Abstract
Persistent pain is a central characteristic of neuropathic pain conditions in humans. Knowing whether rodent models of neuropathic pain produce persistent pain is therefore crucial to their translational applicability. We investigated the Spared Nerve Injury (SNI) model of neuropathic pain and the formalin pain model in rats using Positron Emission Tomography (PET) with the metabolic tracer [18F]fluorodeoxyglucose (FDG) to determine if there is ongoing brain activity suggestive of persistent pain. For the formalin model, under brief anesthesia we injected one hindpaw with 5% formalin and the FDG tracer into a tail vein. We then allowed the animals to awaken and observed pain behavior for 30 min during the FDG uptake period. The rat was then anesthetized and placed in the scanner for static image acquisition, which took place between minutes 45 and 75 post-tracer injection. A single reference rat brain magnetic resonance image (MRI) was used to align the PET images with the Paxinos and Watson rat brain atlas. Increased glucose metabolism was observed in the somatosensory region associated with the injection site (S1 hindlimb contralateral), S1 jaw/upper lip and cingulate cortex. Decreases were observed in the prelimbic cortex and hippocampus. Second, SNI rats were scanned 3 weeks post-surgery using the same scanning paradigm, and region-of-interest analyses revealed increased metabolic activity in the contralateral S1 hindlimb. Finally, a second cohort of SNI rats were scanned while anesthetized during the tracer uptake period, and the S1 hindlimb increase was not observed. Increased brain activity in the somatosensory cortex of SNI rats resembled the activity produced with the injection of formalin, suggesting that the SNI model may produce persistent pain. The lack of increased activity in S1 hindlimb with general anesthetic demonstrates that this effect can be blocked, as well as highlights the importance of investigating brain activity in awake and behaving rodents.
Keywords: microPET, FDG, Neuropathic pain, Spared Nerve Injury, Formalin Model
INTRODUCTION
Neuropathic pain related to peripheral nerve injury results from a variety of causes, including diabetes, shingles (herpes zoster), cancer treatments, and trauma. Neuropathic pain almost always involves sensory abnormalities, such as numbness and/or allodynia and hyperalgesia to touch or temperature (Maier et al., 2010). In addition, patients report pain in the absence of obvious externally applied stimuli. This pain may result from spontaneous activity in nerve fibers, or subtle stimulation resulting from normal daily activities. Thus, persistent pain experienced by patients is likely a mix of stimulus-independent pain and pain provoked by inadvertent stimulation. Neuropathic pain is studied using multiple nerve-injury rodent models (Bennett and Xie, 1988; Decosterd and Woolf, 2000; Kim and Chung, 1992; Seltzer et al., 1990). Unfortunately, assessing persistent pain using these models is difficult, since the animals frequently do not manifest the pain behaviors observed during acute injury. Attempts to measure persistent pain using ultrasonic vocalizations, facial expression, altered locomotion and altered sleep patterns have revealed few positive results (Jourdan et al., 2002; Langford et al., 2010; Mogil et al., 2010; Urban et al., 2011; Wallace et al., 2005). Thus, neuropathic pain models typically rely on measures of mechanical and/or thermal hypersensitivity (D’Amour and Smith, 1941; Le Bars et al., 2001; Woolfe and MacDonald, 1944), which may not reflect the persistent pain reported by chronic pain patients (Backonja and Stacey, 2004; Baron et al., 2009; Gottrup et al., 1998). Based upon behavioral results, it is unclear whether the assessment methods are inadequate or if the rodent models do not produce chronic persistent pain. In contrast, there are rodent pain models that result in overt short lived pain-related behaviors. As an example, the formalin tonic pain model results in a well characterized set of persistent pain-related behaviors that last for approximately one hour (Dubuisson and Dennis, 1977).
In humans, imaging has revealed brain regions commonly activated by pain, including the primary somatosensory cortex of the area affected by pain, secondary somatosensory cortex, prefrontal cortex, insular cortex, anterior cingulate cortex, and thalamus (for reviews see:(Apkarian et al., 2005; Schweinhardt and Bushnell, 2010). These regions are also activated during ongoing, chronic pain in humans (Baliki et al., 2006; Howard et al., 2012). Rodent in vivo brain imaging has revealed activations of homologous brain regions in response to acute noxious stimuli (for reviews see:(Borsook and Becerra, 2011; Thompson and Bushnell, 2012). Using ex vivo CBF imaging, Paulson et al. (2002) showed that 12 weeks after a chronic constriction nerve injury (CCI), somatosensory cortex showed increased CBF in the absence of stimulation. However, no in vivo brain imaging study has evaluated activations related to unstimulated, chronic persistent pain in awake rodents.
The current study tested the hypothesis that rats with a chronic nerve injury that produces cutaneous hypersensitivity also show a pattern of brain activity consistent with persistent pain. To test this hypothesis, positron emission tomography (PET) scans were performed on three cohorts of rats using the metabolic tracer [18F]fluorodeoxyglucose (FDG) (Ido et al., 1978; Kornblum et al., 2000). In the first group, formalin-evoked brain activity was assessed in awake and behaving rats (during the tracer uptake period) to identify the pattern of persistent pain-related activation. In a second group, the same scanning paradigm was used in rats three weeks post-nerve injury to measure ongoing nerve-injury-related brain activity. Finally, to examine whether activations related to nerve injury were influenced by the state of consciousness, a third group of nerve-injured rats were scanned after they had been anesthetized during tracer uptake.
MATERIALS & METHODS
Experimental Animals
Forty-six male Sprague-Dawley rats (150–200 grams, Charles River, QC) were pair housed in temperature controlled (23 +/− 1 C) ventilated racks with a 14-hour light, 10-hour dark cycle with lights on at 07:00. The rats had access to both food (Harlan Teklad 2920X) and water. Ethical treatment of animals was ensured; all procedures were approved by McGill University’s Animal Care Committee.
PET Imaging Acquisition Procedures
[18F] Fluorodeoxyglucose (FDG), an analog of glucose, was used as the PET tracer to yield a relative measure of glucose metabolism in the brain. As shown in Figure 1, for the formalin and awake SNI scanning procedures, the FDG was injected in the tail vein while the rat was briefly anesthetized with sevoflurane (5% induction, 2.5% maintenance for ~3 min). The injection was made 45 min before PET scanning began, since the peak signal in rat brain occurs approximately 1 hour after injection and represents an accumulation of the tracer that occurred from the time of injection (Ido et al., 1978). The anesthesia was quickly removed, the animal awoke, and was awake and behaving for the next 30 minutes before the animals was re-anesthetized and scanned. The use of this delayed scanning allowed us to capture metabolic activity that occurred while the animal was awake and behaving throughout 30 minutes of tracer uptake. Forty minutes after FDG injection, the animal was anesthetized (sevoflurane, 5% induction, 2.5% maintenance throughout the scan), placed in the PET scanner and a static 30-min scan was acquired. A single static scan was chosen over dynamic scanning, since maximizing signal-to-noise ratio was more important for this study than obtaining temporal information. For the SNI anesthetized scan, the rat was anesthetized (isoflurane, 5% induction, 2% maintenance) before the FDG injection and anesthesia was maintained with the rat resting on the scanner bed during the entire period of tracer uptake and scanning. Images were acquired using a microPET R4 (CTI Concorde, Knoxville, TN, USA). The scanner bed was equipped with a breathing rate monitor, rectal thermometer, and heating pad to maintain body temperature at 37°C. Following standard procedures, rats were fasted for approximately 12 hours prior to scanning as blood glucose levels can affect FDG uptake (Lindholm et al., 1993). The FDG tracer was obtained from on-site production at the Montreal Neurological Institute Cyclotron Facility using standard practices for the production of clinical FDG.
Figure 1.
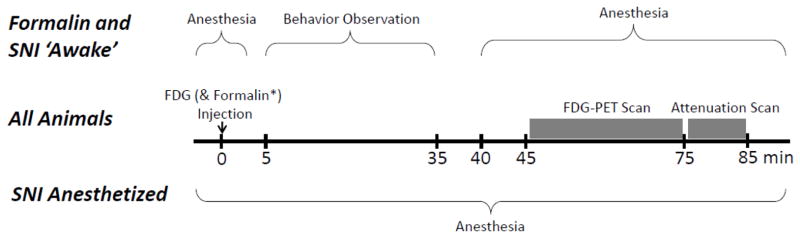
Time course of small animal positron emission tomography (PET) scanning for the 3 experimental groups: formalin unanesthetized during uptake (‘awake’), spared nerve injury (SNI) unanesthetized during uptake (‘awake’) and SNI anesthetized during uptake.
Formalin Pain Model
Sixteen rats in total (8 formalin, 8 controls) were randomly assigned to either a formalin (5%, 50 μL) or control (saline, 50 μL) injection. Injection of formalin results in a well-characterized behavioral response lasting approximately 1 hour (Dubuisson and Dennis, 1977). On the day of the scan, each rat received a tail vein injection of a volume less than 0.2 ml and approximately 0.2 MBq of FDG, and a subcutaneous injection of formalin or saline into the plantar surface of the left hindpaw while briefly anesthetized with 5.0% sevoflurane (minute zero, see figure 1). The anesthetic was immediately removed after injections and the rats were placed in a ventilated clear Plexiglas observation chamber with a clear floor (30 cm × 30 cm × 30 cm). Beneath the floor, a mirror was mounted at a 45-degree angle allowing for an unobstructed view of the paws. Behavior was video recorded from minute 5 to minute 35. Behavior was not recorded minute 0 to 5 to allow for anesthesia to fully lift, nor at minute 35 to 40 because of scanning preparations requiring technician movement and noise, which could have modified behavior. At minute 40, the rat was removed from the observation apparatus, anesthetized with sevoflurane (5.0% for induction, 2.5% for maintenance) and placed on the scanner bed, with scanning starting at minute 45 and ending at minute 85 as shown in figure 1.
Neuropathic Pain Model
Eighteen rats were randomly assigned to either spared nerve injury (SNI) surgery (9 rats) or sham surgery (9 rats, control group). Surgery was performed while the rat was under isoflurane anesthesia (5.0% for induction, 2.0% for maintenance). The SNI model of neuropathic pain involves the ligation and transection of the tibial and common peroneal nerves of the hindlimb, while leaving the sural nerve intact (Decosterd and Woolf, 2000). Each nerve was ligated with 6-0 sterile suture silk in two places with approximately 2 mm separation followed by transection between sutures. Wound closure was performed with 4-0 sterile suture followed by cutaneous application of antibiotic ointment. Sham rats underwent a similar surgical procedure with the exception that tibial and common peroneal nerves were only visualized and no nerve ligation was performed. SNI and sham surgeries were all performed on the left hindlimbs. Rats underwent sensory testing 1 week pre-surgery and 2 weeks post-surgery (as described later). Brain scanning occurred 3 weeks post-surgery following the same unanesthetized procedure as described for the formalin pain model (figure 1). Previous studies from our lab(Low et al., 2012; Seminowicz et al., 2009), as well as the first description of the SNI procedure (Decosterd and Woolf, 2000) showed that the sensory alterations observed at 2 weeks post-surgery continue well beyond 3 weeks post-surgery. Thus, although there was a delay between behavioral testing and scanning, it is reasonable to assume that the sensory abnormalities were similar at these two time points Anesthetic was immediately removed and behavioral assessment and scanning followed the same procedure as described previously and as shown in figure 1.
Neuropathic Pain Model, Anesthetized
A final cohort of twelve rats (6 SNI surgeries, 6 sham surgeries) underwent the surgical procedures and behavioral testing as described in the preceding paragraph. Scanning occurred at 3 weeks post-surgery following a similar procedure with the exception that rats underwent an anesthetized scanning procedure. In brief, each rat was anesthetized with isoflurane (5.0% for induction, 2.0% for maintenance) and then placed on the PET scanner bed. A tail vein injection of a volume less than 0.2 ml and approximately 0.2 MBq of FDG was given at minute 0. The rat remained anesthetized on the scanner bed for the entire procedure as shown in figure 1.
Neuropathic Pain Model, Sensory Testing
Withdrawal responses to mechanical and thermal stimuli were measured both 1 week pre-surgery and 2 weeks post-surgery. Prior to behavioral testing, rats were habituated to the room for 1 hour in their home cages followed by a 30-minute habituation to the testing apparatus. Mechanical sensitivity was measured on both hindpaws using von Frey hairs (Stoelting, IL) and the up-down method adapted from Chaplan et al., (1994). Approximately 30 minutes later, cold sensitivity was measured on both hindpaws by applying 50μL acetone to the plantar surface of the hindpaw and measuring the duration (in seconds) of the response (shaking or licking of the paw) that occurs within 1 minute of acetone application using a stopwatch and lab timer, adapted from Choi et al., (1994). Group differences between nerve-injured rats and sham rats were assessed using unpaired two-tailed t-tests (SPSS, IBM SPSS, version 20.0.0). Results are reported as mean ± standard error.
Behavior Monitoring during Tracer Uptake
Each rat was acclimated for one hour prior to behavioral monitoring. Formalin-injected and nerve-injured rats that were awake and behaving during tracer uptake were video recorded while in a clear Plexiglas observation apparatus so that group behavioral differences (which might confound imaging results) could be monitored. Videos were scored by two observers blinded to experimental group. Behaviors scored included: grooming, hindlimb locomotion, exploring (forelimb and/or hindlimb locomotion), rearing, guarding the injured limb, licking the injured limb, twitch/rapid lift and replacement of the injured limb, and resting (eyes open, but not participating in any apparent action). A weighted pain score was also calculated using a method developed for assessing the formalin test (Coderre et al., 1993). For this score, the amount of time is measured in seconds for three categories of behaviors. For the first category, the amount of time spent when the injured paw has little or no weight on it is multiplied by 1. For the second category, time spent with the injured paw elevated and not in contact with any surface is multiplied by 2. For the third category, time spent licking the injured paw is multiplied by 3. The sum of all of these measures is divided by the total observation time (1800 seconds) which yields a unitless Weighted Pain Behavior score that ranges from 0 to 3. Group differences were analyzed using SPSS (IBM SPSS, version 20.0.0) using an unpaired two-tailed t-test comparing nerve injured rats to sham rats, and formalin to formalin control (saline). Results are reported as mean ± standard error. A blind observer also recorded the presence of abnormal weight bearing during walking and abnormal foot positioning.
Image Data
The PET images were reconstructed per manufacturer’s recommended voxel size of 0.84 × 0.84 × 1.21 mm and 128 × 128 × 63 matrix. Reconstruction was performed using CTI Concorde’s microPET Manager Software using OSEM3D (2 iterations) MAP (18 iterations) with reconstructions from minute 50 to 70 to a single timeframe. PET images were converted to the MINC file format and further processed using MINC tools (http://packages.bic.mni.mcgill.ca/). Activity was normalized using a whole brain metabolic activation index (Casteels et al., 2006; Luyten et al., 2012). Specifically, the radioactivity count was converted to the metabolic activation index by normalizing the whole brain mean to a value of 1.0 making the value of each voxel relative to the whole brain indexed at 1.0.
Image Analysis
Alignment was performed using tools available from the Montreal Neurological Institute Brain Imaging Centre (http://packages.bic.mni.mcgill.ca/) (Collins et al., 1994) with processing scripts developed in-house. The original scans at 128 × 128 × 63 voxels included both the head and upper torso of the rat. To facilitate alignment, a block of 26 × 36 × 31 voxels centered on and containing the whole brain was extracted from each scan. Alignment was performed using an lsq-6 linear registration algorithm, which rotates and translates each brain image to a standard space. Average metabolic index maps were then co-registered to a size-matched anatomical rat MRI from previous work in our lab. For the whole brain analysis, group average differences were calculated using the statistical analysis software R (http://www.r-project.org/) and the RMINC library, using a False Discovery Rate of q=0.05 to correct for multiple comparisons. The mean activity values of significant clusters were correlated with relevant behavior during the FDG uptake period. The two-tailed Pearson’s correlation coefficient was calculated using SPSS (IBM SPSS, version 20.0.0).
Region-of-interest (ROI) analysis was performed to evaluate nerve-injured rats, and brain regions most likely to be activated with persistent pain were selected. The criteria were based upon the overlap between commonly activated brain regions in rodent pain studies (primary somatosensory cortex of the area affected by pain, cingulate area 1 [Cg1, homologous to anterior cingulate cortex in humans], and thalamus; for review see: Thompson and Bushnell, 2012) and the brain regions activated in the formalin pain model as found in this study (see Results section). Based upon these criteria, primary somatosensory cortex hindlimb and Cg1 were selected. Regions were anatomically defined from the co-registered anatomical MRIs using the Paxinos and Watson Atlas (Paxinos and Watson, 2007). Mean activity values within these regions were also used to investigate correlations to behavior. The two-tailed Pearson’s correlation coefficient was calculated using SPSS (IBM SPSS, version 20.0.0).
RESULTS
Sensory Testing of the Neuropathic Pain Model
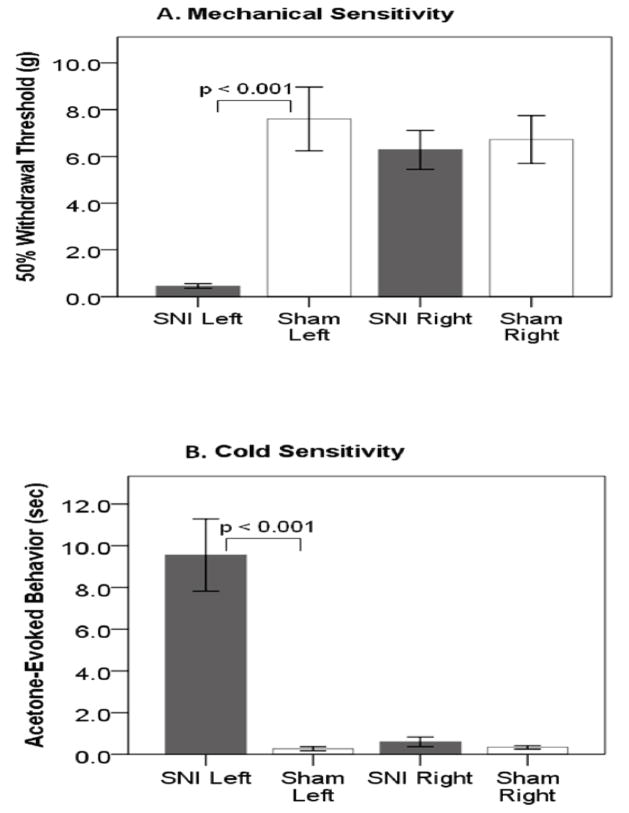
Nerve-injured rats were hypersensitive on the injured hindlimb to both mechanical and cold stimuli as compared to sham rats (figure 2). Von Frey filament stimulation elicited reflex withdrawals at 0.46g±0.10g for nerve-injured rats compared to 7.60g±1.36g for sham rats (t(28)=5.232, p<0.001). Acetone application to the plantar surface produced response times of 9.6s±1.7s for nerve injured compared to 0.3s±0.1s for sham rats (t(28)=5.360, p<0.001). As expected, nerve-injured rats demonstrated signs of thermal and mechanical hypersensitivity.
Figure 2.
Sensory testing of spared nerve injury (SNI) and Sham 2 weeks post-surgery. SNI rats are hypersensitive on the injured limb (left) post-surgery to both (A) mechanical and (B) cold stimuli. Error bars +/− 1 S.E.
Behavior during Tracer Uptake
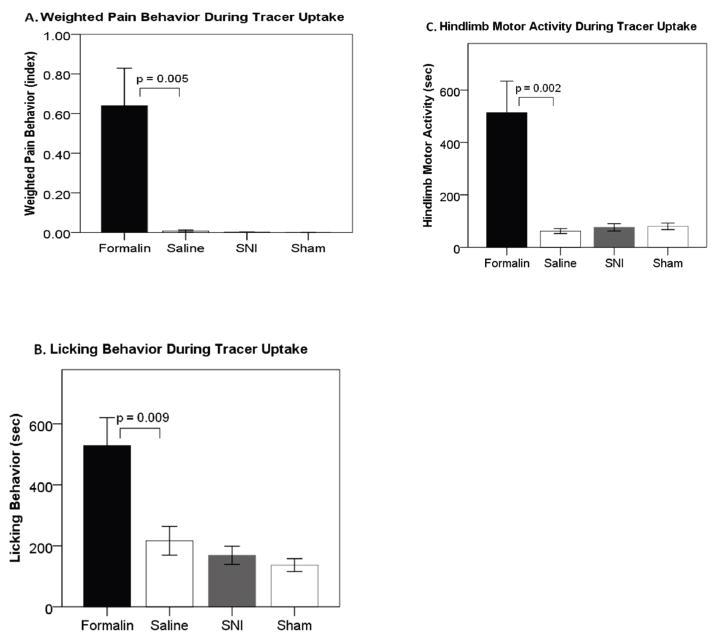
Formalin-injected rats displayed well characterized pain-like behaviors, including guarding and licking of the injected paw. Figure 3A shows that the formalin-injected rats displayed significantly more pain behaviors at 0.64±0.19 (weighted pain behavior index) compared to controls at 0.01±0.00 (t(14)=3.360, p=0.005). Using the same algorithm, pain behaviors were also scored for nerve-injured and sham rats. Nerve-injured rats did not display any pain behaviors at 0.001±0.001, nor did sham rats at 0.000±0.000 (t(16)=1.000, p=0.332). Nevertheless, all rats with the SNI injury displayed abnormal weight bearing and positioning of the injured foot, whereas sham operated rats did not.
Figure 3.
Behavior during tracer uptake for unanesthetized formalin and spared nerve injury (SNI). (A) Pain behaviors are only seen in formalin-injected rats. (B) Formalin-injected rats show more licking behaviors than saline-injected controls, and no significant difference is seen between SNI and Sham. (C) Formalin rats have more hindlimb motor activity than controls, and no significant difference is seen between SNI and Sham. Error bars +/− 1 S.E.
Motor activity was investigated as a potential contributor to enhanced brain activity. As shown in figure 3B, formalin-injected rats spent significantly more time licking and grooming (528.5s± 91.9s) than controls (216.5s±47.2s, t(14)=3.021, p=0.009). This has the potential to enhance brain activity in the jaw- and face-related somatosensory and motor brain regions. Nerve-injured rats did not display different licking and grooming behavior than sham rats (168.9s±29.7s, 136.7s±21.0s, t(16)=0.884, p=0.390). As shown in figure 3C, formalin-injected rats also spent significantly more time performing hindlimb motor activity (hindlimb locomotion, guarding hindlimb, licking hindlimb, twitch of hindlimb) (513.4s±120.6s) than controls (61.5s±9.2s, t(14)=3.733, p=0.002). This behavior has the potential to enhance brain activity in the somatosensory region associated with the injury (S1 hindlimb contralateral). In contrast, nerve-injured rats did not display any significant differences in hindlimb motor activity (73.33s±13.92s) compared to shams (79.78s±12.58s, t(16)=0.343, p=0.736).
Whole Brain Image Analysis of the Formalin Pain Model
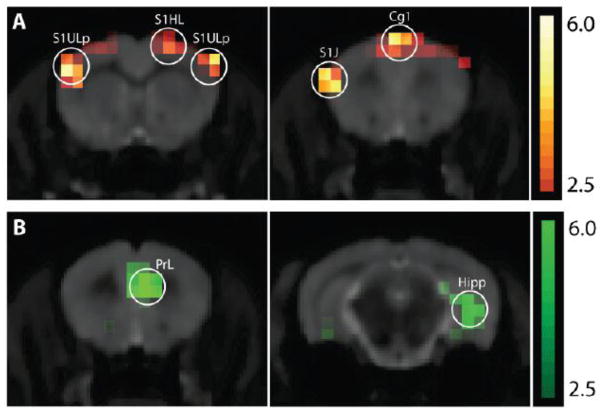
Metabolic activity was found to be significantly different between formalin-injected rats and saline-injected rats in multiple brain regions after correction for multiple comparisons (t-value > 4.367 was calculated for FDR q=0.05, Table 1). Areas with significantly increased metabolic activity are shown in figure 4A and included the somatosensory region associated with the injury (S1 hindlimb contralateral - S1HL, t(14)=4.454), the somatosensory regions associated with the jaw and upper lip (S1 jaw and S1 upper lip - S1J and S1ULp, t(14)=5.705), and cingulate cortex area 1 (Cg1, t(14)=4.575). Since the spatial resolution of PET in this study allows for the resolution of two distinct activations at least 1.8mm apart, we are able to resolve distinct activations in S1 hindlimb and S1 jaw/upper lip, as the peak-to-peak spatial resolution of these regions is 3.6mm. On the other hand, we are not able to resolve possible distinct activations in the upper lip and jaw regions. Areas where the formalin-injected rats had less metabolic activity compared to the control rats are shown in figure 4B and include an area homologous to the human medial prefrontal cortex (prelimbic cortex - PrL at t(14)=5.327), and two regions that are part of the hippocampal formation (t(14)=4.523 and 6.034).
Table 1.
Statistically significant clusters from whole brain image analysis contrasting formalin to control condition. Correction for multiple comparisons with False Discovery Rate at less than 0.05 at t > 4.367.
| Cluster (formalin > control) | Peak t value | Peak location | Bregma | Horiz | Vert |
|---|---|---|---|---|---|
| S1HL contralateral | 4.454 | S1HL contra | −0.24 | 3 | 1.5 |
| S1Jaw & Uplip bilateral | 5.705 | S1ULp ipsi | −0.24 | −5.5 | 6 |
| CG1, medial | 4.575 | CG1, ipsi | 2.76 | −0.5 | 0.8 |
| Cluster (control > formalin) | Peak t value | Peak location | Bregma | Horiz | Vert |
|---|---|---|---|---|---|
| Prelimbic/infralimbic, medial | 5.327 | PrL, contra | 3.24 | 1.1 | 4.2 |
| Hippocampus, contralateral | 4.523 | Rad, contra | −4.92 | 5.5 | 7 |
| Entorhinal Cortex, ipsilateral | 6.034 | Cent, ipsi | −8.76 | −5.4 | 5 |
Figure 4.
Coronal slices of t-stat map contrasting formalin- to saline-injected rats, t > 2.5 shown, overlaid on size matched anatomical magnetic resonance imaging (MRI). (A) Formalin > control with scale shown for t-values 2.5 to 6.0 in orange. (B) Control > formalin with scale shown for t-values 2.5 to 6.0 in green.
Brain Clusters and Behavioral Correlation of the Formalin Pain Model
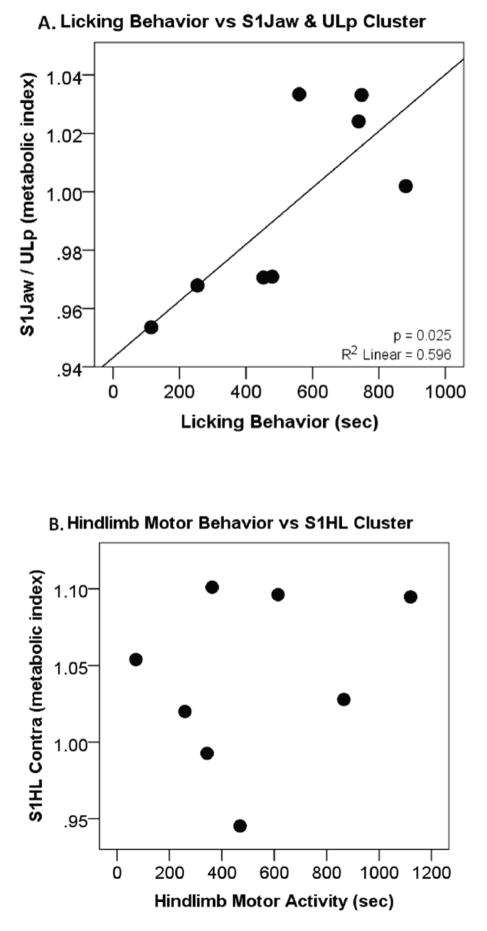
Correlations between motor activity and brain activity were investigated in the formalin-injected rats to test whether increased motor activity contributed to enhanced brain activity. Differences in hindlimb movement and licking behaviors were observed in the formalin-injected rats compared to controls. The licking behavior correlated positively with S1 jaw and S1 upper lip cluster activity (p=0.025, R2 = 0.596, figure 5A). In contrast, no correlation was found between hindlimb movement and S1 hindlimb contralateral cluster activity (p=0.511, R2 = 0.075, figure 5B)) nor between overall motor activity and Cg1 cluster brain activity (p=0.679, R2 = 0.030).
Figure 5.
For formalin-injected rats, (A) a correlation is seen between licking behavior and activity in the brain region associated with that behavior (jaw and upper lip) suggesting that the behavior is reflective of the brain activity. (B) No correlation is seen with hindlimb behavior and the primary somatosensory (S1) hindlimb cluster activity suggesting behavior is not a sole explanation for increased brain activity.
Region of Interest Brain Image Analysis, Neuropathic Pain
S1 hindlimb and Cg1 were selected as ROIs using the criteria described in the methods section. The region of S1 jaw/S1 upper lip was included as a control region where we would not expect to see pain-related increases related to hind-paw stimulation. Because the increase in brain activity in this region was associated with motor activity in formalin-injected rats, and the neuropathic animals did not show increased motor activity compared to the control animals, we anticipated that we would not see any difference in brain activity between nerve-injured rats and sham rats. As a point of reference, ROI analysis was also performed on the formalin-injected rats and is displayed side-by-side with the SNI results.
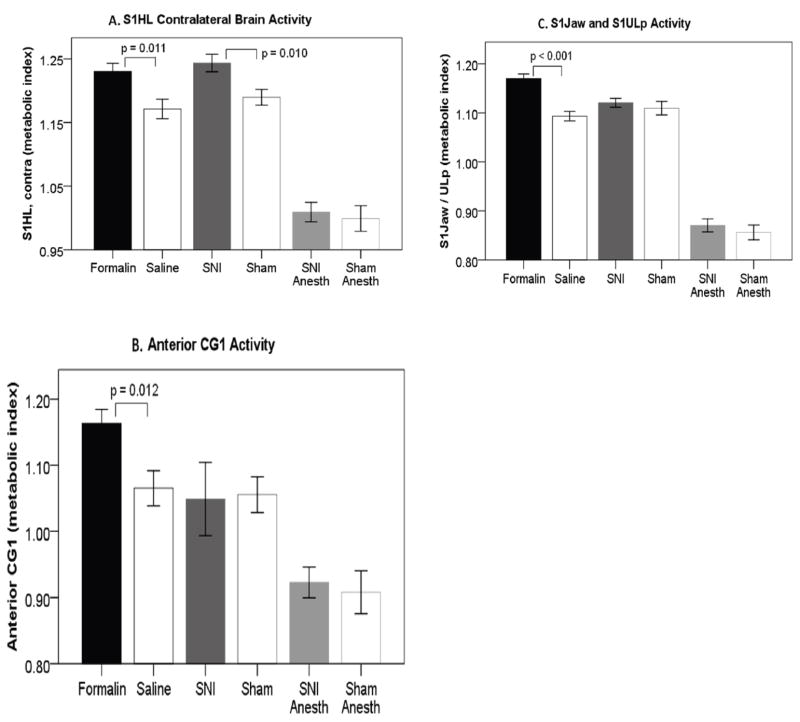
For the contralateral S1 hindlimb, there was a significant increase in activity in formalin compared to control rats (formalin: 1.230±0.013 [metabolic activation index], saline: 1.171±0.015, t(14)=2.919, p = 0.011, figure 6A), as well as nerve-injured to sham rats (SNI: 1.244±0.014, sham: 1.189±0.013, t(16)=2.915, p = 0.010, figure 6A). There was no difference between nerve-injured anesthetized rats and sham anesthetized rats (SNI: 1.009±0.015, sham: 0.999±0.020, t(10)=0.397, p = 0.700). For Cg1, a significant increase in activity was observed between formalin and control rats (formalin: 1.163±0.021, saline: 1.065±0.026, t(14)=2.892, p = 0.012, figure 6B), while neither of the nerve-injured groups differed from their respective controls (unanesthetized SNI: 1.049±0.056, sham: 1.056±0.027, t(16)=0.108, p = 0.915 and anesthetized SNI: 0.922±0.023, sham: 0.908±0.033, t(10)=0.368, p = 0.720, figure 6B). In S1 jaw and S1 upper lip, formalin rats had significantly higher metabolic activity than control rats, as expected from the whole brain results (formalin: 1.170±0.010, saline: 1.093±0.010, t(14)=5.547, p < 0.001, figure 6C), while no difference in activity was observed for either nerve-injured group compared to their respective controls (unanesthetized SNI: 1.121±0.009, sham: 1.110±0.014, t(16)=0.668, p = 0.514 and anesthetized SNI: 0.871±0.013, sham: 0.856±0.015, t(10)=0.706, p = 0.496, figure 6C).
Figure 6.
Anatomically defined regions of interest (ROIs) for formalin, spared nerve injury (SNI), SNI anesthetized, and respective controls. (A) primary somatosensory (S1) hindlimb contralateral brain activity differences are seen in both formalin and SNI, but not SNI anesthetized versus controls. (B) Anterior cingulate area 1 (Cg1) difference is seen in formalin vs. controls, but not in either SNI group versus controls. (C) S1 jaw & S1 upper lip difference is seen in formalin versus controls, but not in either SNI group versus controls. Error bars +/− 1 S.E.
ROI Brain Activity and Behavioral Correlation, Neuropathic Pain
For nerve-injured rats, contralateral S1 hindlimb was the only brain region found to have significantly more activity compared to controls. To investigate whether this activity might be related to motor behavior, correlations to hindlimb behavioral activity were investigated. No correlation was found between hindlimb motor activity and contralateral S1 hindlimb ROI activity (R2 = 0.029, p = 0.293).
DISCUSSION
After formalin injection into the rat hindpaw, brain activity was observed in the contralateral hind paw region of S1 cortex and in cingulate cortex, consistent with pain-related activation patterns observed in both human and rodent studies. This activity did not correlate with limb movement, suggesting that it was not driven by motor activity. In contrast, activity in the S1 lip/jaw region correlated highly with licking, suggesting it was a direct consequence of sensorimotor stimulation of the face rather than ongoing pain. The animals with a chronic nerve injury showed S1 hind limb activation similar to animals with an acute formalin injury, even though the nerve-injured animals did not manifest pain behaviors seen after formalin injection or with other acute pain states. .This finding supports the observations of a previous ex vivo CBF imaging study (Paulson et al., 2002), in which 12 weeks after a hindlimb chronic constriction injury, increased rCBF was observed in hindlimb S1, despite a lack of observable spontaneous pain behaviors at the time point. When the experiment was performed with nerve-injured rats under anesthesia, the increase in S1 hindlimb was eliminated, suggesting that the activation observed in awake animals may relate to a conscious perception. Cingulate cortex, a region involved in affective-motivational aspects of pain (Rainville et al., 1997), was activated during the formalin assay, but not in the chronically nerve-injured rats.
Does the hindpaw S1 activity suggest peripherally or centrally driven persistent pain in rats with nerve injury?
The finding that activation observed in awake nerve-injured animals was absent in anesthetized animals suggests that the activation is likely related to a conscious perception. This idea is supported by the Hofbauer et al (2004) human PET study showing that S1 pain-evoked activity disappeared when subjects lost consciousness. The finding that S1 hind limb contralateral but not ipsilateral to the injury was activated suggests that the activation was related to nociceptive input and not normal tactile input evoked by walking during the tracer uptake period. Thus, it is likely that this activation represents some type of persistent pain in the nerve-injured rats.
The next question is “what is causing the persistent pain—enhanced tactile input or spontaneous activity in peripheral or central nervous system?” Since the rat’s paw was contacting surfaces during the uptake period, tactile or thermal allodynia could clearly contribute to the activation. However, unlike the S1 lip/jaw activation, there was not a correlation between the S1 hindlimb activation and any particular behavior, making it unlikely that the S1 hindpaw activation was solely due to a touch-evoked allodynic input. Spontaneous neural activity may well also have contributed. Several types of evidence support this idea. Single- and multi-unit neuronal studies in nerve-injured rodents find not only heightened touch-evoked activity, but also increased spontaneous discharge, in neurons in the spinal cord dorsal horn (Laird and Bennett, 1993) and primary somatosensory cortex (Guilbaud et al., 1992). Spontaneous discharge has also been documented in cutaneous nerves of patients with painful peripheral neuropathy (Campero et al., 1998; Nordin et al., 1984; Ochoa et al., 2005).
Human brain imaging studies also show that increased S1 activity can correspond to either allodynia or spontaneous pain. Several studies of touch-evoked allodynia in nerve-injured patients show increased activation in multiple cortical regions, including primary somatosensory cortex, when stimulating an injured region compared to the mirrored non-injured site (Becerra et al., 2006; Petrovic et al., 1999; Schweinhardt et al., 2006). However, S1 activity has also been observed during spontaneous fluctuation on back pain, in the absence of a change in stimulation (Baliki et al., 2006).
Behavioral evidence of persistent pain in nerve-injured rodents?
Several studies have attempted to assess persistent pain in chronically nerve-injured rodents using behaviors that are altered in acute pain models, including facial expression, ultrasonic vocalization, asymmetrically directed behaviors and dynamic weight-bearing. However, none of these measures have been found useful for measuring persistent pain in chronic nerve injury models (Jourdan et al., 2002; Langford et al., 2010; Mogil et al., 2010; Wallace et al., 2005). Rodents in our study and in other SNI studies (Mogil et al., 2010) do show abnormal weight bearing on the injured limb. Nevertheless, Mogil et al found that this abnormal behavior was dissociated temporally, pharmacologically and genetically from mechanical allodynia observed in the same animals, suggesting that it more likely represents a motor dysfunction than a pain-related behavior.
Since human chronic pain patients often report altered quality of life, a recent study examined home-cage behaviors in mice, including feeding, drinking and locomotion, as a surrogate for “quality of life.” However, animals with either a chronic nerve injury or chronic inflammation showed no significant abnormalities in their home cage behavior (Urban et al., 2011). Nevertheless, the absence of behavioral alterations in these tests does not necessarily indicate that the animals are not experiencing persistent pain. Human pain patients can report persistent pain without showing significant changes in daily-life functions. Furthermore, as pointed out by Urban et al, since mice are prey animals, there is an evolutionary imperative to refrain from showing signs of weakness or persistent pain.
A more specific behavioral measure of persistent pain may be the test of conditioned place preference (CPP). King et al. (2009) demonstrated that rats with a nerve injury (spinal nerve ligation) spent more time in the chamber of the apparatus where they received an analgesic compared to time spent in the chamber where they received saline. Rats without an injury had no such preference, suggesting that it is the analgesic properties of the drug that are driving the preference. Thus, this measure suggests that rats may be experiencing persistent pain during normal activities after a nerve injury.
What is the role of cingulate cortex activation in rodent pain behavior?
The anterior cingulate cortex is a region that is often activated in pain-related human brain imaging, including those involving neuropathic pain. Nevertheless, in the current study we only found increased cingulate activity in the formalin pain model and not the neuropathic pain model. Evidence from human studies suggests that activation in the cingulate cortex may be related to the affective-motivational aspects of pain processing (Rainville et al., 1997). Thus, our findings of cingulate activation in an acute, but not chronic pain, model could indicate that rodents have a less pronounced emotional response to chronic nerve injury than humans. However, such a comparison is difficult to make, since the human studies involved repeated application of a tactile stimulus, whereas the natural movements of the rats in our studies probably provided a less reliable tactile stimulus. Further, even for strong acute pain stimuli, such as electric shock, rodent studies do not reliably find cingulate activation. Using noxious electrical forepaw stimulation two studies found activations in both S1 forepaw and cingulate cortex (Tuor et al., 2000; Zhao et al., 2012) whereas two other studies using similar stimuli observed S1 forepaw activation, without a corresponding activation in cingulate cortex (Bosshard et al., 2010; Lowe et al., 2007). In fact, Lowe et al. actually observed a deactivation in cingulate cortex of the injured animals compared to controls. In any case, in the current study, the difference in cingulate activation between the acute inflammatory pain stimulus that evoked pain-related behavior and the chronic nerve injury that did not evoked such behavior could be related to the behavioral drive evoked by the noxious stimulus. As previously discussed, there is an evolutionary imperative in rodents to not display pain-related behavior; this drive may be reduced in humans, leading to more reliable pain-related cingulate activation.
Conclusion
The similar somatosensory brain activations in awake formalin-injected rats and nerve-injured rats three weeks post-injury are consistent with the hypothesis that nerve injured rodents may have persistent chronic pain despite the absence of pain-related behaviors observed in response to acute injuries. The finding that S1 activity is independent of pain-related behavior in both the acute and chronic models suggests that such activity is related to the afferent nociceptive signal and not efferent motor activity. Other studies have shown both spontaneous discharge and increased tactile sensitivity within afferent pain pathways of humans and rodents after nerve injury, so that both types of activity may well contribute to persistent pain and S1 hindlimb activation seen in our study. Finally, our findings illustrate the disruptive effect of anesthesia, and the value of moving towards unanesthetized rodent brain imaging methods.
HIGHLIGHTS.
Patterns of rodent brain activity were investigated using microPET-FDG.
Acute paw inflammation produced responses in somatosensory and cingulate cortices.
Somatosensory cortex was activated with chronic nerve injury.
Anesthesia eliminated nerve-injury related cortical activation.
Acknowledgments
This work was partially funded by grants to Dr. Bushnell from AstraZeneca & Pfizer Canada. This research was also partially funded by the Intramural Research Program of the National Center for Complementary and Alternative Medicine, National Institutes of Health. Scott J Thompson is supported by The Louise and Alan Edwards Foundation’s Edwards PhD. Studentship in Pain Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. European journal of pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. The journal of pain : official journal of the American Pain Society. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Tolle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. Pain. 2009;146:34–40. doi: 10.1016/j.pain.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L. CNS animal fMRI in pain and analgesia. Neuroscience and biobehavioral reviews. 2011;35:1125–1143. doi: 10.1016/j.neubiorev.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshard SC, Baltes C, Wyss MT, Mueggler T, Weber B, Rudin M. Assessment of brain responses to innocuous and noxious electrical forepaw stimulation in mice using BOLD fMRI. Pain. 2010;151:655–663. doi: 10.1016/j.pain.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Campero M, Serra J, Marchettini P, Ochoa JL. Ectopic impulse generation and autoexcitation in single myelinated afferent fibers in patients with peripheral neuropathy and positive sensory symptoms. Muscle & nerve. 1998;21:1661–1667. doi: 10.1002/(sici)1097-4598(199812)21:12<1661::aid-mus6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Casteels C, Vermaelen P, Nuyts J, Van Der Linden A, Baekelandt V, Mortelmans L, Bormans G, Van Laere K. Construction and evaluation of multitracer small-animal PET probabilistic atlases for voxel-based functional mapping of the rat brain. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006;47:1858–1866. [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Fundytus ME, McKenna JE, Dalal S, Melzack R. The formalin test: a validation of the weighted-scores method of behavioural pain rating. Pain. 1993;54:43–50. doi: 10.1016/0304-3959(93)90098-A. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of computer assisted tomography. 1994;18:192–205. [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. The Journal of pharmacology and experimental therapeutics. 1941:74–79. [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Gottrup H, Nielsen J, Arendt-Nielsen L, Jensen TS. The relationship between sensory thresholds and mechanical hyperalgesia in nerve injury. Pain. 1998;75:321–329. doi: 10.1016/s0304-3959(98)00011-6. [DOI] [PubMed] [Google Scholar]
- Guilbaud G, Benoist JM, Levante A, Gautron M, Willer JC. Primary somatosensory cortex in rats with pain-related behaviours due to a peripheral mononeuropathy after moderate ligation of one sciatic nerve: neuronal responsivity to somatic stimulation. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1992;92:227–245. doi: 10.1007/BF00227967. [DOI] [PubMed] [Google Scholar]
- Hofbauer RK, Fiset P, Plourde G, Backman SB, Bushnell MC. Dose-dependent effects of propofol on the central processing of thermal pain. Anesthesiology. 2004;100:386–394. doi: 10.1097/00000542-200402000-00031. [DOI] [PubMed] [Google Scholar]
- Howard MA, Sanders D, Krause K, O’Muircheartaigh J, Fotopoulou A, Zelaya F, Thacker M, Massat N, Huggins JP, Vennart W, Choy E, Daniels M, Williams SC. Alterations in resting-state regional cerebral blood flow demonstrate ongoing pain in osteoarthritis: An arterial spin-labeled magnetic resonance imaging study. Arthritis and rheumatism. 2012;64:3936–3946. doi: 10.1002/art.37685. [DOI] [PubMed] [Google Scholar]
- Ido T, Wan CN, Casella V, Fowler JS, Wolf AP, Reivich M, Kuhl DE. Labeled 2-Deoxy-D-Glucose Analogs - F-18-Labeled 2-Deoxy-2-Fluoro-D-Glucose, 2-Deoxy-2-Fluoro-D-Mannose and C-14-2-Deoxy-2-Fluoro-D-Glucose. Journal of Labelled Compounds & Radiopharmaceuticals. 1978;14:175–183. [Google Scholar]
- Jourdan D, Ardid D, Eschalier A. Analysis of ultrasonic vocalisation does not allow chronic pain to be evaluated in rats. Pain. 2002;95:165–173. doi: 10.1016/s0304-3959(01)00394-3. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum HI, Araujo DM, Annala AJ, Tatsukawa KJ, Phelps ME, Cherry SR. In vivo imaging of neuronal activation and plasticity in the rat brain by high resolution positron emission tomography (microPET) Nature biotechnology. 2000;18:655–660. doi: 10.1038/76509. [DOI] [PubMed] [Google Scholar]
- Laird JM, Bennett GJ. An electrophysiological study of dorsal horn neurons in the spinal cord of rats with an experimental peripheral neuropathy. Journal of neurophysiology. 1993;69:2072–2085. doi: 10.1152/jn.1993.69.6.2072. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nature methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacological reviews. 2001;53:597–652. [PubMed] [Google Scholar]
- Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer--a PET study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1993;34:1–6. [PubMed] [Google Scholar]
- Low LA, Millecamps M, Seminowicz DA, Naso L, Thompson SJ, Stone LS, Bushnell MC. Nerve injury causes long-term attentional deficits in rats. Neuroscience letters. 2012 doi: 10.1016/j.neulet.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Lowe AS, Beech JS, Williams SC. Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation. NeuroImage. 2007;35:719–728. doi: 10.1016/j.neuroimage.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Luyten L, Casteels C, Vansteenwegen D, van Kuyck K, Koole M, Van Laere K, Nuttin B. Micro-positron emission tomography imaging of rat brain metabolism during expression of contextual conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:254–263. doi: 10.1523/JNEUROSCI.3701-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Graham AC, Ritchie J, Hughes SF, Austin JS, Schorscher-Petcu A, Langford DJ, Bennett GJ. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Molecular pain. 2010;6:34. doi: 10.1186/1744-8069-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M, Nystrom B, Wallin U, Hagbarth KE. Ectopic sensory discharges and paresthesiae in patients with disorders of peripheral nerves, dorsal roots and dorsal columns. Pain. 1984;20:231–245. doi: 10.1016/0304-3959(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Ochoa JL, Campero M, Serra J, Bostock H. Hyperexcitable polymodal and insensitive nociceptors in painful human neuropathy. Muscle & nerve. 2005;32:459–472. doi: 10.1002/mus.20367. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Casey KL, Morrow TJ. Long-term changes in behavior and regional cerebral blood flow associated with painful peripheral mononeuropathy in the rat. Pain. 2002;95:31–40. doi: 10.1016/s0304-3959(01)00370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press/Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83:459–470. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Bushnell MC. Pain imaging in health and disease--how far have we come? J Clin Invest. 2010;120:3788–3797. doi: 10.1172/JCI43498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P, Glynn C, Brooks J, McQuay H, Jack T, Chessell I, Bountra C, Tracey I. An fMRI study of cerebral processing of brush-evoked allodynia in neuropathic pain patients. NeuroImage. 2006;32:256–265. doi: 10.1016/j.neuroimage.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. NeuroImage. 2009;47:1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SJ, Bushnell MC. Rodent functional and anatomical imaging of pain. Neuroscience letters. 2012;520:131–139. doi: 10.1016/j.neulet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Tuor UI, Malisza K, Foniok T, Papadimitropoulos R, Jarmasz M, Somorjai R, Kozlowski P. Functional magnetic resonance imaging in rats subjected to intense electrical and noxious chemical stimulation of the forepaw. Pain. 2000;87:315–324. doi: 10.1016/S0304-3959(00)00293-1. [DOI] [PubMed] [Google Scholar]
- Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain. 2011;152:990–1000. doi: 10.1016/j.pain.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VC, Norbury TA, Rice AS. Ultrasound vocalisation by rodents does not correlate with behavioural measures of persistent pain. European journal of pain. 2005;9:445–452. doi: 10.1016/j.ejpain.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Woolfe G, MacDonald A. The evaluation of the analgesic action of pethidine hydrochloride (Demerol) The Journal of pharmacology and experimental therapeutics. 1944:300–307. [Google Scholar]
- Zhao F, Welsh D, Williams M, Coimbra A, Urban MO, Hargreaves R, Evelhoch J, Williams DS. fMRI of pain processing in the brain: A within-animal comparative study of BOLD vs. CBV and noxious electrical vs. noxious mechanical stimulation in rat. NeuroImage. 2012;59:1168–1179. doi: 10.1016/j.neuroimage.2011.08.002. [DOI] [PubMed] [Google Scholar]