Abstract
Many studies have examined brain states in an effort to predict individual differences in capacity for learning, with overall moderate results. The present study investigated how measures of cortical network function acquired at rest using dense-array EEG (256 leads) predict subsequent acquisition of a new motor skill. Brain activity was recorded in 17 healthy young subjects during three minutes of wakeful rest prior to a single motor skill training session on a digital version of the pursuit rotor task. Practice was associated with significant gains in task performance (% time on target increased from 24% to 41%, p < 0.0001). Using a partial least squares regression (PLS) model, coherence with the region of the left primary motor area (M1) in resting EEG data was a strong predictor of motor skill acquisition (R2 = 0.81 in a leave-one-out cross-validation analysis), exceeding the information provided by baseline behavior and demographics. Within this PLS model, greater skill acquisition was predicted by higher connectivity between M1 and left parietal cortex, possibly reflecting greater capacity for visuomotor integration, and by lower connectivity between M1 and left frontal-premotor areas, possibly reflecting differences in motor planning strategies. EEG coherence, which reflects functional connectivity, predicts individual motor skill acquisition with a level of accuracy that is remarkably high compared to prior reports using EEG or fMRI measures.
Keywords: EEG, motor learning, coherence, PLS
Introduction
Individuals demonstrate significant variability in motor learning (Ackerman, 1987; King et al., 2012). The ability to predict an individual’s learning skill could have utility in a number of settings, including clinical (Stinear, 2010). Previous studies have identified neural correlates of variability during motor learning (Tomassini et al., 2011), and both structural and functional neuroimaging methods have been evaluated as predictors of motor learning (Mathewson et al., 2012; Vo et al., 2011). However, the ability to accurately predict learning differences, in healthy or diseased populations, remains modest, for example, with fMRI-derived resting-state connectivity accounting for 35% (Wang et al., 2010) to 66% (Baldassarre et al., 2012) of inter-individual variability.
Recent resting-state studies have provided new inroads for measuring differences in brain function in relation to behavior across individual subjects (Deco et al., 2011). Markers of brain function at rest are influenced by experience (Lewis et al., 2009) and reflect the functional organization of brain networks that are selectively engaged during behavioral tasks. Organization of brain networks at rest has also been correlated with subsequent behavioral performance (Hampson et al., 2006; Tambini et al., 2010). However, there is limited study of how inter-individual heterogeneity in brain functional connectivity at rest relates to learning and plasticity.
Combined EEG and fMRI studies have reported that specific combinations of EEG rhythms correspond with low frequency activity of specific resting-state networks (Mantini et al., 2007). Thus, EEG metrics also may be useful for characterizing brain states and relating them to behavioral variance. One potential metric is spectral power, which measures synchronization within cortical regions (Nunez and Srinivasan, 2006). A recent EEG study found that a regional measure of spectral power in a frontal electrode (Fz) and a parietal electrode (Pz) obtained early during training predicted 53% of the variance in subsequent motor learning (Mathewson et al., 2012). An alternate EEG-based metric is spectral coherence, which measures synchronization between regions and thus can capture cortical connectivity. In various studies of motor function using EEG coherence, changes in brain connectivity have been observed in the β (20–30 Hz) frequency range (Deeny et al., 2009; Pfurtscheller et al., 1996; Tropini et al., 2011).
Measures of connectivity, as compared to assessments of focal brain regions, have an improved ability to represent complexity in human cortical processing and as a result have a stronger relationship with many types of behavior (Bullmore and Sporns, 2009). Therefore, the present study hypothesized that EEG coherence measures of motor network connectivity in the β band during wakeful rest would predict subsequent motor skill acquisition in a single motor skill training session. Secondarily, it was hypothesized that a PLS approach for deriving brain-behavior relationships would perform better than an ROI based approach. An additional secondary hypothesis was that β band coherence during movement (in the training session) would also be a predictor of subsequent motor skill learning.
Material and methods
2.1 Experimental Design
Healthy subjects, age 18–30 years and right-handed (Edinburgh Handedness Inventory) were recruited. This study was approved by the University of California, Irvine Institutional Review Board. Each subject gave written informed consent.
The experiment took place across a single session. Participants sat in a chair facing a computer monitor atop a desk. In order to minimize variation across participants, awake resting-state EEG was acquired for three minutes (EEG-Rest) at 1000 Hz prior to any description or practice of the motor task. Next, each individual’s maximum arm movement speed was measured, standardized instructions for the visuomotor skill task were provided, and a baseline assessment of the motor skill task was obtained (Test 1), during which EEG was again recorded (EEG-Test 1). Next, two blocks of practice and two additional blocks of motor skill task testing with EEG recording were completed in an interleaved manner (Figure 1A), from which measures of motor skill learning were obtained. Arm movements were recorded by a USB 8“X6” digitizing pen tablet (Genius MousePen, Taipei, Taiwan).
Figure 1.
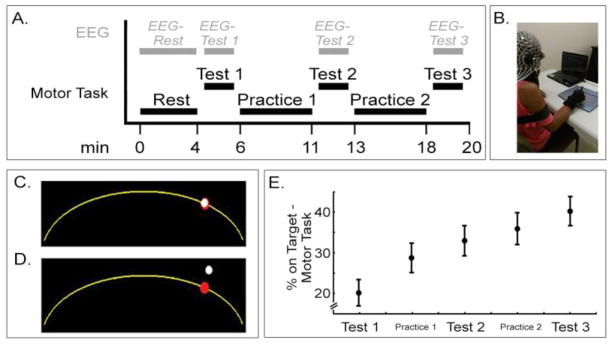
Experimental setup. A. Experiment timeline. B. Digitizing pen tablet and presentation laptop. C. Example of cursor on target. D. Example of cursor off target. E. The % Improvement (Test 3 - Test 1) on the motor task with practice was statistically significant (mean±s.e.; repeated measures ANOVA, F(2,15) = 5.05, p<0.0001).
To measure maximum arm movement speed, two 20-pixel target circles were displayed on the monitor, 1300-pixels apart. Participants were instructed to make horizontal movements between the centers of each circle, as rapidly as possible. The maximum number of targets hit during a 10 second period was recorded, and a maximum movement speed was calculated. The speed test was repeated three times, and the maximum was used to determine the speed that motor task target moved for each individual participant.
The motor skill task used in the current study was a digital version of the classic pursuit rotor task motor learning paradigm (Adams, 1952; Grafton et al., 1994). Subjects viewed a computer monitor on which a target (a 20-pixel diameter red circle) moved, back and forth, along a fixed arc (yellow, spanning a 450-pixel wide and 200-pixel long path), at 50% of each individual’s maximum movement speed. A cursor (15-pixel diameter white circle) was also present, the position of which was controlled by subjects using the digitizing tablet pen held by the right hand (Figure 1B). Subjects were instructed to keep the cursor on the target as the target moved along the arc (Figure 1C).
Participants directed cursor movement by moving the pen tip across the surface of the USB digitizing tablet, maintaining contact of the pen tip on the tablet surface at all times during task performance. To insure arm movements were standardized across participants and were restricted to right shoulder internal/external rotation only, a soft strap was placed on the distal part of the right forearm, minimizing shoulder abduction, and a wrist brace was placed across the distal right arm, minimizing wrist flexion/extension (Figure 1B). Subjects sat with both feet flat on the floor and were not permitted to move at other body joints.
Performance was quantified as percent time that the cursor position was >50% overlapping with target position (% on Target, Figure 1C). A total of three test blocks and two interleaved practice blocks were completed (Figure 1A). Each Test block consisted of a 50 second rest period followed by an 80 second task period. Each Practice block consisted of four 20-second task periods interleaved with three 50-second rest periods. Degree of motor skill acquisition was calculated from absolute change in % on Target from Test 1 to Test 3 (% Improvement).
2.2 EEG Acquisition
Dense-array surface EEG was acquired using a 256-lead Hydrocel net (Electrical Geodesics, Inc., Eugene, OR). Awake resting-state EEG was acquired for three minutes. EEG signal was referenced to Cz during recording and re-referenced to the average of all leads for analysis; an advantage of this approach is that it minimizes common reference effects. A ground electrode was not used. EEG signal was recorded raw with no bandpass filter used.
During EEG-Rest, participants were asked to hold still with the forearms resting on the anterior thigh and to direct their gaze at a fixation cross displayed on the computer monitor. During EEG-Test 1, and subsequent recordings (EEG-Test 2 and EEG-Test 3), participants used their right hand to keep the cursor on the target, as above. Data were collected at 1000 Hz using a high input impedance Net Amp 300 amplifier (Electrical Geodesics) and Net Station 4.5.3 software (Electrical Geodesics).
2.3 EEG Preprocessing
EEG data were exported to Matlab (7.8.0, MathWorks, Inc., Natick, MA) for preprocessing. The continuous EEG signal was low-pass filtered at 50 Hz, segmented into non-overlapping one-second epochs, and detrended. Visual inspection and independent components analysis were used in combination to remove extra-brain artifacts from the EEG. First, epochs were visually inspected for contamination by overt muscle activity, such as from neck or cheek movements, and removed from further analysis. Next, EEG data underwent an Infomax ICA decomposition (EEGLAB (Delorme and Makeig, 2004)). Components that only occurred in one channel were automatically rejected, as were components attributed to muscle artifact (showing high activity at >35 Hz, and generally accompanied by high activity at 4–15 Hz with visual review consistent with muscle artifact). Of the remaining components, amplitude topography, frequency spectra, and component time series were inspected to identify eye blinks, eye movements, and heart rhythms (Delorme et al., 2007), and were removed. Across EEG-Rest and EEG-Test data, 6.8±5.5 of the top 30 components were removed from each EEG recording. Finally, data were transformed back to channel space, and epochs were again visually inspected to insure absence of artifacts. Of all epochs recorded in EEG-Rest and EEG-Test 1 data sets, 92.1±6.8% were retained for further analyses.
2.4 Coherence and Power
EEG coherence was used to estimate functional connectivity (Murias et al., 2007; Nunez and Srinivasan, 2006; Srinivasan et al., 2007). At each frequency band, coherence is a measure of phase consistency between signals recorded at two electrodes. It is reported as a squared correlation coefficient, and expresses the fraction of variance at a given electrode in one frequency band that can be linearly predicted by the signal from another electrode. For a given frequency, a coherence value of 1 indicates EEG signals that have exactly the same phase difference and amplitude ratio on each epoch, while a coherence value of 0 indicates EEG signals that have a random difference in phase. Although EEG coherence is here used as an index of cortico-cortical functional connectivity to represent communication between neural sources, it does not exclude the possibility that increased coherence may also represent increased drive from a common source (Mima et al., 2000; Saltzberg et al., 1986).
Spectral analysis was performed by submitting the time series to a discrete Fast Fourier transform with the MATLAB fft function, and was normalized by epoch length. The frequency resolution was 1 Hz, and no windowing function was used. Average absolute power at each electrode and coherence between each pair of electrodes for β (20–30 Hz) frequencies, a range associated with motor system function (Deeny et al., 2009; Roopun et al., 2006). Due to the central role of the contralateral primary motor cortex in movement execution and motor learning (Halsband and Lange, 2006; Hardwick et al., 2013), EEG coherence with a seed region overlying left hand motor area (M1) was used as the primary metric of interest. The M1 seed region included C3 and the six electrodes immediately surrounding C3, with each surrounding electrode being located approximately 1-cm from C3; this seed region may have included some signal from sensory cortex and other surrounding areas, and so the “M1” label is used to refer to the center of the seed. As a control, a right M1 seed region including C4 and its six immediate neighbors was also examined in separate analyses.
2.5 PLS Analysis
A partial least squares (PLS) regression model of the EEG data, focused on M1 connectivity at rest was used to predict motor skill acquisition. PLS analysis is a multivariate method in which an optimal least-squares fit is computed for a partial correlation matrix between the independent and dependent variables. The PLS algorithm then generates a series of models with successively more components that maximally account for variance in the dependent variable. PLS methods have been used to identify patterns of neural activation related to changes in task content (McIntosh et al., 2004), and has been found useful for defining relationships between measures of brain function and performance in spatial attention tasks (Krishnan et al., 2011).
In the present study, the N-way toolbox (Andersson and Bro, 2000) was used to generate PLS models of resting EEG coherence predicting % Improvement across practice. As a pre-processing step, data were mean-centered. Direct orthogonal signal correction (Westerhuis et al., 2001) was then used to remove the component of the predicting data (coherence) that was most orthogonal to the behavioral data (% Improvement). As with previous studies that used PLS (Esteban-Diez et al., 2004; Krishnan et al., 2013; Svensson et al., 2002; Williams et al., 2009), this step allowed for more efficient PLS models with fewer components. Two separate PLS models were generated for % Improvement with each of the EEG metrics, β frequency bands during EEG-Rest, and during EEG-Test 1. As in a previous study from our group using PLS, as many components as were required to explain at least 80% of fitted behavior variance were used to determine the number of components to retain in the model (Krishnan et al., 2013).
2.6 Predicting motor skill acquisition
A leave-one-out cross-validation procedure was applied to determine predictive value of each of the four PLS models. With this approach, data from one participant is iteratively removed from the PLS model, then this participant’s skill acquisition is predicted from his/her EEG data based on the PLS model and direct orthogonal signal correction generated from the remaining n−1 participants. The resulting cross-validated R2 is a measure of prediction accuracy determined from the ratio of prediction error and total variance in the actual behavioral data.
As control analyses, mean power and coherence were also calculated from regions of interest (ROIs), defined a priori via two methods, to predict subsequent motor skill acquisition. Similar to a recent study by Mathewson and colleagues (Mathewson et al., 2012), mean β power and coherence were calculated for single electrodes, including C3 (left M1), F3 (left frontal-premotor, PM), and P3 (left parietal, Pr). Alternatively, and more akin to fMRI studies (Baldassarre et al., 2012; Wang et al., 2010), regions in left M1, left PM, and left Pr were outlined across several scalp electrodes. Left M1 was defined as above for left M1 seed region in the PLS analyses. Left PM included F3 and the six electrodes that are immediately proximal, and left Pr included P3 and its six immediate neighbors. For both methods, mean power for each region and mean coherence for each pair of regions were then used to predict % Improvement in separate bivariate models. In an additional exploratory secondary analysis, separate PLS prediction models were also calculated using coherence in θ (4–6 Hz), μ (11–14 Hz), lower β (15–19 Hz), and γ (31–50 Hz) frequency bands.
2.7 Statistical analyses
Performance on the motor skill task was subjected to one-way repeated measures ANOVA to establish significance of motor skill acquisition with factor Test. Bivariate analyses of ROI-based brain-behavior relationships used two-tailed parametric linear regression. Statistical significance was set at p < 0.05.
Results
Eighteen healthy, right-handed individuals were recruited, one of whom was excluded due to technical problems during data collection, leaving 17 participants for the current report. For these 17 participants, gender was 9M/8F, and age was 22.1 ± 3.0 (mean ± SD). In addition, EEG-Test 1 data were discarded for one participant due to substantial muscle artifact.
Motor skill performance across participants increased significantly from Test 1 to Test 3 (Figure 1E, repeated measures ANOVA, factor: Test, p < 0.0001), with % Improvement increasing from 24% time on target to 41%.
3.1 Baseline Behavioral Performance and Demographic Data Do Not Correlate With Subsequent Motor Skill Acquisition
Baseline behavioral performance (% on Target at Test 1), age, and handedness by Edinburgh Handedness Inventory were each examined as correlates of subsequent motor skill acquisition (% Improvement), and none was significant (p > 0.05). Participants also completed a motor skills inventory that probed time spent typing, playing video games, playing a musical instrument, playing sports, driving, and other skilled activities of the hands (i.e., sewing, sign language). Although participants showed some range (1–4, max = 6, min = 0), motor skills inventory score also did not predict % Improvement (p > 0.05).
3.2 EEG Coherence At Rest Predicts Subsequent Learning in a PLS model
A fitted PLS model of β coherence with left M1 using the EEG-Rest data found a pattern that strongly correlated with % Improvement (R2 = 0.93, Figure 2A). This was not true in a control analysis of the EEG-Rest data that examined a PLS model of β coherence with right M1 where no significant prediction of % Improvement was found. A second control analysis was also negative, whereby PLS analysis of EEG β power also did not significantly predict % Improvement.
Figure 2.
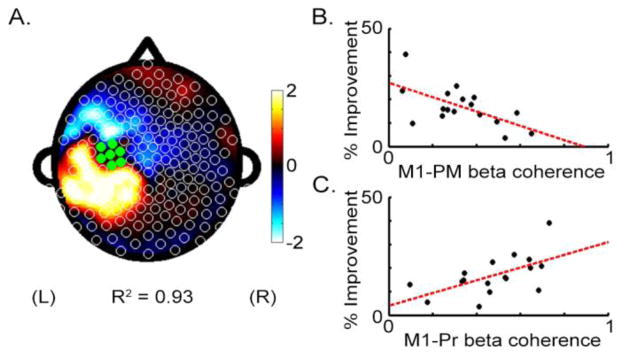
Coherence with left M1 during EEG-Rest predicts % Improvement on the motor task. A. Topographic plot of regression coefficients from the PLS model using M1 β band (20–30 Hz) coherence. B. Mean beta coherence between left M1 and left frontal-premotor regions (M1-PM) is negatively correlated with improvement. C. Mean beta coherence between left M1 and left parietal regions (M1-Pr) is positively correlated with improvement.
The primary method for assessing the predictive strength of the PLS model was a leave-one-out cross-validation approach using the EEG-Rest data acquired prior to training. With this approach, the cross-validated R2 for predicting % Improvement was 0.81. As a secondary method for assessing strength of the PLS model, data from 17-i participants were randomly selected to serve as a training group to generate a PLS model, and data from the remaining i participants were then used as a test group to independently assess prediction. Stepping up from i = 2, i was then increased until the prediction error in the test group exceeded total variance in the training group, and the model was said to have failed validation. The PLS model failed validation at i = 6. At i = 5, cross-validated R2 was 0.75 ± 0.19 across 500 random partitions.
Separate PLS models using coherence in θ (4–6 Hz), μ (11–14 Hz), lower β (15–19 Hz), and γ (31–50 Hz) frequency bands also predicted % Improvement, but with reduced prediction strength as compared to the β (20–30 Hz) frequency band. Fitted R2 was 0.82, 0.87, 0.80, and 0.90 for θ, μ, lower β, and γ coherences, respectively, and cross-validated R2 was 0.71, 0.76, 0.68, and 0.79.
3.2.1 Variation In Premotor and Parietal Connectivity At Rest Demonstrate Different Effects On Learning
From the PLS model of EEG-Rest using β coherence with left M1, the subset of electrodes accounting for >90% of the regression weights on EEG coherence were identified. All were located in left Pr and left frontal-premotor (PM) regions. Focusing on these electrodes, bivariate analysis found that greater % Improvement was associated with higher M1-Pr coherence (Figure 2C, r = 0.58, p < 0.05) and with lower M1-PM coherence (Figure 2B, r = −0.61, p < 0.01). These two effects were independent, as M1-PM coherence and M1-Pr coherence were not significantly related (p>0.05). Note that PM-Pr coherence also was not significantly related to % Improvement (p>0.05).
3.2.2 Power and Coherence at Rest in Predefined M1, Premotor, and Parietal Regions are Not Strong Predictors of Learning
Mean β power and coherence were calculated for predefined regions of interest at left M1 (C3), left PM (F3), and left Pr (P3) regions. When data from individual electrodes were used as correlates of % Improvement, mean power and coherence during EEG-Rest 1 did not correlate with subsequent % Improvement (p > 0.05). When data from groups of electrodes were used for each ROI, mean coherence during EEG-Rest 1 for left M1-left PM, left M1-left Pr, and left PM-left Pr were not significantly related to subsequent % Improvement on the motor skill task (p > 0.05). Mean power during EEG-Rest 1 from left M1, left PM, and left Pr also did not relate significantly with % Improvement (p > 0.05).
3.3 EEG Coherence During Start Of Practice (EEG-Test 1) Also Predicts Learning
A secondary hypothesis was that M1 connectivity during the initial phase of motor skill practice (EEG-Test 1) would also predict skill acquisition. Coherence with left M1 was again a significant predictor of % Improvement. The PLS model of coherence with left M1 during EEG-Test 1 significantly predicted % Improvement (Figure 3, fitted model R2 = 0.91, cross-validated R2 = 0.74), although this relationship was weaker compared to analyses using EEG-Rest.
Figure 3.
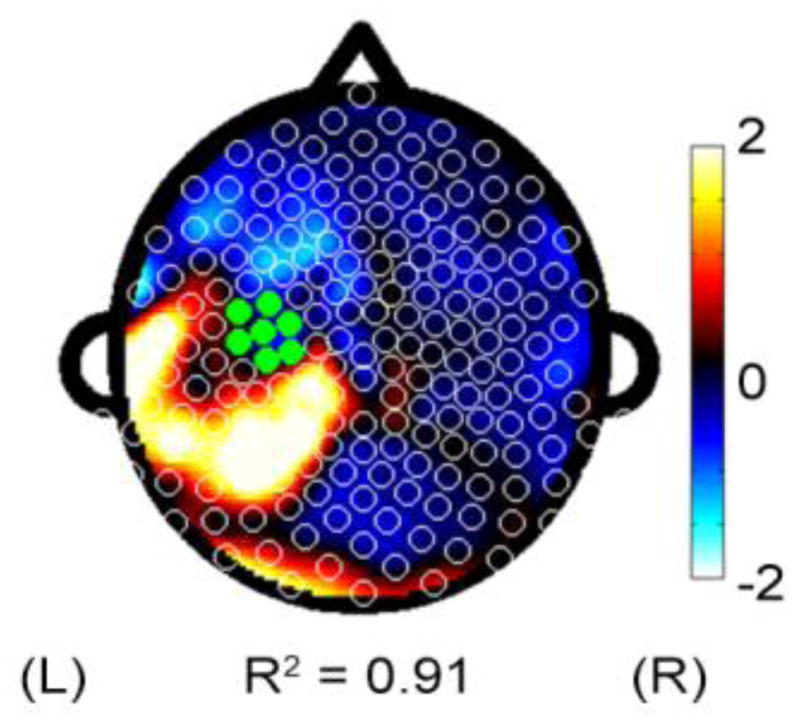
Topographic representation of regression coefficients derived from PLS models using M1 beta coherence during EEG-Test 1.
As above, electrodes of interest were derived from the EEG-Test 1 PLS model with the subset of electrodes accounting for >90% of the regression weights all located in a left Pr cluster. Mean coherence for left M1-left Pr was significantly related to subsequent % Improvement (p = 0.006, r = 0.65). Using the left PM cluster from EEG-Rest 1, left M1-left PM and left PM-left Pr did not correlate with % Improvement. Mean power during EEG-Rest 1 from left M1, left PM, and left Pr also did not have a significant relationship with % Improvement (p > 0.05).
Separate PLS models using left M1 beta coherence during EEG-Test 2 and EEG-Test 3 showed weaker strength at predicting % Improvement compared to what was seen using EEG-Rest or using EEG-Test 1. The fitted R2 was 0.81 and 0.86 for EEG-Test 2 and EEG-Test 3, respectively, and cross-validated R2 was 0.66 and 0.45.
Discussion
Measures of brain function using fMRI activity level or EEG power have been found to predict motor learning better than measures of baseline behavior, but the precision of prediction is modest. The current study examined the ability of a dense-array EEG measure of M1 cortical connectivity to predict individual differences in motor skill acquisition. Motor network connectivity measured during rest, prior to practice, was found to predict a remarkably high fraction of the variance in motor skill acquisition over 20 min of subsequent practice (R2 = 0.81), while pre-practice behavior and demographic data had no predictive value. In comparison, an ROI-based approach showed much reduced predictive value (R2 = 0.43). Current results emphasize the importance of activity in M1, parietal, and frontal-premotor cortical areas to motor learning, and furthermore reveal that M1-premotor connectivity and M1-parietal connectivity predict opposite effects on behavioral improvement.
Measures of resting-state network connectivity are proving highly useful for probing the functional potential of the brain (Deco et al., 2011). This approach was used in the current study and predicted learning in human subjects with greater precision (R2=0.81) than in any prior report of which we are aware. The strength of these findings may be due to several factors. First, use of EEG may offer some advantages for measuring synchronized activity across neural ensembles (Ganguly et al., 2009), as EEG provides higher temporal resolution than many other functional neuroimaging techniques. This might account for differences in prediction strength between the current study and studies using fMRI-based connectivity measures for prediction, e.g., of math skill acquisition (R2=0.61, (Supekar et al., 2013)), performance on an associative memory task (R2=0.35, (Wang et al., 2010)), or a visual discrimination task (R2=0.66, (Baldassarre et al., 2012)), although these are indirect comparisons and these studies differed from the current report in a number of key ways. The high temporal resolution may have been particularly advantageous in the current study as it permits measurement of brain function in high beta frequencies, a range closely associated with function of the cortical motor system (Deeny et al., 2009; Roopun et al., 2006). In the current study, high beta frequencies were shown to be the best biological correlate of motor system function as prediction strength was weaker in θ, μ, lower β, and γ frequency ranges. Second, connectivity-based measures, as compared to focal measures of brain activity, have an improved ability to provide insight into cortical processing underlying complex behaviors (Bullmore and Sporns, 2009). This is directly supported by the observation in the current study that connectivity but not regional spectral power measures predicted learning, and indirectly by comparing the strength of prediction in the current report with that found in a prior EEG study that used regional measures of spectral power to predict learning of a complex motor task (R2=0.53, (Mathewson et al., 2012)). Third, PLS modeling is useful for defining relationships between brain function and behavior (Krishnan et al., 2011; McIntosh and Lobaugh, 2004), for example, decoding behavioral output with high accuracy using fewer variables than with other approaches (Chao et al., 2010). Furthermore, a PLS approach has been found useful for understanding behavioral correlates of EEG in populations with a range of brain-related diagnoses, such as major depression (Khodayari-Rostamabad et al., 2010) and Parkinson’s disease (Chiang et al., 2012).
To facilitate a closer comparison with previous studies, an ROI-based approach, in which regions of interest in frontal-premotor, primary motor, and parietal areas were defined a priori, was also examined. When single electrode ROIs were used, no significant relationships were found between brain data and behavior. This might be due in part to variability in brain morphology and in site of electrode placement across individuals. When regions were defined across several electrodes, some relationships between dEEG metrics and % Improvement were found. However, compared to the PLS approach, the ROI approach still showed reduced predictive value in establishing a brain-behavior relationship between brain function at rest and subsequent motor skill acquisition (R2 range: PLS approach = 0.60 to 0.81, ROI approach = 0.26 to 0.43). The predictive value of the ROI approach in the current study is similar to previous studies that also used an ROI-based approach in previous fMRI and EEG studies (Mathewson et al., 2012; Wang et al., 2010). When comparing the same metric across approaches, such as M1-PM beta coherence to predict % Improvement, the PLS approach (R2 = 0.37) also demonstrates improved prediction strength compared with the ROI approach (R2 = 0.09). The favorable comparison of a PLS approach suggests whole-brain approaches, including graph-theoretical network analysis (Langer et al., 2011) and Independent Component Analysis (Lin et al., 2012), may be more sensitive to inter-individual variations that provide insight into differences in behavior.
The current approach not only predicts learning, but also provides insight into the neural circuits underlying this learning (Supekar et al., 2013). As in previous studies of motor learning (Hardwick et al., 2013), the current results show motor skill acquisition is linked to activity in primary motor cortex, parietal cortex, and frontal-premotor areas. Furthermore, the current results extend previous findings by demonstrating how inter-individual differences in resting-state cortical networks may relate to differences in capacity for skilled motor learning. M1-Pr connectivity was found to have an opposite relationship with predicting skill learning as compared to M1-PM connectivity. Thus, within the model, increased M1-Pr connectivity predicted greater skill acquisition. This may reflect greater capacity for visuomotor integration, as suggested by a recent study that reported that increased functional connectivity between sensorimotor and parietal regions during early motor learning coincided with significant behavioral improvement (Ma et al., 2010). The model also found that increased M1-PM connectivity at rest predicted reduced skill acquisition. This may reflect basal differences in motor system efficiency, as premotor cortex activation has been associated with increased task complexity (O’Shea et al., 2007) and cognitive effort (Kantak et al., 2012). It may be that increased M1-PM connectivity at rest is a marker of a inefficient motor system, similar to the aging motor system, which exhibits over-activity in premotor cortex during simple motor tasks (Ward, 2006). Opposite findings regarding M1-Pr vs. M1-PM connectivity may therefore reflect individual differences in specific processing components that contribute to variability in early motor learning (Grafton et al., 2008).
The current study has a number of limitations. Regarding localization of coherence effects, we acknowledge the use of scalp EEG has limitations in spatial localization. As such, the anatomical relationship between EEG electrodes and specific brain structures is imperfect. However, 256-electrodes systems, as used in the current study, are shown to provide significantly improved spatial resolution compared to traditional 10–20 systems (Luu et al., 2001; Ryynänen et al., 2004). A surface Laplacian could be used to further improve spatial resolution (Nunez and Westdorp, 1994). However, there is evidence that such a transform could erroneously distort coherence across distances in the range of left M1 to left PM and Pr regions (Srinivasan et al., 1998). Although not a focus of the current study, future work on the evolution of connectivity profiles in parallel with behavioral gains might provide additional insights into the mechanisms underying motor learning.
Predicting biological behavior remains a major challenge for understanding variation in healthy subjects and diseased populations. The current results show that a measure of connectivity obtained from three minutes of resting-state dEEG captures individual differences in brain state that are highly related to subsequent behavioral learning. The current dEEG method has high potential clinical utility, as these data can be acquired easily, inexpensively, rapidly, safely, and in complex medical settings. Although it is known that patients demonstrate significant heterogeneity in motor learning, the current methods may be useful in clinical settings related to cortical plasticity, such as acute stroke or traumatic brain injury, where a high need exists for accurate methods of patient stratification (Burke and Cramer, 2013).
Highlights.
Coherence in resting EEG data was a strong predictor of motor skill acquisition
Prediction was very strong with this method (R2 = 0.81)
This method predicted much better than baseline behavior or demographics
Parietal-M1 coherence predicted in opposite direction vs. premotor-M1 coherence
An advantage of this method is its ease of application in complex medical settings
Abbreviations
- PLS
Partial least squares
- M1
primary motor area
- PM
Frontal-Premotor
- Pr
Parietal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman PL. Individual differences in skill learning: An integration of psychometric and information processing perspectives. Psychol Bull. 1987;102:3–27. [Google Scholar]
- Adams J. Warm-up decrement in performance on the pursuit-rotor. Am J Psychol. 1952;65:404–414. [PubMed] [Google Scholar]
- Andersson CA, Bro R. The N-way Toolbox for MATLAB. Chemom Intell Lab Syst. 2000;52:1–4. [Google Scholar]
- Baker S, Kilner J, Pinches E, Lemon R. The role of synchrony and oscillations in the motor output. Exp brain Res. 1999;128:109–117. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- Baldassarre A, Lewis C, Committeri G, Snyder A, Romani G, Corbetta M. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci. 2012;109:3516–3521. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Burke E, Cramer SC. Biomarkers and predictors of restorative therapy effects after stroke. Curr Neurol Neurosci Rep. 2013;13:329. doi: 10.1007/s11910-012-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao ZC, Nagasaka Y, Fujii N. Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkeys. Front Neuroeng. 2010;3:3. doi: 10.3389/fneng.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J, Wang Z, McKeown M. A multiblock PLS model of cortico-cortical and corticomuscular interactions in Parkinson’s disease. Neuroimage. 2012;63:1498–1509. doi: 10.1016/j.neuroimage.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Deeny SP, Haufler AJ, Saffer M, Hatfield BD. Electroencephalographic Coherence During Visuomotor Performance: A Comparison of Cortico-Cortical Communication in Experts and Novices. J Mot Behav. 2009;41:106–116. doi: 10.3200/JMBR.41.2.106-116. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004 doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban-Diez I, Gonzalez-Saiz J, Pizarro C. An evaluation of orthogonal signal correction methods for the characterisation of arabica and robusta coffee varieties by NIRS. Anal Chim Acta. 2004;514:57–67. [Google Scholar]
- Ganguly K, Secundo L, Ranade G, Orsborn A, Chang EF, Dimitrov DF, Wallis JD, Barbaro NM, Knight RT, Carmena JM. Cortical representation of ipsilateral arm movements in monkey and man. J Neurosci. 2009;29:12948–56. doi: 10.1523/JNEUROSCI.2471-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Schmitt P, Van Horn J, Diedrichsen J. Neural substrates of visuomotor learning based on improved feedback control and prediction. Neuroimage. 2008;39:1383–95. doi: 10.1016/j.neuroimage.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Tyszka M. Functional imaging of procedural motor learning: Relating cerebral blood flow with individual subject performance. Hum Brain Mapp. 1994;1:221–234. doi: 10.1002/hbm.460010307. [DOI] [PubMed] [Google Scholar]
- Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J Physiol Paris. 2006;99:414–24. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Skudlarski P, Gore J, Constable R. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick R, Rottschy C, Miall R, Eickhoff S. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2013;67:283–97. doi: 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak SS, Stinear JW, Buch ER, Cohen LG. Rewiring the brain: potential role of the premotor cortex in motor control, learning, and recovery of function following brain injury. Neurorehabil Neural Repair. 2012;26:282–292. doi: 10.1177/1545968311420845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodayari-Rostamabad A, Hasey G, MacCrimmon D, Reilly J, de Bruin H. A pilot study to determine whether machine learning methodologies using pre-treatment electroencephalography can predict the symptomatic response to clozapine therapy. Clin Neurophysiol. 2010;121:1998–2006. doi: 10.1016/j.clinph.2010.05.009. [DOI] [PubMed] [Google Scholar]
- King A, Ranganathan R, Newell K. Individual differences in the exploration of a redundant space-time motor task. Neurosci Lett. 2012;529:144–149. doi: 10.1016/j.neulet.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56:455–75. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Krishnan L, Kang A, Sperling G, Srinivasan R. Neural strategies for selective attention distinguish fast-action video game players. Brain Topogr. 2013;26:83–97. doi: 10.1007/s10548-012-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer N, Pedroni A, Gianotti LRR, Hänggi J, Knoch D, Jäncke L. Functional brain network efficiency predicts intelligence. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21297. 000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Baldassarre A, Committeri G, Romani G, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Shaw FZ, Young KY, Lin CT, Jung TP. EEG correlates of haptic feedback in a visuomotor tracking task. Neuroimage. 2012;60:2258–73. doi: 10.1016/j.neuroimage.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Englander R, Lockfeld A, Lutsep H, Oken B. Localizing acute stroke-related EEG changes: assessing the effects of spatial undersampling. J Clin Neurophysiol. 2001;18:302–17. doi: 10.1097/00004691-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Ma L, Narayana S, DAR, Fox P, Xiong J. Changes occur in resting state network of motor system during 4 weeks of motor skill learning. Neuroimage. 2010;58:226–233. doi: 10.1016/j.neuroimage.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–5. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson K, Basak C, Maclin E, Low K, Boot W, Kramer A, Fabiani M, Gratton G. Different slopes for different folks: Alpha and delta EEG power predict subsequent video game learning rate and improvements in cognitive control tasks. Psychophysiology. 2012;49:1558–1570. doi: 10.1111/j.1469-8986.2012.01474.x. [DOI] [PubMed] [Google Scholar]
- McIntosh A, Chau W, Protzner A. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23:764–75. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- McIntosh A, Lobaugh N. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23(Suppl 1):S250–63. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Mima T, Matsuoka T, Hallett M. Functional coupling of human right and left cortical motor areas demonstrated with partial coherence analysis. Neurosci Lett. 2000;287:93–6. doi: 10.1016/s0304-3940(00)01165-4. [DOI] [PubMed] [Google Scholar]
- Murias M, Swanson JM, Srinivasan R. Functional connectivity of frontal cortex in healthy and ADHD children reflected in EEG coherence. Cereb cortex. 2007;17:1788–1799. doi: 10.1093/cercor/bhl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P, Westdorp A. The surface Laplacian, high resolution EEG and controversies. Brain Topogr. 1994;6:221–6. doi: 10.1007/BF01187712. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain. 2. Oxford University Press; New York, New York: 2006. [Google Scholar]
- O’Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MFS. Functional specificity of human premotor-motor cortical interactions during action selection. Eur J Neurosci. 2007;26:2085–95. doi: 10.1111/j.1460-9568.2007.05795.x. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancák a, Neuper C. Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr Clin Neurophysiol. 1996;98:281–93. doi: 10.1016/0013-4694(95)00258-8. [DOI] [PubMed] [Google Scholar]
- Roopun AK, Middleton SJ, Cunningham MO, LeBeau FEN, Bibbig A, Whittington Ma, Traub RD. A beta2-frequency (20–30 Hz) oscillation in nonsynaptic networks of somatosensory cortex. Proc Natl Acad Sci U S A. 2006;103:15646–50. doi: 10.1073/pnas.0607443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryynänen ORM, Hyttinen JaK, Laarne PH, Malmivuo Ja. Effect of electrode density and measurement noise on the spatial resolution of cortical potential distribution. IEEE Trans bio-medical Eng. 2004;51:1547–54. doi: 10.1109/TBME.2004.828036. [DOI] [PubMed] [Google Scholar]
- Saltzberg B, Burton WD, Burch NR, Fletcher J, Michaels R. Electrophysiological measures of regional neural interactive coupling. Linear and non-linear dependence relationships among multiple channel electroencephalographic recordings. Int J Biomed Comput. 1986;18:77–87. doi: 10.1016/0020-7101(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL, Silberstein RB. Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE Trans Biomed Eng. 1998;45:814–26. doi: 10.1109/10.686789. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Winter WR, Ding J, Nunez PL. EEG and MEG coherence: measures of functional connectivity at distinct spatial scales of neocortical dynamics. J Neurosci Methods. 2007;166:41–52. doi: 10.1016/j.jneumeth.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–32. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]
- Supekar K, Swigart AG, Tenison C, Jolles DD, Rosenberg-lee M, Fuchs L. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc Natl Acad Sci. 2013;110:8230–8235. doi: 10.1073/pnas.1222154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson O, Kourti T, MacGregor J. An investigation of orthogonal signal correction algorithms and their characteristics. J Chemom. 2002;16:176–188. [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Kincses ZT, Bosnell R, Douaud G, Pozzilli C, Matthews PM, Johansen-Berg H. Structural and functional bases for individual differences in motor learning. Hum Brain Mapp. 2011;32:494–508. doi: 10.1002/hbm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropini G, Chiang J, Wang ZJ, Ty E, McKeown MJ. Altered directional connectivity in Parkinson’s disease during performance of a visually guided task. Neuroimage. 2011;56:2144–56. doi: 10.1016/j.neuroimage.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Vo L, Walther D, Kramer A, Erickson K, Boot W, Voss M, Prakash R, Lee H, Fabiani M, Gratton G, Simons D, Sutton B, Wang M. Predicting individuals’ learning success from patterns of pre-learning MRI activity. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk K, Pihlajamäki M, Dickerson B, Sperling R. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51:910–7. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev. 2006;5:239–54. doi: 10.1016/j.arr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Westerhuis JA, de Jong S, Smilde AK. Direct orthogonal signal correction. Chemom Intell Lab Syst. 2001;56:13–25. [Google Scholar]
- Williams H, Cox I, Walker D, North B, Patel V, Marshall S, Jewell D, Ghosh S, Thomas H, Teare J, Jakobovits S, Zeki S, Welsh K, Taylor-Robinson S, Orchard T. Characterization of Inflammatory Bowel Disease With Urinary Metabolic Profiling. Am J Gastroenterol. 2009;104:1435–1444. doi: 10.1038/ajg.2009.175. [DOI] [PubMed] [Google Scholar]


