Abstract
Study Objective
The objective of this study was to derive a clinical decision instrument with a sensitivity of at least 95% (with upper and lower bounds of the 95% CIs within a 5% range) to identify adult emergency department patients with mild traumatic intracranial hemorrhage (tICH) who are at low risk for requiring critical care resources during hospitalization and thus may not need admission to the ICU.
Methods
This was a prospective, observational study of adult patients with mild tICH (initial Glasgow Coma Scale [GCS] score 13 to 15 with tICH) presenting to a Level 1 trauma center from July 2009 to February 2013. The need for ICU admission was defined as the presence of an acute critical care intervention (intubation, neurosurgical intervention, blood product transfusion, vasopressor or inotrope administration, invasive monitoring for hemodynamic instability, emergent treatment for arrhythmia or cardiopulmonary resuscitation, therapeutic angiography). We derived the clinical decision instrument using binary recursive partitioning (with a misclassification cost of 20 to 1). The accuracy of the decision instrument was compared to the treating physician’s (emergency medicine faculty) clinical impression.
Results
A total of 600 patients with mild tICH were enrolled; 116 patients (19%) had a critical care intervention. The derived instrument consisted of four predictor variables: admission GCS score less than 15, non-isolated head injury, age 65 years or older, and evidence of swelling or shift on initial cranial computed tomography scan. The decision instrument identified 114 of 116 patients requiring an acute critical care intervention (sensitivity 98.3%; 95% CI 93.9–99.5%) if at least one variable was present, and 192 of 484 patients that did not have an acute critical care intervention (specificity 39.7%; 95% CI 35.4–44.1%) if no variables were present. Physician clinical impression was slightly less sensitive (90.1%; 95% CI 83.1–94.4%) but overall similar to the clinical decision instrument.
Conclusion
We derived a clinical decision instrument which identifies a subset of patients with mild tICH that are at low risk for acute critical care intervention and thus may not require ICU admission. Physician clinical impression had similar test characteristics as the decision instrument. Since the results are based on single center data without a validation cohort, external validation is required.
Introduction
Background
Patients with mild traumatic brain injury (Glasgow Coma Scale [GCS] score 13 to 15) with intracranial hemorrhage are often admitted to the intensive care unit (ICU) for early detection of secondary brain injury from cerebral edema, increased intracranial pressure, and cerebral ischemia.1 These secondary insults are the leading cause of inpatient death following traumatic intracranial hemorrhage (tICH).2 Most patients with mild tICH, however, do not develop hemorrhage progression or need acute neurosurgical intervention.3–5 While guidelines suggest the need for in-patient observation and serial neurological evaluations, there are no clear recommendations addressing the need for ICU admission in this group of patients.6–9 Moreover, there is wide variability of ICU use among trauma centers.10
Importance
With escalating health care costs, there is increasing demand to improve the efficiency and effectiveness of health care delivery. Particularly pertinent is the utilization of ICU resources, which is both costly (one-third of acute hospital charges) and limited (8% of hospital beds).11 Appropriate utilization of ICU resources is important to provide safe and efficient health care. ICU admission decisions are highly variable and subjective, often leading to inappropriate admissions to both the ICU and hospital ward. Patients mis-triaged to a non-ICU setting, who then decompensate and require in-hospital transfer to the ICU, have increased mortality.12–16 Patients initially admitted to the ICU but never requiring critical care resources are a poor utilization of health care resources. These inappropriate admissions lead to ICU and emergency department (ED) crowding, prolonged ED boarding times, and adverse patient outcomes.17,18
Goals of this Investigation
The objective of this study was to derive a clinical decision instrument to identify adult ED patients with mild tICH (defined as GCS score 13 to 15 with tICH) who are at low risk for requiring an acute critical care intervention. We hypothesized that a clinical decision instrument with a sensitivity of at least 95% (with upper and lower bounds of the 95% confidence intervals [CI] within a 5% range) can identify a group of patients with mild tICH that may be safely managed in a non-ICU setting. The accuracy of the decision instrument was compared to the treating physician’s (emergency medicine faculty) clinical impression.
METHODS
Study Design and Setting
We conducted a single-center, prospective cohort study of adult trauma patients who sustained mild tICH and were evaluated at a Level 1 trauma center from July 2009 to February 2013. The center has an annual ED census of 65,000 patients of which 4,700 are evaluated by the trauma service. The institutional review board at the study site approved the study.
Selection of Participants
We included consecutive patients 18 years of age and older with radiographic evidence of acute tICH on initial ED cranial computed tomography (CT) and an initial ED GCS score of 13 to 15. Radiographic evidence of tICH included subarachnoid hemorrhage, epidural hematoma, subdural hematoma, intraventricular hemorrhage, intraparenchymal hemorrhage/contusion, or diffuse axonal injury (with or without hemorrhage) on cranial CT scan as interpreted by the faculty neuroradiologist. We excluded patients with documented pre-existing “Do-Not-Resuscitate” (DNR) orders and patients with pre-injury anticoagulation use (at study site it is standard practice to reverse anticoagulated patients with tICH with blood products [critical care intervention]). At the study site, patients with mild tICH are routinely admitted under the trauma or neurological surgical service to the hospital for neurologic monitoring for a minimum of 24 hours following injury. Admission decisions at the study site are made collectively by input from EM, trauma surgery, and neurological surgery.
Study Protocol
We collected ED data using a standardized data collection form. Data were coded as present, unknown, or missing. Variables included age, sex, co-morbidities, pre-injury antiplatelet use (aspirin or clopidogrel), mechanism of injury, ED vital signs (initial and range) including systolic blood pressure, heart rate, respiratory rate, oxygen saturation, and the initial ED and admission GCS scores. ED laboratory variables collected included initial platelet count, international normalized ratio (INR) level, and hematocrit. We abstracted demographic (e.g., age, sex) and laboratory data from electronic medical records (EMR) while clinical data (e.g., GCS score, mechanism of injury) were obtained directly from the treating board-certified emergency medicine (EM) faculty physicians. We designed and modified the ED data collection form and survey using input from EM physicians and trauma surgeons. This data collection form was pilot tested and modified prior to the start of the study. All data collection was facilitated by research associates who are undergraduate students with specific research training for data collection. Research associates completed study specific training sessions every two months. Data abstractors were not blinded to study objectives.
Two trained research coordinators collected additional radiographic and hospital course variables. We coded radiograph and CT imaging into anatomical categorical variables based on attending radiologist text report. We also recorded hospital and ICU length of stay (days).
To describe injury severity, an Abbreviated Injury Score (AIS) for head and neck, face, chest, abdomen, extremities, and external body regions, and an Injury Severity Score (ISS) were calculated for each patient.19 The AIS and ISS are scoring systems developed to measure injury severity based on anatomical injuries divided by body regions.19 Injury severity calculations were conducted by research coordinators who received initial training and direct supervision for the first twenty patients by one of the study authors (DN).
To measure inter-observer reliability of the variables considered for inclusion into the clinical decision instrument, a second independent physician completed the same data form within one hour of the initial survey on a convenience sample of 6% of the patients. Raters were masked to each other’s results. To evaluate for enrollment bias, we compared demographic, injury severity, ED disposition, and outcomes of the enrolled patients to those eligible but not enrolled.
To assess clinician impression for the patient’s need for ICU level of care, we surveyed the treating EM faculty physician at the time of hospital admission. Survey questions included the physician impression that 1) the patient will need ICU level of care (yes/no), and 2) the patient will require an acute critical care intervention within 48 hours from hospital admission (categorized as < 1%, 1 to 5%, > 5% to 10%, > 10 to 20%, > 20% to 50%, and > 50%). Categories at lower levels of risk had a smaller range since we believed clinician threshold for admission to the ICU was generally at lower levels of risk.
Outcome Measures
We defined the need for ICU admission as the presence of an acute critical care intervention within 48 hours of ED arrival (Table 1). The list of critical care interventions was derived and modified from the Task Force of American College of Critical Care Medicine Guidelines for ICU admission.20 This list is based on a combination of prior literature and expert opinion. We chose a 48-hour endpoint as this was considered a reasonable timeframe to evaluate for neurological deterioration from a tICH. Prior studies suggest that critical care interventions after 48 hours were not due to neurological deterioration from tICH but rather other causes (e.g., intubation for hospital acquired infection).3,21 This time frame of 48 hours for neurological observation is also consistent with prior literature4,22 and published guidelines.6
Table 1.
List of critical care interventions
| Critical care intervention | Definition |
|---|---|
| Mechanical ventilation | Use of mechanical ventilation for acute respiratory failure |
| Neurosurgical intervention | Craniotomy/craniectomy, burr hole evaluation of hematoma, placement of a subdural drain, placement of an intracranial pressure monitor/intraventricular catheter/intracranial oxygen probe, or treatment with mannitol or hypertonic saline |
| Vasopressor or inotrope use | Use of dopamine, norepinephrine, epinephrine, dobutamine, phenylephrine, or vasopressin for hemodynamic instability |
| RBC transfusion | Transfusion of packed red blood cells |
| FFP transfusion | Transfusion of fresh frozen plasma |
| Invasive monitoring | Use of central venous catheter to measure central venous pressure (not for venous access alone), or the use an arterial line to measure blood pressure, or the use of a pulmonary artery catheter to measure pulmonary artery wedge pressure |
| Cardiac arrest or arrhythmia | Cardiac arrest requiring cardiopulmonary resuscitation or non-sinus arrhythmia less than 40 or greater than 120 beats/minute with the need for urgent intervention |
| Interventional angiography | Use of interventional angiography for therapeutic purposes |
Data Analysis
We entered data into a Microsoft Access 2003 database (Microsoft, Redmond, WA) and analyzed data using STATA 11.0 statistical software (STATA Corp, College Station, TX). We described the study population using descriptive statistics.
We derived the decision instrument with binary recursive partitioning using Classification and Regression Trees (CART) software (Salford Systems, San Diego, CA).23 We used the Ginni splitting function in CART and set the misclassification cost for missing a patient with a critical care intervention at 20:1. This represents the cost of misclassifying twenty patients who did not receive a critical care intervention for one patient who did receive a critical care intervention.
We considered variables for inclusion into the clinical decision instrument, based on prior literature and biological/physiological plausibility and determined clinically sensible cutoff values of predictor variables a priori. We considered the following variables for inclusion into the decision instrument: age 65 years or older, non-fall from standing mechanism of injury (fall from height, motor vehicle collision, pedestrian/bicyclist struck, direct blow to the head, other or unknown mechanism of injury), pre-injury antiplatelet use (aspirin or clopidogrel), the presence of any pre-defined high risk co-morbidity (atrial fibrillation or atrial flutter, bleeding disorder, congestive heart failure, coronary artery disease, end stage liver disease, pulmonary disease requring home oxygen, and end stage renal disease requiring dialysis) GCS score less than 15 at the time of admission, hypotension (systolic blood pressure less than 90 mmHg at any point in the ED), hypoxia (pulse oximetry reading less than 95% at any point in the ED), presence of intracranial swelling (cisterns are compressed or absent) or midline shift on initial cranial CT, presence of a depressed skull fracture, and non-isolated head injury. Our definition of non-isolated head injury was modified from the AIS severity scoring system (injuries associated with AIS scores 3 or greater) and included spinal injuries (any spinal vertebral fractures or cord injury), clinically important facial fractures (LeFort II or III fractures), serious thoracic injuries (≥ 2 rib fractures, pulmonary contusion, vascular injury, pneumothorax/hemothorax), intra-abdominal injuries (injuries to spleen, liver, urinary tract, gastrointestinal tract, pancreas, gallbladder, adrenal gland, intra-abdominal vascular structure, or traumatic fascial defect), any femur or pelvic fracture, and severe burns (>20% body surface area of 2nd or 3rd degree burns).19 Relative risk (RR) ratios were calculated for variables considered for inclusion in the clinical decision instrument.
To measure the accuracy of clinician impression that the patient would require ICU admission, we calculated sensitivity, specificity, positive and negative predictive values, and likelihood ratios based on the two by two table generated by treating EM faculty response to the clinical impression question, “Do you think this patient needs to be admitted to the ICU?”. We also calculated test characteristics based on the question, “What is the probability of the patient requiring an ICU intervention during the first 48 hours of hospitalization? Responses marked 1% or greater (or if the physician document the patient had already received a critical care intervention) were considered to require ICU admission by clinician suspicion and responses marked <1% were considered to not require ICU admission by clinician suspicion. In addition, we calculated the prevalence of acute critical care interventions for each of the different levels of risk as specified by the treating EM faculty.
We conducted a sensitivity analysis to evaluate the test characteristics (i.e., sensitivity and specificity) of the derived clinical decision instrument and the physician clinical impression to predict intubation or neurosurgical intervention within 48 hours of ED arrival since these two critical care interventions were most likely directly associated with brain injury.
Based on prior retrospective pilot data (estimated prevalence of critical care intervention of 20% and sensitivity of decision instrument of 98%), we estimated a sample size of approximately 500 patients to derive a clinical decision instrument with a sensitivity with sufficiently narrow confidence intervals (upper and lower bounds within a 5% range).3,24
Results
Characteristics of study subjects
A total of 600 patients with mild tICH on cranial CT were enrolled (Figure 1). The mean age was 52 years old (SD 22) and 425 were male (70.8%). Three patients (0.5%) died within the first 48 hours after ED triage. All patients were either admitted to the ICU (558, 93.0%) or the floor (42, 7.0%). The most common mechanisms of injury were fall from standing (197, 32.8%) and direct blow to the head (125, 20.8%). The majority of patients (406, 67.7%) had an initial ED GCS score of 15; 32 (5.3%) of patients had a GCS score of 13. The most common cranial CT findings were subdural hematoma (282, 47.0%), subarachnoid hemorrhage (275, 45.8%), and intraparenchymal hemorrhage/contusion (221, 36.8%). Table 2 provides complete patient characteristics. A total of 116 (19.3%) patients had an acute critical care intervention (195 interventions overall, median of one intervention) with RBC transfusion (55, 9.2%), mechanical ventilation (50, 8.3%), and neurosurgical intervention (39, 6.5%) the most common types of critical care interventions (Table 3).
Figure 1.
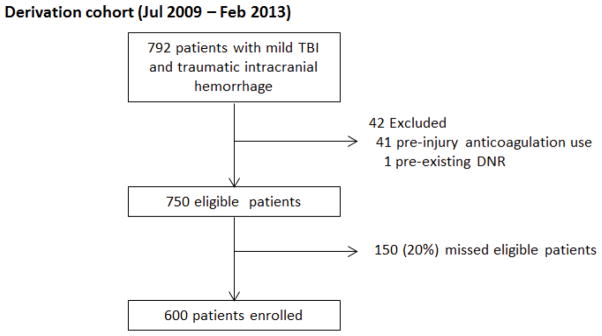
Study flowchart
Table 2.
Patient characteristics, n=600
| Characteristic | n (%) |
|---|---|
| Patient history | |
| Age, mean (SD) | 52 (22) |
| Male | 425 (70.8%) |
| History of antiplatelet use | 79 (13.2%) |
| History of arrhythmia | 17 (2.8%) |
| History of bleeding disorder | 0 (0%) |
| History of congestive heart failure | 19 (3.2%) |
| History of coronary artery disease | 56 (9.3%) |
| History of end stage liver disease | 4 (0.7%) |
| History of pulmonary disease requiring home oxygen | 2 (0.3%) |
| History of end stage renal disease | 8 (1.3%) |
| History of any comorbidity | 85 (14.2%) |
| Mechanism of injury | |
| Fall from standing height or less | 197 (32.8%) |
| Fall from greater than standing height | 68 (11.3%) |
| Motor vehicle collision | 118 (19.7%) |
| Pedestrian/bicyclist struck | 42 (7.0%) |
| Direct blow to the head | 125 (20.8%) |
| Unknown mechanism of injury | 19 (3.2%) |
| Other mechanism of injury | 31 (3.4%) |
| Initial vital signs/GCS/laboratory results | |
| Initial systolic blood pressure (mmHg), mean (SD) | 137 (SD 23) |
| Initial heart rate (beats per min), mean (SD) | 87 (SD 18) |
| Initial respiratory rate (breaths per min), mean (SD) | 18 (SD 4) |
| Initial ED GCS score, median (IQR) | 15 (IQR 14–15) |
| Initial platelet count (per ml), median (IQR) | 213 (IQR 177–257) |
| Initial INR, median (IQR) | 1.03 (IQR 0.97–1.08) |
| Initial hematocrit (%), median (IQR) | 40 (IQR 36–43) |
| Alcohol level 80–200 mg/dl | 59 (9.8%) |
| Alcohol level >200 mg/dl | 84 (14.0%) |
| Injury severity | |
| Initial ED GCS score 13 | 32 (5.3%) |
| Initial ED GCS score 14 | 162 (27.0%) |
| Initial ED GCS score 15 | 406 (67.7%) |
| Admission GCS score 15 | 396 (66.0%) |
| Isolated head injurya | 443 (73.7%) |
| Abbreviated injury score for head and neck, median (IQR) | 4 (IQR 3–4) |
| Injury severity score, median (IQR) | 16 (IQR 10–20) |
| Mortality at 48 hours | 3 (0.5%) |
| Cranial CT scan findings | |
| Depressed skull fracture | 20 (3.3%) |
| Nondepressed skull fracture | 99 (16.5%) |
| Intraparenchymal hemorrhage/contusion | 221 (36.8%) |
| Subdural hematoma | 282 (47.0%) |
| Epidural hematoma | 52 (8.7%) |
| Subarachnoid hemorrhage | 275 (45.8%) |
| Interventricular hemorrhage | 24 (4.0%) |
| Diffuse axonal injury | 0 (0%) |
| Presence of cerebral shift | 52 (8.7%) |
| Presence of cerebral mass effect | 47 (7.8%) |
| Presence of herniation | 2 (0.3%) |
| ED disposition and hospital length of stay | |
| Admission to the ICU | 558 (93.0%) |
| Admission to the floor | 42 (7.0%) |
| Hospital length of stay (days), median (IQR) | 3 (2–6) |
| ICU length of stay (days), median (IQR) | 1 (1–2) |
Abbreviations: SD, standard deviation; GCS, Glasgow Coma Scale; IQR, Interquartile range; INR; international normalized ratio; CT, computed tomography; ICU, intensive care unit
see text for definition
Table 3.
Critical care interventions, n=600
| Critical care interventiona | n (%) |
|---|---|
| RBC transfusion | 55 (9.2%) |
| Mechanical ventilation | 50 (8.3%) |
| Neurosurgical intervention | 39 (6.5%) |
| FFP transfusion | 30 (5.0%) |
| Invasive monitoring | 12 (2.0%) |
| Cardiac arrest or arrhythmias requiring treatment | 5 (0.8%) |
| Vasopressor or inotrope use | 2 (0.3%) |
| Interventional angiography | 2 (0.3%) |
Abbreviations: RBC, red blood cell; FFP, fresh frozen plasma
see Table 1 for definitions of critical care intervention
Comparison of patients enrolled and those eligible but not enrolled (20%) demonstrated similar patient characteristics (age, isolated head injury), injury severity (GCS score, AIS for head and neck, ISS, mortality at 48 hours), ED disposition, and outcome (acute critical care intervention) (eTable 1).
Main results
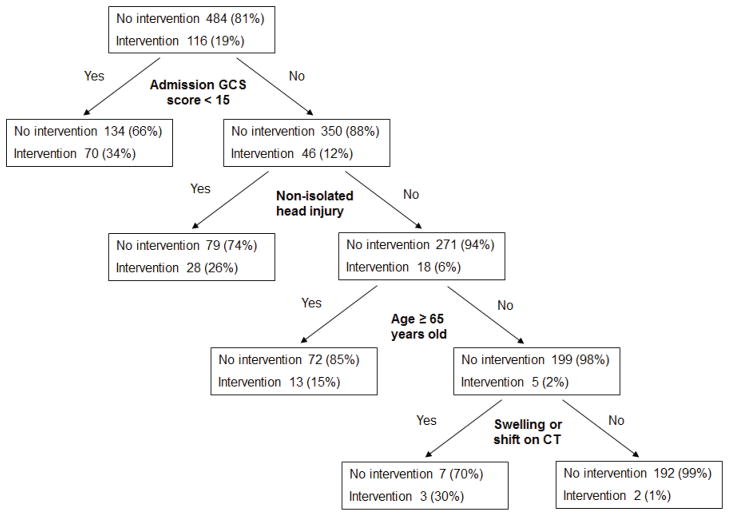
Clinical decision instrument
Binary recursive partitioning derived a decision instrument with the following four predictor variables for requiring an acute critical care intervention: admission GCS less than 15 (RR 2.95; 95% CI 2.12–4.12), non-isolated head injury (RR 2.74; 95% CI 1.99–3.78), age 65 years or older (RR 1.46; 95% CI 1.05–2.03), and the presence of swelling or shift on initial cranial CT (RR 4.11; 95% CI 3.08–5.48) (Figure 2 and eTable 2). The test characteristics of the clinical decision instrument for identifying patients requiring an acute critical care intervention is presented in Table 4. We measured inter-rater agreement of the clinical decision instrument variables in 37 patients (6.2%). All variables except for the presence of hypotension prior to admission had substantial agreement (eTable 3).25
Figure 2.
Derived model from binary recursive partitioning
Table 4.
Test performance of the clinical decision instrument to identify patients with an acute critical care intervention, n=600
| n | % (95% CI) | |
|---|---|---|
| Sensitivity | 114/116 | 98.3% (93.9–99.5%) |
| Specificity | 192/484 | 39.7% (35.4–44.1%) |
| Positive predictive value | 114/406 | 28.1% (23.9–32.6%) |
| Negative predictive value | 292/294 | 99.0% (96.3–99.7%) |
Abbreviations: CI, confidence interval
Two patients were misclassified by the decision instrument (determined to be low risk but had an acute critical care intervention) (Table 5). Neither patient required a neurosurgical intervention. One patient received a unit of fresh frozen plasma (FFP) and no other interventions and one patient was intubated as his actions were a danger to himself. Both patients, misclassified by the decision instrument, survived to hospital discharge.
Table 5.
Patients misclassified as low risk by the clinical decision instrument (n=600)
| Age, gender | Mechanism | Cranial CT findings | Critical care intervention (CCI) | Time to CCI after ED triage | Comments | LOS in days (ICU, hospital) |
|---|---|---|---|---|---|---|
| 55, M | Fall from bicycle | Small IPH | FFP transfusion (1 unit) | 4 hours | Pre-injury clopidogrel use, also transfused one unit of platelets, INR 1.07 | 5, 5 |
| 62, M | Pedestrian struck | SDH | Intubation | 4 hours | Patient was a danger to himself in the ED, refusing to wear a cervical collar and walking around. Intubated for patient safety. Extubated five hours after intubation. | 11, 15 |
Abbreviations: CT, computed tomography; ED, emergency department; LOS, length of stay; ICU, intensive care unit; IPH, intraparenchymal hemorrhage; MVC, motor vehicle collision; SDH, subdural hematoma; FFP, fresh frozen plasma; INR, international normalized ratio
Physician clinical impression
Compared to the clinical decision instrument, physician clinical impression had a slightly lower sensitivity (Table 6). Based on the question, “Do you think this patient needs to be admitted to the ICU?”, eleven patients were not identified by physician clinical impression (eTable 4). The prevalence of acute critical care interventions was roughly similar to physician clinical impression, with higher prevalence rates seen in higher levels of risk (eFigure).
Table 6.
Test characteristics of physician clinical impression compared to the clinical decision instrument based on the outcome of acute critical care interventiona
| Clinician | Sensitivity | Specificity | LR+ | LR− |
|---|---|---|---|---|
| Clinical decision instrument | 114/116, 98.3% (93.9–99.5%) | 192/484, 39.7% (35.4–44.1%) | 1.63 (1.51–1.76) | 0.04 (0.01–0.17) |
| “Do you think this patient needs to be admitted to the ICU?”b | 100/111, 90.1% (83.1–94.4%) | 224/455, 49.2% (44.7–53.8%) | 1.78 (1.59–1.98) | 0.20 (0.11–0.36) |
| “In your best estimation, what is the probability of the patient requiring an ICU intervention during the first 48 hours of this patient’s hospitalization?”c | 101/111, 91.0% (84.2–95.0%) | 179/453, 39.5% (35.1–44.1%) | 1.50 (1.37–1.65) | 0.23 (0.13–0.42) |
Abbreviations: LR, likelihood ratio
The treating EM faculty was surveyed at the time of admission to evaluate clinical impression (566 of 600 patients).
Responses were reported on 566 patients. Responses marked “yes” were considered to require ICU admission and responses marked “no” were considered not to require ICU admission.
Responses were reported on 564 patients. The responses were categorized as: <1%, 1–5%, >5–10%, >10–50%, >50%, and already received a critical care intervention. Responses marked 1% or greater (or the patient already received a critical care intervention) were considered to require ICU admission and responses marked <1% were considered to not require ICU admission.
Sensitivity analysis based on the outcome of intubation or neurological intervention demonstrated similar test characteristics of the clinical decision instrument (sensitivity 98.6%, specificity 36.6%) and physician clinical impression (sensitivity 93.0%, specificity 46.5%) (eTable 5).
Limitations
This study should be interpreted in the context of several limitations. It was conducted at a single, Level 1 trauma center where severity of injury, hospital resources, and management of mild tICH might not be generalizable to other settings. The clinical decision instrument and clinician impression should be externally evaluated in a different patient population to ensure generalizability of the rule.24 At the study site, the large majority of patients were admitted to the ICU and thus we are unable to appropriately evaluate patients with mild tICH that were managed in a non-ICU setting. Moreover, ICU admission may have unexpected advantages (i.e., more frequent nursing care) that prevented a critical care intervention from occurring and thus would not be identified in our study. Since we did not collect data on individual physicians, we were unable to adjust the results of physician clinical impression for clustering by provider. We determined the presence of tICH based on the faculty neuroradiologist cranial CT interpretation. CT scan interpretations that diagnosed very minor tICH however, may be subjective (i.e., read as negative for tICH by another radiologist).
Discussion
In this study we derived a clinical decision instrument to identify a subset of patients with mild tICH who are at low risk for requiring an acute critical care intervention. These low-risk patients represented 31% of all ICU admissions among the study cohort, suggesting the potential for the decision instrument to substantially improve ICU resource utilization.
An important component in clinical decision instrument development is comparison of the instrument to physician clinical impression.24 In this study, physician clinical impression performed similarly to the clinical decision instrument, though slightly less sensitive and more specific. This implies our clinical decision instrument may not have an advantage over ED faculty clinical impression in identifying low risk patients with tICH who do not need ICU admission.
Physician clinical impression, however, was inconsistent with actual admission practice. The large majority of patients (85%) considered by treating physicians to not need an ICU admission, were nonetheless admitted to the ICU. This suggests ICU admission in the setting of mild tICH may depend on a number of factors independent of clinical suspicion, such as local policy and practice, hospital resources, patient and family preferences, and legal liability.18,26 Given the complexity of factors involved in ICU admission decisions in adult patients with mild tICH, the potential role of this clinical decision instrument may be to objectively reaffirm clinical judgment or as an aid to be used in conjunction with clinical judgment.
Prior studies have evaluated baseline characteristics to predict outcome in traumatic patients with tICH.27–29 Trauma scoring systems including the ISS 30 and the Revised Trauma Score (RTS)31 predict mortality and other functional outcomes32 but are not designed as an ICU triage tool as they are calculated after all injuries are identified and the patient is discharged from the hospital. A number of other prediction models specific to traumatic brain injury exist, however the methodology is limited, and they are not clinically practical.32
Some authors have suggested that patients with a GCS score of 13 be excluded from the “mild” category due to their increased risk for clinically important intracranial injuries.33 We included patients with an initial ED GCS score of 13 to 15 since some of these patients with an initial ED GCS score of 13 or 14 may improve during their ED course. Patients with improving GCS scores tend have better outcomes compared to patients with GCS scores that remain low33 In our study, 24% (46 of 194) of patients with an initial ED GCS score less than 15, improved to a GCS score of 15 at the time of admission.
These four variables (non-isolated head injury, admission GCS less than 15, age 65 years or older, and the presence of swelling or shift on initial cranial CT) are known to be prognostic in patients with tICH,32,34–37 but they have not been formally studied to predict the need for ICU admission. Only a single study attempts to predict the need for specialized ICU admission in patients with tICH using variables prior to disposition decision-making.28 In that study, patient age and pupillary reactivity were significant predictors for the need of ICU admission, however, the authors were unable to develop a sufficiently accurate prediction model. This study, however, only evaluated patients with GCS scores less than 13 and had a high threshold for defining the need for ICU admission (as their outcome variable was raised intracranial pressure and neurosurgical intervention). Compared to these prior trauma scoring systems and prediction models, our decision instrument focuses on patients with mild tICH in the ED and uses data readily available to clinicians at the time of decision-making.
Our decision instrument failed to identify two patients who ultimately underwent a critical care intervention, a finding potentially causing concern among some clinicians. Closer inspection of these cases, however, indicates that the failure of the clinical decision instrument to identify these patients would likely have little impact on the patients’ actual outcomes. One patient received one unit of FFP that was likely of limited clinical importance and probably not recommended as the patient’s INR was normal. The other patient was endotracheally intubated in the ED as he was a danger to himself and combative. He was extubated five hours after intubation. On review of the chart, the patient did not appear to have neurological deterioration and did not require any neurosurgical interventions.
The list of critical care interventions was adapted from prior published guidelines that broadly defined disease and physiological conditions that warrant ICU admission.20 This list was narrowed to represent critical care interventions specific for the injured patient. While most critical care interventions are universally equated to requiring ICU admission (i.e., mechanical ventilation, neurosurgical intervention, invasive monitoring) certain interventions (specifically RBC and FFP transfusion) may not require ICU admission. We opted to include RBC and FFP transfusion as an outcome measure to ensure a conservative model. However due to the lack of clear guidelines for platelet transfusion we did not include it as a critical care intervention.38 A sensitivity analysis evaluating the derived clinical decision instrument and the physician clinical impression to predict intubation or neurosurgical intervention (critical care interventions most likely directly associated with brain injury) demonstrated similar test characteristics to the primary analysis.
In conclusion, we were able to derive a clinical decision instrument which accurately identifies a subset of patients with mild tICH that are at low risk for acute critical care intervention and thus may not require ICU admission. Physician clinical impression had similar test characteristics as the decision instrument. Since the results are based on single center data without a validation cohort, external validation is required.
Supplementary Material
Acknowledgments
DN was supported through a Mentored Clinical Research Training Program Award (Grant Number UL1TR000002 and linked award KL2TR000134) from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. NCATS and NIH had no role in the design and conduct of the study, in the analysis or interpretation of the data, or in the preparation of the data. The views expressed in this article are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or the NIH. Information on NCATS is available at http://www.ncats.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
Conflict of interest:
None
Author Contributions:
All authors helped conceive, design, and supervise the study. DN, MS, JH, JG, and JL conducted data collection. DN and JH analyzed the data. DN drafted the manuscript and all authors contributed to its revision. DN takes responsibility of the paper as a whole.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham DI, Ford I, Adams JH, et al. Ischaemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry. 1989;52(3):346–50. doi: 10.1136/jnnp.52.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall LF, Klauber MR. The outcome of severe closed head injury. J Neurosurg. 1991;75:S28–36. [Google Scholar]
- 3.Nishijima DK, Shahlaie K, Echeverri A, Holmes JF. A clinical decision rule to predict adult patients with traumatic intracranial haemorrhage who do not require intensive care unit admission. Injury. 2012;43(11):1827–1832. doi: 10.1016/j.injury.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huynh T, Jacobs DG, Dix S, Sing RF, Miles WS, Thomason MH. Utility of neurosurgical consultation for mild traumatic brain injury. Am Surg. 2006;72(12):1162–5. [PubMed] [Google Scholar]
- 5.Sifri ZC, Livingston DH, Lavery RF, Homnick AT, Mosenthal AC, Mohr AM, et al. Value of repeat cranial computed axial tomography scanning in patients with minimal head injury. Am J Surg. 2004;187(3):338–42. doi: 10.1016/j.amjsurg.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Vos PE, Battistin L, Birbamer G, Gerstenbrand F, Potapov A, Prevec T, et al. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9(3):207–19. doi: 10.1046/j.1468-1331.2002.00407.x. [DOI] [PubMed] [Google Scholar]
- 7.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58(3 Suppl):S25–46. doi: 10.1227/01.NEU.0000210365.36914.E3. [DOI] [PubMed] [Google Scholar]
- 8.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(3 Suppl):S16–24. [PubMed] [Google Scholar]
- 9.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute epidural hematomas. Neurosurgery. 2006;58(3 Suppl):S7–15. [PubMed] [Google Scholar]
- 10.Nishijima DK, Haukoos JS, Newgard CD, Staudenmayer K, White N, Slattery D, et al. Variability of ICU use in adult patients with minor traumatic intracranial hemorrhage. Ann Emerg Med. 2013;61(5):509–17. doi: 10.1016/j.annemergmed.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groeger JS, Guntupalli KK, Strosberg M, et al. Descriptive analysis of critical care units in the United States: patient characteristics and intensive care unit utilization. Crit Care Med. 1993;21(2):279–91. doi: 10.1097/00003246-199302000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Frost SA, Alexandrou E, Bogdanovski T, Salamonson Y, Parr MJ, Hillman KM. Unplanned admission to intensive care after emergency hospitalisation: risk factors and development of a nomogram for individualising risk. Resuscitation. 2009;80(2):224–30. doi: 10.1016/j.resuscitation.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Escarce JJ, Kelley MA. Admission source to the medical intensive care unit predicts hospital death independent of APACHE II score. JAMA. 1990;264(18):2389–94. [PubMed] [Google Scholar]
- 14.Sax FL, Charlson ME. Medical patients at high risk for catastrophic deterioration. Crit Care Med. 1987;15(5):510–5. doi: 10.1097/00003246-198705000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Goldhill DR, Sumner A. Outcome of intensive care patients in a group of British intensive care units. Crit Care Med. 1998;26(8):1337–45. doi: 10.1097/00003246-199808000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Hillman KM, Bristow PJ, Chey T, et al. Duration of life-threatening antecedents prior to intensive care admission. Intensive Care Med. 2002;28(11):1629–34. doi: 10.1007/s00134-002-1496-y. [DOI] [PubMed] [Google Scholar]
- 17.Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35(6):1477–83. doi: 10.1097/01.CCM.0000266585.74905.5A. [DOI] [PubMed] [Google Scholar]
- 18.Escher M, Perneger TV, Chevrolet JC. National questionnaire survey on what influences doctors’ decisions about admission to intensive care. BMJ. 2004;329(7463):425. doi: 10.1136/bmj.329.7463.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copes WS, Champion HR, Sacco WJ, Lawnick MM, Keast SL, Bain LW. The Injury Severity Score revisited. J Trauma. 1988;28(1):69–77. doi: 10.1097/00005373-198801000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27(3):633–8. [PubMed] [Google Scholar]
- 21.Nishijima DK, Sena MJ, Holmes JF. Identification of low-risk patients with traumatic brain injury and intracranial hemorrhage who do not need intensive care unit admission. J Trauma. 2011;70(6):E101–7. doi: 10.1097/TA.0b013e3181e88bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang MC, Linnau KF, Tirschwell DL, Hollingworth W. Utility of repeat head computed tomography after blunt head trauma: a systematic review. J Trauma. 2006;61(1):226–33. doi: 10.1097/01.ta.0000197385.18452.89. [DOI] [PubMed] [Google Scholar]
- 23.Brieman LFJ, Olshen RA. Classification and Regression Trees. Washington, DC: Chapman & Hall; 1984. [Google Scholar]
- 24.Stiell IG, Wells GA. Methodologic standards for the development of clinical decision rules in emergency medicine. Ann Emerg Med. 1999;33(4):437–47. doi: 10.1016/s0196-0644(99)70309-4. [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 26.Truog RD, Brock DW, Cook DJ, et al. Rationing in the intensive care unit. Crit Care Med. 2006;34(4):958–63. doi: 10.1097/01.CCM.0000206116.10417.D9. [DOI] [PubMed] [Google Scholar]
- 27.Andrews PJ, Sleeman DH, Statham PF, et al. Predicting recovery in patients suffering from traumatic brain injury by using admission variables and physiological data: a comparison between decision tree analysis and logistic regression. J Neurosurg. 2002;97(2):326–36. doi: 10.3171/jns.2002.97.2.0326. [DOI] [PubMed] [Google Scholar]
- 28.Hukkelhoven CW, Steyerberg EW, Habbema JD, Maas AI. Admission of patients with severe and moderate traumatic brain injury to specialized ICU facilities: a search for triage criteria. Intensive Care Med. 2005;31(6):799–806. doi: 10.1007/s00134-005-2628-y. [DOI] [PubMed] [Google Scholar]
- 29.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):329–37. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 30.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–96. [PubMed] [Google Scholar]
- 31.Champion HR, Sacco WJ, Copes WS, Gann DS, Gennarelli TA, Flanagan ME. A revision of the Trauma Score. J Trauma. 1989;29(5):623–9. doi: 10.1097/00005373-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Perel P, Edwards P, Wentz R, Roberts I. Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak. 2006;6:38. doi: 10.1186/1472-6947-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagoda AS, Bazarian JJ, Bruns JJ, Jr, et al. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med Dec. 2008;52(6):714–748. doi: 10.1016/j.annemergmed.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Brown AW, Malec JF, McClelland RL, Diehl NN, Englander J, Cifu DX. Clinical elements that predict outcome after traumatic brain injury: a prospective multicenter recursive partitioning (decision-tree) analysis. J Neurotrauma. 2005;22(10):1040–51. doi: 10.1089/neu.2005.22.1040. [DOI] [PubMed] [Google Scholar]
- 35.Demetriades D, Kuncir E, Murray J, Velmahos GC, Rhee P, Chan L. Mortality prediction of head Abbreviated Injury Score and Glasgow Coma Scale: analysis of 7,764 head injuries. J Am Coll Surg. 2004;199(2):216–22. doi: 10.1016/j.jamcollsurg.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Hukkelhoven CW, Steyerberg EW, Habbema JD, et al. Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. J Neurotrauma. 2005;22(10):1025–39. doi: 10.1089/neu.2005.22.1025. [DOI] [PubMed] [Google Scholar]
- 37.Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336(7641):425–9. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishijima DK, Zehtabchi S, Berrong J, Legome E. Utility of platelet transfusion in adult patients with traumatic intracranial hemorrhage and preinjury antiplatelet use: a systematic review. J Trauma Acute Care Surg. 2012;72(6):1658–63. doi: 10.1097/TA.0b013e318256dfc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.