency in the rat anterior pituitary cells in culture (RAP) assay.
Astressin differs from [DPhe12,Nle21,38]-hCRF(12–41) in that a lactam bridge encompassing residues -Glu30-Ala-His-Lys33- was introduced in lieu of -Gln30-Ala-His-Ser33- in the parent [DPhe12,Nle21,38]-hCRF(12–41).
Astressin B, a non-selective, potent, long-acting antagonist, differs from astressin, a nonselective, potent, short-acting antagonist, in that astressin B was elongated at the N-terminal by Ac-Asp-Leu-Thr that may confer resistance to amino peptidases and CαMeLeu27,40 that may confer additonal conformational stability.
Astressin2-B, an 8–40 fragment of sauvagine and a CRFR2 selective potent, long-acting antagonist, differs from sauvagine in that residues Leu12, Leu14, Met18, Ala32, Arg35, Thr40 (CRF numbering) are substituted by the corresponding DPhe12, CαMeLeu14, Nle18, Glu32, Lys35, CαMeLeu40. We hypothesized that positioning of the lactam bridge (Glu32, Xaa, Xbb, Lys35] is in part responsible for CRFR2 selectivity.
Anti-sauvagine-30 differs from sauvagine (12–41) (CRF numbering) in that Leu12-Glu13 are substituted by DPhe12-His13.
Table 2.
Binding affinities and in vitro relative potencies
| hCRF R1 EC50 nM |
mCRF R2β EC50 nM |
Relative Potenciesa,b RAP assay |
|
|---|---|---|---|
| oCRFa | 1.2 (0.9 – 1.6) (Rivier et al., 2007) | 52 (21 – 128) (Rivier et al., 2007) | 1.0 |
| r/hCRFa | 1.0 (0.2 – 4.6) (Rivier et al., 2007) | 6.2 (2.0 – 19) (Rivier et al., 2007) | 1.0 (Gulyas et al., 1995) |
| hUcn 1a | 01. (0.1 – 0.2)* | 0.5 (0.3 – 0.7)* | ND |
| hUcn 2a | >100 (Lewis et al., 2001) | 0.5 (0.2 – 1.2) (Lewis et al., 2001) | ND |
| hUcn 3a | >100 (Lewis et al., 2001) | 13.5 (9.2 – 19.7) (Lewis et al., 2001) | ND |
| Sauvaginea | 0.9 (0.5 – 1.7) (Rivier et al., 2002) | 1.6 (0.7 – 3.8) (Rivier et al., 2002) | ND |
| Stressin1a | 1.7 (0.5 – 6.2) (Rivier et al., 2007) | 222 (137 – 361) (Rivier et al., 2007) | 1.1(0.53 – 2.2) (Rivier et al., 2007) |
| α-helical CRF(1–41)a | 2.5 (1.1 – 5.5)* | 0.4 (0.3 – 0.6) | 2.0 – 3.0 (Rivier et al., 1984) |
| α-helical CRF(9–41)b | 19 (5.5 – 66) (Rivier et al., 2002) | 1.1 (1.0 – 1.3) (Rivier et al., 2002) | 0.03(0.02–0.05) (Gulyas et al., 1995) |
| [DPhe12,Nle21,38]-hCRF(12–41)b | 19.2 (13.4 – 27.5) (Rivier et al., 2002) | 4.4 (2.0 – 9.6) (Rivier et al., 2002) | 1.0 (Gulyas et al., 1995) |
| [DPhe12,Nle21,38,CαMe Leu37]-hCRF(12–41)b | 2.7 (1.2–5.9)* 7.7 (6.0–9.8) |
2.8 (2.0–3.9)* 4.9 (3.7–6.5) |
1.0 (0.5 – 2.0) (Hernandez et al., 1993) |
| Astressinb | 0.7 (0.3 – 1.8) (Rivier et al., 2002) | 0.6 (0.5 – 0.8) (Rivier et al., 2002) | 32.5(12–82) (Gulyas et al., 1995) |
| Astressin Bb | 0.3 (0.1 – 0.8)* | 1.2 (0.9 – 1.6)* | 1.1 (0.50 – 2.5) (Rivier et al., 1999) |
| Astressin2-Bb | >500 (Rivier et al., 2002) | 1.3 (1.0 – 1.7) (Rivier et al., 2002) | ND |
| Anti-sauvagine 30b | 400 (Rivier et al., 2002) | 1.1 (0.6 – 2.2) (Rivier et al., 2002) | ND |
CRF agonists were tested in an in vitro assay measuring stimulation of ACTH release by rat anterior pituitary cells in culture (RAP assay) when using hCRF or oCRF as the standard compound (Gulyas et al., 1995; Rivier et al., 2007).
CRF antagonists were tested measuring alteration of CRF-induced release of ACTH by rat anterior pituitary cells in culture relative to that of [DPhe12,Nle21,38]-h/rCRF(12–41) as the standard compound (Hernandez et al., 1993).
Unpublished data; assay carried out by C. Miller under the supervision of Dr. M. Perrin.
Figure 1.
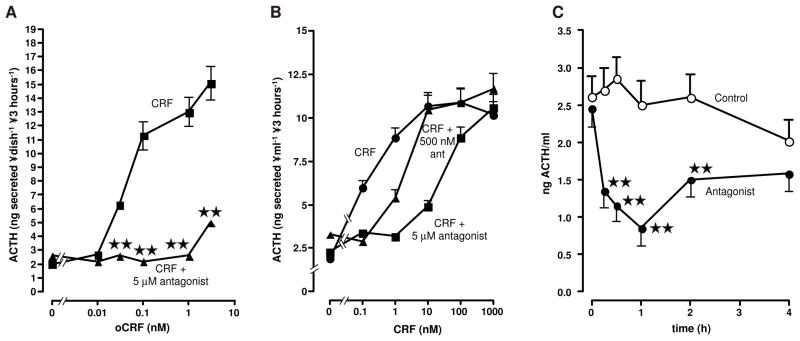
(A) Interaction between 1 nM CRF (■) and increasing doses of α-helical CRF(9–41) (▲) on ACTH secretion by rat anterior pituitary cells in monolayer culture. (B) Effect of increasing doses of CRF (●) on ACTH release in the presence of 500 nM (▲) or 5 μM (■) α-helical CRF(9–41) on ACTH secretion by rat anterior pituitary cells in monolayer culture. (C) Effect of the iv injection of the vehicle (○) or 1 mg (0.6 μmole/kg) α-helical CRF(9–41) (●) on ACTH release by non-stressed, adrenalectomized male rats. Each point represents the mean + SEM of 6 animals. **, P<0.01 vs. vehicle. Results reproduced from (Rivier et al., 1984) by permission.
b) α-helical CRF(9–41): Additional in vivo studies (Fisher et al., 1991)
Studies were then performed in conscious unrestrained rats to compare the ability of the CRF receptor antagonist α-helical CRF(9–41) to inhibit the actions of CRF in three in vivo bioassay systems. When both peptides were administered intracerebroventricularly (icv), an antagonist:agonist ratio between 6:1 and 12:1 was required to abolish CRF-induced elevations of plasma catecholamine levels. When both peptides were administered intravenously (iv), CRF-induced hypotension and tachycardia were completely prevented by an antagonist:agonist ratio of 6:1, while total blockade of CRF-induced elevations of plasma ACTH and β-endorphin levels required an antagonist:agonist ratio of 3,000:1. These results demonstrated a marked difference in the ability of α-helical CRF(9–41) to antagonize various biological actions of CRF, and supported the existence of multiple CRF receptor subtypes. The effectiveness of blocking CRF receptors within the brain was further illustrated by the ability of icv infusion of relatively low doses of α-helical CRF(9–41) to block the central effect of CRF (Lee and Sarna, 1997), or that of swim stress (Coskun et al., 1997) on gastric emptying, as well as stress-induced fighting (Tazi et al., 1987).
c) Ala scan of oCRF (Kornreich et al., 1992)
As mentioned earlier, there are precedents for the use of peptide alanine scans in order to gain an appreciation of the critical role played by certain amino acids side chains in a bioactive peptide (Kornreich et al., 1992). oCRF analogs that are monosubstituted by Ala have therefore been synthesized that were from 4.5 times more potent, to <0.001% as potent as native oCRF. The Ala substitutions which showed the greatest loss of potency (<1% of oCRF), consisted in replacing hydrophobic residues ([Ile6], [Leu8], [Leu14], and [Leu38]), while the Ala analogs which showed the greatest increase in potency were obtained by replacing hydrophilic residues ([Thr22] and [His32]). The next, and yet unproven, step will be to identify whether some or all of the recognized, beneficial substitutions can be introduced in the same molecule, and be additive in generating potent analogs. As deletion of the first 12 residues will generate peptides that are competitive antagonists, and as all Ala substitutions that yielded more potent analogs than oCRF are beyond residue 19, CRF antagonists that incorporate selected Ala substitutions, might become useful to treat CRF-dependent pathologies in the periphery.
d) Single point D-substitution of oCRF (Rivier et al., 1993)
oCRF analogs with monosubstitutions by D-amino acids were similarly synthesized and found to be between two times as potent, to less than 0.005% as potent as CRF (Rivier et al., 1993). Out of 37 analogs in this series, three (DPhe12, DGlu20 and DAla24) were equipotent to or twice as potent as oCRF, twelve (DLeu10, DLeu15, DGlu17, DVal18, DAsp25, DGln26, DLeu27, DAla28, DGln29, DHis32, DSer33, and DAsp39) had relative potencies in the range from 10 to 60% that of oCRF, twenty-one (DPro5, DIle6, DSer7, DLeu8, DAsp9, DThr11, DHis13, DLeu14, DArg16, DMet21, DThr22, DLys23, DGln30, DAla31, DAsn34, DArg35, DLys38, DLeu37, DLeu38, DLeu40, and DAla41) had potencies ranging from inactive to 10% of that of CRF. From this series, we identified the DPhe12 substitution used in further improved agonists and antagonists. Indeed, increased potencies of these molecules in vitro led to increased duration of action of both agonists and antagonists (Figures 3 and 4) (Rivier et al., 1993). While such systematic studies lack the luster of hypothesis-directed research, it is still the most efficient approach to identify which residues within the sequence of a biologically active peptide are most sensitive to alteration.
Figure 3.
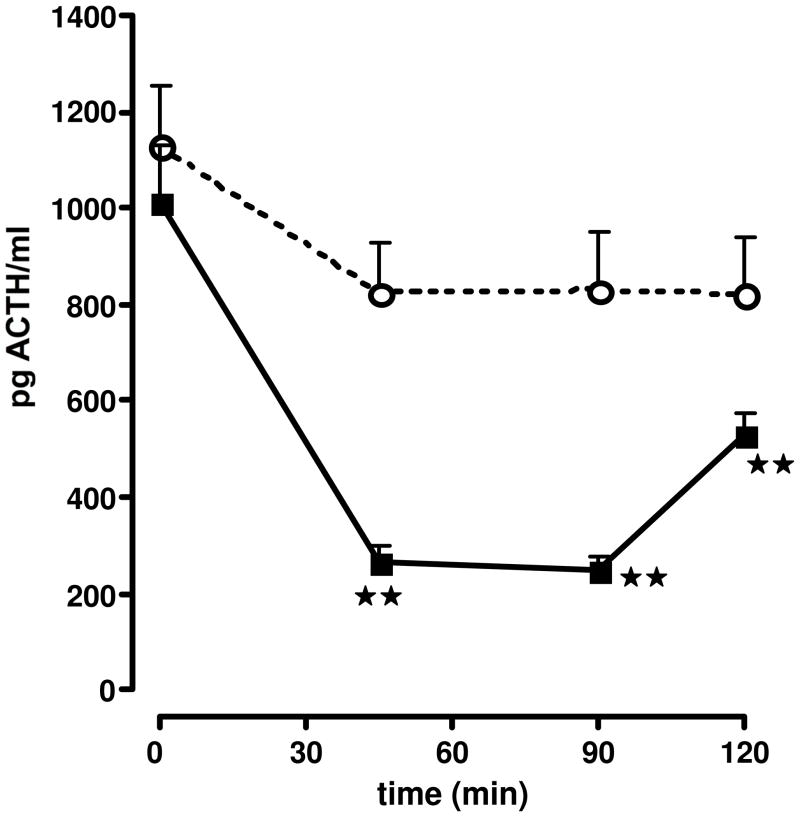
Effect of the iv injection of the vehicle ○ or astressin ■, diluted in aqueous solvent, on ACTH secretion by non-stress adx male rats. Each point represents the mean + SEM of 5–6 animals. **, P<0.01 vs. vehicle. Results reproduced from (Gulyas et al., 1995) by permission.
Figure 4.
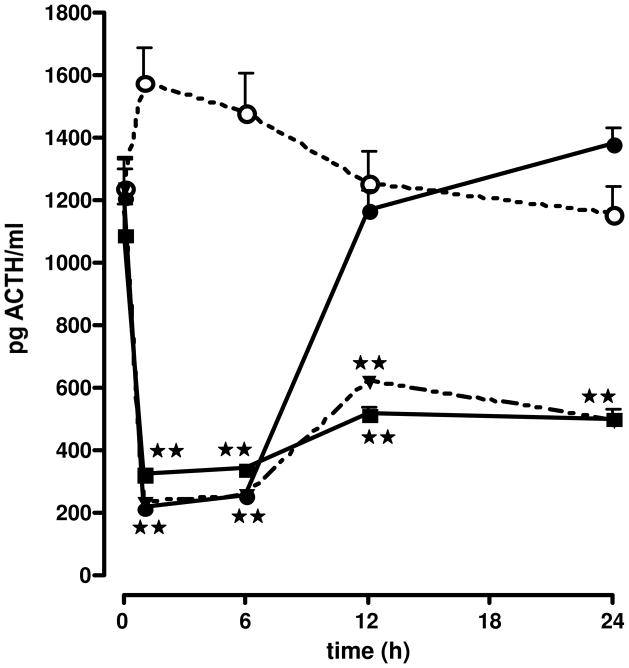
Effect of the injection of the vehicle ○ or astressin B (●, 400 μg/kg iv in aqueous solvent; ▼, 1.2 mg/kg sc in aqueous solvent; ■, 120 μg/kg sc in oil), on ACTH secretion in non-stressed adx male rats. Each point represents the mean + SEM of 5–6 animals. **, P<0.01 vs. vehicle. Results reproduced from (Rivier et al., 1999) by permission.
e) [DPhe12,Nle21,38,CαMeLeu37]-r/h CRF(12–41) CαMeLeu and Aib partial scan (Hernandez et al., 1993)
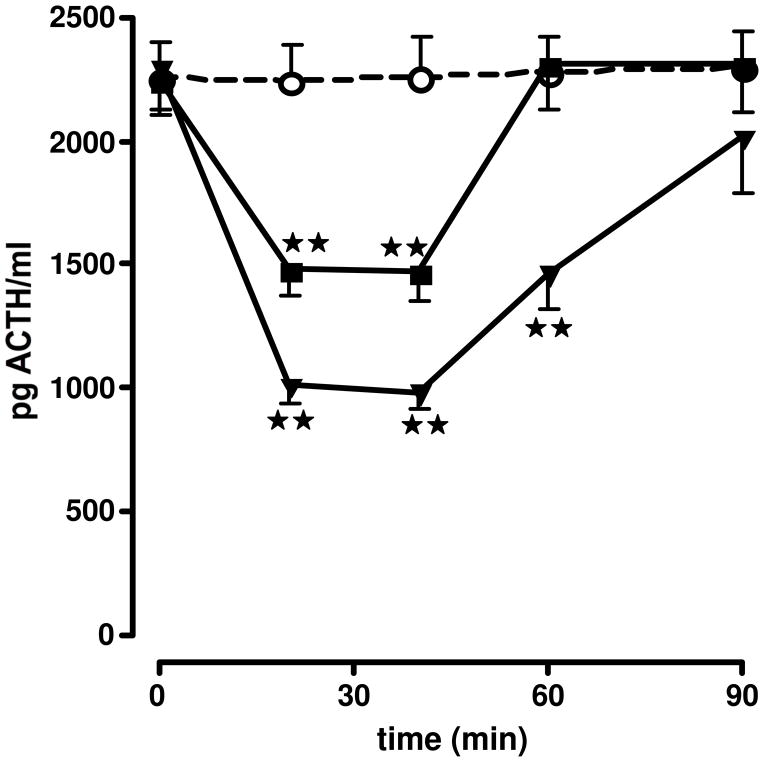
The strategy behind the design of [DPhe12,Nle21,38,CαMeLeu37]-r/h CRF(12–41) (also referred to as DPhe12 CRF in the literature; Table 1) was to introduce in the same molecule, substitutions known to improve in vitro potency [i.e., DPhe12 was identified from a D-AA scan of oCRF (Rivier et al., 1993)]. Of interest was the fact that the CαMeLeu substitution did not bring detectable conformational changes in the expected way (i.e., stabilization of helical structure as monitored by CD). On the other hand, introduction of NαMe amino acids resulted in drastic conformational changes (loss of α-helicity) and biological activity. We used DPhe12 CRF as a standard in our in vitro RAP assay, and >20 publications showing its inhibitory activity and potency resulted from the use of this analog (Baram et al., 1996; Chen et al., 2012; Curtis et al., 1994; Curtis et al., 1997; Hernandez et al., 1993; Jones et al., 1999; Lowery and Thiele, 2010; Macey et al., 2000; Martinez et al., 1999; Menzaghi et al., 1994; Rodriguez de Fonseca et al., 1996; Tache and Brunnhuber, 2008; Zislis et al., 2007). Nle at positions 21,38 to replace the Methionines conferred stability against oxidation. In addition, we scanned the sequence of [DPhe12,Nle21,38]-r/h CRF(12–41) with CαMeLeu at positions 14, 15, 37 and Glu17 or Aib at positions 20, 22, 24, 26, 28, 30, 31, 32, 33, 34, 39, and 40, respectively. Most analogs retained relative potencies close to that of DPhe12 CRF with the exception of Aib26. In addition, we found that CαMeLeu37 increased local helicity and enzymatic stability. As illustrated in Figure 2, the combined substitutions yielded the first antagonist that inhibited ACTH secretion in vivo for more than one hour at the doses tested. This antagonist was also tested in a variety of other in vivo systems. For example, the central administration of [DPhe12,Nle21,38,CαMeLeu37]-r/h CRF(12–41) antagonized defensive behavior responses to swim stress (Fonseca et al., 1996), and prevented gastroparesis-induced by surgery (Barquist et al., 1996; Hernandez et al., 1993). [For a more complete discussion see (Stengel and Tache, 2010; Tache et al., 2001) for blockade of visceral responses to stress, and for blockade of various stress-related behaviors, see (Koob, 2008b; a).]
Figure 2.
Effect of the iv injection of the vehicle ○, [DPhe12,Nle21,38]h/rCRF12–41 ■ or [DPhe12,Nle21,38,CαMeLeu37]h/rCRF12–41 ▼, diluted in aqueous solvent, on ACTH secretion in non-stressed adx male rats. Each point represents the mean + SEM of 5–6 animals. **, P<0.01 vs. vehicle. Results reproduced from (Hernandez et al., 1993) by permission.
The next contribution from our laboratory was to increase in vivo and in vitro potencies through the introduction of backbone constraints generated by the use of side chain to side chain bridging, thus constraining the tertiary structure.
f) Cyclo(30–33)[DPhe12,Nle21,38,Glu20,Lys23/Orn23]-r/h CRF(12–41) (Miranda et al., 1994)
With limited literature reference as to where to position lactam bridges as well as their size and chirality, we systematically addressed several hypotheses related to the optimal bridging configuration. First, can bridgeheads be located at positions where a D-residue is allowed, provided that bridge length, chirality, and dipole direction are optimized? Second, can we suggest that any improvement in receptor affinity is mediated through stabilization of an α- helical structure, a hypothesis that is compatible with the observation that all of these biologically active structures can assume an α-helical conformation in the presence of TFE? Third, by assuming an α-helical bioactive structure for CRF, will a simple predictive tool based on Cα-Cα distances in an ideal polyalanine α-helical model be developed and used with some predictive value? We found an analog with a cycle centered around residue Glu20 ([cyclo(20–23)[DPhe12,Glu20,Lys23,Nle21,38]h/rCRF(12–41)]) with an in vitro potency slightly greater than that of DPhe12 CRF. This suggested that restricted conformational freedom is compatible with strong antagonist potency (Miranda et al., 1994).
g) Cyclo(30–33)[DPhe12,Nle21,38,Glu30,Lys33]-r/h CRF(12–41) (astressin) (Gulyas et al., 1995)
It took several additional trials before we identified the cyclo (Glui-Lysi+3), and not (Lysi-Glui+3), as the best ring size and composition (Gulyas et al., 1995; Miranda et al., 1994) for side chain stabilization, at a time when published work used mostly cyclo(Xaai-Xbbi+4) scaffolds (Grace et al., 2007a). The discovery of astressin with increased in vitro potency (32.5 times that of DPhe12CRF or DPhe12CαMeLeu37, RAP assay (Table 2) and binding affinity to both CRFR1 and CRFR2 over that of [DPhe12,Nle21,38]-r/h CRF(12–41) and [DPhe12,Nle21,38,CαMeLeu37]-r/h CRF(12–41) (Table 2), resulted from constraining the structure of [DPhe12,Nle21,38]-r/h CRF(12–41) further through the introduction of a lactam bridge (see Table 1 and below). We then chose to scan the whole sequence of the CRF antagonist [D-Phe12,Nle21,38]r/hCRF(12–41) with an i-(i + 3) bridge consisting of the Glu-Xaa-Xbb-Lys lactam scaffold. We found the nonselective CRFR1 and CRFR2 antagonist astressin {cyclo(30–33) [DPhe12,Nle21,38,Glu30,Lys33] r/hCRF(12–41)} to be approximately 30 times more potent than [DPhe12,Nle21,38]r/hCRF(12–41) see Table 2, and 300 times more potent than the corresponding linear analog in an in vitro RAP assay. When we used radioiodinated [D-125I-Tyr12]astressin (CRF numbering) as a reliable ligand for binding assays, we observed that astressin had low affinity for the CRF binding protein (Behan et al., 1995) and high affinity (Ki = 2 nM) for the cloned pituitary receptor. In vivo, astressin (Figure 3) was found significantly more potent than any previously tested antagonists in reducing ACTH secretion in stressed adrenal-intact, as well as non-stressed adrenalectomized rats, and completely blocked the inhibitory effect of CRF and other members of the CRF family on gastric emptying (Martinez et al., 1998). The observation that by contrast, the CRFR1-selective antagonist NBI-27914 was ineffective in this system, suggested the participation of CRFR2 receptors in mediating gastric empyting. The cyclo(30–33) [Ac-Pro4,DPhe 12,Nle21,38,Glu30,Lys33]r/hCRF(4–41) agonist and its linear analog are nearly equipotent, while the antagonist astressin and its linear form vary greatly in their potencies. This suggests that the 30–33 lactam cyclization reinstates a structural constraint in the antagonists that is normally induced by the N terminus of the agonist.
Prior generations of CRF antagonists had been administered at high concentrations in the central nervous system, and shown to effectively blunt endogenous CRF actions; however, antagonists that would be potent and long acting on ACTH secretion were still lacking. As illustrated above, astressin was a significant improvement over previously available CRF antagonists due to its high potency, low intrinsic activity, high receptor affinity, and high solubility in neutral aqueous solutions. Because astressin is effective at low doses, the impact of its limited intrinsic activity at high concentrations in vitro may be of little consequence in vivo. Also, the availability of a high affinity antagonist radioligand has allowed further advances in receptor pharmacology (Perrin et al., 1999). These results illustrated the role that secondary and tertiary structures may play in controlling biological signaling through protein-ligand interactions. To our knowledge, there is no documented evidence that such long distance induction/stabilization (imparted by residues 4–11) of α-helix formation could be restored by a single bridging element 20 residues down the sequence, upon deletion of these residues. We have shown that Glu/Lys i-(i + 3) and i-(i + 4) lactam bridges may impart the proper geometry for the stabilization of an α-helical backbone (Miranda et al., 1994).
h)Cyclo(30–33)[DPhe12,Nle21,38,MeLeu27,40,Glu30,Lys33]-Ac-r/h CRF(9–41) (astressin B) (Rivier et al., 1998)
Next, we identified specific modifications and substitutions of CRF that led to the discovery of antagonists with extended duration of action as compared to that of astressin (Rivier et al., 1998). These additional modifications included elongation of the peptide chain by three residues at the N-terminus, its acetylation, and the [CαMeLeu37] substitution to yield cyclo(30–33)[DPhe12,Nle21,Glu30,Lys33,CαMeLeu37,Nle38]Ac-hCRF(9–41). To further increase the efficiency (potency, duration of action, and bioavailability) of this family of antagonists, we introduced two or more CαMe-leucine residues at positions shown in earlier studies to be favorable (Hernandez et al., 1993). Whereas the introduction of CαMe-leucine residues at positions 27 and either 18, 37, or 40 resulted in dramatic increases in duration of inhibitory action in adx rats after iv injection, the same substitution at positions 27 and either 15, 17, 19, or 41 led to short acting analogs. Other substitutions by CαMeLeu at positions 27 and either 10, 13, 14, 21, 24, 36, or 38, yielded analogs with duration of action intermediate between those mentioned above. Cyclo(30–33)[DPhe12, Nle21,CαMeLeu27,Glu30,Lys33,Nle38,CαMeLeu40]Ac-hCRF(9–41) (astressin B) was one of the most efficacious analogs of this series (>4 h inhibition of ACTH secretion at 25 μg/adx rat). It was found to be even longer acting via subcutaneous (sc) administration in either an aqueous (>24 h inhibition of ACTH secretion at 100 μg/adx rat) or lipid milieu (DMSO/peanut oil, >24 h inhibition of ACTH secretion at 30 μg/adx rat) than after iv administration (<12 h inhibition of ACTH secretion at 100 μg/adx rat) (Figure 4). The effectiveness of astressin B was also demonstrated in other systems, such as its ability to significantly interfere with CRF-induced ACTH release in the Rhesus monkey (Broadbear et al., 2004), the prevention of CRF-induced changes in intestinal permeability in the isolated ileum (Overman et al., 2012), or intestinal barrier dysfuntion caused by early weaning in piglets (Smith et al., 2010). These results demonstrated the efficacy of this antagonist in a variety of stress-induced conditions, as well as across species. We concluded that Cα-methylation at some positions may favor a bioactive conformation while also preventing degradation and/or elimination, resulting in significant extension of duration of action.
i) Short analogs of astressin (Yamada et al., 2004)
An important contribution was made by Yamada et al. (Yamada et al., 2004), who discovered that short C-terminus fragment 19–30 of astressin (astressin numbering) retained high binding affinity. Additional SAR studies of that peptide led to improved binding affinity. The authors assumed that a particular surface of the alpha-helix was important for binding to the receptor. The small peptide containing DAla31, Asn34 and cyclohexylalanine (Chx38) on that surface as well as Glu32 and Asp39 on an adjacent surface was as potent as astressin in binding to the CRF receptor and showed significant ACTH suppression when administered to rats.
j) Astressin2-B (Rivier et al., 2002), anti-sauvagine 30 (Rühmann et al., 1998)
We then reported that moving the lactam bridge by two residues toward the C-terminus, led to CRFR2 selectivity (astressin2-B) (Rivier et al., 2002) (Tables 1 and 2), and that therefore members of the CRF family assumed distinct structures when interacting with the CRF1 and CRF2 receptors. Predictive methods, physicochemical measurements, and structure-activity relationship studies have suggested that CRF, its family members, and competitive antagonists such as astressin {cyclo(30–33)-[DPhe12,Nle21,Glu30,Lys33,Nle38]hCRF(12–41)}, assume an α- helical conformation when interacting with their receptors (Grace et al., 2010; Grace et al., 2007b; Koerber et al., 1998). As indicated earlier, we had shown that α-helical CRF(9–41) and sauvagine exhibited some selectivity for CRF receptors other than that responsible for ACTH secretion, and later for CRF2 binding (Perrin et al., 1999). We then suggested the possibility of a helix-turn-helix motif around a turn encompassing residues 30–33 (Koerber et al., 1998), that would confer high affinity for both CRF1 and CRF2 (Perrin et al., 1999; Rühmann et al., 1998) in agonists as well as antagonists of all members of the CRF family (Koerber et al., 1998). On the other hand, the substitutions that conferred ca. 100-fold CRF2 selectivity to the antagonist antisauvagine- 30 {[DPhe11,His12]-sauvagine(11–40)}, did not confer such property to the corresponding N-terminally extended agonists. We observed that a Glu32-Lys35 side chain to side chain covalent lactam constraint in hCRF and the corresponding Glu31-Lys34 side chain to side chain covalent lactam constraint in sauvagine, yielded potent ligands that were selective for CRF2. Additionally, we introduced deletions and substitutions known to increase duration of action to yield antagonists such as cyclo(31–34)[DPhe11,His12,CαMeLeu13,39,Nle17,Glu31,Lys34]Ac-sauvagine(8–40) (astressin2-B) with CRF2 selectivities greater than 100-fold. CRF receptor autoradiography was performed in rat tissue known to express CRF2 and CRF1 in order to confirm that astressin2-B could indeed bind to established CRF2, but not CRF1 receptor-expressing tissues. Extended duration of action of astressin2-B versus that of anti-sauvagine-30 was also demonstrated in the CRF2-mediated animal model, whereby the inhibition of gastric emptying of a solid meal in mice by urocortin administered intraperitoneally at time zero was antagonized by the administration of astressin2-B but not by anti-sauvagine-30 at times -3 and -6 h. Both peptides were effective when given 10 min before urocortin (Martinez et al., 1998).
k) Stressin1 (Rivier et al., 2007)
As described above, the potencies and selectivity of peptide CRF antagonists is increased through structural constraints, suggesting that the resulting ligands assume distinct conformations when interacting with CRF1 and CRF2 receptors. To develop selective CRF receptor agonists, we have scanned the sequence -Gln-Ala-His-Ser-Asn-Arg-(residues 30–35 of [DPhe12,Nle21,38]Ac-hCRF(4–41)) with an i-(i+3) bridge consisting of the Glui-Xaa-Xbb-Lysi+3 scaffold, where residues i = AA30, AA31, and AA32. When i = AA31, stressin1, a potent CRF1 receptor-selective agonist was generated (Table 1). In vitro (RAP assay), stressin1 was equipotent to h/rCRF to release ACTH (Table 2). Astressin1 showed a low nanomolar affinity for CRF1 receptor (Ki = 1.7 nM) and greater than 100-fold selectivity versus CRF2 receptor (Ki = 222 nM). Stressin1 released slightly less ACTH than oCRF in adult adrenal-intact male rats, with increased duration of action. Interestingly, stressin1, injected intraperitoneally in rats, induced fecal pellet output (a CRF1 receptor-mediated response) and did not influence gastric emptying and blood pressure (CRF2 receptor-mediated responses).
D. Pharmacological use of long acting peptide CRF antagonists
As discussed above, CRF receptors are present in many peripheral organs, including the heart, the GI tract, the skin and the gonads, as well as in specialized cells of the immune system. It is therefore reasonnable to predict that a variety of stress-related pathologies might be alleviated by CRF antagonists. Indeed, many investigators have used CRF antagonists in their own system, and collectively this work suggests that these analogs have an important potential as clinical tools. What follows is a short list, without discussion, of some of these findings.
Tache et al. have suggested a role for CRF in functional gastrointestinal disorders (Tache et al., 2009), and for CRF antagonists as a potential drugs for future therapies in gastroenterology (Taché, 2004). More specifically, these investigators have emphasized the role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function and integrities (Taché and Perdue, 2004). Independently, Risbrough et al. suggest that CRF receptors exert both additive and opposing influences on startle defensive behavior (Risbrough et al., 2004). Moeser et al. reported that early weaning stress impairs development of mucosal barrier function in the porcine intestine (Smith et al., 2010), while CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha (Overman et al., 2012). Ferrin et al. showed that astressin B prevents the inhibitory effect of ghrelin on luteinizing hormone pulse frequency in the ovariectomized Rhesus monkey (Vulliémoz et al., 2008), while the nonpeptidic antagonist Antalarmin show differing profiles of activity in Rhesus monkeys (Broadbear et al., 2004).
In humans, mood disorders such as depression are associated with significant pathological changes in the activity of the HPA axis [see for example (Dinan, 2001; Kandel, 1999; Keller et al., 2006; Slattery et al., 2004; Tichomirowa et al., 2005)]. Consequently, it was hypothesized that compounds that would block endogenous CRF activity in the brain might have interesting and useful anti-depressant activity. As a result of years of design of CRF ligands, several of these peptides have become extremely useful reagents in defining the complex role of CRF’s family members (Table 1). However, except for α–helCRF(9–41), no CRF peptide antagonist has yet been tested in humans (Fukudo, 2007; Fukudo et al., 1998). We explain this lack of interest by academia for peptide ligands to CRFRs, by the complexity of the syntheses and the significant costs associated with the use of a quantitative in vitro assay. While the peripheral administration of peptide CRF analogs offers clinical opportunities listed in part above, it is important to note that these antagonists are not able to penetrate the brain, and therefore do not access CRF neurons in the CNS. An alternate approach would be to use nasal administration to circumvent the blood-brain barrier (BBB) (Bethlehem et al., 2012; Zhu et al., 2012), an approach that has been successfully used for the delivery of oxytocin to patients with schizophrenia (Berardis et al., 2013) and fear extinction deficit (Acheson et al., 2013). On the other hand, the clinical potential of non peptidic CRFR1 antagonists that would be orally active and would penetrate the BBB, has not escaped the pharmaceutical industry, which has pursued the quest for such small molecules very energetically. However to date, non peptidic antagonists capable of targeting the CNS following peripheral administration, have not yet proven clinically useful (Griebel and Holsboer, 2012).
E. Conclusion
The purpose of this brief review was to describe the history of the development of CRF peptidic antagonists, including the rationale used for the synthesis of long-acting analogs. In view of the presence of CRF receptors in many organs, it seems reasonable to predict that these compounds may play a major role in the treatment of chronic stress-induced pathologies in the periphery. Indeed, there is animal (Chatzaki et al., 2013; Moeser et al., 2007; Smith et al., 2009) and clinical (Fukudo, 2007; Fukudo et al., 1998) evidence for the potential of CRF peptide antagonists for the treatment of gastrointestinal dysfunctions such as irritable bowel syndrome, as well as disorders of the skin (Slominski et al., 2013) and reproductive system (Xiao et al., 2007). Additionally, if one can develop intranasal modes of delivery, it is possible that CRF antagonists may also become useful in the treatment of brain pathologies such as post-traumatic stress syndrome and depression. Obviously much remains to be done in this regard, but mounting evidence for the involvement of endogenous CRF in a variety of stress-releated diseases, suggests that developing means to block the receptors that mediate the effects of this peptide, may address as yet unmet clinical needs.
Highlights.
CRFs are mediators of gut, heart, skin, immune and CNS homeostasis
First antagonist α-helical CRF(9–41) is potent in the brain, less though in the periphery
CRF antagonists will likely play a major role in stress-related diseases
Astressin2-B was designed to be a CRFR2 selective long acting antagonist
Stressin1 was designed to be a CRFR1selective long acting agonist
Acknowledgments
The work described was supported by Award Number P01 DK026741 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. J.E.R. is the Dr. Frederik Paulsen Chair in Neurosciences Professor.
I, Jean Rivier, first met Wylie Vale at Rice University in Houston (Texas) for a game of tennis in August 1969. To tell the truth, our skills did not match our aspirations. Wylie’s tenacity (and the weather) generally decided the outcome. A year later, we had moved to the Salk Institute in La Jolla and benefitted from Wylie’s intimate knowledge of the American way of life, which Catherine and I quickly embraced (he rented a house, we rented an apartment on the beach; he bought a house and so did we). Betty and he, and Catherine and I, also started a family around the same time. On the work front, we quickly learned to collaborate in the laboratory in a way that respected each other’s boundaries: for him it was whole animal physiology and neuroendocrinology, while I developed analytical, synthetic and structural peptide expertise. Our combined skills led to fascinating results. I miss a friend, a colleague, and the challenges he would bestow upon the members of the peptide biology laboratories
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson D, Feifil D, de Wilde S, Mckinney R, Lhor J, Risbrough V. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in healthy human sample. Psychopharmacology. 2013;229:199–208. doi: 10.1007/s00213-013-3099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T, Vale W. CRF and CRF receptors: Role in stress responsivity and other behaviors. In: Cho AK, editor. Annual Review of Pharmacology and Toxicology, Annual Reviews. 2004. pp. 525–557. [DOI] [PubMed] [Google Scholar]
- Barquist E, Bonaz B, Martinez V, Rivier J, Zinner M, Taché Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am J Physiol. 1996:R888–R894. doi: 10.1152/ajpregu.1996.270.4.R888. [DOI] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: A novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Berardis Dd, Marini S, Iasevoli F, Tomassetti C, Bartolomeis Ad, Mazza M, Valchera A, Fornaro M, Cavuto M, Srinivasan V, Sepede G, Martinotti G, Giannantonio Md. The role of intranasal oxytocin in the treatment of patients with schizophrenia: A systemic review. CNS Neurol Disord Drug Targets. 2013;12:252–264. doi: 10.2174/1871527311312020012. [DOI] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: A review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Beyermann M, Fechner K, Furkert J, Krause E, Bienert M. A single-point slight alteration set as a tool for structure-activity relationship studies of ovine corticotropin releasing factor. J Med Chem. 1996;39:3324–3330. doi: 10.1021/jm960116z. [DOI] [PubMed] [Google Scholar]
- Beyermann M, Heinrich N, Fechner K, Furkert J, Zhang W, Kraetke O, Bienert M, Berger H. Achieving signalling selectivity of ligands for the corticotropin-releasing factor type 1 receptor by modifying the agonist’s signalling domain. Br J Pharmacol. 2007;151:851–859. doi: 10.1038/sj.bjp.0707293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH. Corticotropin-releasing hormone in nonhuman primates. Front Biosci. 2006;11:2303–35. doi: 10.2741/1972. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Rivier JE, Rice KC, Woods JH. Corticotropin-releasing hormone antagonists, astressin B and antalarmin: differing profiles of activity in rhesus monkeys. Neuropsychopharmacology. 2004;29:1112–21. doi: 10.1038/sj.npp.1300410. [DOI] [PubMed] [Google Scholar]
- Broqua P, Riviere P, Conn PM, Rivier JE, Aubert MK, Junien JL. Pharmacological profile of a new, potent and long-acting gonadotropin-releasing hormone antagonist: Degarelix. J Pharmacol Exp Ther. 2002;301:95–102. doi: 10.1124/jpet.301.1.95. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, DeSouza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CP, Pearse RV, II, O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- Chatzaki E, Anton PA, Million M, Lambropoulou M, Constantinidis T, Kolios G, Tache Y, Grigoriadis DE. Corticotropin-releasing factor receptor subtype 2 in human colonic mucosa: down-regulation in ulcerative colitis. World J Gastroenterol. 2013;19:1416–23. doi: 10.3748/wjg.v19.i9.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin releasing factor (CRF) receptor. Proc Natl Acad Sci USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou PY, Fasman GD. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Chou PY, Fasman GD. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol. 1987;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Coskun T, Bozkurt A, Alican I, Ozkutlu U, Kurtel H, Yegen BC. Pathways mediating CRF-induced inhibition of gastric emptying in rats. Regul Pept. 1997;69:113–20. doi: 10.1016/s0167-0115(96)02066-6. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Dinan TG. Novel approaches to the treatment of depression by modulating the hypothalamic-pituitary-adrenal axis. Human Psychopharmacol Clin Exper. 2001;16:89–93. doi: 10.1002/hup.188. [DOI] [PubMed] [Google Scholar]
- Farrokhi CB, Tovote P, Blanchard RJ, Blanchard DC, Litvin Y, Spiess J. Cortagine: behavioral and autonomic function of the selective CRF receptor subtype 1 agonist. CNS Drug Rev. 2007;13:423–43. doi: 10.1111/j.1527-3458.2007.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Rivier C, Rivier J, Brown M. Differential antagonist activity of α-helical CRF(9–41) in three bioassay systems. Endocrinology. 1991;129:1312–1316. doi: 10.1210/endo-129-3-1312. [DOI] [PubMed] [Google Scholar]
- Fonseca FRd, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob G, Navarro M. Corticotropini-releasing factor (CRF) antagonist [D-Phe12, Nle21,38, CαMeLeu37]CRF attenuates the acute actions of te highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996;276:56–64. [PubMed] [Google Scholar]
- Fujino M, Shinagawa S, Yamazaki I, Kobayashi S, Obayashi M, Fukuda T, Nakayama R, White WF, Rippel RH. [Des-Gly-NH210, Pro-ethylamide9]-LH-RH: A highly potent analog of luteinizing hormone releasing hormone. Arch Biochem Biophys (Communication) 1973;154:488–489. doi: 10.1016/0003-9861(73)90083-0. [DOI] [PubMed] [Google Scholar]
- Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42(Suppl 17):48–51. doi: 10.1007/s00535-006-1942-7. [DOI] [PubMed] [Google Scholar]
- Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Kling MA, Khan I, Calabrese JR, Kalogeras K, Post RM, Avgerinos PC, Loriaux DL, Chrousos GP. Corticotropin releasing hormone: relevance to normal physiology and to the pathophysiology and differential diagnosis of hypercortisolism and adrenal insufficiency. In: Nerozzi D, Goodwin FK, Costa E, editors. Hypothalamic Dysfunction in Neuropsychiatric Disorders. Raven Press; New York: 1987. pp. 183–200. [PubMed] [Google Scholar]
- Goldman ME, McKee RL, Caulfield MP, Reagan JE, Levy JJ, Gay CT, DeHaven PA, Rosenblatt M, Chorev M. A new highly potent parathyroid hormone antagonist: [D-Trp12,Tyr34]bPTH-(7–34)NH2. Endocrinology. 1988;123:2597–9. doi: 10.1210/endo-123-5-2597. [DOI] [PubMed] [Google Scholar]
- Grace CRR, Cervini L, Gulyas J, Rivier J, Riek R. Astressin-amide and astressin-acid are structurally different in DMSO. Biopolymers. 2007;87:196–205. doi: 10.1002/bip.20818. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holsboer F. Neuropeptide receptor ligans as drugs for phsychiatric diseases: the end of the beginning? Nature Rev. 2012;11:462–478. doi: 10.1038/nrd3702. [DOI] [PubMed] [Google Scholar]
- Gulyas J, Rivier C, Perrin M, Koerber SC, Sutton S, Corrigan A, Lahrichi SL, Craig AG, Vale WW, Rivier J. Potent, structurally constrained agonists and competitive antagonists of corticotropin releasing factor (CRF) Proc Natl Acad Sci USA. 1995;92:10575–10579. doi: 10.1073/pnas.92.23.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. vii–viii. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–43. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JF, Kornreich W, Rivier C, Miranda A, Yamamoto G, Andrews J, Taché Y, Vale WW, Rivier JE. Synthesis and relative potencies of new constrained CRF antagonists. J Med Chem. 1993;36:2860–2867. doi: 10.1021/jm00072a004. [DOI] [PubMed] [Google Scholar]
- Hoeger C, Galyean R, Boublik J, McClintock R, Rivier J. Preparative reversed phase high performance liquid chromatography. II Effects of buffer pH on the purification of synthetic peptides. Biochromatography. 1987;2:134–142. [Google Scholar]
- Horiuchi N, Holick MF, Potts JT, Jr, Rosenblatt M. A parathyroid hormone inhibitor in vivo: design and biological evaluation of a hormone analog. Science. 1983;220:1053–5. doi: 10.1126/science.6302844. [DOI] [PubMed] [Google Scholar]
- Jiang G, Stalewski J, Galyean R, Dykert J, Schteingart C, Broqua P, Aebi A, Aubert ML, Semple G, Robson P, Akinsanya K, Haigh R, Riviere P, Trojnar J, Junien JL, Rivier JE. GnRH antagonists: a new generation of long acting analogues incorporating p-ureido-phenylalanines at positions 5 and 6. J Med Chem. 2001;44:453–467. doi: 10.1021/jm0003900. [DOI] [PubMed] [Google Scholar]
- Kandel ER. Biology and the future of psychoanalysis: A new intellectual framework for psychiatry revisited. Am J Psychiat. 1999;156:505–524. doi: 10.1176/ajp.156.4.505. [DOI] [PubMed] [Google Scholar]
- Karten MJ, Hoeger CA, Hook WA, Lindbert MC, Naqvi RH. The development of safer GnRH antagonists: strategy and status. In: Bouchard P, Haour F, Franchimont P, Schatz B, editors. Recent Progress on GnRH and Gonadal Peptides. Elsevier; Paris, France: 1990. pp. 147–158. [Google Scholar]
- Karten MJ, Rivier JE. Gonadotropin-releasing hormone analog design. Structure-function studies toward the development of agonists and antagonists: Rationale and perspective. Endocr Rev. 1986;7:44–66. doi: 10.1210/edrv-7-1-44. [DOI] [PubMed] [Google Scholar]
- Keller PA, McCluskey A, Morgan J, O’Connor SM. The role of the HPA axis in psychiatric disorders and CRF antagonists as potential treatments. Arch Pharm (Weinheim) 2006;339:346–355. doi: 10.1002/ardp.200600021. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Pearse RV, II, Lin CR, Rosenfeld MG. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci USA. 1995;92:1108–1112. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber SC, Gulyas J, Lahrichi SL, Corrigan A, Craig AG, Rivier C, Vale WW, Rivier J. Constrained corticotropin releasing factor (CRF) agonists and antagonists with i-(i+3) Glu-Xaa-DXbb-Lys bridges. J Med Chem. 1998;41:5002–5011. doi: 10.1021/jm980350k. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008a;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism. Proc Natl Acad Sci USA. 2008b;105:8809–10. doi: 10.1073/pnas.0804354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich WD, Galyean R, Hernandez JF, Craig AG, Donaldson CJ, Yamamoto G, Rivier C, Vale WW, Rivier JE. Alanine series of ovine corticotropin releasing factor (oCRF): a structure-activity relationship study. J Med Chem. 1992;35:1870–1876. doi: 10.1021/jm00088a024. [DOI] [PubMed] [Google Scholar]
- Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: The CRF2gamma receptor. Mol Endocrinol. 1998;12:1077–1085. doi: 10.1210/mend.12.8.0145. [DOI] [PubMed] [Google Scholar]
- Krohg K, Hageman I, Jorgensen MB. Corticotropin-releasing factor (CRF) in stress and disease: a review of literature and treatment perspectives with special emphasis on psychiatric disorders. Nord J Psychiatry. 2008;62:8–16. doi: 10.1080/08039480801983588. [DOI] [PubMed] [Google Scholar]
- Lau SH, Rivier J, Vale W, Kaiser ET, Kezdy FJ. Surface properties of an amphiphilic peptide hormone and of its analog: Corticotropin-releasing factor and sauvagine. Proc Natl Acad Sci USA. 1983;80:7070–7074. doi: 10.1073/pnas.80.23.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederis K, Letter A, McMaster D, Moore G. Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science. 1982;218:162–164. doi: 10.1126/science.6981844. [DOI] [PubMed] [Google Scholar]
- Lee C, Sarna SK. Central regulation of gastric emptying of solid nutrient meals by corticotropin releasing factor. Neurogastroenterol Motil. 1997;9:221–9. doi: 10.1046/j.1365-2982.1997.d01-58.x. [DOI] [PubMed] [Google Scholar]
- Liapakis G, Venihaki M, Margioris A, Grigoriadis D, Gkountelias K. Members of CRF family and their receptors: from past to future. Curr Med Chem. 2011;18:2583–600. doi: 10.2174/092986711795933704. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, DeSouza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci, USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Märki W, Spiess J, Taché Y, Brown M, Rivier JE. Total solid phase synthesis of porcine gut gastrin releasing peptide (GRP), a mammalian bombesin. J Am Chem Soc. 1981;103:3178–3185. [Google Scholar]
- Martinez V, Barquist E, Rivier J, Taché Y. Central CRF inhibits gastric empyting of a nutrient solid meal in rats: The role of CRF2 receptors. Am J Physiol. 1998;274:G965–G970. doi: 10.1152/ajpgi.1998.274.5.G965. [DOI] [PubMed] [Google Scholar]
- Mazur AW, Wang F, Tscheiner M, Donnelly E, Isfort RJ. Sauvagine analogs selective for corticotropin releasing factor 2 receptor: effect of substitutions at positions 35 and 39 on CRF2R selectivity. Peptides. 2005;26:887–891. doi: 10.1016/j.peptides.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Miller C, Rivier J. Peptide chemistry: Development of high-performance liquid chromatography and capillary zone electrophoresis. Biopolymers Pept Sci. 1996;40:265–317. doi: 10.1002/(SICI)1097-0282(1996)40:3%3C265::AID-BIP2%3E3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Miranda A, Koerber SC, Gulyas J, Lahrichi S, Craig AG, Corrigan A, Hagler A, Rivier C, Vale WW, Rivier JE. Conformationally restricted competitive antagonists of human/rat corticotropin-releasing factor. J Med Chem. 1994;37:1450–1459. doi: 10.1021/jm00036a010. [DOI] [PubMed] [Google Scholar]
- Moeser AJ, Ryan KA, Nighot PK, Blikslager AT. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G413–G421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- Monahan M, Amoss M, Anderson H, Vale W. Synthetic analogs of the hypothalamic luteinizing hormone releasing factor with increased agonist or antagonist properties. Biochemistry. 1973;12:4616–4620. doi: 10.1021/bi00747a012. [DOI] [PubMed] [Google Scholar]
- Montecucchi PC, Gozzini L. Secondary structure prediction of sauvagine, a novel biologically active polypeptide from a frog. Int J Peptide Protein Res. 1982;20:139–143. doi: 10.1111/j.1399-3011.1982.tb02666.x. [DOI] [PubMed] [Google Scholar]
- Montecucchi PC, Henschen A. Amino acid composition and sequence analysis of sauvagine, a new active peptide from skin of Phyllomedusa sauvagei. Int J Peptide Protein Res. 1981;18:113–120. doi: 10.1111/j.1399-3011.1981.tb02047.x. [DOI] [PubMed] [Google Scholar]
- Overman E, Rivier J, Moeser A. CRF induces intestinal epithelila barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One. 2012;7:1–9. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallai PV, Mabilia M, Goodman M, Vale W, Rivier J. Structural homology of corticotropin-releasing factor, sauvagine, and urotensin I: Circular dichroism and prediction studies. Proc Natl Acad Sci USA. 1983;80:6770–6774. doi: 10.1073/pnas.80.22.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro N, Dallman MF, Warne JP, Ginsberg AB, Laugero KD, la Fleur SE, Houshyar H, Gomez F, Bhargava A, Akana SF. From Malthus to motive: how the HPA axis engineers the phenotype, yoking needs to wants. Prog Neurobiol. 2006;79:247–340. doi: 10.1016/j.pneurobio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale WW. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci USA. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MH, Sutton SW, Cervini L, Rivier JE, Vale WW. Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of CRF receptors. J Pharmacol Exp Ther. 1999;288:729–734. [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale WW. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts A, Vale WW, Geyer MA. CRF receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Brownstein M, Spiess J, Rivier J, Vale W. In vivo CRF-induced secretion of ACTH, β-endorphin and corticosterone. Endocrinology. 1982a;110:272–278. doi: 10.1210/endo-110-1-272. [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivier J, Vale W. Inhibition of adrenocorticotropic hormone secretion in the rat by immunoneutralization of corticotropin-releasing factor. Science. 1982b;218:377–379. doi: 10.1126/science.6289439. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W, Guillemin R. An in vivo corticotropin-releasing factor (CRF) assay based on plasma levels of radio-immunoassayable ACTH. Proc Soc Exp Biol Med. 1973a;142:842–845. doi: 10.3181/00379727-142-37129. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W, Rivier J. Effects of gonadotropin releasing hormone/agonists and antagonists on reproductive functions. J Med Chem. 1983a;26:1545–1550. doi: 10.1021/jm00365a001. [DOI] [PubMed] [Google Scholar]
- Rivier J, Amoss M, Rivier C, Vale W. Synthetic luteinizing hormone releasing factor. Short chain analogs. J Med Chem. 1974;17:230–233. doi: 10.1021/jm00248a019. [DOI] [PubMed] [Google Scholar]
- Rivier J, Brown M, Vale W. [D-Trp8]-somatostatin: An analog of somatostatin more potent than the native molecule. Biochem Biophys Res Commun. 1975;65:746–751. doi: 10.1016/s0006-291x(75)80208-7. [DOI] [PubMed] [Google Scholar]
- Rivier J, Burgus R, Vale W. 3-methyl-TRF, a synthetic analogue with specific activity greater than that of TRF, The Endocrine Society Meeting. Endocrinology. 1971 doi: 10.1210/endo-89-6-1485. [DOI] [PubMed] [Google Scholar]
- Rivier J, Gulyas J, Corrigan A, Craig AG, Martinez V, Taché Y, Vale W, Rivier C. Astressin analogues (CRF antagonists) with extended duration of action in the rat. J Med Chem. 1998;41:5012–5019. doi: 10.1021/jm980426c. [DOI] [PubMed] [Google Scholar]
- Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martínez V, Wang LX, Taché Y, Vale W. Potent and long acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- Rivier J, Gulyas J, Kunitake K, DiGruccio M, Cantle JP, Perrin MH, Donaldson C, Vaughan J, Million M, Gourcerol G, Adelson DW, Rivier C, Taché Y, Vale W. Stressin1-A, a potent corticotropin releasing factor Receptor 1 (CRF1)-selective peptide agonist. J Med Chem. 2007;50:1668–1674. doi: 10.1021/jm0613875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J, McClintock . The Use of HPLC in Receptor Biochemistry. Alan R. Liss, Inc; 1989. Isolation and characterization of biologically active peptides and proteins using reversed-phase HPLC; pp. 77–105. [Google Scholar]
- Rivier J, McClintock R, Eksteen R, Karger BL. Reversed phase HPLC semipreparative purification of unprotected biologically active peptides: Application to synthetic mixtures. Proceedings of the 1982 FDA-USP Workshop on Drug and Reference Standards for Insulins, Somatropins and Thyroid-axis Drugs; Rockville, MD: The United States Pharmacopeial Convention, Inc; 1982c. pp. 554–564. [Google Scholar]
- Rivier J, Rivier C, Galyean R, Miranda A, Miller C, Craig AG, Yamamoto G, Brown M, Vale W. Single point D-substituted corticotropin releasing factor analogues: Effects on potency and physicochemical characteristics. J Med Chem. 1993;36:2851–2859. doi: 10.1021/jm00072a003. [DOI] [PubMed] [Google Scholar]
- Rivier J, Rivier C, Vale W. Synthetic competitive antagonists of corticotropin releasing factor: Effect on ACTH secretion in the rat. Science. 1984;224:889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- Rivier J, Spiess J, Vale W. Characterization of rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci USA. 1983b;80:4851–4855. doi: 10.1073/pnas.80.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J, Vale W, Burgus R, Ling N, Amoss M, Blackwell R, Guillemin R. Synthetic luteinizing hormone releasing factor analogs. Series of short-chain amide LRF homologs converging to the amino terminus. J Med Chem. 1973b;16:545–549. doi: 10.1021/jm00263a031. [DOI] [PubMed] [Google Scholar]
- Rivier JE, Kirby DA, Lahrichi SL, Corrigan A, Vale WW, Rivier CL. Constrained corticotropin releasing factor (CRF) antagonists (Astressin analogues) with long duration of action in the rat. J Med Chem. 1999;42:3175–3182. doi: 10.1021/jm9902133. [DOI] [PubMed] [Google Scholar]
- Rizo J, Sutton RB, Breslau J, Koerber SC, Porter J, Hagler AT, Rivier J, Gierasch LM. A novel conformation in a highly potent, constrained gonadotropin releasing hormone antagonist. J Am Chem Soc. 1996;118:970–976. [Google Scholar]
- Rosenblatt M, Segre GV, Potts JT., Jr Synthesis of a fragment of parathyroid hormone, bPTH-(28–48): an inhibitor of hormone cleavage in vivo. Biochemistry. 1977;16:2811–6. doi: 10.1021/bi00632a001. [DOI] [PubMed] [Google Scholar]
- Rosenblatt M, Shepard GL, Tyler GA, Potts JT., Jr Modification of the arginines in parathyroid hormone: effect on biological activity. Biochemistry. 1978;17:3188–3191. doi: 10.1021/bi00609a003. [DOI] [PubMed] [Google Scholar]
- Rühmann A, Bonk I, Lin CR, Rosenfeld MG, Spiess J. Structural requirements for peptidic antagonists of the corticotropin-releasing factor receptor (CRFR): Development of CRFR2β-selective antisauvagine-30. Proc Natl Acad Sci USA. 1998;95:15264–15269. doi: 10.1073/pnas.95.26.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress hormones: good and bad. Neurobiol Dis. 2000;7:540–542. doi: 10.1006/nbdi.2000.0350. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Hudson AL, Nutt DJ. Invited review: the evolution of antidepressant mechanisms. Fundam Clin Pharmacol. 2004;18:1–21. doi: 10.1111/j.1472-8206.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–2248. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key Role of CRF in the Skin Stress Response System. Endocr Rev. 2013 doi: 10.1210/er.2012-1092. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F, Clark JE, Overman BL, Tozel CC, Huang JH, Rivier JE, Blikslager AT, Moeser AJ. Early Weaning Stress Impairs Development of Mucosal Barrier Function in the Porcine Intestine. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00081.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F, Clark JE, Overman BL, Tozel CC, Huang JH, Rivier JE, Blisklager AT, Moeser AJ. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:G352–G363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold L, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, an impaired stress response and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Spiess J, Dautzenberg FM, Sydow S, Hauger RL, Rühmann A, Blank T, Radulovic J. Molecular properties of the CRF receptor. Trends Endocrinol Metabol. 1998;9:140–145. doi: 10.1016/s1043-2760(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci USA. 1981;78:6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J, Rivier J, Vale W. Sequence analysis of rat hypothalamic corticotropin-releasing factor with the o-Phthalaldehyde strategy. Biochemistry. 1983;22:4341–4346. doi: 10.1021/bi00287a027. [DOI] [PubMed] [Google Scholar]
- Stengel A, Tache Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp Biol Med (Maywood) 2010;235:1168–78. doi: 10.1258/ebm.2010.009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel P, Kasterson R, Yeung W, Cone RD, Rittenberg MB, Stenzel-Poore MP. Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol Endocrinol. 1995;9:637–645. doi: 10.1210/mend.9.5.7565810. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Emotions and disease: From balance of humors to balance of molecules. Nature Med. 1997;3:264–267. doi: 10.1038/nm0397-264. [DOI] [PubMed] [Google Scholar]
- Taché Y. Corticotropin releasing factor receptor antagonists: potential future therapy in gastroenterology? Gut. 2004;53:919–921. doi: 10.1136/gut.2003.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2009;11:270–7. doi: 10.1007/s11894-009-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G173–7. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- Taché Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Tazi A, Dantzer R, LeMoal M, Rivier J, Vale W, Koob GF. Corticotropin releasing factor antagonist blocks stress-induced fighting in rats. Regul Pept. 1987;18:37–42. doi: 10.1016/0167-0115(87)90048-6. [DOI] [PubMed] [Google Scholar]
- Tichomirowa MA, Keck ME, Schneider HJ, Paez-Pereda M, Renner U, Holsboer F, Stalla GK. Endocrine disturbances in depression. J Endocrinol Invest. 2005;28:89–99. doi: 10.1007/BF03345535. [DOI] [PubMed] [Google Scholar]
- Vale W, Grant G, Amoss M, Blackwell R, Guillemin R. Culture of enzymatically dispersed anterior pituitary cells. Functional validation of a method. Endocrinology. 1972a;91:562–572. doi: 10.1210/endo-91-2-562. [DOI] [PubMed] [Google Scholar]
- Vale W, Grant G, Rivier J, Monahan M, Amoss M, Blackwell R, Burgus R, Guillemin R. Synthetic polypeptide antagonists of the hypothalamic luteinizing hormone releasing factor. Science. 1972b;176:933–934. doi: 10.1126/science.176.4037.933. [DOI] [PubMed] [Google Scholar]
- Vale W, Rivier C, Brown M, Leppaluoto J, Ling N, Monahan M, Rivier J. Pharmacology of hypothalamic regulatory peptides. Clin Endocrinol. 1976;5:261s–273s. doi: 10.1111/j.1365-2265.1976.tb03834.x. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates the secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vita N, Laurent P, Lefort S, Chalon P, Lelias JM, Kaghad M, Le FG, Caput D, Ferrara P. Primary structure and functional expression of mouse pituitary and human brain corticotropin releasing factor receptors. FEBS Lett. 1993;335:1–5. doi: 10.1016/0014-5793(93)80427-v. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Vulliemoz N, Rivier J, Ferin M. Astressin B, a corticotropin-releasing hormone receptor antagonist, accelerates the return to normal luteal function after an inflammatory-like stress challenge in the rhesus monkey. Endocrinology. 2007;148:841–848. doi: 10.1210/en.2006-1074. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Mizutani K, Mizusawa Y, Hantani Y, Tanaka M, Tanaka Y, Tomimoto M, Sugawara M, Imai N, Yamada H, Okajima N, Haruta J. New class of corticotropin-releasing factor (CRF) antagonists: small peptides having high binding affinity for CRF receptor. J Med Chem. 2004;47:1075–1078. doi: 10.1021/jm034180+. [DOI] [PubMed] [Google Scholar]
- Zhu J, Jiang Y, Xu G, Liu X. Intranasal administration: a potential solution for cross-BBB delivering neurotrophic factors. Histol Histopathol. 2012;27:537–548. doi: 10.14670/HH-27.537. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]