Abstract
BACKGROUND & AIMS:
High mobility group box 1 (HMGB1) is an abundant protein that regulates chromosome architecture and also functions as a damage-associated molecular pattern molecule. Little is known about its intracellular roles in response to tissue injury or during subsequent local and systemic inflammatory responses. We investigated the function of Hmgb1 in mice following induction of acute pancreatitis.
METHODS:
We utilized a Cre/LoxP system to create mice with pancreas-specific disruption in Hmbg1 (Pdx1-Cre; HMGB1flox/flox mice). Acute pancreatitis was induced in these mice (HMGB1flox/flox mice served as controls) following injection of L-arginine or cerulein. Pancreatic tissues and acinar cells were collected and analyzed by histologic, immunoblot, and immunohistochemical analyses.
RESULTS:
Following injection of L-arginine or cerulein, Pdx1-Cre; HMGB1flox/flox mice developed acute pancreatitis more rapidly than controls, with increased mortality. Pancreatic tissues of these mice also had higher levels of serum amylase, acinar cell death, leukocyte infiltration, and interstitial edema than controls. Pancreatic tissues and acinar cells collected from the Pdx1-Cre; HMGB1flox/flox mice following L-arginine- or cerulein injection demonstrated nuclear catastrophe with greater nucleosome release when compared with controls, along with increased phosphorylation/activation of RELA Nfκb, degradation of Iκb, and phosphorylation of Mapk. Inhibitors of reactive oxygen species (N-acetyl-L-cysteine) blocked L-arginine–induced DNA damage, necrosis, apoptosis, release of nucleosomes, and activation of Nfκb in pancreatic tissues and acinar cells from Pdx1-Cre; HMGB1flox/flox and control mice. Exogenous genomic DNA and recombinant histone H3 proteins significantly induced release of HMGB1 from mouse macrophages; administration of antibodies against H3 to mice reduced serum levels of HMGB1 and increased survival following L-arginine injection.
CONCLUSIONS:
In 2 mouse models of acute pancreatitis, intracellular HMGB1 appeared to prevent nuclear catastrophe and release of inflammatory nucleosomes to block inflammation. These findings indicate a role for the innate immune response in tissue damage.
Keywords: DNA damage, pancreatitis, oxidative stress, Nfκb
Introduction
Innate immune cells orchestrate both the physiologic and pathologic inflammatory immune response following engagement by pattern-recognition receptors including pathogen-associated molecular pattern (PAMPs) or damage-associated molecular pattern (DAMPs) molecules 1-3. Many DAMPs are derived from the nucleus, and are thus collectively termed nuclear DAMPs (nDAMPs). Examples of nDAMPs include high mobility group box 1 (HMGB1) 4, 5 and components of the nucleosome (e.g., DNA 6 and histones 7). Within the nucleus, HMGB1 maintains chromosomal structure and regulates DNA damage responses 8. Under a variety of stressful situations, however, HMGB1 translocates to the cytosol, where it sustains autophagy, and then is released into the extracellular space. There, it coordinates inflammation, immunity, and other local cellular processes 9. Pathologic nDAMP release is increasingly being recognized as an etiology for a variety of human inflammatory diseases and represents an emergent target for therapy 10, 11. A better understanding of the intricate mechanisms underlying the release and response to nDAMPs will aid in this effort.
Acute pancreatitis (AP) is a poorly understood inflammatory disease, responsible for significant human morbidity and mortality each year worldwide 12. In patients and animals with AP, serum levels of HMGB1 are significantly increased and positively correlate with the severity of the disease 13-15. Inhibiting the release or cytokine activity of HMGB1 confers protection against experimental AP 16-18. The precise role of HMGB1 during acute pancreatitis-induced tissue injury and subsequent local and systemic inflammation is poorly understood. Here, we show, in contrast to neutralization of HMGB1 in the serum, that conditional knockout of HMGB1 in the pancreas rendered mice dramatically more susceptible to experimental AP. Deficiency of endogenous pancreatic HMGB1 resulted in accelerated tissue injury and lethality. This enhanced severity was associated with increased nuclear catastrophe and nucleosome (histone and DNA) release and increased recruitment and activation of inflammatory cells. Interestingly, the subsequent activation of innate immune effectors was associated with increased HMGB1 release into the circulation. Neutralizing extracellular histone and/or HMGB1 conferred protection against AP in conditional pancreas-specific HMGB1 knockout mice. Thus, intracellular nuclear HMGB1 serves as a previously underappreciated negative regulator of inflammation-limiting nuclear catastrophe following injury with resultant release of other nDAMPs. This work improves our understanding of the complex role of HMGB1 in protecting against cellular injury and subsequent activation of innate immune responses following sterile tissue damage.
Materials and Methods
Reagents
The antibodies to p-RELA, RELA (also known as p65), p-Iκb, Iκb, IKKα, IKKβ, IKKγ, p100, RELB, p-MAPK14, MAPK14 (also known as p38), p-MAPK8, MAPK8 (also known as JNK), p-MAPK1, MAPK1 (also known as ERK), H3, H4, γ-H2AX, C-Casp3, C-PARP, and actin were obtained from Cell Signaling Technology (Danvers, MA, USA). The antibody to HMGB1 came from Novus (Littleton, CO, USA). The antibody to 4-Hydroxy-2-nonenal came from Alpha Diagnostic (San Antonio, TX, USA). Mouse genomic DNA and DNase I came from New England Biolabs (Ipswich, MA, USA). Recombinant calf thymus histone H3 came from Roche (Madison, WI, USA). Recombinant HMGB1 proteins were generated as previously described 19. Contaminating endotoxin was removed from protein by Triton X-114 extraction. Mouse HMGB1 neutralizing antibody (IgG2B) was generated as previously described 20. Rabbit H3 neutralizing antibody came from Abcam. Control mouse IgG2B and rabbit IgG came from R&D Systems (Minneapolis, MN, USA). Unless otherwise stated, all other reagents were purchased from Sigma (St. Louis, MO, USA).
Mouse strains
The protocol for animal use was reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Pancreatic specific HMGB1 knockout mice were prepared and bred in our laboratory by crossing floxed HMGB1 (HMGB1flox/flox) and Pdx1-Cre transgenic mice. In the floxed mice, exons 2 and 3 of HMGB1 gene were flanked by two lox/p sites that enable the recombination of the HMGB1 loci in the presence of cre recombinase (Fig.S1A). Pdx1-Cre transgenic mice on C57BL/6 background were obtained from the MMHCC/NCI Mouse Repository. As both mouse strains were on the B6 background, all progeny used in our study were generated with B6 mice with pure genetic backgrounds. Specifically, F1 offspring of an initial Pdx1-Cre × HMGB1flox/flox intercross mating were genotyped to confirm the presence of both the Pdx1-Cre and floxed HMGB1 alleles by standard polymerase chain reaction (PCR). To generate mice homozygous for the floxed HMGB1 allele, F1 generation mice were backcrossed with the HMGB1flox/flox line (Fig.S1B). Pdx1-Cre; HMGB1flox/flox (termed CH mice) offspring were identified by genotyping for the presence of both the Pdx1-Cre and floxed HMGB1 alleles and the absence of the wild-type HMGB1 allele. Recombination/deletion of the HMGB1 gene in pancreatic cells was confirmed by genotyping analysis (Fig.S1C). The presence of the Pdx1-Cre transgene was detected by PCR amplification with primers 5’-CTGGACTACATCTTGAGTTGC-3′ and 5′-GGTGTACGGTCAGTAAATTTG-3′; the identification of floxed (700bp) and wild type (635bp) HMGB1 with 5′-TGATGCGAACACGGCGTGCTCTA′ and 5′-GCACAAAGAATGCATATGAGGAC-3′. In parallel, HMGB1 level in pancreatic tissue or extracts from the pancreatic acinar cells of CH mice was assayed by immunofluorescent staining (Fig.S1D) and Western blot (Fig.S1E), respectively.
Experimental animal models of acute pancreatitis
For L-arginine-induced pancreatitis, a sterile solution of L-arginine hydrochloride (8%; Sigma) was prepared in normal saline and the pH was adjusted to 7.0. Mice received two hourly intraperitoneal (i.p.) injections of L-arginine (4 g/kg), while controls were administered saline i.p. as a control as described previously 21. For cerulein-induced pancreatitis, mice received seven hourly i.p. injections of 50 μg/kg cerulein (Sigma) in sterile saline, while controls were given saline as described previously 22. Animals were sacrificed at the indicated time by CO2 asphyxia, and a blood sample and tissue were collected. Blood samples were collected in heparinized syringes and centrifuged at 10,000 g for 10 minutes at 4°C. Following centrifugation, the plasma was aspirated and used for measurement of amylase, lactate dehydrogenase (LDH), nucleosomes, HMGB1, and other cytokines by ELISA. Tissue samples were collected, snap frozen in liquid nitrogen, and stored at −80°C for analysis of myeloperoxidase (MPO) activity. Formalin-fixed pancreas samples were processed, and 5 μm thick paraffin sections were stained with hematoxylin and eosin (H&E) for histological analysis.
Results
Pancreas-Derived HMGB1 Protects Against Experimental Acute Pancreatitis
Because global HMGB1 knockout mice die shortly following birth 23, we generated transgenic mice with conditional knockout of HMGB1 within the pancreas (CH mice) (Fig. S1A-1C). The expression of HMGB1 is essentially missing from pancreatic tissue (Fig.S1D) or cultured pancreatic acinar cells from CH mice (Fig.S1E). CH mice demonstrated phenotypes similar to wild type and/or HMGB1flox/flox control mice (F/F mice) in pancreas size (Fig.S1F), exocrine function (serum amylase level and pancreatic trypsin activity) (Fig.S1G-H), endocrine function (blood glucose level) (Fig.S1I), and pancreatic histology (Fig. S1J). These findings suggest that HMGB1 does not itself affect pancreatic development.
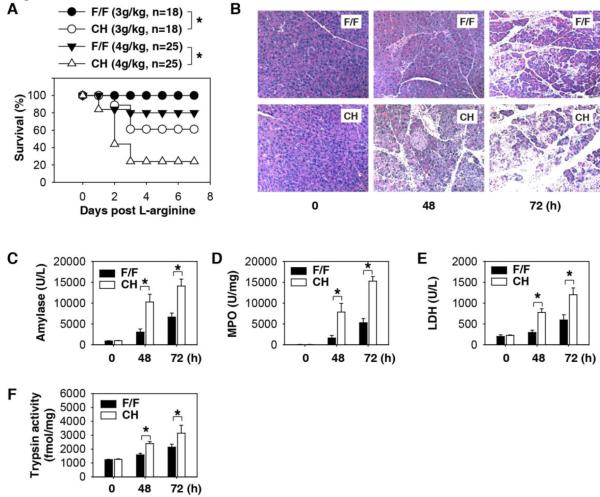
Severe AP was induced with i.p. injection of L-arginine or cerulein as previously described 21, 22. The CH mice were substantially more susceptible to AP with significantly higher mortality rates compared to F/F mice (Fig. 1A). Histological assessment of pancreatic damage revealed exaggerated acinar cell death, leukocyte infiltration, and interstitial edema in the CH mice compared to F/F mice (Fig. 1B). The level of serum amylase, the most commonly used biochemical marker of established AP, was also significantly higher in CH mice (Fig. 1C). Consistently, pancreatic neutrophil recruitment (as measured by the pancreatic MPO activity, Fig. 1D) and pancreatic necrosis (as reflected by the serum LDH activity, Fig. 1E) were significantly higher in the CH mice as well. Intrapancreatic conversion of trypsinogen to trypsin is an important step in the development of acute pancreatitis 12. The loss of HMGB1 within pancreatic tissue from CH mice accelerated L-arginine-induced pancreatic trypsin activity when compared with F/F mice (Fig. 1F). Severe AP is often associated with acute lung injury, a significant cause of morbidity and mortality in this disease. L-arginine-induced, AP-associated acute lung injury was worse in CH mice (Fig. S2). Similarly, cerulein-induced experimental AP was also associated with increased acinar cell injury (Fig. S3A), increased serum amylase (Fig. S3B), increased pancreatic MPO activity (Fig. S3C), and increased pancreatic trypsin activity (Fig. S3D) in CH mice when compared with F/F control mice. These results demonstrate that, in contrast to neutralization or prevention of HMGB1 release within the serum, loss of endogenous HMGB1 within the injured pancreatic acinar cell worsens the severity of experimental AP.
Fig.1. HMGB1 protects against L-arginine-induced acute severe pancreatitis.
(A) HMGB1flox/flox (“F/F”) and pancreas-specific conditional HMGB1 knockout mice (“CH”) received a lethal L-arginine dose (3-4 g/kg × 2, i.p.). The Kaplan-Meyer method was used to compare the differences in survival rates between groups. *, P<0.05. (B-D) Hematoxylin and eosin -stained pancreatic sections (B), plasma amylase activity (C), pancreatic MPO activity (D), plasma LDH activity (E), and pancreatic trypsin activity (F) at the indicated time points following administration of L-arginine (4 g/kg× 2, n=3-5 mice/group, *, P<0.05).
Pancreas-Derived HMGB1 Inhibits Inflammatory Response in Acute Pancreatitis
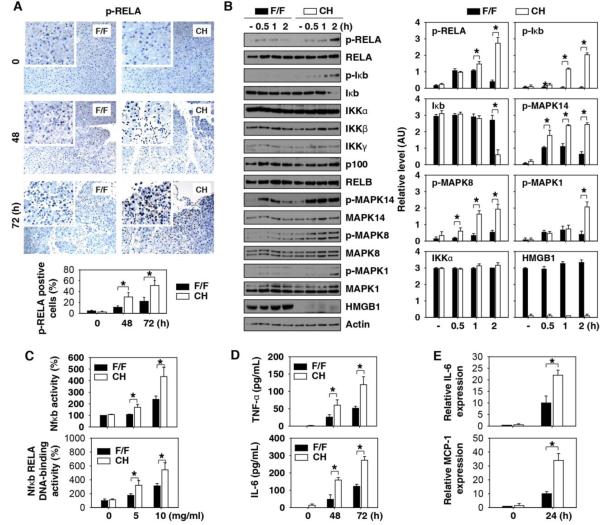
Excessive inflammation contributes to the pathogenesis of AP through activation of multiple signal transduction pathways involving nuclear factor κB (Nfκb) and mitogen-activated protein kinases (MAPKs) 24, 25. Loss of HMGB1 in the pancreas led to a significant enhancement of L-arginine-induced Nfκb RELA phosphorylation/activation (Fig. 2A, 2B, 2C), Iκb degradation (Fig. 2B), and MAPK phosphorylation (Fig. 2B and Fig. S4) in the pancreas or pancreas extracts. In parallel, the serum levels of TNF-α and IL-6 were significantly increased at 48 and 72 hours in the CH mice when compared with F/F control mice following administration of L-arginine (Fig. 2D) or cerulein (Fig. S3E and 3F). The intra-pancreatic increase in cytokines and chemokines are early and much more pronounced than found in the blood. Loss of HMGB1 in the pancreas led to a significant enhancement of L-arginine-induced intra-pancreatic cytokine (e.g., IL-6) and chemokine (monocyte chemoattractant protein 1, MCP-1) expression at 24 hours (Fig. 2E). Thus, loss of HMGB1 in the acinar cell created an exaggerated local and systemic inflammatory response.
Fig.2. Loss of HMGB1 promotes Nfκb and MAPK inflammatory signaling pathways in vitro and in vivo.
(A) Immunohistochemistry and quantitative analysis of phosphorylation of RELA in pancreatic tissue sections from F/F and CH mice at indicated times following administration of L-arginine (4 g/kg× 2). (B) Western blot analysis of the indicated proteins in L-arginine (10 mg/ml) treated pancreatic acinar cells from F/F and CH mice in vitro. (C) Nfκb transcriptional activity and Nfκb RELA DNA-binding activity analysis in L-arginine (10 mg/ml) treated pancreatic acinar cells from F/F and CH mice in vitro (n=3, *, P<0.05). (D) Serum TNF- α and IL-6 levels in F/F and CH mice at indicated time points following administration of L-arginine (4 g/kg× 2, n=3-5 mice/group, *, P<0.05). (E) Intra-pancreatic IL-6 and MCP-1 protein expression levels in F/F and CH mice at 24 h following administration of L-arginine (4 g/kg× 2, n=3-5 mice/group, *, P<0.05).
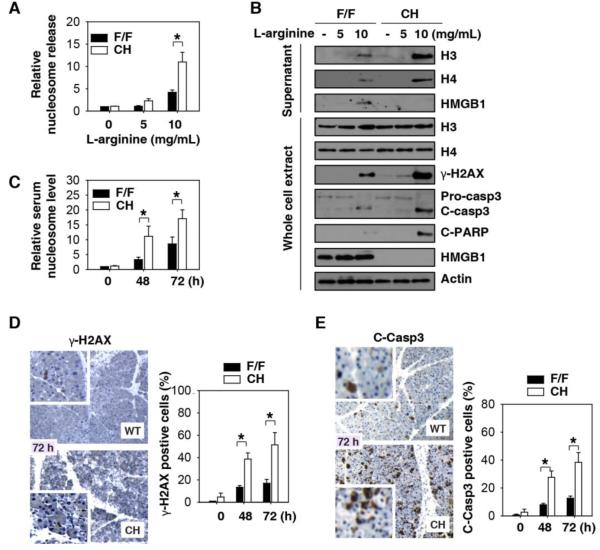
Fig.3. Loss of HMGB1 promotes DNA damage, cell death, and nDAMP release in vitro and in vivo.
(A) Relative extracellular nucleosome levels in L-arginine (24 hours) treated pancreatic acinar cells from F/F and CH mice in vitro (n=3, *, P<0.05). (B) Western blot analysis of whole- cell lysates and supernatants from L-arginine (24 hours) treated pancreatic acinar cells from F/F and CH mice. γ-H2AX is a marker of DNA damage. Cleaved-caspase 3 (“C-casp3”) and cleaved-PARP (“C-PARP”) are markers of apoptosis. (C-E) Relative serum nucleosome level (C), pancreatic γ-H2AX (D), and C-casp3(C) in F/F and CH mice following administration of L-arginine (4 g/kg× 2, n=3-5 mice/group, *, P<0.05).
Intracellular HMGB1 Prevents DNA Damage, Cell Death, and nDAMP Release in Acute Pancreatitis
Nuclear HMGB1 is a high affinity DNA-binding and bending protein that directly interacts with the nucleosome to maintain chromosomal structure, function, and stability 26-30. The extracellular release of HMGB1 and other nDAMPs (including individual nucleosomal components including DNA and histone proteins H3 and H4) triggers rigorous inflammatory responses 6, 7, 31-34. Following L-arginine stimulation, a large number of HMGB1-nucleosomal complexes were observed within the nuclei of pancreatic acinar cells from F/F control mice (Fig. S5), which may represent sites of DNA damage and repair. Notably, the release of nDAMPs, including the nucleosomal octamer histone proteins H3 and H4, was significantly higher in primary cultured CH pancreatic acinar cells following stimulation with L-arginine (Fig. 3A and 3B) or cerulein (Fig. S6). Consistent with this finding, exposure of CH mice to L-arginine produced significantly higher serum nucleosome levels (Fig. 3C). Since DNA damage promotes nDAMP release, we investigated whether the loss of HMGB1 would exaggerate DNA damage in AP. The loss of HMGB1 within pancreatic acinar cells accelerated L-arginine-induced phosphorylation of H2AX (namely γ-H2AX), a specific marker for DNA damage (Fig. 3B and 3D). Consistently, both necrosis (Fig.1E and Fig. S7) and apoptosis (Fig. 3B, 3E and Fig. S7) were significantly increased in pancreatic tissue and/or acinar cells from CH mice in response to L-arginine. These findings suggest that intracellular HMGB1 prevents DNA damage, cell death, and nDAMP release in the setting of AP.
Oxidative Stress Mediates Experimental Acute Pancreatitis in HMGB1 Conditional Knockout Mice
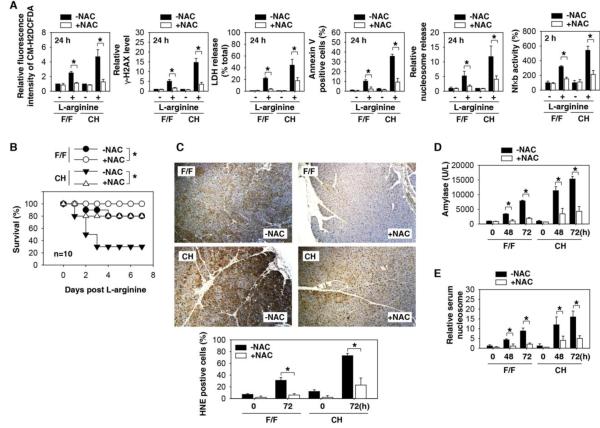
The generation of reactive oxygen species (ROS) directly or indirectly provokes additional DNA damage 35, thereby resulting in the so-called mitotic catastrophe 36. In addition, ROS is an important mediator of NFκb activation during pancreatitis 37, 38. We have previously demonstrated that HMGB1 is essential for mitochondrial quality control, and loss of HMGB1 leads to excessive ROS production 39. Consistent with these previous observations, ROS inhibitors (e.g., N-acetyl-L-cysteine, NAC) effectively blocked L-arginine-induced DNA damage, necrosis, and apoptosis, as well as the release of nucleosomes and Nfκb activation by pancreatic acinar cells derived from F/F and CH mice (Fig. 4A). NAC protected against L-arginine-induced AP in F/F and CH mice (Fig. 4B), as indicated by a decrease in oxidative injury (Fig. 4C) and serum amylase (Fig. 4D) or nucleosomes (Fig. 4E). Similarly, administration of NAC also decreased cerulein-induced oxidative injury (Fig. S8A), serum amylase (Fig. S8B), or nucleosome release (Fig. S8C) in both F/F and CH mice. Thus, oxidative stress is a critical component of experimental AP.
Fig.4. NAC protects against L-arginine-induced AP.
(A) The fluorescence intensity of CM- H2DCFDA, γ-H2AX level, LDH release, percentage of annexin V-positive cells, nucleosome release, and Nfκb activation in L-arginine (10mg/mL) treated cultured pancreatic acinar cells derived from F/F and CH mice with or without addition of 50 mM NAC (n=3, *, P<0.05). (B) F/F and CH mice received a lethal L-arginine dose (4 g/kg × 2, i.p.) with or without NAC (300 mg/kg i.p.). The Kaplan-Meyer method was used to compare the differences in survival rates between groups (n=10 mice/group, *, P<0.05). (C-E) In parallel, oxidative injury by staining for HNE (the marker of oxidative damage) (C), plasma amylase activity (D), and relative level of serum nucleosome (E) at indicated time points were assayed (*, P<0.05).
Neutralization of Extracellular Histone and HMGB1 Confers Protection against Acute Pancreatitis in HMGB1 Conditional Knockout Mice
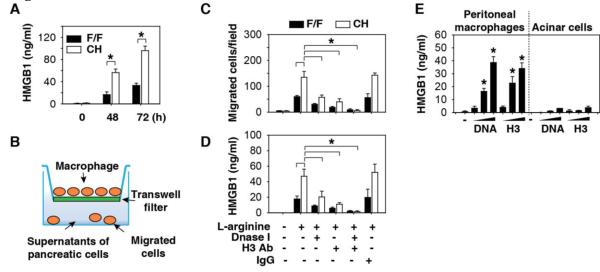
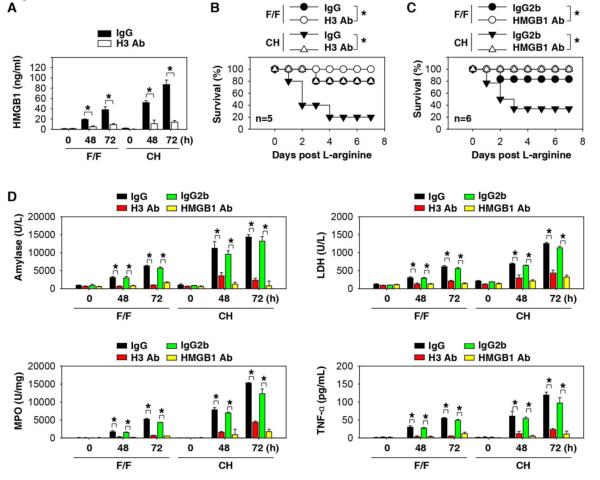
We anticipated that CH mice would demonstrate lower serum HMGB1 levels following administration of L-arginine as a consequence of diminished release from injured pancreatic acinar cells deficient in the molecule (Fig. 3B). We observed, however, higher serum levels of HMGB1 accumulation in the CH mice when compared to controls (Fig. 5A). HMGB1 is released utilizing two predominant pathways: 1) passive release by dying or stressed cells 5, 40, or 2) active secretion by innate immune or parenchymal cells 4, 41, 42. To identify the source of circulating HMGB1 in CH mice, we evaluated the effect of pancreatic acinar cell-derived products on macrophage cell migration using a trans-well system (Fig. 5B). The supernatant of L-arginine-treated acinar cells from CH mice was significantly more potent in inducing macrophage migration (Fig. 5C) and HMGB1 release (Fig. 5D) when compared to F/F control acinar cells. Addition of a DNA inhibitor (Dnase I) or H3-neutralizing antibodies significantly impaired macrophage migration (Fig. 5C) and HMGB1 release (Fig. 5D), suggesting that the release of pancreatic nucleosomal DNA and histone may be important in triggering HMGB1 release by infiltrated leukocytes. As expected, exogenous genomic DNA and recombinant histone H3 proteins significantly induced HMGB1 release in mouse primary peritoneal macrophages (Fig. 5E) and the macrophage-like RAW 264.7 cell line (Fig. S9). However, exogenous genomic DNA and recombinant histone H3 proteins did not induce HMGB1 release in acinar cells from F/F mice (Fig. 5E), suggesting that activated immune cells, but not acinar cells, release HMGB1 into the extracellular space during nDAMP stimuli. Consistent with these in vitro observations (Fig. 5D), anti-H3 antibody significantly suppressed serum HMGB1 levels (Fig. 6A) and improved survival following L-arginine administration in F/F and CH mice (Fig. 6B). To determine whether serum HMGB1 is responsible for L-arginine-induced pancreatitis in CH mice, we injected anti-HMGB1 neutralizing antibody two hours after L-arginine (followed by additional doses at 12 and 24 hours after L-arginine administration). This delayed anti-HMGB1 treatment conferred significant and long-lasting protection against lethal pancreatitis (Fig. 6C). Consistently, serum levels of tissue enzymes (amylase, LDH, and MPO) and the pro- inflammatory cytokine TNF-α were significantly reduced by repetitive administration of anti-HMGB1 or anti-histone H3 antibodies (Fig. 6D).
Fig.5. Extracellular histone and DNA induce HMGB1 release in macrophages.
(A) Serum HMGB1 levels in F/F and CH mice following administration of L-arginine (4 g/kg× 2, n=3-5 mice/group, *, P<0.05). (B) Schematic diagram of the transwell assay. (C) Transwell assay shows the number of mouse RAW264.7 cells that migrated towards the supernatants of L-arginine treated pancreatic cells obtained from F/F and CH mice with or without treatment with Dnase I (10 U/ml), H3 antibody (10 μ g/ml), and control IgG (n=3, *, P<0.05). (D) In parallel, HMGB1 release by RAW264.7 cells was analyzed by ELISA (n=3, *, P<0.05). (E) Peritoneal macrophages or acinar cells from F/F mice were treated with genomic DNA and recombinant histone H3 proteins for 24 hours. HMGB1 release was analyzed by ELISA (n=3, *, P<0.05).
Fig.6. Neutralization of extracellular histone and HMGB1 confer protection against acute pancreatitis.
(A, B) F/F and CH mice received anti-histone H3 (20 mg/kg) or rabbit isotype control IgG by i.p. injection two hours after completion of the L-arginine-induced pancreatitis protocol. Serum HMGB1 levels (A) and animal survival rates (B) were assayed (*, P<0.05). (C) F/F and CH mice received anti-HMGB1 (20 mg/kg) or mouse isotype control IgG2B by i.p. injection two hours after the end of the L-arginine-induced pancreatitis procedure (followed by additional doses at 12 and 24 hours after L-arginine). The Kaplan-Meyer method was used to compare the differences in survival rates between groups. *, P<0.05. (D) In parallel, plasma amylase activity, plasma LDH activity, pancreatic MPO activity, and serum TNF-α levels at the indicated time points after administration of L-arginine with or without indicated antibody in F/F and CH mice were assayed (n=3-5 mice/group, *, P<0.05).
Discussion
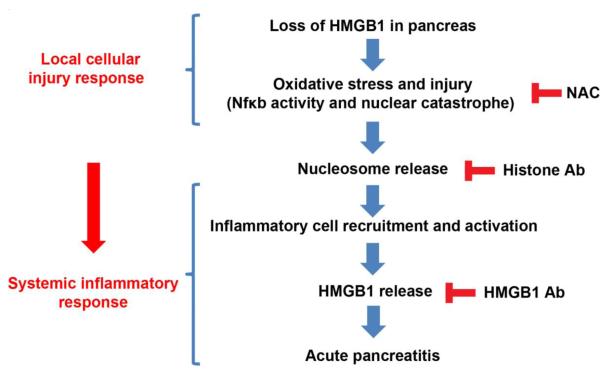
Our current knowledge of the development of pancreatitis can be described as progression from an initial injury of the exocrine pancreas which then progresses to local and systemic inflammatory responses 43. Our findings demonstrate that HMGB1 plays two major pathological roles in experimental AP: 1) Pancreatic-derived intracellular HMGB1 limits the severity of the disease by protecting cells from Nfκb activation, DNA damage, cell death, and release of nucleosomes from injured acinar cells and 2) Extracellular HMGB1 released from innate immune cells exacerbates the disease by enhancing pathologic inflammatory responses (Fig. 7).
Fig.7. Endogenous HMGB1 protects against severe acute pancreatitis by preventing nuclear catastrophe and proinflammatory nucleosome release.
Illustrated is our current model of the role of HMGB1 in limiting nucleosomal release and the subsequent inflammatory response.
In experimental models of AP, acinar cells die as a consequence of both necrosis and apoptosis 12. Our findings demonstrate that loss of HMGB1 in pancreatic tissues increased cell death in vivo and in vitro. HMGB1 is the most abundant non-histone protein within the nucleus and is demonstrably a DNA damage sensor during stress 8. Loss of HMGB1 in pancreatic acinar cells increases Nfκb activation and DNA damage. This enhanced Nfκb activation and DNA damage is mediated in part by ROS signaling, as the free radical scavenger NAC reverses the effects of HMGB1 loss on cellular injury in vivo and in vitro. Oxidative stress plays a pivotal role in the development of AP. The functional relationship between ROS and AP is admittedly complex. In many AP cases, ROS is responsible for cell death . In some cases, ROS generation within acinar cells may be a protective response as NAC stimulates necrosis in models of AP 44. The key molecular events that eventually determine whether ROS is protective or destructive still remain largely unknown.
Nfκb is responsible for regulating the transcription of a wide variety of genes involved in inflammation and cell death in the development and progression of AP 45. Mechanisms mediating the upstream signal of Nfκb activation involve calcium, ROS, and MAPK activation. Loss of HMGB1 in the pancreatic acinar cells led to a significant enhancement of L-arginine-induced Nfκb phosphorylation/activation, suggesting that both inflammatory cell-dependent and -independent mechanisms may contribute to the effects of HMGB1. A number of studies have demonstrated that pharmacologic inhibition of Nfκb activation (e.g., Bay 11-7082 and NAC) diminish the inflammatory response and limit the severity of pancreatitis 38. Of note, Nfκb activation may function as a stress-induced self-defense mechanism to protect AP. Thus, blocking of Nfκb activation (e.g., pyrrolidine dithiocarbamate and NAC) increased cerulean-induced injury 37. Activation of the pancreas-specific Nfκb pathway in transgenic mice by overexpressing Nfκb RELA (termed LSL-RELA/Cre mice) or ablation of Iκbα (termed IκbαΔpanc mice) had distinct phenotypes in AP. Cerulein- and L-arginine-induced AP is protected in IκbαΔpanc mice 46, whereas it is aggravated in LSL-RELA/Cre mice 47. These studies suggest that the key components of the Nfκb pathway may have differing roles in the regulation of AP development. For this reason, further exploration of these pathways should be carried out.
Despite the loss of HMGB1 in pancreatic tissues, CH mice with experimentally induced AP demonstrate enhanced disease severity that is neutralized by anti-HMGB1 antibodies. In fact, serum levels of HMGB1 were higher in CH mice than in control mice. Indeed, tissue leukocyte infiltration and serum levels of pro-inflammatory cytokines (e.g., TNF-α and IL-6) were significantly higher in CH mice compared to their wild-type control littermates. Thus, in our model, extracellular HMGB1 acts as a pro-inflammatory cytokine and its release by innate immune effectors is enhanced in the setting of increased nDAMP release. A recent study showed that extracellular DAMP mediated inflammation in AP partly through TLR9 signaling48. RAGE may also contribute to DAMP-TLR9 signaling, serving as a vehicle for internalization of DNA/protein complexes, which has been described in the setting of necrosis in plasmacytoid dendritic cells 49. Thus, the functional interplay between DAMP molecules and their individual receptors may provide clues to developing new therapeutic strategies for patients with AP.
While it has been well established that DAMPs contribute to cell death-induced inflammation1-3, it has not been previously demonstrated that these same molecules can also serve as intracellular mediators of cellular stress. Many DAMPs are nuclear or cytosolic proteins. Our observation that loss of HMGB1 in pancreatic acinar cells enhanced cell death and promoted release of other nDAMP such as nucleosomes (DNA and histone) suggests a new paradigm whereby the same molecules that serve to protect cells against cellular stress also serve as extracellular signaling molecules when recognized by innate immune effectors. While there is not likely to be a physiologic setting where cells will have complete loss of HMGB1, we have previously shown that inflammation and stress induce HMGB1 translocation from the nucleus to the cytosol 50, 51. We hypothesize that relative loss of HMGB1 from the nucleus will render cells more sensitive to DNA damage and subsequent cell death. This observation may explain increased DNA damage in the setting of inflammation and cancer.
In summary, our observations describe for the first time the existence of an HMGB1-mediated nuclear regulatory mechanism for the control of nuclear homeostasis and nDAMP release that modulates the magnitude of the subsequent inflammatory response following tissue injury. Significant loss of nuclear HMGB1 could result in a rapid escalation of local inflammation through destabilization of the nucleus and enable rapid DNA and histone release. These findings significantly add to our understanding of the complex regulatory mechanisms and functional consequences of the innate immune response to tissue damage mediated through nDAMPs. In addition, to our knowledge, our CH model is the first HMGB1 conditional knockout disease model, which will accelerate the study of HMGB1 function and provide direct evidence for its role in vivo.
Supplementary Material
Acknowledgments
We thank Christine Heiner (Department of Surgery, University of Pittsburgh) for her critical reading of the manuscript. We also thank Drs. Donna Stolz and Simon Watkins for making available the confocal microscopy facilities at the Center for Biologic Imaging at University of Pittsburgh School of Medicine.
Grant Support: This work was supported by the National Institutes of Health (NIH - R01CA160417 to D.T.), Departmental funding from the University of Pittsburgh (to D.T., M.T.L, and H.J.Z), and a grant from the American Association for Cancer Research-Pancreatic Cancer Action Network (to D.T.). This project used University of Pittsburgh Cancer Institute shared resources including the Cyometry and Imaging Cores that are supported in part by award P30CA047904.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Full Methods and any associated references are available in the SUPPLEMENTAL MATERIALS AND METHODS.
References
- 1.Tang D, Kang R, Coyne CB, et al. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–75. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 5.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 6.Marichal T, Ohata K, Bedoret D, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange SS, Mitchell DL, Vasquez KM. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc Natl Acad Sci U S A. 2008;105:10320–5. doi: 10.1073/pnas.0803181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 10.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nature reviews. Rheumatology. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 12.Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–51. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 13.Yasuda T, Ueda T, Takeyama Y, et al. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33:359–63. doi: 10.1097/01.mpa.0000236741.15477.8b. [DOI] [PubMed] [Google Scholar]
- 14.Kocsis AK, Szabolcs A, Hofner P, et al. Plasma concentrations of high-mobility group box protein 1, soluble receptor for advanced glycation end-products and circulating DNA in patients with acute pancreatitis. Pancreatology. 2009;9:383–91. doi: 10.1159/000181172. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda T, Ueda T, Shinzeki M, et al. Increase of high-mobility group box chromosomal protein 1 in blood and injured organs in experimental severe acute pancreatitis. Pancreas. 2007;34:487–8. doi: 10.1097/MPA.0b013e31804154e4. [DOI] [PubMed] [Google Scholar]
- 16.Cheng BQ, Liu CT, Li WJ, et al. Ethyl pyruvate improves survival and ameliorates distant organ injury in rats with severe acute pancreatitis. Pancreas. 2007;35:256–61. doi: 10.1097/MPA.0b013e318064678a. [DOI] [PubMed] [Google Scholar]
- 17.Sawa H, Ueda T, Takeyama Y, et al. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666–70. doi: 10.3748/wjg.v12.i47.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan H, Jin X, Sun J, et al. Protective effect of HMGB1 a box on organ injury of acute pancreatitis in mice. Pancreas. 2009;38:143–8. doi: 10.1097/MPA.0b013e31818166b4. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Wang H, Mason JM, et al. Recombinant HMGB1 with cytokine-stimulating activity. J Immunol Methods. 2004;289:211–23. doi: 10.1016/j.jim.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Hreggvidsdottir HS, Palmblad K, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawra R, Sharif R, Phillips P, et al. Development of a new mouse model of acute pancreatitis induced by administration of L-arginine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1009–18. doi: 10.1152/ajpgi.00167.2006. [DOI] [PubMed] [Google Scholar]
- 22.Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–55. doi: 10.1172/JCI38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calogero S, Grassi F, Aguzzi A, et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet. 1999;22:276–80. doi: 10.1038/10338. [DOI] [PubMed] [Google Scholar]
- 24.Altavilla D, Famulari C, Passaniti M, et al. Attenuated cerulein-induced pancreatitis in nuclear factor-kappaB-deficient mice. Lab Invest. 2003;83:1723–32. doi: 10.1097/01.lab.0000101734.82054.be. [DOI] [PubMed] [Google Scholar]
- 25.Liu HS, Pan CE, Liu QG, et al. Effect of NF-kappaB and p38 MAPK in activated monocytes/macrophages on pro-inflammatory cytokines of rats with acute pancreatitis. World J Gastroenterol. 2003;9:2513–8. doi: 10.3748/wjg.v9.i11.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawase T, Sato K, Ueda T, et al. Distinct domains in HMGB1 are involved in specific intramolecular and nucleosomal interactions. Biochemistry. 2008;47:13991–6. doi: 10.1021/bi8013449. [DOI] [PubMed] [Google Scholar]
- 27.Cato L, Stott K, Watson M, et al. The interaction of HMGB1 and linker histones occurs through their acidic and basic tails. J Mol Biol. 2008;384:1262–72. doi: 10.1016/j.jmb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Giavara S, Kosmidou E, Hande MP, et al. Yeast Nhp6A/B and mammalian Hmgb1 facilitate the maintenance of genome stability. Curr Biol. 2005;15:68–72. doi: 10.1016/j.cub.2004.12.065. [DOI] [PubMed] [Google Scholar]
- 29.Celona B, Weiner A, Di Felice F, et al. Substantial histone reduction modulates genomewide nucleosomal occupancy and global transcriptional output. PLoS Biol. 2011;9:e1001086. doi: 10.1371/journal.pbio.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonaldi T, Langst G, Strohner R, et al. The DNA chaperone HMGB1 facilitates ACF/CHRAC-dependent nucleosome sliding. Embo J. 2002;21:6865–73. doi: 10.1093/emboj/cdf692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palumbo R, Galvez BG, Pusterla T, et al. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allam R, Scherbaum CR, Darisipudi MN, et al. Histones from Dying Renal Cells Aggravate Kidney Injury via TLR2 and TLR4. J Am Soc Nephrol. 2012;23:1375–88. doi: 10.1681/ASN.2011111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urbonaviciute V, Furnrohr BG, Meister S, et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–18. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–28. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooke MS, Evans MD, Dizdaroglu M, et al. Oxidative DNA damage: mechanisms, mutation, and disease. Faseb J. 2003;17:1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 36.Vitale I, Galluzzi L, Castedo M, et al. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–92. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- 37.Steinle AU, Weidenbach H, Wagner M, et al. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420–30. doi: 10.1016/s0016-5085(99)70140-x. [DOI] [PubMed] [Google Scholar]
- 38.Gukovsky I, Gukovskaya AS, Blinman TA, et al. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–14. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 39.Tang D, Kang R, Livesey KM, et al. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011;13:701–11. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu B, Nakamura T, Inouye K, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–4. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsung A, Klune JR, Zhang X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–23. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hegyi P, Pandol S, Venglovecz V, et al. The acinar-ductal tango in the pathogenesis of acute pancreatitis. Gut. 2011;60:544–52. doi: 10.1136/gut.2010.218461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Booth DM, Murphy JA, Mukherjee R, et al. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140:2116–25. doi: 10.1053/j.gastro.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 45.Rakonczay Z, Jr., Hegyi P, Takacs T, et al. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–67. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 46.Neuhofer P, Liang S, Einwachter H, et al. Deletion of IkappaBalpha Activates RelA to Reduce Acute Pancreatitis in Mice Through Up-regulation of Spi2A. Gastroenterology. 2012;144:192–201. doi: 10.1053/j.gastro.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 47.Huang H, Liu Y, Daniluk J, et al. Activation of Nuclear Factor-kappaB in Acinar Cells Increases the Severity of Pancreatitis in Mice. Gastroenterology. 2012;144:202–10. doi: 10.1053/j.gastro.2012.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 2011;141:358–69. doi: 10.1053/j.gastro.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 50.Kang R, Tang D, Schapiro NE, et al. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2013 doi: 10.1038/onc.2012.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang D, Kang R, Livesey KM, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 190:881–92. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.