Abstract
Objective
Long, uninterrupted bouts of sedentary behavior are thought to negatively influence postprandial glucose and insulin concentrations. We examined the effects of a 1-h bout of morning exercise versus intermittent walking bouts of short duration on glucose excursions and insulin secretion over 12-h.
Materials/Methods
Eleven young, obese individuals (18–35y, BMI>30kg/m2) with impaired glucose tolerance were studied on three 12-h study days: 1) sedentary behavior (SED); 2) sedentary behavior with 1-h morning exercise (EX) at 60–65% VO2peak; and 3) sedentary behavior with 12-hourly, 5-min intervals of exercise (INT) at 60–65% VO2peak. Meals (1046 kJ/meal) were provided every 2-h. Blood samples were collected every 10 min and measured for glucose, insulin, and c-peptide concentrations.
Results
Glucose iAUC (12-h) was attenuated in the INT and SED conditions compared to the EX condition (P<0.05). Glucose concentrations were higher in the EX compared to the SED condition for ~150 min (20% of the study day), and comparison of the EX-INT study days revealed that glucose concentrations were greater for ~ 240 minutes (~1/3 of the 12-h day). In the SED condition, the 12-h insulin iAUC was ~15% higher (P<0.05) compared to the INT and EX conditions. Insulin production rate was found to increase ~20% with INT exercise vs. the SED and EX condition (P<0.05).
Conclusions
Short, frequent periods of exercise attenuated glucose excursions and insulin concentrations in obese individuals to a greater degree than an equal amount of exercise performed continuously in the morning.
Keywords: obesity, insulin, glucose, sedentary, physical activity
Introduction
Type 2 diabetes is characterized by repeated hyperglycemic periods throughout the day that can eventually result in numerous health complications. Increased physical activity has been shown to reduce these hyperglycemic excursions (1), and is known to reduce the risk of complications of type 2 diabetes. Muscle glucose transport and reduced insulin secretion are seen both acutely and chronically with physical activity in both lean and obese individuals with impaired fasting glucose concentrations (2, 3). The reduced insulin demand is closely associated with the contraction-mediated GLUT-4 translocation in skeletal muscle, resulting in increased glucose uptake during and following exercise. One study demonstrated a 51% reduction in insulin concentrations, which corresponded with a 48% reduction in the secretory rate of insulin following an hour-long bout of low-intensity (40% VO2peak) exercise (4).
Recent investigations also point to the negative aspects of accumulating long periods of sedentary behavior regardless of adherence to physical activity guidelines, and recommend the use of short bouts of activity to break up sedentary periods throughout the day (5, 6). An increased number of breaks in sedentary behavior, corresponding with short active bursts, are associated with reduced 2-h plasma glucose concentrations in middle-aged, healthy individuals following an oral glucose tolerance test (7). Dunstan and colleagues (8) recently reported lower glucose and insulin responses to a single test drink in obese adults with the addition of light- and moderate-intensity walking during the 5 h testing period. These findings (7, 8) raised the question of whether short, frequent bouts of exercise would be more beneficial than 1-h of acute morning exercise in modulating insulin secretion and glucose excursions when multiple meals are consumed over the course of an entire day.
The purpose of this study was to determine the effect of exercise on glucose and insulin concentrations during a 12-h study period in obese individuals with impaired fasting glucose concentrations. It was hypothesized that 1-h of walking in the morning or accumulated through short, frequent bouts throughout the 12-h study period would attenuate glucose excursions and insulin secretion to multiple meals over the course of a day as compared to a sedentary condition. We also hypothesized that the short, frequent walking bouts would improve glucose control more so than the single 1-h exercise bout, and this would be independent of insulin concentrations. Further previous studies have only examined postprandial glucose or insulin responses to a single OGTT, and did not examine the hormone responses over the course of an entire day when multiple meals are consumed. Since the glucose and insulin responses to the first meal are not replicated in subsequent meals (known as the second-meal phenomenon) (9–11), and that there is evidence that glucose tolerance (12, 13) exhibits diurnal patterns, it is possible that postprandial glucose and insulin responses may also demonstrate altered responses with subsequent meals, and that this response may be altered through various patterns of physical activity. This study allowed us to examine the hormonal response across a 12 h day, and with frequent blood sampling identify how responses differ with different exercise patterns.
Methodology
Study subjects
All subjects completed a written informed consent document prior to participation in this study which was approved by the Syracuse University Institutional Review Board. Details of this study have been published previously (14). Subjects were young (18–35 years old), obese (BMI >30 kg/m2) individuals with impaired fasting glucose concentrations (>5.55 mmol/L following a 12-h fast). Inclusion criteria were non-hypertensive; total cholesterol <11.1mmol/L and low-density lipoprotein cholesterol <8.88mmol/L; with no known cardiovascular disease. Exclusion criteria included weight loss or gain in the prior 3 months, gastrointestinal problems, type 2 diabetes, or orthopedic limitations to normal walking activity. All subjects engaged in light to moderate walking no more than five times per week. Female subjects did not use oral contraceptive agents, and were consistently tested within the first eight days of their menstrual cycle.
Experimental Design
Each subject reported to the Human Performance Lab on three separate occasions for 12-h of meal testing, beginning at 0700 h. Each subject completed all three conditions, including sedentary (SED), exercise (EX; 60–65% VO2 peak; 1-h continuous bout from 0705–0805 h), and intermittent exercise protocols (INT; 12 bouts of 5 min duration, hourly). Subjects were randomly allocated to each intervention (14). Exercise duration and intensity were matched between the EX and INT study days.
Protocols
After completing written informed consent, each subject’s habitual dietary intake and meal frequency, general health, physical activity, and physical inactivity levels were obtained using questionnaires. Height and weight were obtained. Body composition was assessed using air-displacement plethysmography (BODPOD system, Life Measurement, Inc. Concorde, CA). Aerobic capacity was assessed using a continuous treadmill exercise stress test (15) in accordance with American College of Sports Medicine guidelines(16). The results were used for assigning exercise intensity during the study days.
Study Day
Subjects reported to the Human Performance Laboratory at 0700, were fasted, abstained from caffeine consumption for the previous 12-h, and from alcohol and structured exercise for at least 24-h. Subjects recorded their dietary intake for 3 days prior to each testing day, and this was used as a guide to consume similar foods and amounts in the days leading up to the two remaining visits. Upon arrival to the lab, subjects had a catheter inserted in their antecubital vein. Baseline blood samples were drawn prior to the ingestion of the first meal, and at 10 min intervals thereafter for the remainder of the 12-h study day. Liquid meals were used for each trial and were matched for energy content (14). The energy-matched meal conditions consisted of 6276 kJ of a high carbohydrate liquid (15% protein (PRO), 65% carbohydrate (CHO), 20% fat (FAT)), consumed in evenly-spaced intervals as six small meals (~1046 kJ/meal). The macronutrient composition presented was very similar to that used in previous work (17, 18) (Wegmans, Rochester, NY, USA). All subjects sat quietly throughout the day in the lab, with extremely limited physical activity outside of designated experimental protocols.
On the EX study day, the exercise bout (1-h) was performed immediately following the baseline blood sample and the first liquid meal; on the INT study day the first bout of exercise (5 min) was also immediately following the baseline blood sample and first liquid meal, and then subsequently 5 min bouts of exercise hourly for the remainder of the day. Treadmill grade and speed were monitored to produce a workload of 60–65% of the VO2 peak value from initial aerobic testing, and this workload was confirmed during a familiarization visit prior to the initial study day.
Blood samples were centrifuged, and stored at −80°C for subsequent analysis. Samples were assayed in duplicate for serum glucose using glucose oxidase assay (Sigma-Aldrich Corp., St. Louis, MO). Serum insulin and c-peptide concentrations were measured using Luminex xMap Technology (Linco Research, St. Charles, MO) on a Luminex 100/200 platform (Luminex Corporation, Austin, TX) following manufacturer’s instructions (Millipore, Billerica, MA) with quality controls. Inter-assay and intra-assay coefficients for insulin and c-peptide were 4.9 and 8.5%, and 6.0 and 8.9%, respectively. The lowest limits of detection in this assay were 137 and 69 pg/ml for insulin and c-peptide, respectively. All samples for a given subject were run in the same assay series.
Statistical Analysis
The c-peptide pulse profile was analyzed using a multi-parameter deconvolution technique (AutoDecon, Pulse_XP software; University of Virginia) designed to derive quantitative estimates of attributes of c-peptide secretory pulses and half-life from measured c-peptide concentrations (19, 20). Basal secretion was estimated for each condition, and assumed not to change during the exercise days (19). C-peptide pulses were considered significant if they were able to be distinguished from zero with a 95% statistical significance as previously reported (19, 21). Each analysis determined five secretory parameters, including 1) the number and temporal location of each discrete secretory burst, 2) half-width of each secretory pulse 3) pulse amplitude (peak per secretory burst), 4) pulse mass (total c-peptide release), and 5) production rate (pulse mass/secretory pulses) (22). Basal secretion was estimated for the control day and was assumed not to change during the exercise days (21). Similar protocols have been utilized to characterize the deconvolution of hormonal patterns during exercise (19, 21).
Incremental area under the curve (iAUC; 12-h and 2-h) for serum glucose, insulin and c-peptide were calculated as the total area corrected for baseline values using the trapezoidal method for all three study conditions (GraphPad Prism version 3.0 for Windows, GraphPad Software, San Diego, California, USA). In addition, 2-h iAUC was calculated as the 2-h period around each meal, and the time point just prior to the meal was used as the new baseline value. A one-way ANOVA with repeated measures assessed the differences in the baseline, peak, 12-h iAUC for glucose, insulin and c-peptide concentrations, and c-peptide deconvolution analysis parameters between study conditions. A two-way ANOVA (time*condition) with Bonferroni adjustments was used to assess differences between 2 h iAUC values for glucose, insulin and c-peptide.
Comparing the spontaneous changes in glucose and insulin concentrations over the course of the day using AUC only can result in a misleading interpretation of the data as the pattern of response is not considered. Thus to objectively assess the glucose and insulin release by exercise against a background of spontaneous pulsatile secretion modulated by diurnal and circadian rhythms, we normalized each subject’s exercise glucose and insulin concentration time trends to the subject’s sedentary concentration time trends, respectively (23). For each study day, we subtracted each subject’s serum hormone concentration at each time point during the SED day from their hormone concentration at the same time point during the EX or INT day (e.g. EX day – SED day day). A curve was derived for the change in serum hormone concentrations over time with simultaneous 95% confidence bands. The 95% confidence regions were derived using bootstrapping technique. The use of this 95% confidence region avoids the problem of multiple point-wise comparisons. A significant response to EX or INT was defined as occurring when the lower 95% confidence limit for the curve was more than zero, and significant suppression of hormone release was defined as occurring when the upper 95% confidence limit for the regression curve was less than zero (23). A graphical comparison of the EX to INT day was also generated.
Significance levels in all statistical tests were accepted at α=0.05. Statistical analyses were performed with SPSS for Windows (version 16.0, SPSS, Inc., Chicago, USA), and all data are reported as mean ± standard error of the mean.
Results
Subject Characteristics
Eleven subjects completed all study visits (3 women, 8 men). The mean age was 25 y, BMI 34 kg/m2, HOMA-IR 0.42±0.12, and QUICKI 2.97±0.10. Female subjects had a higher percent body fat (42.0±3.3 v. 28.9±2.7%), total cholesterol (181±2.4 v. 127.3±2.6 mg/dl) and LDL cholesterol (91.5±10.2 v. 51.3±5.7 mg/dl) than male subjects (P<0.05). The mean resting BP value across subjects was slightly elevated (122/68 mmHg) and the mean fasting blood glucose value was 6.3±0.3 mmol/L. Across testing days, there were no significant differences in body weight in any of the subjects. According to self-report dietary logs and physical activity questionnaires, subjects consumed meals an average of 4.7±0.38 times/day, and participated in walking activity 3.3±0.57 times/wk at a Borg RPE (6–20) level of 13.4±0.5.
VO2 Max Testing and Exercise Intensity
The female subjects had a VO2 peak of 25.5±1.8 ml/kg/min during the exercise stress test, while the male subjects achieved a VO2 peak of 32.6±2.5 ml/kg/min (P>0.05). During the study days, treadmill speed ranged from 3–3.5 mph (3.2±0.1 mph), with grade ranging from 0–8% (2.9±1.0%).
Glucose Concentrations
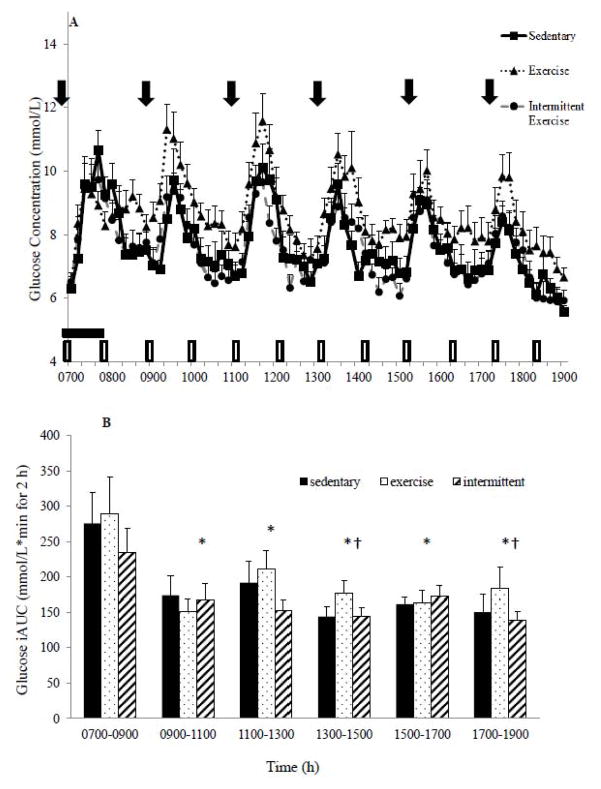
Fasting glucose concentrations and the pattern of glucose response were not significantly different across conditions (Figure 1A). However, there were significant differences in the 12-h glucose iAUC (P=0.021) with glucose concentrations highest in the EX group: SED vs. EX (5536.9±255.3 vs. 6249.6±286.3 mmol/L*min for 12-h; P=0.042); INT vs. EX conditions (5457.0±238.8 6249.6±286.3 mmol/L*min for 12-h; P=0.048). There were also significant differences in the 2-h glucose iAUC over time. The glucose iAUC showed no differences by condition but there were differences across the time of day (P<0.05). The first interval (0700–0900 h) resulted in higher glucose concentrations than any other interval throughout the day (P<0.05), and from 1100–1300 h there was significantly greater glucose than observed at 1300–1500 and 1700–1900 h time periods (Figure 1B; P<0.05).
Figure 1.
A: Pattern of glucose response over the 12 h study day by condition. ⬇ Indicates meals.
 1-h Exercise.
1-h Exercise.
 Intermittent exercise condition; hourly 5-minute walking bouts. B: Two-hour glucose incremental area under the curve (iAUC) across the 12 h study day. Mean±SEM. *P<0.05 vs. 0700–0900h. †P<0.05 vs. 1100–1300h
Intermittent exercise condition; hourly 5-minute walking bouts. B: Two-hour glucose incremental area under the curve (iAUC) across the 12 h study day. Mean±SEM. *P<0.05 vs. 0700–0900h. †P<0.05 vs. 1100–1300h
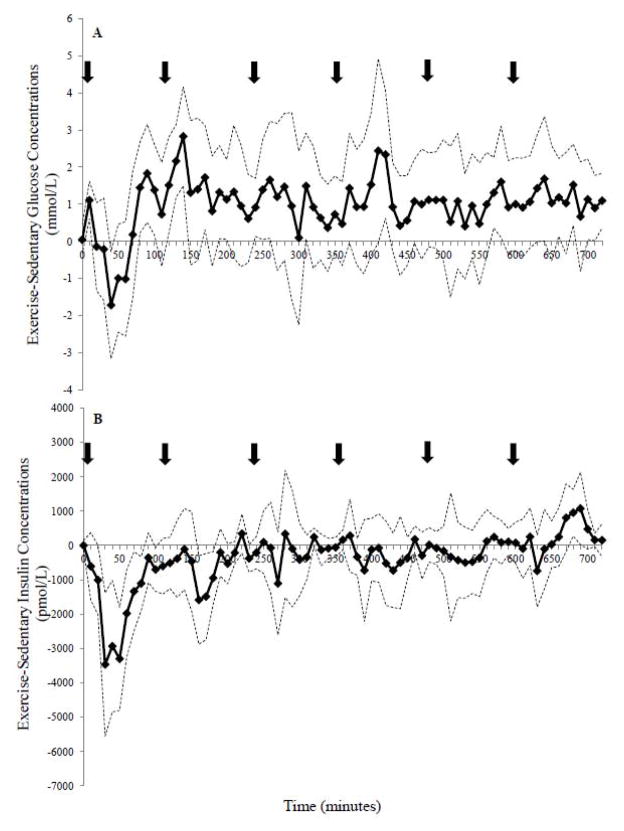
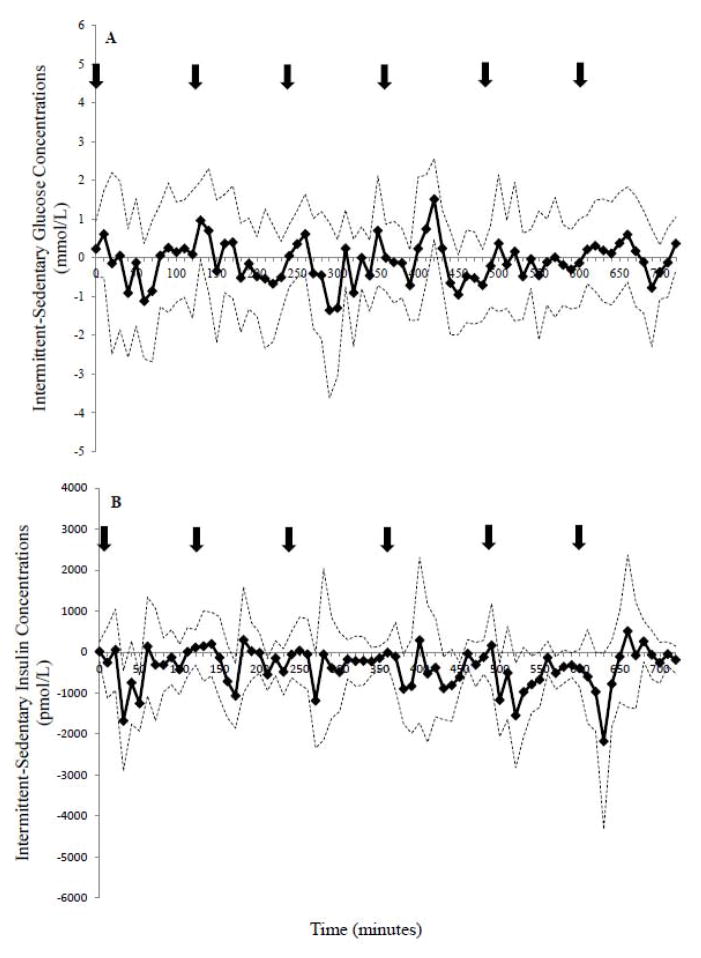
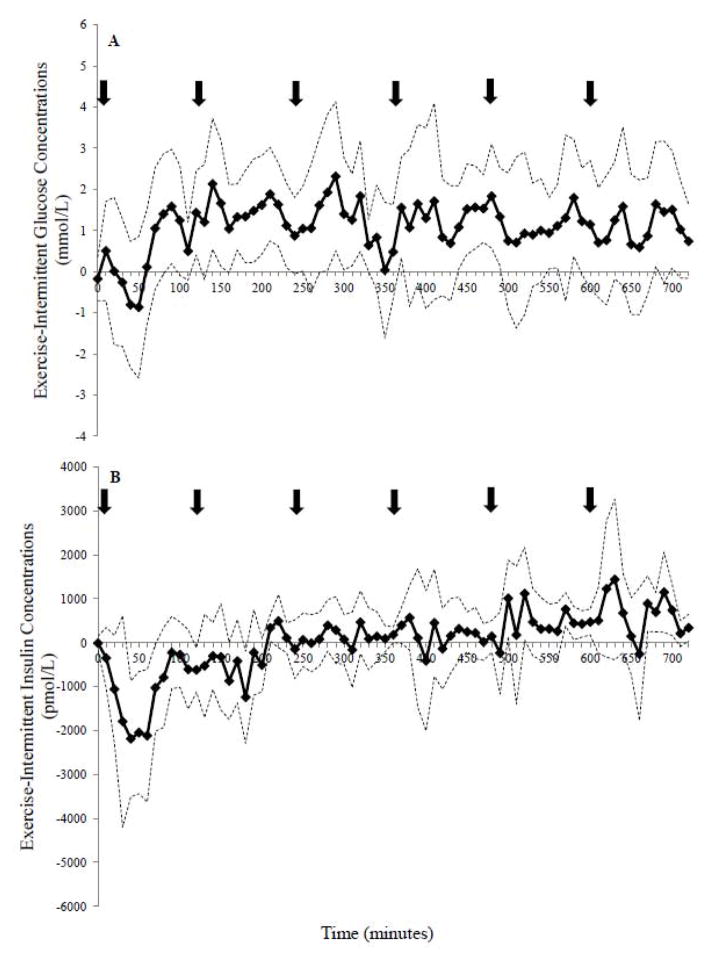
Bootstrapping analysis revealed that glucose concentrations were higher in the EX compared to the SED condition (~150 minutes; 20% of the study day), with the 95% CI above zero during the 10–20, 80–100, 120–150, 170–180, 190–200, 240–260, 410–430, 570–580, 680–690, and 710–720 minute intervals (Figure 3A). In contrast, glucose was only higher between 420–430 minutes with INT-SED comparison (Figure 4A). The EX-INT analysis revealed that glucose concentrations were greater for ~ 240 minutes (~one-third of the 12-h day) (Figure 5A). The 95% CI for the EX-INT was above zero during the 80–100, 120–130, 140–150, 160–230, 280–330, 370–380, 440–490, 550–560, 580–590, and 680–690 minute intervals (Figure 5A).
Figure 3.
The comparison of the exercise-sedentary day for A. glucose and B. insulin concentrations over 12-h. The solid line represents the difference between study days and simultaneous 95% confidence limits (dotted line) are shown. Significant stimulation of glucose or insulin release by exercise over the sedentary day conditions occurred when the lower 95% confidence limit was more than zero. Significant suppression of glucose or insulin release occurred when the upper 95% confidence limit was less than zero.
Figure 4.
Comparison of the magnitudes of the A. glucose and B. insulin response to intermittent exercise over sedentary conditions for the 12 h period. Refer to Fig 3 for more detail.
Figure 5.
The differences in response between the exercise and intermittent exercise day for A) glucose and B): insulin concentrations over 12-h. Refer to Fig 3 for further details.
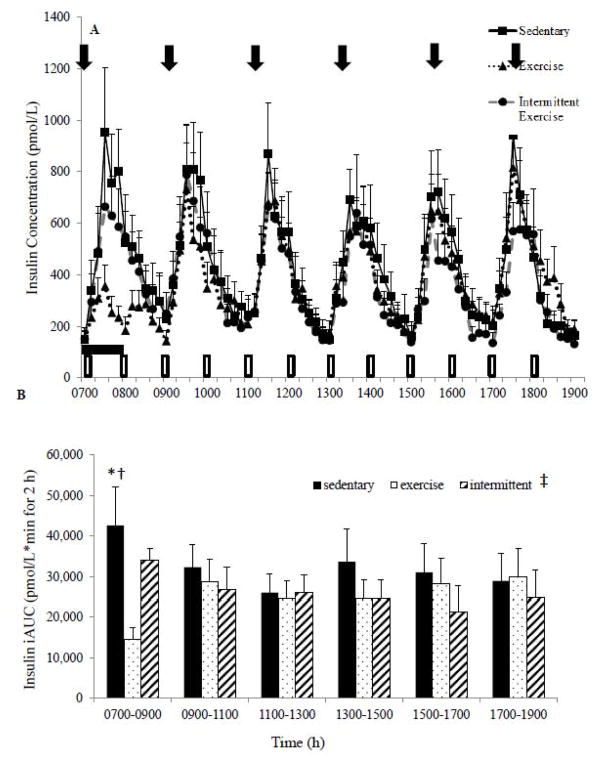
Insulin Concentrations
Fasting insulin concentrations were not significantly different across conditions (Figure 2A). In the SED condition, the 12-h insulin iAUC was higher (P<0.05) compared to the INT and EX conditions. However, no significant differences were observed in the 12-h insulin iAUC response between the EX and INT conditions (P=0.13). Examining the 2-h iAUC revealed a significant condition effect (P=0.02) when collapsed across all 2-h time intervals but no significant difference between intervals (Figure 2B). The SED condition demonstrated higher insulin iAUC than either the EX or SED condition (P=0.01). Examining just the first 2-h interval (0700–0900 h) revealed that the SED condition was significantly higher than the EX condition (P=0.007) and approached significance compared with the INT condition (P=0.06).
Figure 2.
A: Pattern of insulin glucose response over the 12 h study day by condition. ⬇ Indicates meals.
 1-h Exercise.
1-h Exercise.
 Intermittent exercise condition; hourly 5-minute walking bouts.
Intermittent exercise condition; hourly 5-minute walking bouts.
B: Two-hour serum insulin iAUC across the 12 h study day. Mean±SEM. ‡P<0.05 between conditions (SED vs EX P=0.01, SED vs INT P=0.015); no significant difference between the 2-h time intervals; 0700–0900 *P=.007 SED vs EX, †P=0.06 SED vs INT.
The bootstrapping analysis demonstrated lower insulin concentrations in the EX condition than the SED condition early in the day (20–90, 100–180 minute interval) and higher later in the day (Figure 3B). Insulin was shown to be lower in the INT compared to the SED condition during several brief intervals during the comparison (30–40, 50–60, 440–460, 520–530, 530–540, and 570–580 minutes; Figure 4B). Further, insulin concentrations were significantly lower on the EX day compared to INT day between 40–70 min during the exercise bout (Figure 5B). However, towards the end of the 12 h, insulin was higher in the EX (580–610, 680–710 minute intervals; Figure 5B) compared to the INT condition.
C-Peptide Concentrations
Baseline c-peptide concentrations following the overnight fast, and at the start of each 2-h interval during the day, were not different across the three experimental conditions. A condition*time interaction was demonstrated where c-peptide concentrations were lower in the EX condition compared to the SED and INT conditions (4.7±1.3 v. 11.8±1.5 and 9.6±1.8 nmol/L*min for 2-h; P<0.014) during the 0700–0900 time block, the time during which one hour of continuous exercise was performed. In addition, the 2-h c-peptide iAUC in the EX and INT conditions were significantly attenuated when compared to the SED condition across all time blocks (P<0.05).
Deconvolution analysis was employed to estimate insulin secretion from measured c-peptide concentrations (Table 1). There were no significant differences in the number of secretory pulses in 12-h, half-width, half-life or mass of c-peptide secretion. The peak amplitude was significantly lower in the INT condition than the EX and SED condition (P<0.05), while the production rate was significantly higher with INT exercise than the EX condition.
Table 1.
Deconvolution Parameters for C-Peptide
| Sedentary | Exercise | Intermittent Exercise | |
|---|---|---|---|
| Secretory Pulses (pulses/12 h) | 5.7±0.6 | 5.3±0.7 | 7.3±0.8 |
| Half Width (nmol/L) | 22.3±6.5 | 18.4±8.4 | 28.0±4.7 |
| Half Life (min) | 48.8±5.2 | 41.6±6.5 | 47.0±7.9 |
| Mass (nmol/L/pulse) | 2.2±0.3 | 1.8±0.1 | 2.2±0.3 |
| Peak Amplitude (nmol/L/pulse) | 0.33±0.1 | 0.37±0.1 | 0.12±0.1† |
| Production Rate (mass/pulse) | 11.7±4.5 | 10.0±5.5 | 14.5±6.5* |
All values reported as means and standard error of the mean.
P<0.05 EX vs. SED condition.
P<0.05 INT vs. EX condition
Discussion
Chronically elevated glucose concentrations, and a subsequent increase in insulin secretion are characteristic of the progression towards type 2 diabetes. Increased levels of physical activity and exercise are recommended to help with glycemic control by improving insulin resistance. No prior studies have compared intermittent bouts of exercise with a continuous bout of exercise in controlling the glycemic excursions and insulin secretion over the course of 12-h, and while meals are being consumed. The major findings of this study were: 1) 1-h of morning exercise compared to 5 min bouts of exercise hourly resulted in higher glucose concentrations throughout much of the day; 2) glucose concentrations were higher with the EX condition than the INT condition for about 240 min of the 12 h period (or 1/3 of the time); 3) 2-h iAUC for insulin and c-peptide was reduced in the INT and EX conditions, compared to the SED condition; and 4) intermittent exercise altered insulin secretory parameters compared with the EX and SED condition.
Our findings of increased glucose concentrations (20% greater on EX day than SED day) were unexpected, particularly since 1-h of exercise meets or exceeds recommendations designed to reduce hyperglycemia in individuals with or at risk for T2D (16). Further the observation that glucose concentrations were higher for more than 240 min on the EX day than INT was also unexpected. The elevated glucose levels with morning exercise may be related to the exercise demands for glucose as well as the previous 12-h period of fasting and relatively small carbohydrate intake (~40g) prior to undertaking the 1-h exercise (24). Under these conditions, there appears to be increased hepatic gluconeogenesis and hepatic glycogenolysis. Supporting this, Trimmer et al (25) reported that highly trained males responded to 90 minutes of moderate-intensity exercise in a fasted state, with a 128% increase in gluconeogenesis, and 203% increase in hepatic glycogenolysis (characterized by a 135% increase in glucose production). A similar increase in glucose production (25) may explain the increased glucose iAUC seen in the EX condition in our obese subjects following exercise. This is plausible given the low insulin concentrations during and immediately following exercise, indicating a favorable physiological environment for gluconeogenesis and hepatic glycogeneolysis.
The increased glucose concentration in the EX condition may also relate to an increased oxidation of free fatty acids when compared to glucose. A similar exercise protocol in healthy, obese individuals demonstrated that 1-h of exercise (50% VO2max) increased plasma catecholamine concentrations and markers of lipid oxidation well into the recovery period (26). Furthermore, Borer et al (27) noted a 20% increase in glucose concentrations when glucose-tolerant, obese females performed 2-h of low-intensity walking (40–45% VO2max) an hour after a high-carbohydrate meal. Considering the lack of research examining the interaction between meal consumption and exercise on insulin secretion, substrate metabolism, and glucose excursions in obese individuals, we can only postulate that the meals used in the current study may have exacerbated the glucose excursions observed in the EX condition.
The response of insulin to exercise performed either as one continuous morning session, or twelve short bouts designed to break up the duration of sedentary behavior, is noteworthy. When the EX and INT conditions were compared to SED, there was ~15% reduction in insulin iAUC over the 12-h day. The prevailing insulin concentrations following each individual meal were lower in the INT condition compared to the SED condition throughout the 12-h day, while in the EX condition, there was an expected reduction in the insulin concentrations during the 1-h of exercise which did not persist throughout the remainder of the day. Thus overall, insulin iAUC rose following the initial 2-h interval in the EX condition with the subsequent meal stimuli, while the short, frequent bouts of activity maintained lower insulin excursions throughout the day. The work of Healy et al (7) demonstrated that indices of impaired glucose tolerance are inversely related to the daily number of breaks in sedentary time. Individuals with metabolic syndrome tend to spend longer periods of the day in uninterrupted sedentary behavior when compared to their healthy peers (28). The current findings highlight the importance of interrupting daily sedentary time with light ambulatory activity on reducing circulating insulin levels, either as a stand-alone therapy, or in concert with a structured physical activity program (7, 8, 28).
A major strength of the present study is the frequent blood samples obtained, allowing for study of the pattern of response as well as deconvolution analysis of the c-peptide concentrations to model insulin secretion over the course of the 12-h period. As insulin and c-peptide are secreted in a 1:1 ratio, the measurement of c-peptide allows an accurate estimation of the pattern of insulin secretion (19). While no significant difference was found, the number of c-peptide pulses increased by 1.6–2 pulses/12-h during the INT condition. The INT exercise condition increased insulin production rate by 20–30%, while peak amplitude decreased by ~60% with the INT condition compared to the SED or EX days. Despite no significant difference in c-peptide half-life between conditions, the half-life of c-peptide clearance in the EX condition was 17% lower than the SED and 13% lower than the INT conditions, respectively. Thus this study demonstrated that short bouts of exercise can alter insulin secretion patterns, and in particular lower peak amplitude while increasing production rate.
Additional strengths of this study include studying our subjects in response to multiple meals (vs. a single meal) over the course of 12-h so that the pattern of the glucose and insulin response could be carefully studied. Further bootstrapping analysis employed allowed us to more clearly establish the changes in insulin and glucose concentrations over time.
There are important experimental considerations regarding the present study that should be acknowledged. Large inter-individual variability in glycemic control is a hallmark in this population group. A recent study (8)with a similar design indicated that a sample size of 8 subjects would yield a power of .85 and an alpha of 0.05. Therefore, while the sample size of 11 should have provided adequate power (0.80), the findings in the present study are reflective only of the specific subject population recruited to the study, namely healthy, young obese individuals without evidence of overt chronic diseases. Further the exercise intervention was acute in nature and the long term implications can only be speculated. However, our findings do indicate that interrupting sedentary behavior with 5 min of activity will enhance glycemic control. Further we had subjects consume frequent liquid meals and possibly a different response may be observed if only 3 meals/day were consumed.
Our findings support the hypothesis that short, frequent periods of exercise over the course of a day attenuate glucose excursions and reduce insulin concentrations in obese individuals to a greater degree than an equal amount of exercise performed continuously in the morning. These short bouts of exercise affected insulin secretion and may have altered insulin clearance patterns, while 1-h of morning exercise followed by a day of sedentary behavior may exacerbate glucose responses to meal consumption in this population. However caution should be heeded that these findings are a reflection of an acute response to one day of exercise, and further research is needed to evaluate the long-term effects of continuous versus intermittent periods of exercise on glycemic control and the prevention of diabetes.
Acknowledgments
We thank the individuals who participated in this study. This study was supported by a NIH R21DK084467-01 grant.
Abbreviations
- BMI
body mass index
- SED
sedentary behavior
- EX
1-h of morning exercise
- INT
12 hourly, 5 min intervals of exercise
- VO2peak
measure of aerobic capacity- peak oxygen consumption
- T2D
type 2 diabetes
- iAUC
incremental area under the curve
- HOMA
Homeostasis Model Assessment (HOMA) estimates steady state beta cell function (%B) and insulin sensitivity (%S)
- QUICKI
Quantitative Insulin Sensitivity Check Index
- PRO
protein
- CHO
carbohydrate
- OGTT
oral glucose tolerance test
Footnotes
There were no conflicts of interest for any of the authors of this manuscript.
Disclosure statement: There is nothing to disclose for any of the authors.
- Holmstrup - design and conduct of the study, data collection and analysis, data interpretation and manuscript writing.
- Keslacy - conduct of the study, data interpretation and manuscript writing.
- Fairchild - design of the study, data analysis, data interpretation and manuscript writing.
- Weinstock - data interpretation and manuscript writing.
- Kanaley - design and conduct of the study, data collection and analysis, data interpretation and manuscript writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Dijk JW, Tummers K, Stehouwer CD, Hartgens F, van Loon LJ. Exercise therapy in type 2 diabetes: is daily exercise required to optimize glycemic control? Diabetes Care. 2012;35(5):948–54. doi: 10.2337/dc11-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol. 1997;273(6 Pt 1):E1039–51. doi: 10.1152/ajpendo.1997.273.6.E1039. [DOI] [PubMed] [Google Scholar]
- 3.LeBlanc J, Nadeau A, Richard D, Tramblay A. Studies on the sparing effect of exercise on insulin requirements in human subjects. metabolism. 1981;30:1119–24. doi: 10.1016/0026-0495(81)90057-3. [DOI] [PubMed] [Google Scholar]
- 4.Levitt NS, Hirsch L, Rubenstein AH, Polonsky KS. Quantitative evaluation of the effect of low-intensity exercise on insulin secretion in man. Metabolism. 1993;42(7):829–33. doi: 10.1016/0026-0495(93)90054-r. [DOI] [PubMed] [Google Scholar]
- 5.Hu F, Stampfer M, Solomon C, Liu S, Colditz G, Speizer F, et al. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;134(2):96–104. doi: 10.7326/0003-4819-134-2-200101160-00009. [DOI] [PubMed] [Google Scholar]
- 6.Magliano DJ, Barr EL, Zimmet PZ, Cameron AJ, Dunstan DW, Colagiuri S, et al. Glucose indices, health behaviors, and incidence of diabetes in Australia: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2008;31(2):267–72. doi: 10.2337/dc07-0912. [DOI] [PubMed] [Google Scholar]
- 7.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–6. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 8.Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–83. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins DJ, Wolever TM, Buckley G, Lam KY, Giudici S, Kalmusky J, et al. Low-glycemic-index starchy foods in the diabetic diet. Am J Clin Nutr. 1988;48(2):248–54. doi: 10.1093/ajcn/48.2.248. [DOI] [PubMed] [Google Scholar]
- 10.Jovanovic A, Gerrard J, Taylor R. The second-meal phenomenon in type 2 diabetes. Diabetes Care. 2009;32(7):1199–201. doi: 10.2337/dc08-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolever TMS, Jenkins DJA, Vuksan V, Jenkins AL, Bucklen GC, Wong GS. Beneficial effect of a low glycemic index diet in type II diabetes. Diabetic med. 1992;9:451–8. doi: 10.1111/j.1464-5491.1992.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 12.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61(11):2691–700. doi: 10.2337/db11-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262(4 Pt 1):E467–75. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- 14.Holmstrup ME, Fairchild TJ, Keslacy S, Weinstock RS, Kanaley JA. Satiety, but not total PYY, Is increased with continuous and intermittent exercise. Obesity (Silver Spring) 2013;21(10):2014–20. doi: 10.1002/oby.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanaley JA, Goulopoulou S, Franklin RM, Baynard T, Holmstrup ME, Carhart R, Jr, et al. Plasticity of heart rate signalling and complexity with exercise training in obese individuals with and without type 2 diabetes. Int J Obes (Lond) 2009;33(10):1198–206. doi: 10.1038/ijo.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ACSM’s Guidelines for Exercise Testing and Prescription. 8. Baltimore: Lippincott, Williams and Wilkins; 2010. [Google Scholar]
- 17.James O, Day C. Non-alcoholic steatohepatitis: another disease of affluence. Lancet. 1999;353(9165):1634–6. doi: 10.1016/S0140-6736(99)00163-4. [DOI] [PubMed] [Google Scholar]
- 18.Solomon TP, Chambers ES, Jeukendrup AE, Toogood AA, Blannin AK. The effect of feeding frequency on insulin and ghrelin responses in human subjects. Br J Nutr. 2008:1–10. doi: 10.1017/S000711450896757X. [DOI] [PubMed] [Google Scholar]
- 19.Engdahl JH, Veldhuis JD, Farrell PA. Altered pulsatile insulin secretion associated with endurance training. J Appl Physiol. 1995;79(6):1977–85. doi: 10.1152/jappl.1995.79.6.1977. [DOI] [PubMed] [Google Scholar]
- 20.Johnson ML, Veldhuis PP, Grimmichova T, Farhy LS, Evans WS. Validation of a deconvolution procedure (AutoDecon) for identification and characterization of fasting insulin secretory bursts. J Diabetes Sci Technol. 2010;4(5):1205–13. doi: 10.1177/193229681000400521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritzlaff CJ, Wideman L, Weltman JY, Abbott RD, Gutgesell ME, Hartman ML, et al. Impact of acute exercise intensity on pulsatile growth hormone release in men. J Appl Physiol. 1999;87:498–504. doi: 10.1152/jappl.1999.87.2.498. [DOI] [PubMed] [Google Scholar]
- 22.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54(6):1649–56. doi: 10.2337/diabetes.54.6.1649. [DOI] [PubMed] [Google Scholar]
- 23.Kanaley JA, Weltman JY, Pieper KS, Weltman A, Hartman ML. Cortisol and growth hormone responses to exercise at different times of day. J Clin Endocrinol Metab. 2001;86:2881–9. doi: 10.1210/jcem.86.6.7566. [DOI] [PubMed] [Google Scholar]
- 24.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29(7):939–60. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 25.Trimmer JK, Schwarz JM, Casazza GA, Horning MA, Rodriguez N, Brooks GA. Measurement of gluconeogenesis in exercising men by mass isotopomer distribution analysis. J Appl Physiol. 2002;93(1):233–41. doi: 10.1152/japplphysiol.01050.2001. [DOI] [PubMed] [Google Scholar]
- 26.Marion-Latard F, Crampes F, Zakaroff-Girard A, De Glisezinski I, Harant I, Stich V, et al. Post-exercise increase of lipid oxidation after a moderate exercise bout in untrained healthy obese men. Horm Metab Res. 2003;35(2):97–103. doi: 10.1055/s-2003-39051. [DOI] [PubMed] [Google Scholar]
- 27.Borer KT, Wuorinen EC, Lukos JR, Denver JW, Porges SW, Burant CF. Two bouts of exercise before meals, but not after meals, lower fasting blood glucose. Med Sci Sports Exerc. 2009;41(8):1606–14. doi: 10.1249/MSS.0b013e31819dfe14. [DOI] [PubMed] [Google Scholar]
- 28.Bankoski A, Harris TB, McClain JJ, Brychta RJ, Caserotti P, Chen KY, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34(2):497–503. doi: 10.2337/dc10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]