Abstract
Chlamydia trachomatis is the most common sexually transmitted bacterial pathogen in the World and there is a need for a vaccine. To enhance the immunogenicity of a vaccine formulated with the Chlamydia muridarum (Cm) mouse pneumonitis recombinant major outer membrane protein (MOMP), we used combinations of Pam2CSK4+CpG-1826 and Montanide ISA 720 VG+CpG-1826 as adjuvants. Neisseria gonorrhoeae recombinant porin B (Ng-PorB) was used as the antigen control with the same adjuvants. Female BALB/c mice were primed twice in the nares (i.n.) or in the colon (cl.) and were boosted twice by the intramuscular plus subcutaneous (i.m.+s.c.) routes. Based on the IgG2a/IgG1 ratio in sera, mice immunized with MOMP+Pam2CSK4+CpG-1826 showed a strong Th2 response while animals vaccinated with MOMP+Montanide ISA 720 VG+CpG-1826 had a Th1 response. Both groups of mice also developed robust Cm-specific T cell proliferation and high levels of IFN-γ. Four weeks after the last immunization, the mice were challenged i.n. with 104 inclusion-forming units (IFU) of Cm. Using changes in body weight and number of IFU recovered from the lungs at 10 days post-challenge mice immunized i.n.+i.m./s.c. with MOMP+Pam2CSK4+CpG-1826 were better protected than other groups. In conclusion, MOMP adjuvanted with Pam2CSK4+CpG-1826, elicits strong humoral and cellular immune responses and induces significant protection against Chlamydia.
Keywords: Chlamydia trachomatis, Chlamydia muridarum, vaccine, routes of immunization, Toll-like receptors, CpG-1826, Montanide ISA 720 VG, Pam2CSK4
1. Introduction
C. trachomatis is worldwide the leading cause of bacterial sexually transmitted diseases and can also produce respiratory, gastrointestinal and ocular infections. Genital infections affect particularly young sexually active individuals [1–4]. Newborns become infected in the birth canal and contract ocular and respiratory infections [2, 4, 5]. Adult immunocompromised individuals can also suffer from respiratory infections [6]. Antibiotic therapy is available but, due to the high percentage of asymptomatic patients, or inappropriate treatment, long-term sequelae can develop including abdominal pain, infertility, ectopic pregnancy and blindness [3, 4, 7, 8]. Countries that have established screening programs for genital infections, followed by antibiotic therapy, have observed an increase in the prevalence of the infection [9, 10]. This increase is thought to be due to a block in the development of natural immunity as a result of the antibiotic therapy [9]. Thus, a vaccine will be the best approach to control chlamydial infections [11–13].
Current vaccines, formulated with live or inactivated whole pathogens, possess intrinsic adjuvant activity as they contain “pathogen-associated molecular patterns” (PAMPs), such as LPS and nucleic acids that activate in the host immune cells “pattern recognition receptors” (PRRs), such as toll-like receptors (TLR) [14, 15]. Therefore, the vaccinated individual, upon exposure to the pathogen, is ready to mount a robust immune response. The use of highly purified antigens, including synthetic peptides or recombinant proteins that, for the most part, lack inherent adjuvanticity, has resulted in the need to develop adjuvants to enhance the immune responses [16–18].
Several TLR agonists were recently screened individually for their ability to serve as adjuvants in a vaccine formulated with the Chlamydia. muridarum (Cm) recombinant major outer membrane protein (MOMP) [19]. It was found that Pam2CSK4, a TLR2 ligand, and CpG-1826, a ligand for TLR9, were effective adjuvants for enhancing protection against a chlamydial respiratory challenge [19–21]. Protection against a secondary chlamydial infection is dependent on CD4 Th1 cells, B cells and antibodies [22]. CpG-1826 elicits a strong Th1 response while Pam2CSK4 enhances antibody responses [23, 24]. Therefore, here we tested a vaccine with recombinant MOMP and CpG-1826+Pam2CSK4, or CpG-1826+Montanide ISA 720 VG as adjuvants, to determine which combination elicits the most robust protection against a C. muridarum challenge [25].
2. Materials and methods
2.1. Chlamydia muridarum stocks
The C. muridarum [Cm; previously called C. trachomatis mouse pneumonitis (MoPn) strain Nigg II], was grown in HeLa-229 cells and elementary bodies (EB) were purified as described [26].
2.2. Cloning and purification of the recombinant C. muridarum MOMP and the N. gonorrhoeae porin B (Ng-PorB)
Genomic DNA from C. muridarum and N. gonorrhoeae strain FA 1090 (ATCC) were extracted using the Wizard genomic DNA Purification Kit (Promega, Madison, WI) [27]. The Cm MOMP (GenBank, accession no. AE002272, X63409) and Ng-PorB genes (36 kDa, GenBank ID: AAW90430) were amplified as previously described and E. coli BL21 (DE3) competent cells were used for protein expression [27]. The recombinant proteins were purified using a Sephacryl-S-300 column [25, 27, 28].
The purity of MOMP and Ng-PorB was determined by 10% tricine-SDS-PAGE [29]. Using the limulus amoebocyte assay (BioWhittaker, Inc., Walkersville, MD), both proteins were found to have less than 0.05 EU of LPS/mg of protein.
2.3. Immunization protocols
Three-week-old female BALB/c (H-2d) mice (Charles River Laboratories; Wilmington, MA) were housed at the University of California, Irvine, Vivarium. The UCI, Animal Care and Use Committee approved all animal protocols.
Agonists of TLR2 (Pam2CSK4, InvivoGen, San Diego, CA; 5 µg/mouse/immunization) and TLR9 (CpG-1826, TriLink, San Diego, CA; 10 µg/mouse/immunization) were directly mixed with the antigens (10 µg/mouse/immunization) as published [19, 26]. The TLR-independent adjuvant Montanide ISA 720 VG (Seppic Inc, Fairfield, NJ; at a concentration of 70% of the total volume of the vaccine formulation) was also used. CpG-1826 (C) and Pam2CSK4 (P) were delivered by the mucosal and systemic routes while Montanide ISA 720 VG was only used systemically (Ms) since it is not a mucosal adjuvant [30].
Following anesthesia mice (n=14 to 20) were vaccinated according to Schedule 1: twice by a mucosal route [intranasal (i.n.)] followed twice systemic routes [intramuscular plus subcutaneous (i.m./s.c.)] or Schedule 2: twice colonic (cl.) followed twice i.m./s.c. All immunizations were given at 2-week intervals (Table 1). As a negative antigen control, mice were vaccinated with the Ng-PorB using the same adjuvants, delivery routes and immunization schedules. The antigens were equally divided among three sites: bilateral quadrices femoris muscles (i.m.) and base of the tail (s.c.). The positive control mice were immunized i.n. once with 104 inclusion forming units (IFU) of Cm in 20 µl of Eagle's MEM without serum [(MEM-0); Invitrogen, Carlsbad, CA]. A negative control group was inoculated once i.n. with 20 µl MEM-0 [31]. These last two control groups were immunized once at the same time when the other animals received their first immunization. All animals were of the same age at the time of challenge.
Table 1.
Serum antibody geometric mean titers (GMT; range) the day before the i.n. challenge with C. muridarum.
| Vaccine | Routes and number of immunizations |
No. of mice | IgG1 | IgG2a | IgG2a/ IgG1 |
IgA |
|---|---|---|---|---|---|---|
| MOMP/P/C-1 | 2× i.n. + 2× i.m./s.c. | 20 | 25,600 (12,800 – 51,200) | 2540 (1600 – 3200) | 0.10 | 1008 (800 – 1600) |
| MOMP/Ms/C-1 | 2× i.n. + 2× i.m./s.c. | 20 | 16,127 (6400 – 25,600) | 51,200 (25,600 – 102,400) | 3.17 | 4032 (3200 – 6400) |
| Ng-PorB/P/C-1 | 2× i.n. + 2× i.m./s.c. | 19 | <100 | <100 | --- | <100 |
| Ng-PorB/Ms/C-1 | 2× i.n. + 2× i.m./s.c. | 20 | <100 | <100 | --- | <100 |
| MOMP/P/C-2 | 2× cl. + 2× i.m./s.c. | 17 | 81,275 (51,200 – 102,400) | 16,127 (12,800 – 25,600) | 0.20 | 504 (400 – 800) |
| MOMP/Ms/C-2 | 2× cl. + 2× i.m./s.c. | 14 | 32,254 (25,600 – 51,200) | 102,400 (51,200 – 204,800) | 3.17 | 800 (200 – 1600) |
| Ng-PorB/P/C-2 | 2× cl. + 2× i.m./s.c. | 19 | <100 | <100 | --- | <100 |
| Ng-PorB/Ms/C-2 | 2× cl. + 2× i.m./s.c. | 20 | <100 | <100 | --- | <100 |
| EB | 1 × i.n. | 20 | 1600 (800 – 3200) | 12,800 (6400 – 25,600) | 8 | 2016 (1600 – 3200) |
| MEM-0 | 1 × i.n. | 19 | <100 | <100 | --- | <100 |
P: Pam2CKS4
C: CpG-1826
Ms: Montanide was only used by systemic routes (im./sc.)
1: Schedule 1= 2× in. + 2× im./sc.
2: Schedule 2= 2× cl. + 2× im./sc.
2.4. Characterization of the humoral and cell mediated immune responses
To measure Cm-specific antibodies, blood was collected from each mouse the day before the challenge. ELISA and the Western blot were performed as previously described with Cm EB as the antigen [25].
To assess the T cell memory responses an in vitro lymphoproliferative assay was performed before the i.n. challenge as previously described [31, 32]. Levels of IFN-γ in supernatants from splenic T cells stimulated with UV-inactivated EB for 48 hrs were determined using ELISA kits (BD Pharmingen, San Diego, CA) [33].
2.5. Intranasal challenge
After the last immunization the animals were rested for 4 weeks and then challenged i.n. with 104 IFU of Cm under Xylazine/Ketamine anesthesia [34]. At day 10 post-challenge (p.c.) the mice were euthanized, their lungs homogenized in 5 ml of SPG, and serial 10-fold dilutions were inoculated onto Hela-229 cells. The chlamydial inclusions were stained with a cocktail of monoclonal antibodies and were counted [32]. The limit of detection per pair of lungs was 50 IFU. To determine the local humoral and cellular immune responses the titers of Cm-specific IgA and levels of IFN-γ in lungs at D10 p.c. were determined by an ELISA, as described above.
2.6. Statistical analyses
Statistical analyses were performed with the SigmaStat and SAS software. The two-tailed unpaired Student's t-test, the Mann–Whitney U-test, and repeated measures ANOVA were employed to determine the significance of the differences between groups. A pairwise Pearson’s correlation analysis was performed to investigate the association between the observations of interest [31]. Differences were considered significant for values of p≤0.05.
3. Results
3.1. Humoral immune responses following vaccination
Mice immunized with MOMP/P/C developed a Th2-biased response, independent of the routes of vaccination used, as shown by higher geometric mean titers (GMT) of IgG1 in comparison to IgG2a (IgG1=25,600 and IgG2a=2540, IgG2a/IgG1=0.10 for MOMP/P/C-1 immunization; IgG1=81,275 and IgG2a=16,127, IgG2a/IgG1=0.20 for MOMP/P/C-2 immunization; Table 1). In contrast, mice immunized with MOMP/Ms/C mounted a Th1 response (IgG1=16,127 and IgG2a=51,200, IgG2a/IgG1=3.17 for MOMP/Ms/C-1 immunization; IgG1=32,254 and IgG2a=102,400, IgG2a/IgG1=3.17 for MOMP/Ms/C-2 immunization). Live EB elicited a strong Th1-based response (IgG1=1600 and IgG2a=12,800, IgG2a/IgG1=8). Mice vaccinated with MOMP/P/C-1 and MOMP/Ms/C-1 showed high serum IgA titers (IgA=1008 and IgA=4032) compared to those immunized with MOMP/P/C-2 and MOMP/Ms/C-2 (IgA=504 and IgA=800). The differences for the IgA titers were not statistically significant among the groups immunized with MOMP or with live EB (IgA=2016).
The Western blot shows that mice vaccinated with the MOMP developed antibodies only against this protein (MW 40 kDa; Supplemental Fig. 1). Animals immunized i.n. with EB produced antibodies against components with a molecular mass higher than 100 kDa, the 60-kDa cysteine rich protein (crp), the 60-kDa-heat shock protein (hsp), MOMP and the 28-kDa protein.
3.2. Cell mediated immune responses following vaccination
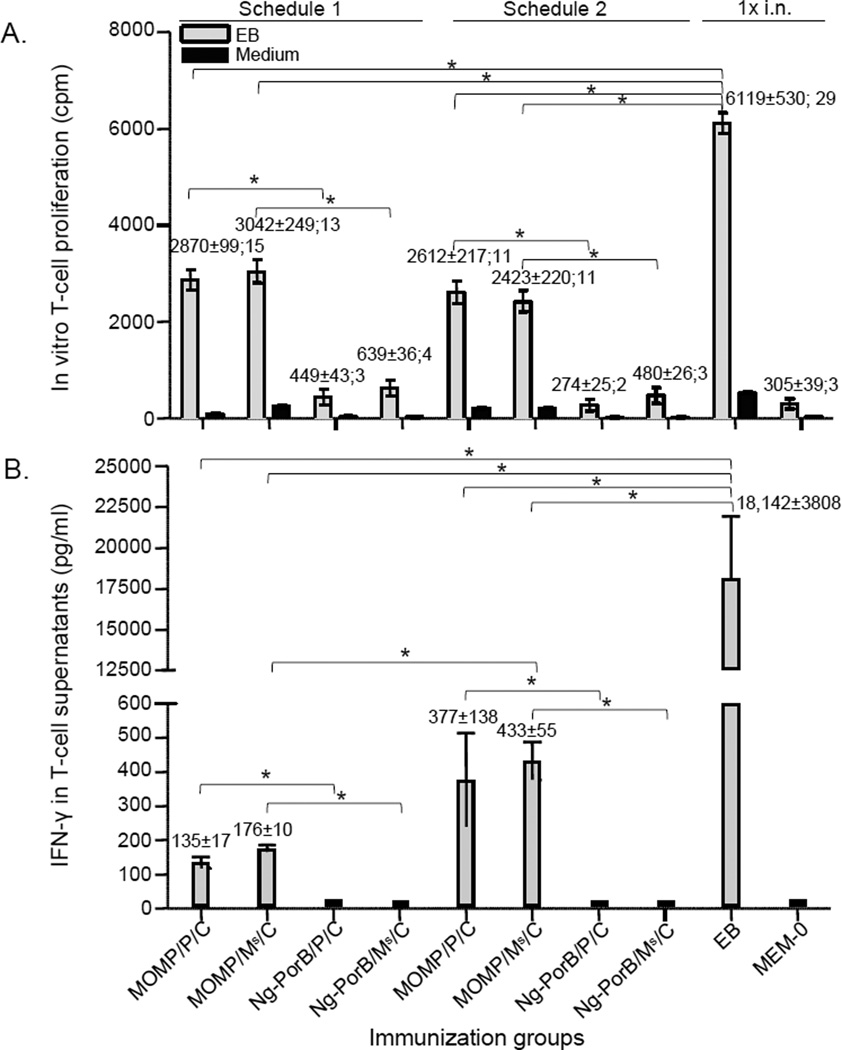
Overall, the response of splenic T-cells, stimulated with UV-inactivated EB, from mice immunized with MOMP, was significantly stronger than their respective negative controls immunized with Ng-PorB or MEM-0 (Fig. 1A, p<0.05). The proliferative response in mice immunized with MOMP/P/C-1 [2870±99 cpm, stimulation index (SI=cpm from EB stimulation/cpm from medium stimulation) =15] was similar to the response of mice immunized with MOMP/P/C-2 (2612±217 cpm, SI=11). A similar response was also found in mice immunized with MOMP/Ms/C-1 (3042±249 cpm, SI=13) in comparison to animals immunized with MOMP/Ms/C-2 (2423±220 cpm, SI=11) (p > 0.05). The most robust lymphoproliferative response was observed in control mice immunized i.n. with EB (6119±530 cpm, SI=29).
Fig. 1. In vitro splenic T-cell responses before the i.n. challenge.
A. Splenic T-cell proliferation (mean cpm ± 1SE; SI). T-cells were stimulated with UV-treated Cm EB, or medium as a control, the day before the i.n. Cm challenge. *, p < 0.05 as determined by the two-tailed unpaired Student’s t-test.
B. Levels of IFN-γ (mean pg/ml ± 1SE) detected in supernatants of proliferating splenic T-cells stimulated with UV-treated Cm EB. *, p < 0.05 as determined by the two-tailed unpaired Student’s t-test.
The level of IFN-γ (Fig. 1B) produced by EB-stimulated splenic T-cells of mice immunized with MOMP/Ms/C-2 (433±55 pg/ml) was significantly higher than that from mice immunized with MOMP/Ms/C-1 (176±10 pg/ml, p < 0.05). No significant difference was found between mice immunized with MOMP/P/C-2 (377±138 pg/ml) and those immunized with MOMP/P/C-1 (135±17 pg/ml; p>0.05). The highest level of IFN-γ was detected in supernatants of activated T cells from mice vaccinated i.n. with EB (18,142±3808 pg/ml). This level was significantly higher in comparison to any group immunized with MOMP (p<0.05).
3.3. Changes in body weight following the i.n. challenge
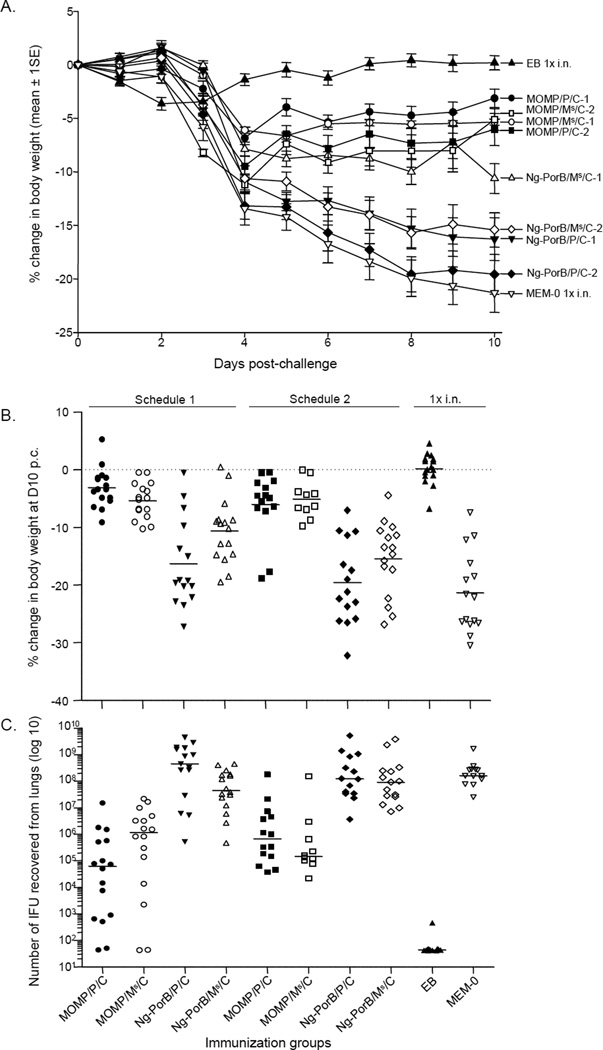
All mice, except those immunized i.n. with EB, lost weight for the first 4 days p.c. (Fig. 2A). Afterward, mice immunized with MOMP regained or maintained the weight up to the end of the observation at D10 p.c. Mice immunized with MOMP/P/C-1 lost less body weight during the 10 days than mice immunized with MOMP/P/C-2, MOMP/Ms/C-1 or MOMP/Ms/C-2 (p<0.05 by repeated measured ANOVA). In contrast, mice immunized with Ng-PorB or MEM-0 consistently lost body weight during the 10 days of observation. Animals immunized with Ng-PorB/Ms/C-1 showed significant less body weight loss over the 10 days of observation in comparison to other negative control groups (p<0.05 by repeated measured ANOVA) probably reflecting a non-specific protection.
Fig. 2. Changes in body weight and Cm yields from the lungs at day 10 following the i.n. challenge.
A. Percentage change in body weight of each group following the i.n. Cm challenge.
B. Percentage change in body weight at day 10 after the i.n. Cm challenge. The mean is indicated as a horizontal line. Each symbol represents a single animal.
C. Number of Cm IFU recovered from the lungs at day 10 after the i.n. challenge. The median is shown as a horizontal line. Each symbol represents a single animal.
As shown in Fig. 2B and Table 2, at D10 p.c., control mice inoculated i.n. with MEM-0 and those immunized with Ng-PorB had lost significantly more body weight in comparison to animals immunized with MOMP (p<0.05). Mice immunized with MOMP/P/C-1 had only lost 3.12±0.85% of their initial mean body weight. However, no significant differences were found among this group and the other three groups of mice immunized with MOMP/P/C-2 (6.03±1.50%), MOMP/Ms/C-1 (5.35±0.78%) or MOMP/Ms/C-2 (5.11±1.01%) (p>0.05).
Table 2.
Disease burden, yields of C. muridarum IFU recovered from the lungs and Cm-specific IgA and IFN-γ in the lungs at day 10 p.c.
| Vaccine | D10 BWC^ (%) Mean ± 1SE |
IFN-γ in lungs (pg/ml) Mean ± 1SE |
IgA in lungs (OD405nm) Mean ± 1SE |
#IFU in lungs Median (Min-Max) ×106 |
|---|---|---|---|---|
| MOMP/P/C-1 | −3.12 ± 0.85a | 387 ± 169a, e | 1.699 ± 0.177a, e, f | 0.74 (BLD* – 15)h, i, j |
| MOMP/Ms/C-1 | −5.35 ± 0.78b | 1875 ± 418b | 0.496 ± 0.017b | 1.6 (BLD – 21.5)k |
| Ng-PorB/P/C-1 | −17.41 ± 1.71 | 12,620 ± 2160 | 0.349 ± 0.011 | 442 (0.52 – 4366) |
| Ng-PorB/Ms/C-1 | −10.62 ± 1.39 | 11,557 ± 2285 | 0.358 ± 0.01 | 44.9 (0.46 – 436.6) |
| MOMP/P/C-2 | −6.03 ± 1.50c | 584 ± 151c | 0.809 ± 0.032c | 0.66 (0.37 – 177.6)l |
| MOMP/Ms/C-2 | −5.11 ± 1.01d | 201 ± 40d, g | 0.674 ± 0.039d, g | 0.14 (0.02 – 146)m |
| Ng-PorB/P/C-2 | −19.57 ± 1.93 | 5736 ± 1872 | 0.286 ± 0.013 | 125.8 (3.6 – 5161.5) |
| Ng-PorB/Ms/C-2 | −15.43 ± 1.59 | 2686 ± 429 | 0.298 ± 0.01 | 94.4 (7 – 3792.5) |
| EB | 0.19 ± 0.66 | 94.4 ± 11.9 | 2.315 ± 0.083 | BLD (BLD – 0.00045) |
| MEM-0 | −22.31 ± 1.62 | 1929 ± 315 | 0.241 ± 0.020 | 209 (24.8 – 2442) |
, BWC: body weight change;
, BLD: below limit of detection.
, p < 0.05 by the Student's t-test compared with the group immunized with Ng-PorB/P/C-1.
, p < 0.05 by the Student's t-test compared with the group immunized with Ng-PorB/Ms/C-1.
, p < 0.05 by the Student's t-test compared with the group immunized with Ng-PorB/P/C-2.
, p < 0.05 by the Student's t-test compared with the group immunized with Ng-PorB/Ms/C-2.
, p < 0.05 by the Student's t-test compared with the group immunized with MOMP/Ms/C-1.
, p < 0.05 by the Student's t-test compared with the group immunized with MOMP/P/C-2.
, p < 0.05 by the Student's t-test compared with the groups immunized with MOMP/Ms/C-1 or with MOMP/P/C-2.
, p < 0.05 by the Mann Whitney's U-test compared with the groups immunized with Ng-PorB/P/C-1 or with MOMP/P/C-2.
, p =0.05 by the Mann Whitney's U-test compared with the group immunized with MOMP/Ms/C-1.
, p =0.069 by the Mann Whitney's U-test compared with the group immunized with MOMP/Ms/C-2.
, p < 0.05 by the Mann Whitney's U-test compared with the group immunized with Ng-PorB/Ms/C-1.
, p < 0.05 by the Mann Whitney's U-test compared with the group immunized with Ng-PorB/P/C-2.
, p < 0.05 by the Mann Whitney's U-test compared with the group immunized with Ng-PorB/Ms/C-2.
3.4. Burden of C. muridarum infection in the lungs
The number of Cm IFU from the lungs of mice immunized with MOMP was significantly lower than from animals inoculated i.n. with MEM-0 or with Ng-PorB (p<0.05); (Fig. 2C; Table 2). The median number of IFU recovered from lungs of mice vaccinated with MOMP/P/C-1 was 0.74 [range: below limit of detection (BLD)–15] × 106 IFU. The number of IFU in this group was significantly less than in mice immunized with MOMP/P/C-2 [0.66 (range: 0.37–177.6) × 106 IFU; p<0.05], or from mice immunized with MOMP/Ms/C-1 [1.6 (range: BLD–21.5) × 106 IFU; p=0.05]. In contrast, the median number of IFU recovered from lungs of mice immunized with MOMP/Ms/C-2 [0.14 (range:<0.02–146) × 106 IFU] was not significantly different than in mice immunized with MOMP/Ms/C-1 (p>0.05), or mice immunized by the same routes with MOMP/P/C-2 [0.66 (range: 0.37–177.6) × 106 IFU; p>0.05]. The difference in the number of IFU recovered from mice immunized with MOMP/P/C-1 and animals immunized with MOMP/Ms/C-2 was approaching statistical significance (p=0.069).
3.5. Immune responses in lungs at 10 days p.c
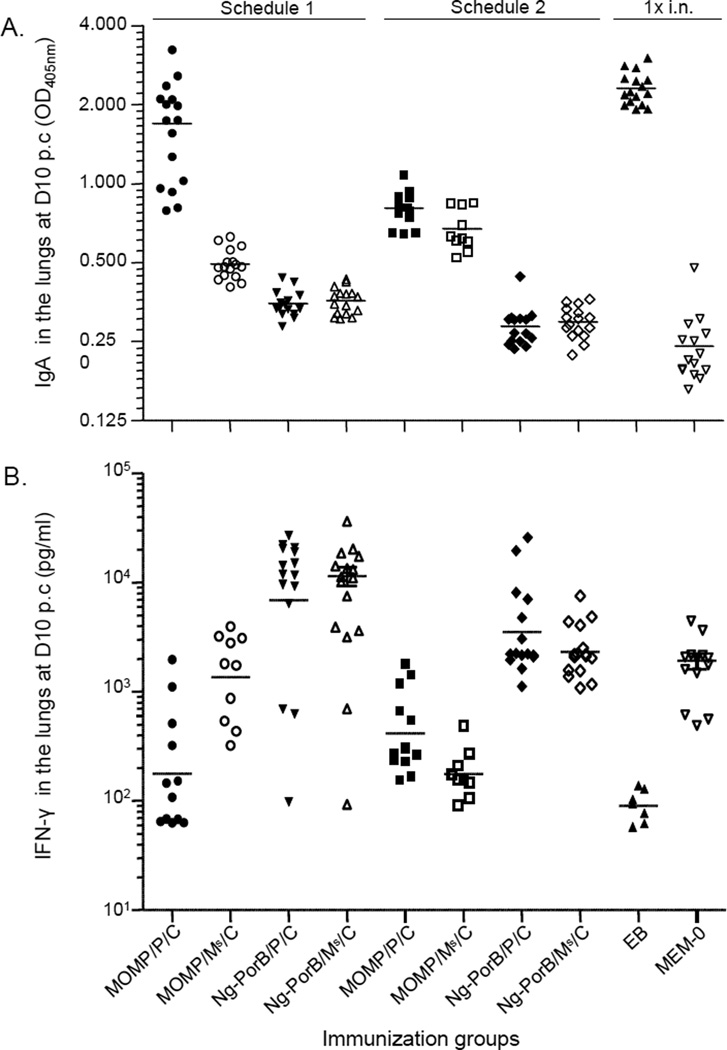
The levels of Cm-specific IgA in the lungs of mice inoculated i.n. with MEM-0 or Ng-PorB were significantly lower than in any other group immunized with MOMP (p<0.05; Fig. 3A; Table 2). The mean OD405nm of Cm-specific IgA in mice immunized with MOMP/P/C-1 was 1.699±0.177. This was significantly higher than that of mice immunized with MOMP/P/C-2 (0.809±0.032), MOMP/Ms/C-1 (0.496±0.017) or MOMP/Ms/C-2 (0.674±0.039) (p<0.05). Furthermore, the level of Cm-specific IgA in mice immunized with MOMP/P/C-2 was statistically higher than in mice immunized with MOMP/Ms/C-2 (p<0.05). As expected, mice inoculated i.n. with EB showed the highest level of Cm-specific IgA (2.315±0.083) in the lungs in comparison to any other group (p<0.05).
Fig. 3. Levels of Cm-specific IgA and IFN-γ detected in the lungs at day 10 following the i.n. challenge.
A. Levels of Cm-specific IgA (405nm OD) detected in the lungs at day 10 after the i.n. Cm challenge. The mean is shown as a horizontal line. Each symbol represents a single animal.
B. Levels of IFN-γ (pg/ml) detected in the lungs at day 10 after the i.n. Cm challenge. The mean is shown as a horizontal line. Each symbol represents a single animal.
The levels of IFN-γ detected in lungs at 10 days p.c. are shown in Fig. 3B and Table 2. Overall, there was an inverse correlation between the levels of IFN-γ and those of Cm-specific IgA. Mice immunized with MOMP/P/C-1, MOMP/P/C-2, MOMP/Ms/C-1 or MOMP/Ms/C-2 showed significantly lower level of IFN-γ (387±169 pg/ml; 584±151 pg/ml; 1875±418 pg/ml; 201±40 pg/ml) in comparison to their respective controls immunized with Ng-PorB and the same adjuvants (12,620±2160 pg/ml; 5736±1872 pg/ml; 11,557±2285 pg/ml; 2686±429 pg/ml; p<0.05). As expected, the lowest level of IFN-γ was detected in mice inoculated i.n. with live EB (94.4±11.9 pg/ml; p<0.05). The relatively low level of IFN-γ in mice inoculated with MEM-0 (1929±315 pg/ml) in comparison to those immunized with Ng-PorB, is likely a reflection of the severity of the Cm infection.
3.6. Correlation analyses between the observations
Pairwise Pearson’s correlation analysis was performed and showed that the five determinations: in vitro splenic T-cell proliferative responses (cpm) before challenge, percentage change in mean body weight p.c., number of Cm IFU recovered from the lungs at D10 p.c., and the levels of Cm-specific IgA (OD405nm) and IFN-γ (pg/ml) in the lungs at 10 days p.c., were significantly correlated to each other (p<0.05; Table 3).
Table 3.
Pairwise Pearson’s correlation assay between the observations.
| Correlation | T-cell proliferation (cpm) |
IgA in lungs (OD405nm) |
IFN-γ in lungs (log of pg/ml) |
D10 BWC* (%) | No. of Cm IFU in lungs |
|---|---|---|---|---|---|
| T-cell proliferation (cpm) | --- | 0.0006807 | 0.0029899 | 0.0007669 | 0.0000024 |
| IgA in lungs (OD405nm) | --- | --- | 0.0099292 | 0.0112737 | 0.0011700 |
| IFN-γ in lungs (log of pg/ml) | --- | --- | --- | 0.0104718 | 0.0008410 |
| D10 BWC* (%) | --- | --- | --- | --- | 0.0011100 |
| No. of Cm IFU in the lungs | --- | --- | --- | --- | --- |
BWC: body weight change.
p value conducted by pairwise Pearson’s correlation analysis between the in vitro proliferative response (cpm) of splenic T-cells before challenge, the levels of IgA and IFN-γ detected in the lungs, the percentage in body weight change, and the number of C. muridarum IFU recovered from the lungs at day 10 p.c. The statistical significant level is set to 0.05. All the p values showed the statistical significance in the pairwise Pearson’s correlation analysis.
4. Discussion
In this study we compared the protective efficacy of vaccines formulated with the MOMP using P/C, or Ms/C, as adjuvants delivered by a combination of mucosal and systemic routes. Robust humoral and cellular immune responses were elicited in BALB/c by all vaccine formulations. As determine by the local immune response in the lungs, body weight changes and number of IFU recovered from lungs, mice vaccinated with MOMP/P/C-1 had a more robust local immune response and were better protected against a respiratory challenge with Cm than the other groups.
Optimally adjuvants used for vaccination should utilize the same signaling pathways that the pathogen will activate at the time of a natural infection. During a Chlamydia infection TLR and Nod 1 mediated activation occurs [35–38]. Several chlamydial PAMPs, including LPS, MOMP, macrophage infectivity potentiator (Mip), heat shock protein-60 (hsp60) and peptidoglycan are involved in this process resulting in signaling mainly through TLR2 and TLR4 while the role of TLR-9 in chlamydial infections is under investigation [39, 40]. Thus, to enhance the immune response of a vaccine formulated with MOMP, we tested a combination of Pam2CSK4, a TLR-2 and CpG-1826, a TLR-9, adjuvants [18, 19, 41, 42].
TLR expression varies depending on the type of tissues and therefore, TLR agonists produce different responses depending upon the route of administration [43]. Protection against a chlamydial genital challenge is enhanced by combining mucosal and systemic routes for immunization [44]. For these reasons here, BALB/c mice were vaccinated using combinations of mucosal (Schedule 1: i.n. or Schedule 2: cl.) and systemic routes (i.m/s.c.) to determine if they will elicit robust immune responses and protection against a respiratory challenge. Up to now, of the mucosal routes, i.n. immunization has been shown to be the best approach for inducing protection against respiratory and genital chlamydial challenges [32, 45]. The use of the i.n. route for immunization however, can result in significant negative secondary effects [46]. For these reasons, we decided to also explore the cl. route [47, 48]. Colonic immunization may be an appropriate vaccination target since it has been shown to induce higher vaginal IgA levels than oral and even i.m. immunization, as well as higher serum IgA levels than the oral route [48]. This may be highly relevant for sexually transmitted infections like C. trachomatis since the colon is a source of IgA plasma effector for the vaginal tract [48].
Both adjuvants formulations elicited high Cm-specific antibody titers. As determined by the IgG2a/IgG1 ratio, animals vaccinated with MOMP/P/C had a Th2-biased immune response while the groups immunized using MOMP/Ms/C had strong Th1 responses. This confirms previous findings showing that, based on the IgG2a/IgG1 ratio in sera, Pam2CSK elicits a Th2 biased immune response. Interestingly, Pam2CSK stimulated the production of IFN-γ in T lymphocytes indicative of a mixed Th1/Th2 immune response [49]. Similar results were obtained following i.n. vaccination of BALB/c mice with the recombinant HIV-1 p17 protein adjuvanted with synthetic MALP-2 [50]. This formulation, as determined by IgG2a/IgG1 ratio, elicited a Th2-biased immune response but a Th1-biased cellular response based on the frequency of IFN-γ-secreting spleen cells. MALP-2, like Pam2CSK, is a diacylated lipopeptide that also signal through TLR-2 and therefore, both compounds likely have similar adjuvant properties.
Following the i.n. challenge, the changes in body weight and number of MoPn IFU recovered from the lungs demonstrated that mice immunized with MOMP/P/C were better protected than the other groups. Also, this protection was more robust than that previously reported when using formulations with single adjuvants [19]. Furthermore, these parameters of protection correlated with the levels of Cm-specific IgA and IFN-γ in the lungs. Significantly higher levels of Cm-specific IgA were detected in the lungs of mice immunized with MOMP/P/C in comparison to animals immunized with MOMP/Ms/C. This was more evident in mice vaccinated using i.n. priming rather than cl. indicating that i.n. immunization is very effective at eliciting strong local humoral immune responses in the lungs. These results are similar to those observed with MALP-2 that also induced high levels of p17-specific IgA in the lungs and vaginal washes in BALB/c mice vaccinated with this recombinant HIV-1 protein [50]. Furthermore, lower levels of IFN-γ, indicative of resolution of the infection at 10 days p.c., were detected in the lungs of the mice immunized with MOMP/P/C-1 in comparison to the mice vaccinated with MOMP/Ms/C-1.
In conclusion, MOMP formulated with Pam2CSK4 plus CpG-1826 and delivered by a combination of i.n. and i.m.+s.c. routes (MOMP/P/C-1), induced in mice strong humoral and cell mediated immune responses and the animals showed a robust protection against a respiratory challenge with C. muridarum. Now it will be interesting to test this vaccine formulation against a genital challenge. Comparing the i.n. versus the cl. routes of vaccination will be important since the regional lymph nodes of the genital tract are in close proximity and may be shared, with those of the gastrointestinal system.
Supplementary Material
Cm EB were used as the antigen. 1) Lane 1, molecular weight standards. Lanes 2 to 12, sera from mice immunized with: 2) MOMP/P/C-1; 3) MOMP/Ms/C-1; 4) Ng-PorB/P/C-1; 5) Ng-PorB/Ms/C-1; 6) MOMP/P/C-2; 7) MOMP/Ms/C-2; 8) Ng-PorB/P/C-2; 9) Ng-PorB/Ms/C-2; 10) Cm EB; 11) MEM-0 and 12) Pre-immunization sera.
Acknowledgments
This work was supported by Public Health Service grant AI-67888 from the National Institute of Allergy and Infectious Diseases and Dr. Z Jia was supported by the UCI SPECS program grant NIH UO1CA114810; the UCI NCI Early Detection Research Network: NIH UO1CAUO1CA152738 and by a grant from the Chao Family Comprehensive Cancer Center funded by NCI P30CA062203.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, Cohen MS, Harris KM, Udry JR. Prevalence of chlamydial and gonococcal infections among young adults in the United States. Jama. 2004;291:2229–2236. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- 2.Schachter J, Dawson C. Human chlamydial infections. Littleton: PSG Publishing Co; 1978. [Google Scholar]
- 3.Ness RB, Smith KJ, Chang CC, Schisterman EF, Bass DC. Prediction of pelvic inflammatory disease among young, single, sexually active women. Sex Transm Dis. 2006;33:137–142. doi: 10.1097/01.olq.0000187205.67390.d1. [DOI] [PubMed] [Google Scholar]
- 4.Stamm W. Chlamydia trachomatis infections of the adult. In: Holmes PS KK, Stamm WE, Piot P, Wasserheit JW, Corey L, Cohen MS, Watts DH, editors. Sexually transmitted diseases. New York: McGrawHill Book Co.; 2008. pp. 575–593. [Google Scholar]
- 5.Darville T. Chlamydia trachomatis infections in neonates and young children. Semin Pediatr Infect Dis. 2005;16:235–244. doi: 10.1053/j.spid.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Stutman HR, Rettig PJ, Reyes S. Chlamydia trachomatis as a cause of pneumonitis and pleural effusion. J Pediatr. 1984;104:588–591. doi: 10.1016/s0022-3476(84)80554-5. [DOI] [PubMed] [Google Scholar]
- 7.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–192. [PubMed] [Google Scholar]
- 8.Taylor HR. Trachoma: a blinding scourge from the Bronze Age to the twenty-first century. Victoria, Australia: Haddington Press Pry Ltd; 2008. [Google Scholar]
- 9.Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192:1836–1844. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 10.Gotz H, Lindback J, Ripa T, Arneborn M, Ramsted K, Ekdahl K. Is the increase in notifications of Chlamydia trachomatis infections in Sweden the result of changes in prevalence, sampling frequency or diagnostic methods? Scand J Infect Dis. 2002;34:28–34. doi: 10.1080/00365540110077001. [DOI] [PubMed] [Google Scholar]
- 11.de la Maza MA, de la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119–127. doi: 10.1016/0264-410x(95)80022-6. [DOI] [PubMed] [Google Scholar]
- 12.de la Maza LM, Peterson EM. Vaccines for Chlamydia trachomatis infections. Curr Opin Investig Drugs. 2002;3:980–986. [PubMed] [Google Scholar]
- 13.Farris CM, Morrison RP. Vaccination against Chlamydia Genital Infection Utilizing the Murine C. muridarum Model. Infect Immun. 2011;79:986–996. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 15.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 16.Harandi AM, Davies G, Olesen OF. Vaccine adjuvants: scientific challenges and strategic initiatives. Expert Rev Vaccines. 2009;8:293–298. doi: 10.1586/14760584.8.3.293. [DOI] [PubMed] [Google Scholar]
- 17.Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine. 2003;21(Suppl 2):S89–S95. doi: 10.1016/s0264-410x(03)00206-8. [DOI] [PubMed] [Google Scholar]
- 18.Hui GS, Hashimoto CN. Adjuvant formulations possess differing efficacy in the potentiation of antibody and cell mediated responses to a human malaria vaccine under selective immune genes knockout environment. Int Immunopharmacol. 2008;8:1012–1022. doi: 10.1016/j.intimp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng C, Jain P, Bettahi I, Pal S, Tifrea D, de la Maza LM. A TLR2 agonist is a more effective adjuvant for a Chlamydia major outer membrane protein vaccine than ligands to other TLR and NOD receptors. Vaccine. 2011;29:6641–6649. doi: 10.1016/j.vaccine.2011.06.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur J Immunol. 2005;35:282–289. doi: 10.1002/eji.200424955. [DOI] [PubMed] [Google Scholar]
- 21.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Morrison SG, Farris CM, Sturdevant GL, Whitmire WM, Morrison RP. Murine Chlamydia trachomatis Genital Infection Is Unaltered by Depletion of CD4+ T cells and Diminished Adaptive Immunity. J Infect Dis. 2011 doi: 10.1093/infdis/jiq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis HL, Weeratna R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 24.Weeratna RD, McCluskie MJ, Xu Y, Davis HL. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine. 2000;18:1755–1762. doi: 10.1016/s0264-410x(99)00526-5. [DOI] [PubMed] [Google Scholar]
- 25.Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun. 2005;73:8153–8160. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun G, Pal S, Weiland J, Peterson EM, de la Maza LM. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine. 2009;27:5020–5025. doi: 10.1016/j.vaccine.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi HL, Tai JY, Blake MS. Expression of large amounts of neisserial porin proteins in Escherichia coli and refolding of the proteins into native trimers. Infect Immun. 1994;62:2432–2439. doi: 10.1128/iai.62.6.2432-2439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 30.Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clinical and experimental vaccine research. 2012;1:50–63. doi: 10.7774/cevr.2012.1.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng C, Bettahi I, Cruz-Fisher MI, Pal S, Jain P, Jia Z, Holmgren J, Harandi AM, de la Maza LM. Induction of protective immunity by vaccination against Chlamydia trachomatis using the major outer membrane protein adjuvanted with CpG oligodeoxynucleotide coupled to the nontoxic B subunit of cholera toxin. Vaccine. 2009;27:6239–6246. doi: 10.1016/j.vaccine.2009.07.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–3362. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ezoe H, Akeda Y, Piao Z, Aoshi T, Koyama S, Tanimoto T, Ishii KJ, Oishi K. Intranasal vaccination with pneumococcal surface protein A plus poly(I:C) protects against secondary pneumococcal pneumonia in mice. Vaccine. 29:1754–1761. doi: 10.1016/j.vaccine.2010.12.117. [DOI] [PubMed] [Google Scholar]
- Pal S, Davis HL, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect Immun. 2002;70:4812–4817. doi: 10.1128/IAI.70.9.4812-4817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Netea MG, Kullberg BJ, Galama JM, Stalenhoef AF, Dinarello CA, Van der Meer JW. Non-LPS components of Chlamydia pneumoniae stimulate cytokine production through Toll-like receptor 2-dependent pathways. Eur J Immunol. 2002;32:1188–1195. doi: 10.1002/1521-4141(200204)32:4<1188::AID-IMMU1188>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 36.Bas S, Neff L, Vuillet M, Spenato U, Seya T, Matsumoto M, Gabay C. The proinflammatory cytokine response to Chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14. J Immunol. 2008;180:1158–1168. doi: 10.4049/jimmunol.180.2.1158. [DOI] [PubMed] [Google Scholar]
- 37.O'Connell CM, AbdelRahman YM, Green E, Darville HK, Saira K, Smith B, Darville T, Scurlock AM, Meyer CR, Belland RJ. Toll-like receptor 2 activation by Chlamydia trachomatis is plasmid dependent, and plasmid-responsive chromosomal loci are coordinately regulated in response to glucose limitation by C. trachomatis but not by C. muridarum. Infect Immun. 79:1044–1056. doi: 10.1128/IAI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagarajan UM, Ojcius DM, Stahl L, Rank RG, Darville T. Chlamydia trachomatis induces expression of IFN-gamma-inducible protein 10 and IFN-beta independent of TLR2 and TLR4, but largely dependent on MyD88. J Immunol. 2005;175:450–460. doi: 10.4049/jimmunol.175.1.450. [DOI] [PubMed] [Google Scholar]
- 39.Ouburg S, Lyons JM, Land JA, den Hartog JE, Fennema JS, de Vries HJ, Bruggeman CA, Ito JI, Pena AS, Lundberg PS, Morre SA. TLR9 KO mice, haplotypes and CPG indices in Chlamydia trachomatis infection. Drugs Today (Barc) 2009;45(Suppl B):83–93. [PubMed] [Google Scholar]
- 40.Massari P, Toussi DN, Tifrea DF, de la Maza LM. TLR2-dependent activity of Chlamydia trachomatis native major outer membrane protein proteosomes. Infect Immun. 2013 doi: 10.1128/IAI.01062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan AC, Mifsud EJ, Zeng W, Edenborough K, McVernon J, Brown LE, Jackson DC. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol Pharm. 2012;9:2710–2718. doi: 10.1021/mp300257x. [DOI] [PubMed] [Google Scholar]
- 42.Harandi AM, Holmgren J. CpG DNA as a potent inducer of mucosal immunity: implications for immunoprophylaxis and immunotherapy of mucosal infections. Curr Opin Investig Drugs. 2004;5:141–145. [PubMed] [Google Scholar]
- 43.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carmichael JR, Pal S, Tifrea D, de la Maza LM. Induction of protection against vaginal shedding and infertility by a recombinant Chlamydia vaccine. Vaccine. 2011;29:5276–5283. doi: 10.1016/j.vaccine.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal S, Peterson EM, de la Maza LM. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect Immun. 1996;64:5341–5348. doi: 10.1128/iai.64.12.5341-5348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 47.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, Czerkinsky C, Kweon MN. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A. 2008;105:1644–1649. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McConnell EL, Basit AW, Murdan S. Colonic antigen administration induces significantly higher humoral levels of colonic and vaginal IgA, serum IgG compared to oral administration. Vaccine. 2008;26:639–646. doi: 10.1016/j.vaccine.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 49.Cheng C, Cruz-Fisher MI, Tifrea D, Pal S, Wizel B, de la Maza LM. Induction of protection in mice against a respiratory challenge by a vaccine formulated with the Chlamydia major outer membrane protein adjuvanted with IC31(R) Vaccine. 2011;29:2437–2443. doi: 10.1016/j.vaccine.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 50.Becker PD, Fiorentini S, Link C, Tosti G, Ebensen T, Caruso A, Guzman CA. The HIV-1 matrix protein p17 can be efficiently delivered by intranasal route in mice using the TLR 2/6 agonist MALP-2 as mucosal adjuvant. Vaccine. 2006;24:5269–5276. doi: 10.1016/j.vaccine.2005.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cm EB were used as the antigen. 1) Lane 1, molecular weight standards. Lanes 2 to 12, sera from mice immunized with: 2) MOMP/P/C-1; 3) MOMP/Ms/C-1; 4) Ng-PorB/P/C-1; 5) Ng-PorB/Ms/C-1; 6) MOMP/P/C-2; 7) MOMP/Ms/C-2; 8) Ng-PorB/P/C-2; 9) Ng-PorB/Ms/C-2; 10) Cm EB; 11) MEM-0 and 12) Pre-immunization sera.